Published online Aug 15, 2021. doi: 10.4251/wjgo.v13.i8.893
Peer-review started: May 20, 2021
First decision: June 12, 2021
Revised: June 17, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 15, 2021
Processing time: 86 Days and 2.6 Hours
Esophageal squamous cell carcinoma (ESCC) is one of the most common ma
To investigate the antitumor effect of DHTS in ESCC and the underlying mecha
CCK-8 assay and cell cycle analysis were used to detect proliferation and cell cycle in ESCC cells. Annexin V-PE/7-AAD double staining assay and Hoechst 33258 staining were used to detect apoptosis in ESCC cells. Western blot was used to detect the expression of proteins associated with the mitochondrial pathway. Immunofluorescence was used to detect the expression of phosphorylated STAT3 (pSTAT3) in DHTS-treated ESCC cells. ESCC cells with STAT3 knockdown and overexpression were constructed to verify the role of STAT3 in DHTS induced apoptosis. A xenograft tumor model in nude mice was used to evaluate the antitumor effect of DHTS in vivo.
After treatment with DHTS, the proliferation of ESCC cells was inhibited in a dose- and time-dependent manner. Moreover, DHTS induced cell cycle arrest in the G0/1 phase. Annexin V-PE/7-AAD double staining assay and Hoechst 33258 staining revealed that DHTS induced obvious apoptosis in KYSE30 and Eca109 cells. At the molecular level, DHTS treatment reduced the expression of pSTAT3 and anti-apoptotic proteins, while increasing the expression of pro-apoptotic proteins in ESCC cells. STAT3 knockdown in ESCC cells markedly promoted the activation of the mitochondrial pathway while STAT3 overexpression blocked the activation of the mitochondrial pathway. Additionally, DHTS inhibited tumor cell proliferation and induced apoptosis in a xenograft tumor mouse model.
DHTS exerts antitumor effect in ESCC via STAT3-mediated activation of the mitochondrial pathway. DHTS may be a novel therapeutic agent for ESCC.
Core Tip: Esophageal squamous cell carcinoma (ESCC) is one of the most common malignancies with a poor prognosis. Our research found that dihydrotanshinone I (DHTS) suppresses proliferation and induces apoptosis in ESCC cells. Besides, STAT3-mediated activation of the mitochondrial pathway plays a vital role in DHTS-induced apoptosis. DHTS may be a promising candidate for the treatment of ESCC.
- Citation: Qi MM, He PZ, Zhang L, Dong WG. STAT3-mediated activation of mitochondrial pathway contributes to antitumor effect of dihydrotanshinone I in esophageal squamous cell carcinoma cells. World J Gastrointest Oncol 2021; 13(8): 893-914
- URL: https://www.wjgnet.com/1948-5204/full/v13/i8/893.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i8.893
Esophageal cancer (EC) is one of the most common malignancies, ranking seventh in terms of incidence and sixth in terms of mortality worldwide. Esophageal squamous cell carcinoma (ESCC) is the main histologic subtype of EC in parts of Asia and Sub-Saharan Africa, and accounts for over 90% of all EC cases[1]. Many patients with ESCC are diagnosed in the advanced stage of disease and have a great need for chemothe
Studies have shown that traditional Chinese medicine has good preventive or therapeutic effect for various malignant tumors[4]. Dihydrotanshinone I (DHTS) is a natural component isolated from the roots of a well-known traditional Chinese me
STAT3 is reported to be associated with cell proliferation, migration, and apoptosis, and is aberrantly hyperactivated in many types of cancer[13-15]. The STAT3 cascade is associated with the promotion of tumor growth and immunosuppression[16]. In the inactive state, STAT3 exists as a monomer found in the cytoplasm. Once activated, the phosphorylation of Tyr705 results in dimerization of the STAT3 protein and translocation of the STAT3 dimer to the nucleus. In the nucleus, phosphorylated STAT3 (pSTAT3) may mediate the induction of key target genes that regulate cellular proliferation and survival, suppress apoptosis, and promote angiogenesis, invasiveness, and/or metastasis[17,18]. It has been proven that STAT3 is involved in the progression of many kinds of tumors, and treatments that target STAT3 promise therapeutic bene
In this study, we treated ESCC cells with DHTS to investigate whether DHTS could exert antitumor effect in ESCC cells, as well as the possible molecular mechanism involved, trying to provide a new strategy for the treatment of ESCC.
The human ESCC cell lines KYSE30, Eca109, KYSE450, and KYSE510 and human normal esophageal epithelial cell line Het-1A were donated by the China Center for Type Culture Collection. The ESCC cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, United States) with 10% fetal bovine serum (FBS) (Gibco, United States) while Het-1A cells in Dulbecco’s modified Eagle medium (DMEM) (Gibco, United States) with 10% FBS in a humidified incubator at 37 °C containing 5% CO2. DHTS (Sigma-Aldrich, United States) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, United States) at a stock concentration of 10 mmol/L and was diluted with RPMI-1640 medium or DMEM medium to perform assays. As a result, the final concentration of DMSO in the medium was no more than 10 μL/mL in all assays and would not show obvious toxicity to cells.
Cells were seeded in triplicate in 96-well plates at a density of 8000 cells per well. After overnight incubation, the cells were exposed to different concentrations of DHTS for 12 h, 24 h, or 36 h. Cell viability was assessed using a Cell Counting Kit-8 (CCK-8, Beyotime, China) according to the manufacturer’s protocol. The absorbance at 450 nm was measured using a microplate spectrophotometer (Victor3 1420 Multilabel Coun
Cells were seeded at a density of 5 × 105 per well in six-well plates and incubated at 37 °C overnight. After culturing in RPMI 1640 medium without fetal bovine serum for 24 h, the cells were cultured in RPMI 1640 medium with 10% FBS and treated with DHTS for 24 h and then collected after trypsinization and fixed in ice-cold 75% ethanol overnight at 4 °C. The cells were then washed with PBS and incubated with PI (50 μg/mL, Antgene, China) and RNAse (Yeasen, China) for 30 min. Cell cycle distribution was detected using the BD TM LSRΙΙ flow cytometer (BD Biosciences, San Jose, CA, United States). The resultant cell cycle profiles were analyzed using ModFit software (Verity Software House, Topshem, ME, United States).
The annexin V-PE/7-AAD kit (BD Pharmingen, United States) was used to quantify the percentage of apoptotic cells using flow cytometry (FACS Calibur, Becton Dic
Cells were seeded into a six-well plate at a density of 2 × 105 per well and incubated at 37 °C overnight. The cells were then treated with different concentrations of DHTS for 24 h. Cells were then fixed with 4% paraformaldehyde for 15 min at room temperature and then washed twice with PBS. After that, the cells were stained with 50 ng/mL Hoechst 33258 (Beyotime, China) for 10 min. Apoptotic morphological features (chro
pLVX-Puro-STAT3-shRNA- and pLVX-Puro-STAT3-expressing lentiviruses were designed and provided by Genomeditech (Shanghai, China). KYSE30 cells and Eca109 cells were transfected with the lentiviral vectors, while control cells were transfected with empty vectors according to the manufacturer’s instructions. To establish stable cell lines, 2 μg/mL puromycin dihydrochloride (Beyotime, China) was used daily for 1 wk after transfection with lentiviral vectors. Knockdown and overexpression effici
Total RNA was extracted using TRIzol Reagent (Invitrogen, United States) as descri
ESCC cells treated with DHTS were used to isolate proteins. The proteins were se
KYSE30 and Eca109 cells were seeded onto glass coverslips in a 24-well plate over
All animal research procedures were approved by the Ethics Committee of Renmin Hospital of Wuhan University. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised in 1978). Male BALB/c nude mice (5 wk old) were purchased from Beijing Life River Experimental Animal Technology Co. Ltd. (China). The collected KYSE30 cells (1 × 106) were washed in serum-free RPMI 1640, suspended in 200 μL of PBS, and subcutaneously implanted into the right flank in the dorsal region of nude mice. When the longest diameter of the tumor was about 5 mm, nude mice were randomly divided into the following four groups (n = 6 each group): Control (normal saline), 5 mg/kg DHTS, 10 mg/kg DHTS, and 15 mg/kg DHTS. Mice were injected intraperitoneally once every other day. There was no significant di
Xenograft tumors from vehicle-treated and DHTS-treated mice were fixed in 10% neutral buffered formalin, embedded in paraffin, and used to prepare 4 μm-thick sections. Apoptotic cells were detected using a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay performed with an in situ apoptosis detection kit (Roche Applied Science, Basel, Switzerland). An optical microscope (Olympus, BX51) was used to observe the specimens. Positive cells were identified, counted, and analyzed.
Immunohistochemistry staining was performed using an UltraSensitive TM SP kit and DAB kit (China Fuzhou Maixin Biotechnology) according to the manufacturer’s ins
SPSS software, version 25.0, was used for all analyses. Data are expressed as the mean ± SD. Analysis of variance was used to analyze differences between groups. A P value of less than 0.05 was considered statistically significant.
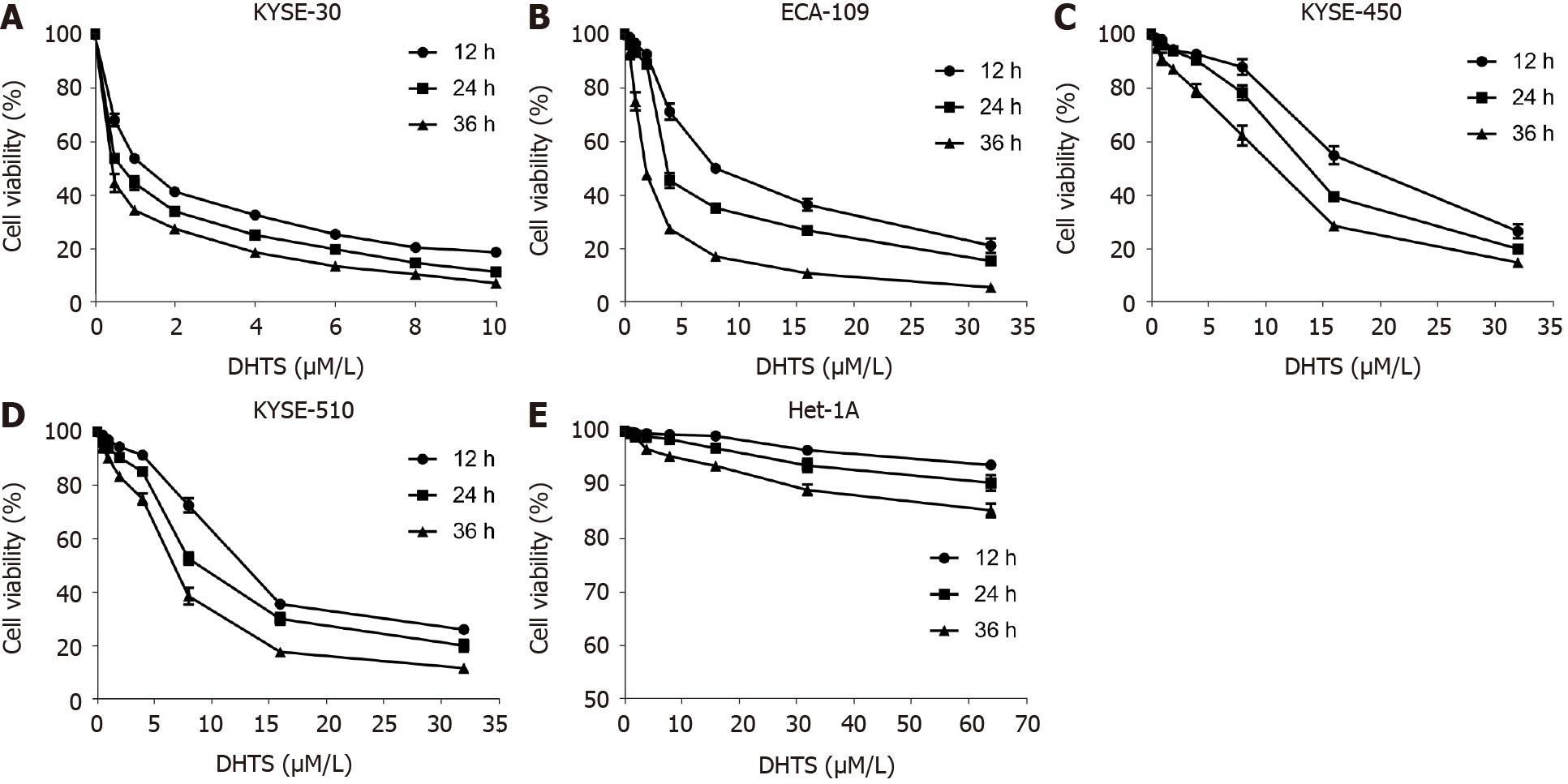
To identify whether DHTS exerts antitumor effect in ESCC cells, CCK8 was used to detect the cell viability of ESCC cells. After treatment with different concentrations of DHTS for 12 h, 24 h, or 36 h, the proliferation of ESCC cells was inhibited in a dose- and time-dependent manner (Figure 1A-D). After treatment with DHTS for 24 h, the IC50 of DHTS in KYSE30, Eca109, KYSE450, and KYSE510 cells was 0.697 (95% confi
| Time of treatment | IC50 (μmol/L) | 95%CI (μmol/L) | |
| KYSE30 | 12 h | 1.313 | 0.962-1.675 |
| 24 h | 0.697 | 0.438-0.961 | |
| 36 h | 0.407 | 0.219-0.607 | |
| Eca109 | 12 h | 9.750 | 8.363-11.499 |
| 24 h | 5.895 | 3.766-9.633 | |
| 36 h | 2.279 | 1.700-2.988 | |
| KYSE450 | 12 h | 18.890 | 13.967-28.640 |
| 24 h | 13.878 | 10.287-20.148 | |
| 36 h | 9.196 | 6.849-12.921 | |
| KYSE510 | 12 h | 13.962 | 12.063-16.416 |
| 24 h | 9.943 | 7.675-13.496 | |
| 36 h | 6.325 | 4.831-8.423 | |
| Het-1A | 12 h | 778.956 | 216.388-175793.136 |
| 24 h | 758.751 | 238.424-20168.744 | |
| 36 h | 632.324 | 227.536-6313.869 |
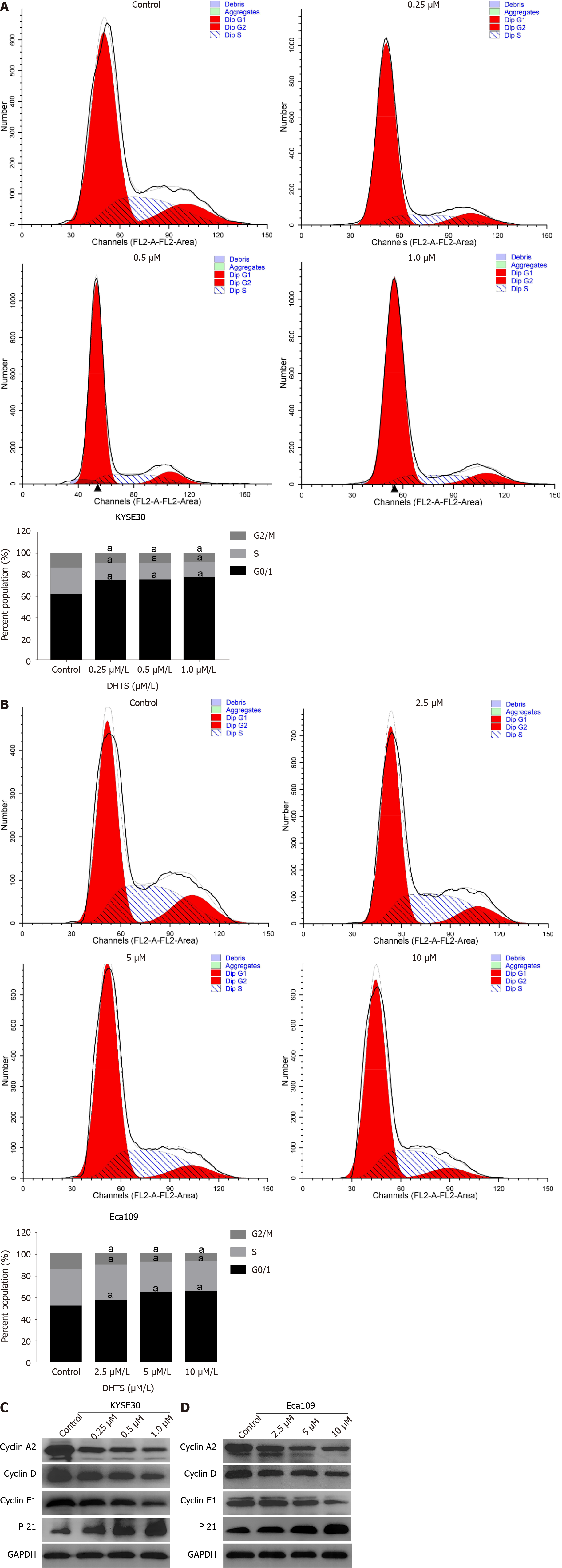
To decipher the mechanisms underlying DHTS-mediated inhibition of proliferation, we analyzed the cell cycle distribution using flow cytometry. KYSE30 cells were treated with 0 μmol/L, 0.25 μmol/L, 0.5 μmol/L, or 1.0 μmol/L DHTS, while Eca109 cells were treated with 0 μmol/L, 2.5 μmol/L, 5 μmol/L, or 10 μmol/L DHTS. Follo
Western blot analysis revealed that DHTS treatment significantly regulated the expression of proteins associated with the cell cycle. Cyclin A2, cyclin E1, and cyclin D1, which are involved in the transition from G1 to S phase, decreased significantly with an increase in DHTS concentration (Figure 2C and D) (P < 0.05). Meanwhile, the expression of P21 increased after DHTS treatment (Figure 2C and D) (P < 0.05). These data indicated that the number of cells in the G0/1 phase increased greatly while the number of cells in the S and G2/M phases decreased significantly after DHTS treat
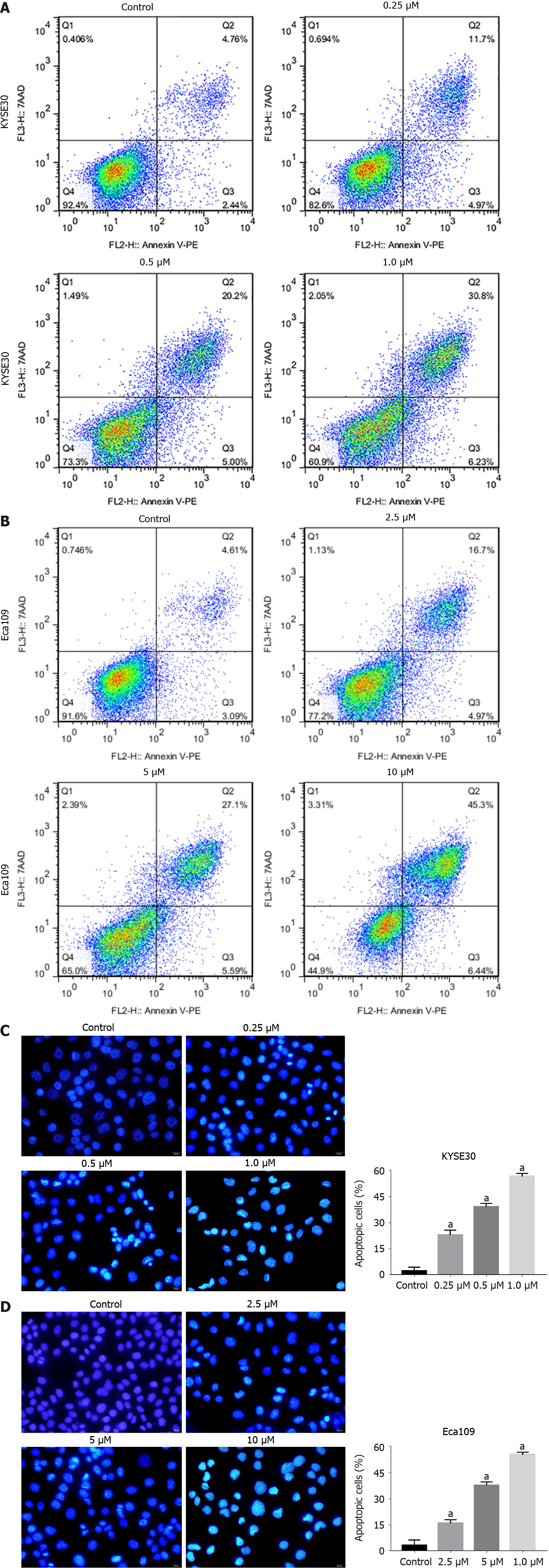
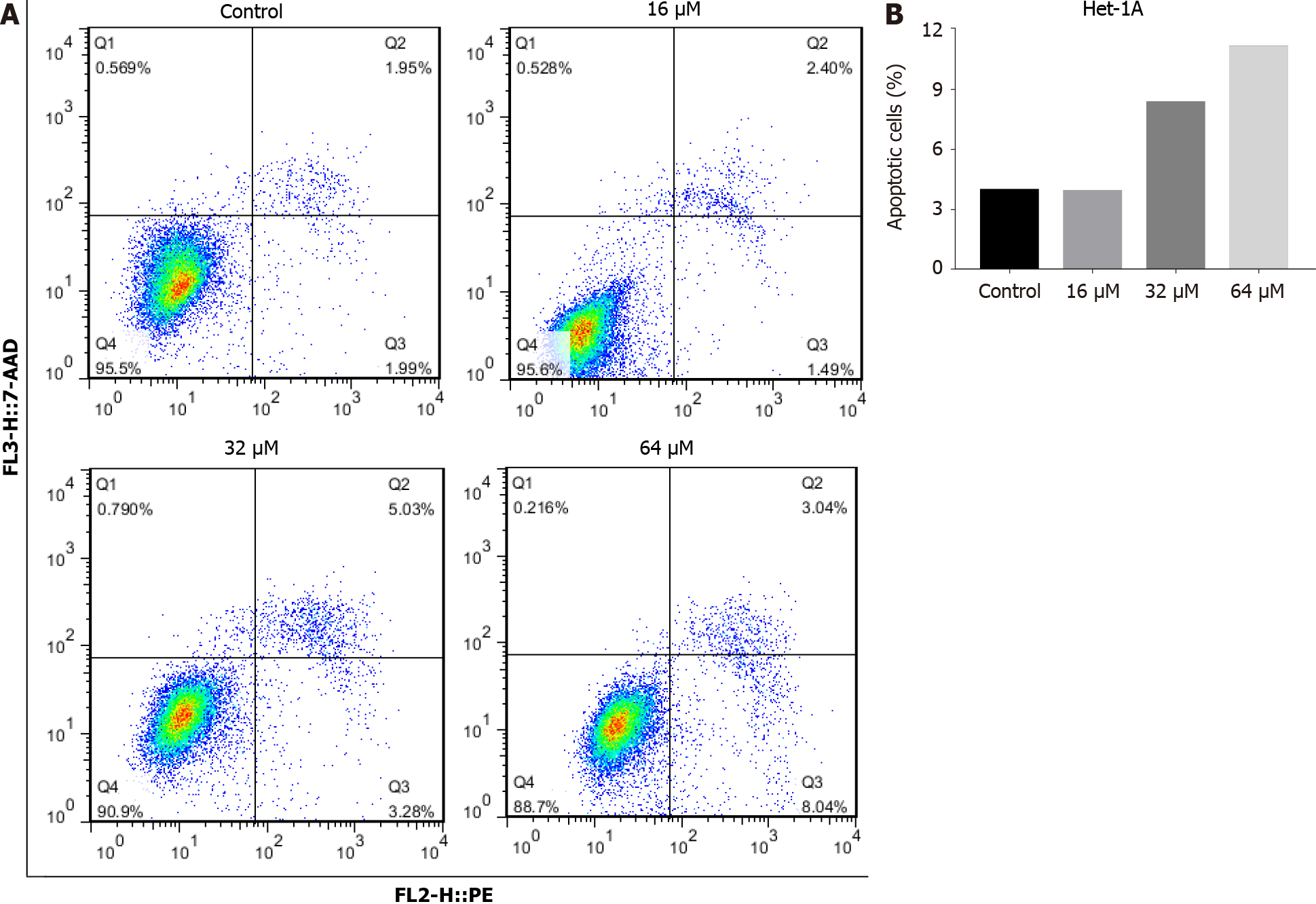
To detect whether DHTS induces apoptosis in ESCC cells, annexin PE/7-AAD staining was used to detect cell apoptosis. The proportion of early and late apoptotic cells increased significantly for DHTS-treated KYSE30 cells and Eca109 cells with the in
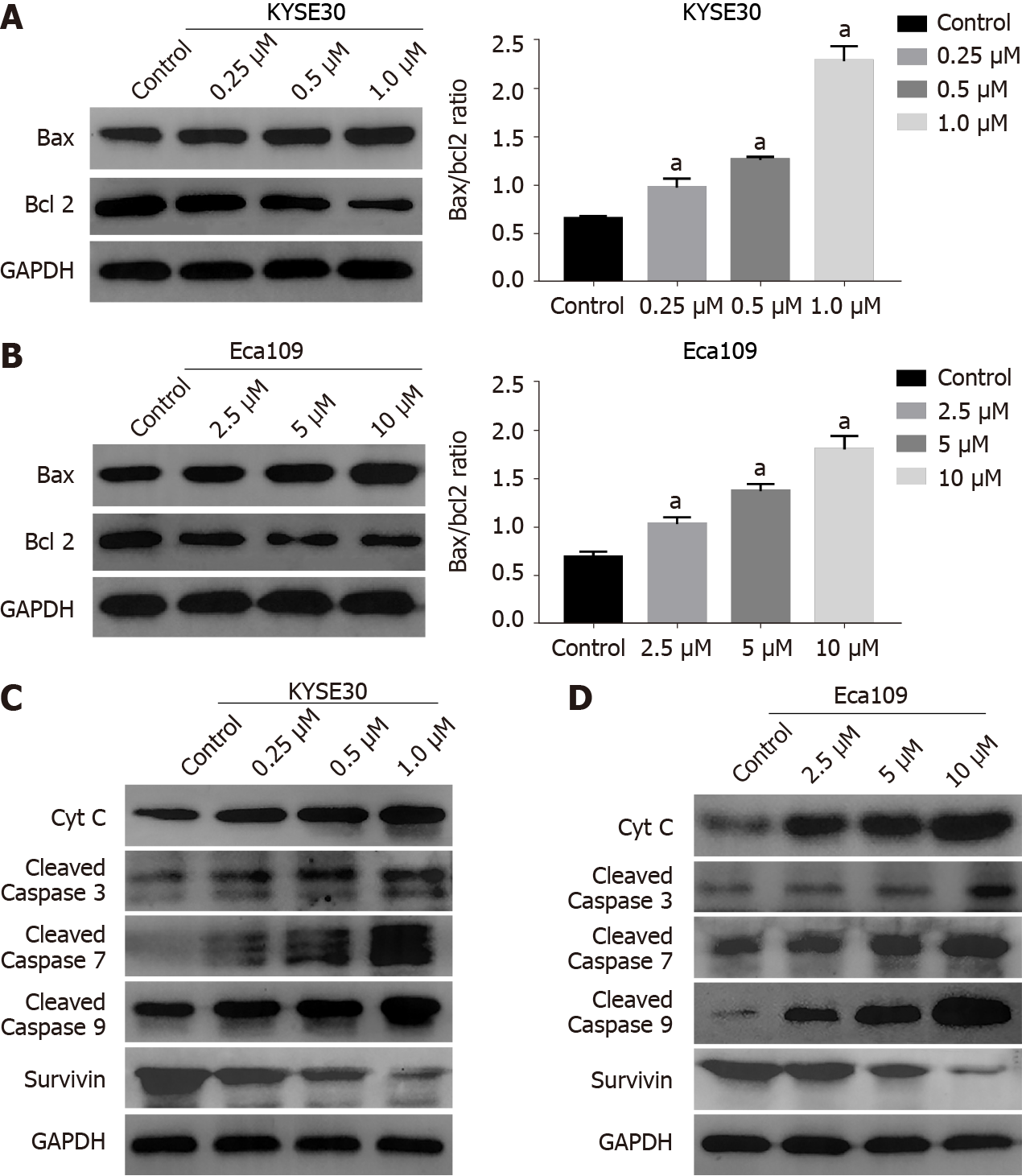
The mitochondrial pathway plays an important role in the apoptosis of tumor cells[20]. To test whether DHTS exerts antitumor effect through the mitochondrial path
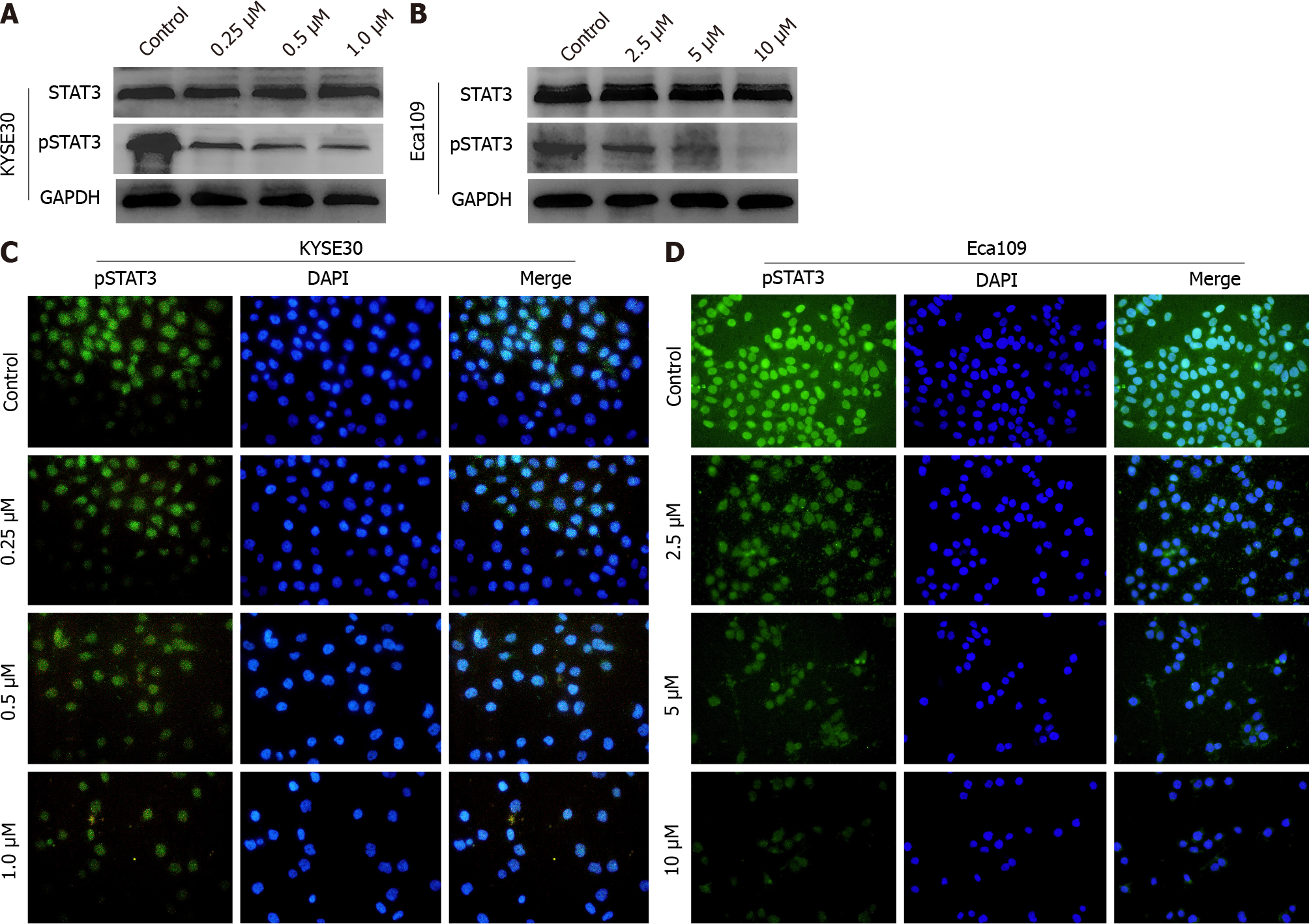
Previous studies have demonstrated that STAT3 plays an essential role in the proliferation of ESCC cells[21]. Hence, we speculated that DHTS may induce apoptosis through STAT3 in ESCC cells. After DHTS treatment for 24 h, the protein expression of STAT3 in both KYSE30 and Eca109 cells did not change significantly, but the ex
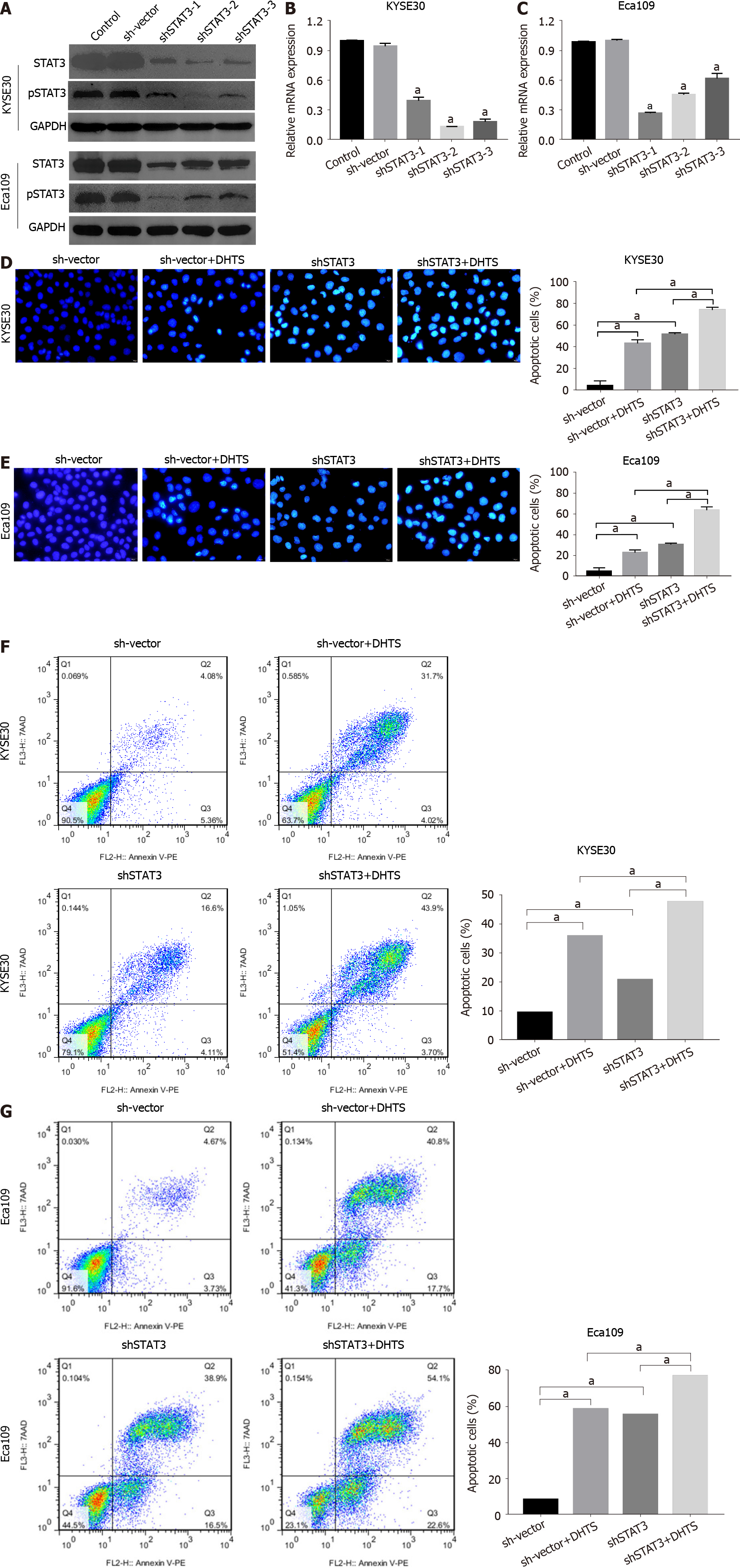
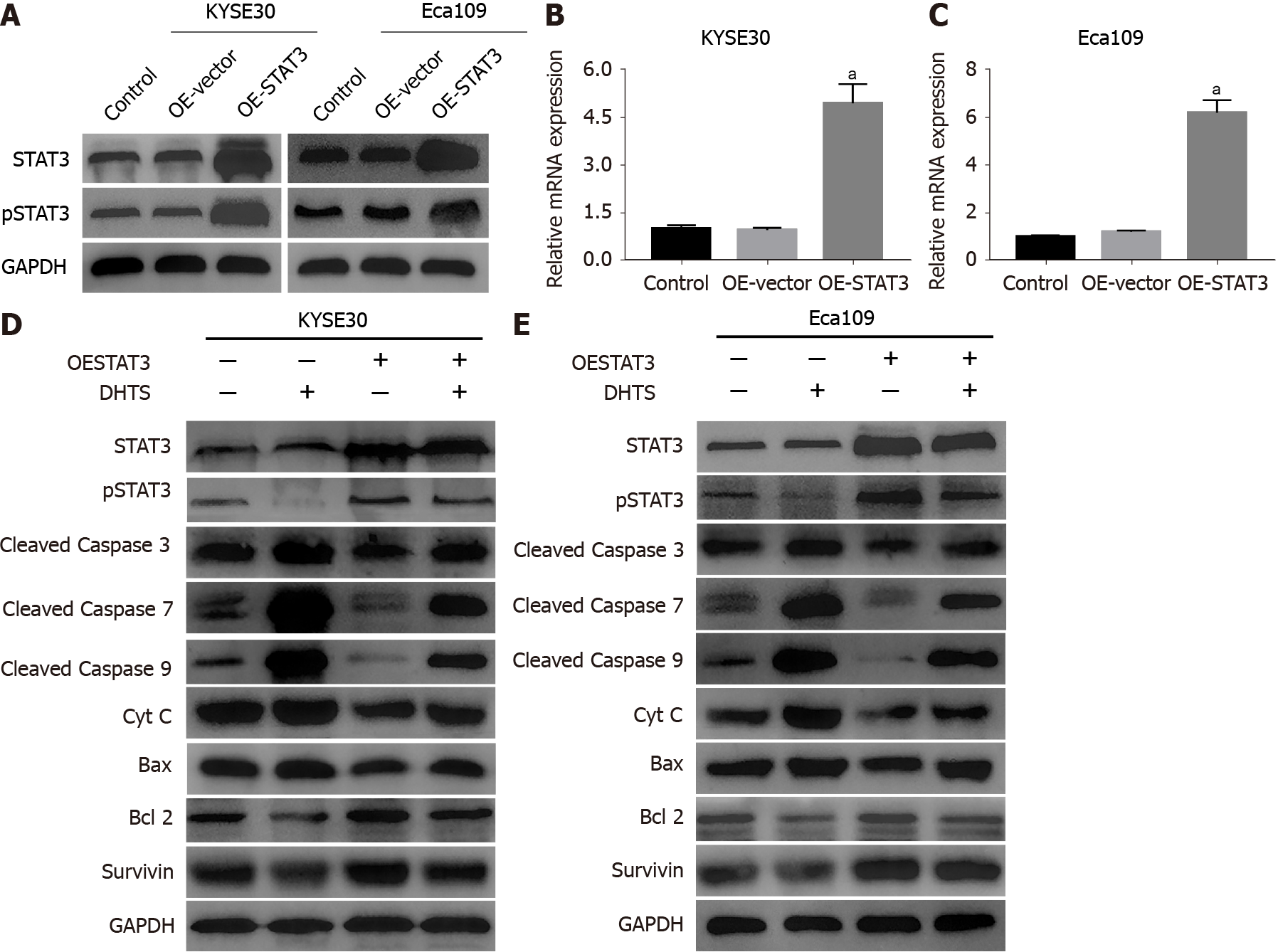
To clarify the role of STAT3 in DHTS-induced apoptosis, we constructed STAT3-knockdown ESCC cell lines. Western blot and RT-qPCR were used to detect the efficiency of STAT3 knockdown (Figure 7A-C). Western blot analysis showed that the level of pSTAT3 was consistent with that of STAT3 (Figure 7A). To verify the role of STAT3 in ESCC cells, cell lines with the best STAT3 knockdown efficiency were used in the following experiments. Hoechst 33258 staining showed that STAT3 knockdown not only induced apoptosis but also showed synergy with DHTS in both KYSE30 and Eca109 cells; STAT3 knockdown and DHTS treatment showed a significant pro-apoptotic effect (Figure 7D and E). Annexin PE/7-AAD staining showed the same results (Figure 7F and G). In addition, the expression proteins associated with the mitochondrial pathway were detected in STAT3-knockdown ESCC cells. In both KYSE30 and Eca109 cells, STAT3 knockdown increased the expression of pro-apop
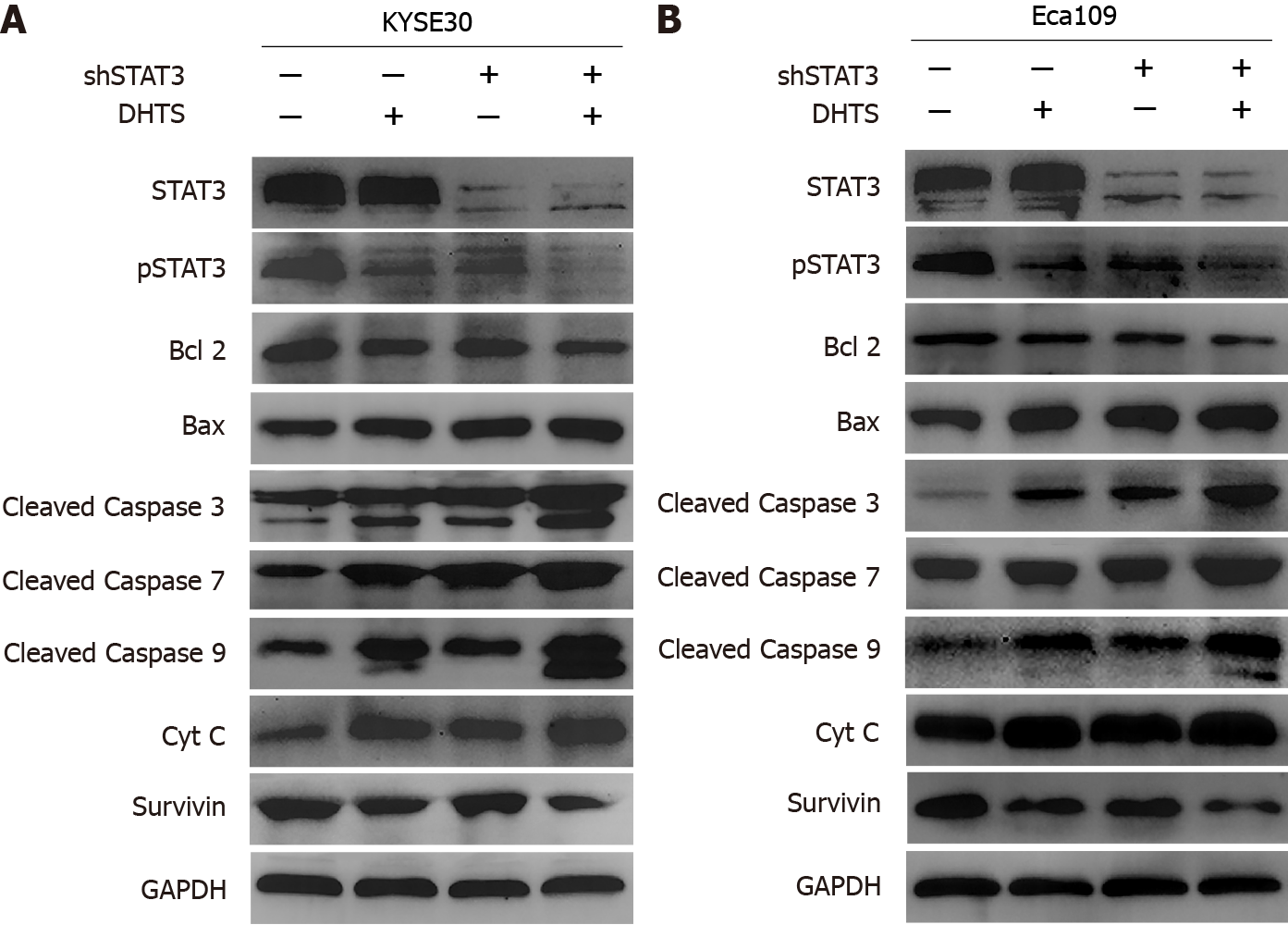
Although STAT3 knockdown promotes the activation of the mitochondrial pathway, we still cannot determine whether DHTS exerts antitumor effect through STAT3 for other molecules that may play a role in the process. To prove that STAT3 is involved in DHTS-induced apoptosis, STAT3-overexpressing ESCC cell lines were used to detect apoptosis-associated proteins. STAT3 overexpression efficiency was verified by Wes
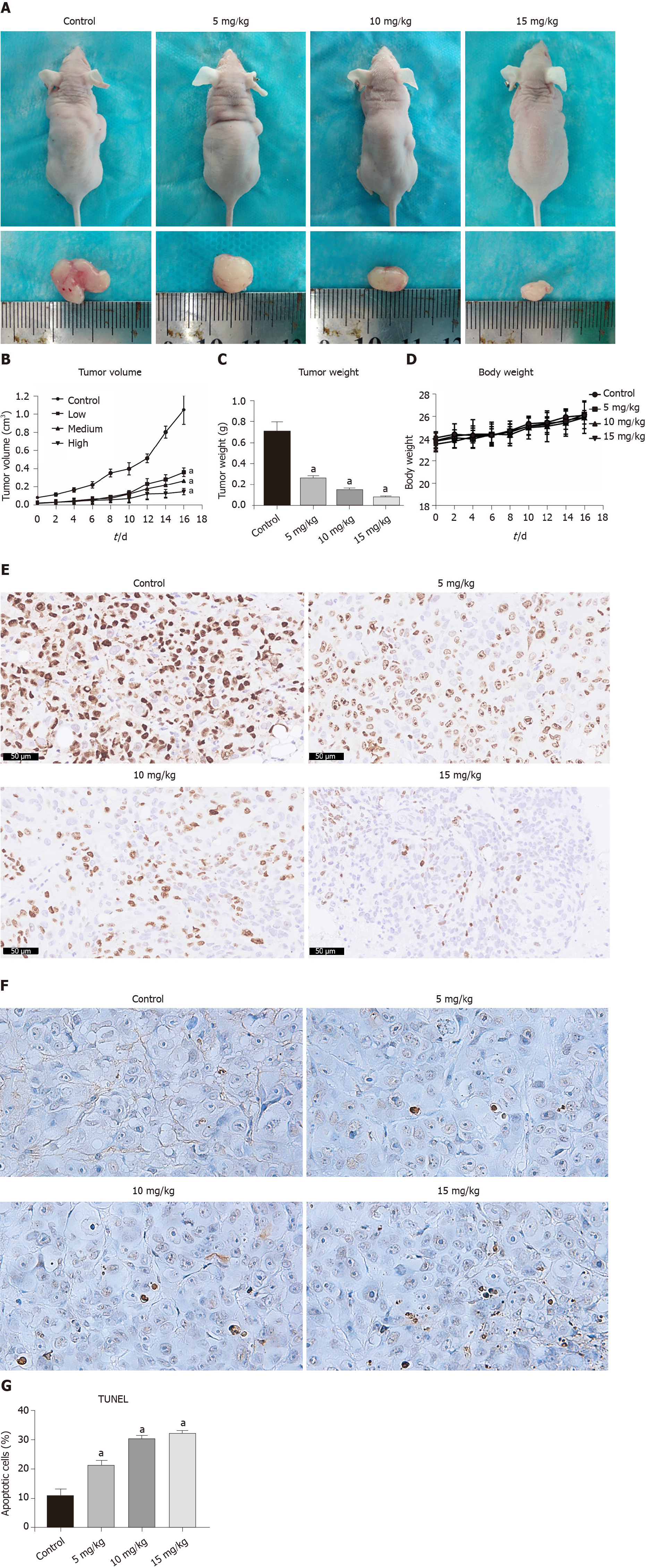
Based on the data in vitro above, we further investigated the antitumor effect of DHTS on xenograft tumors in vivo. The transplanted tumors grew rapidly in the control group (1.047 ± 0.143 cm3) but were suppressed in the treatment group in a dose-dependent manner (0.364 ± 0.042 cm3 in the 5 mg/kg group, 0.270 ± 0.012 cm3 in the 10 mg/kg group, and 0.150 ± 0.030 cm3 in the 15 mg/kg group) (Figure 10A and B) (P < 0.05). The weight of tumors in the DHTS treatment groups also decreased in a dose-dependent manner (Figure 10C) (P < 0.05), while the body weight of mice in the four groups showed no difference during the treatment of DHTS (Figure 10D). Immunohistochemistry staining of tumors showed that the expression of Ki67 decreased signi
ESCC is one of the most common cancers in south African countries and some Asian countries[22]. Although early detection and treatment have been gradually popula
DHTS, a natural lipophilic constituent, has been shown to exert antitumor effect in several cancers without obvious toxicity[10,11]. In the present study, we demonstrated that DHTS significantly inhibited the proliferation of ESCC cells and induced cell cycle arrest in the G0/1 phase in a dose-dependent manner. We further demonstrated that DHTS induced apoptosis via STAT3-mediated activation of the mitochondrial apop
Induction of cell cycle arrest has been proposed as an effective therapeutic approach for cancers[26,27]. Progression through G1 and entry into S-phase is tightly regulated by cyclin A/cyclin E–CDK2 complexes[28,29]. Cyclin D1 is also required for progre
As a programmed form of cell death, apoptosis plays a pivotal role in cancer and therapies inducing cancer cell apoptosis have been proven to be effective strategies[32]. Apoptosis is induced through two main routes involving either the intrinsic mitochondrial pathway or the activation of death receptors (the extrinsic pathway)[33]. In the current study, DHTS treatment increased the expression of Bax while decreasing the levels of Bcl2. The Bax/Bcl2 ratio plays a vital role in determining mitochondrial membrane permeability and activation, which mediates the cell apop
STAT3 has been reported as a key regulator of cell proliferation, survival, and apoptosis and is constitutively activated in many cancers[13,42,43]. It is a cytoplasmic transcription factor characterized by six functionally conserved domains, including the amino-terminal domain, the coiled-coil domain, the DNA-binding domain, the linker domain, the SRC homology 2 domain, and the carboxyl-terminal transactivation domain[44]. STAT3 maintains an inactive state in the cytoplasm in an unstimulated cell while becomes activated mainly by direct phosphorylation when there is stimulus. The phosphorylation induces dimerization of pSTAT3 and translocation of pSTAT3 dimer into the nucleus, and eventually execution of their nuclear functions[45]. Under physiological conditions, transient activation of STAT3 mediates cell proliferation, differentiation, apoptosis, survival, immune function, angiogenesis, and other impor
In summary, our data experimentally showed that DHTS has potent antitumor activity against ESCC by reducing cell proliferation and inducing apoptosis in vitro and in vivo. We further identified that STAT3-mediated activation of the mitochondrial pathway contributes to the antitumor effect of DHTS in ESCC cells. Overall, our results strongly suggest that DHTS is a promising candidate for the treatment of ESCC, which may build a foundation for the clinical trials of this novel therapeutic agent.
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignancies with a poor prognosis. Dihydrotanshinone I (DHTS) has been reported to exert anti
This study investigated the role of DHTS in ESCC and the underlying mechanisms, and explored novel therapeutic agents for ESCC.
The aim of this study was to investigate the antitumor effect of DHTS in ESCC and the underlying mechanisms.
CCK-8 assay was used to detect proliferation and cell cycle analysis was used to detect cell cycle in ESCC cells. Annexin V-PE/7-AAD double staining assay and Hoechst 33258 staining were used to detect apoptosis. Western blot was used to detect the expression of proteins associated with proliferation and the mitochondrial pathway. Immunofluorescence was used to detect the expression of phosphorylated STAT3 (pSTAT3) in DHTS-treated ESCC cells. ESCC cells with STAT3 knockdown and over
After treatment with DHTS, the proliferation of ESCC cells was inhibited in a dose- and time-dependent manner. Moreover, DHTS induced cell cycle arrest in the G0/1 phase. Annexin V-PE/7-AAD double staining assay and Hoechst 33258 staining revealed that DHTS induced obvious apoptosis in KYSE30 and Eca109 cells. At the molecular level, DHTS treatment reduced the expression of pSTAT3 and anti-apop
DHTS exerts antitumor effect in ESCC via STAT3-mediated activation of the mito
In the future, additional research will be carried out to further explore the important role of DHTS and whether DHTS treatment can be employed to improve the prognosis of ESCC patients.
We are grateful to Mrs. Ming-Xia Fan from the Animal Laboratory Center and Mrs. Qiong Ding from the Central Laboratory of Renmin Hospital of Wuhan University for their support.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caba O S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55762] [Article Influence: 7966.0] [Reference Citation Analysis (132)] |
| 2. | Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 790] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 3. | Hou S, Jin W, Xiao W, Deng B, Wu D, Zhi J, Wu K, Cao X, Chen S, Ding Y, Shi H. Integrin α5 promotes migration and cisplatin resistance in esophageal squamous cell carcinoma cells. Am J Cancer Res. 2019;9:2774-2788. [PubMed] |
| 4. | Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, Dai Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121:109570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 5. | Li Y, Hou Z, Su F, Chen J, Zhang X, Xu L, Yang D, Liang Z. Quantitative Determination and Validation of Four Ketones in Salvia miltiorrhiza Bunge Using Quantitative Proton Nuclear Magnetic Resonance Spectroscopy. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Chen X, Yu J, Zhong B, Lu J, Lu JJ, Li S, Lu Y. Pharmacological activities of dihydrotanshinone I, a natural product from Salvia miltiorrhiza Bunge. Pharmacol Res. 2019;145:104254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Jiang L, Zeng H, Ni L, Qi L, Xu Y, Xia L, Yu Y, Liu B, Yang H, Hao H, Li P. HIF-1α Preconditioning Potentiates Antioxidant Activity in Ischemic Injury: The Role of Sequential Administration of Dihydrotanshinone I and Protocatechuic Aldehyde in Cardioprotection. Antioxid Redox Signal. 2019;31:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Yuan R, Huang L, Du LJ, Feng JF, Li J, Luo YY, Xu QM, Yang SL, Gao H, Feng YL. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization. Pharmacol Res. 2019;142:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Zhang XZ, Qian SS, Zhang YJ, Wang RQ. Salvia miltiorrhiza: A source for anti-Alzheimer's disease drugs. Pharm Biol. 2016;54:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Kim SL, Choi HS, Kim JH, Jeong DK, Kim KS, Lee DS. Dihydrotanshinone-Induced NOX5 Activation Inhibits Breast Cancer Stem Cell through the ROS/Stat3 Signaling Pathway. Oxid Med Cell Longev. 2019;2019:9296439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Tan T, Chen J, Hu Y, Wang N, Chen Y, Yu T, Lin D, Yang S, Luo J, Luo X. Dihydrotanshinone I inhibits the growth of osteosarcoma through the Wnt/β-catenin signaling pathway. Onco Targets Ther. 2019;12:5111-5122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Cai Y, Lv F, Kaldybayeva N, Zhamilya A, Wu Z, Wu Y. 15, 16-Dihydrotanshinone I Inhibits Hemangiomas through Inducing Pro-apoptotic and Anti-angiogenic Mechanisms in Vitro and in Vivo. Front Pharmacol. 2018;9:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Laudisi F, Cherubini F, Di Grazia A, Dinallo V, Di Fusco D, Franzè E, Ortenzi A, Salvatori I, Scaricamazza S, Monteleone I, Sakamoto N, Monteleone G, Stolfi C. Progranulin sustains STAT3 hyper-activation and oncogenic function in colorectal cancer cells. Mol Oncol. 2019;13:2142-2159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Yang Y, Fang E, Luo J, Wu H, Jiang Y, Liu Y, Tong S, Wang Z, Zhou R, Tong Q. The Antioxidant Alpha-Lipoic Acid Inhibits Proliferation and Invasion of Human Gastric Cancer Cells via Suppression of STAT3-Mediated MUC4 Gene Expression. Oxid Med Cell Longev. 2019;2019:3643715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Sen M, Kindsfather A, Danilova L, Zhang F, Colombo R, LaPorte MG, Kurland BF, Huryn DM, Wipf P, Herman JG. PTPRT epigenetic silencing defines lung cancer with STAT3 activation and can direct STAT3 targeted therapies. Epigenetics. 2020;15:604-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 3389] [Article Influence: 211.8] [Reference Citation Analysis (0)] |
| 17. | Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1813] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 18. | Bournazou E, Bromberg J. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT. 2013;2:e23828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 19. | Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 2086] [Article Influence: 298.0] [Reference Citation Analysis (0)] |
| 20. | Kühn B, Brat C, Fettel J, Hellmuth N, Maucher IV, Bulut U, Hock KJ, Grimmer J, Manolikakes G, Rühl M, Kühn A, Zacharowski K, Matrone C, Urbschat A, Roos J, Steinhilber D, Maier TJ. Anti-inflammatory nitro-fatty acids suppress tumor growth by triggering mitochondrial dysfunction and activation of the intrinsic apoptotic pathway in colorectal cancer cells. Biochem Pharmacol. 2018;155:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Zhi Y, Song H, Zong M, Yi J, Mao G, Chen L, Huang G. S1PR1 promotes proliferation and inhibits apoptosis of esophageal squamous cell carcinoma through activating STAT3 pathway. J Exp Clin Cancer Res. 2019;38:369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11915] [Article Influence: 2978.8] [Reference Citation Analysis (4)] |
| 23. | Nakajo K, Abe S, Oda I, Ishihara R, Tanaka M, Yoshio T, Katada C, Yano T. Impact of the Charlson Comorbidity Index on the treatment strategy and survival in elderly patients after non-curative endoscopic submucosal dissection for esophageal squamous cell carcinoma: a multicenter retrospective study. J Gastroenterol. 2019;54:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Lin YH, Ou CY, Lee WT, Lee Y-, Chang T-, Yen YT. Treatment outcomes for one-stage concurrent surgical resection and reconstruction of synchronous esophageal and head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2019;276:2929-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Zhao W, Li C, Gao H, Wu Q, Shi J, Chen X. Dihydrotanshinone I Attenuates Atherosclerosis in ApoE-Deficient Mice: Role of NOX4/NF-κB Mediated Lectin-Like Oxidized LDL Receptor-1 (LOX-1) of the Endothelium. Front Pharmacol. 2016;7:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Wang L, Hu T, Shen J, Zhang L, Chan RL, Lu L, Li M, Cho CH, Wu WK. Dihydrotanshinone I induced apoptosis and autophagy through caspase dependent pathway in colon cancer. Phytomedicine. 2015;22:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Luo J, Meng X, Su J, Ma H, Wang W, Fang L, Zheng H, Qin Y, Chen T. Biotin-Modified Polylactic- co-Glycolic Acid Nanoparticles with Improved Antiproliferative Activity of 15,16-Dihydrotanshinone I in Human Cervical Cancer Cells. J Agric Food Chem. 2018;66:9219-9230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999;19:6183-6194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 228] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Lacy S, Whyte P. Identification of a p130 domain mediating interactions with cyclin A/cdk 2 and cyclin E/cdk 2 complexes. Oncogene. 1997;14:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | E Hermosilla V, Salgado G, Riffo E, Escobar D, Hepp MI, Farkas C, Galindo M, Morín V, García-Robles MA, Castro AF, Pincheira R. SALL2 represses cyclins D1 and E1 expression and restrains G1/S cell cycle transition and cancer-related phenotypes. Mol Oncol. 2018;12:1026-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Mansilla SF, de la Vega MB, Calzetta NL, Siri SO, Gottifredi V. CDK-Independent and PCNA-Dependent Functions of p21 in DNA Replication. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 32. | Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016;8:603-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 607] [Cited by in RCA: 1114] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 33. | Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1570] [Cited by in RCA: 1438] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 34. | Tan C, Dlugosz PJ, Peng J, Zhang Z, Lapolla SM, Plafker SM, Andrews DW, Lin J. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem. 2006;281:14764-14775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Yan X, Jiang Z, Bi L, Yang Y, Chen W. Salvianolic acid A attenuates TNF-α- and D-GalN-induced ER stress-mediated and mitochondrial-dependent apoptosis by modulating Bax/Bcl-2 ratio and calcium release in hepatocyte LO2 cells. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:817-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Dorstyn L, Akey CW, Kumar S. New insights into apoptosome structure and function. Cell Death Differ. 2018;25:1194-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 37. | Eldering E, Mackus WJ, Derks IA, Evers LM, Beuling E, Teeling P, Lens SM, van Oers MH, van Lier RA. Apoptosis via the B cell antigen receptor requires Bax translocation and involves mitochondrial depolarization, cytochrome C release, and caspase-9 activation. Eur J Immunol. 2004;34:1950-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Wang Q, Zhang L, Yuan X, Ou Y, Zhu X, Cheng Z, Zhang P, Wu X, Meng Y. The Relationship between the Bcl-2/Bax Proteins and the Mitochondria-Mediated Apoptosis Pathway in the Differentiation of Adipose-Derived Stromal Cells into Neurons. PLoS One. 2016;11:e0163327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Germain M, Affar EB, D'Amours D, Dixit VM, Salvesen GS, Poirier GG. Cleavage of automodified poly(ADP-ribose) polymerase during apoptosis. Evidence for involvement of caspase-7. J Biol Chem. 1999;274:28379-28384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 361] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 40. | Nassar A, Lawson D, Cotsonis G, Cohen C. Survivin and caspase-3 expression in breast cancer: correlation with prognostic parameters, proliferation, angiogenesis, and outcome. Appl Immunohistochem Mol Morphol. 2008;16:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Mikami D, Kobayashi M, Uwada J, Yazawa T, Kamiyama K, Nishimori K, Nishikawa Y, Nishikawa S, Yokoi S, Taniguchi T, Iwano M. β-Hydroxybutyrate enhances the cytotoxic effect of cisplatin via the inhibition of HDAC/survivin axis in human hepatocellular carcinoma cells. J Pharmacol Sci. 2020;142:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Siersbæk R, Scabia V, Nagarajan S, Chernukhin I, Papachristou EK, Broome R, Johnston SJ, Joosten SEP, Green AR, Kumar S, Jones J, Omarjee S, Alvarez-Fernandez R, Glont S, Aitken SJ, Kishore K, Cheeseman D, Rakha EA, D'Santos C, Zwart W, Russell A, Brisken C, Carroll JS. IL6/STAT3 Signaling Hijacks Estrogen Receptor α Enhancers to Drive Breast Cancer Metastasis. Cancer Cell. 2020;38:412-423.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 43. | Zhou C, Ma J, Su M, Shao D, Zhao J, Zhao T, Song Z, Meng Y, Jiao P. Down-regulation of STAT3 induces the apoptosis and G1 cell cycle arrest in esophageal carcinoma ECA109 cells. Cancer Cell Int. 2018;18:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Gharibi T, Babaloo Z, Hosseini A, Abdollahpour-Alitappeh M, Hashemi V, Marofi F, Nejati K, Baradaran B. Targeting STAT3 in cancer and autoimmune diseases. Eur J Pharmacol. 2020;878:173107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 45. | Ma JH, Qin L, Li X. Role of STAT3 signaling pathway in breast cancer. Cell Commun Signal. 2020;18:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 46. | Huang Q, Zhong Y, Dong H, Zheng Q, Shi S, Zhu K, Qu X, Hu W, Zhang X, Wang Y. Revisiting signal transducer and activator of transcription 3 (STAT3) as an anticancer target and its inhibitor discovery: Where are we and where should we go? Eur J Med Chem. 2020;187:111922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, Wang X, Zhang J, Guo L, Chen Z, Wang Z, Xiang S. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020;11:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 48. | Hindupur SV, Schmid SC, Koch JA, Youssef A, Baur EM, Wang D, Horn T, Slotta-Huspenina J, Gschwend JE, Holm PS, Nawroth R. STAT3/5 Inhibitors Suppress Proliferation in Bladder Cancer and Enhance Oncolytic Adenovirus Therapy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 49. | Jing B, Wang T, Sun B, Xu J, Xu D, Liao Y, Song H, Guo W, Li K, Hu M, Zhang S, Ling J, Kuang Y, Zhang T, Zhou BP, Yao F, Deng J. IL6/STAT3 Signaling Orchestrates Premetastatic Niche Formation and Immunosuppressive Traits in Lung. Cancer Res. 2020;80:784-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 50. | Herrmann A, Kortylewski M, Kujawski M, Zhang C, Reckamp K, Armstrong B, Wang L, Kowolik C, Deng J, Figlin R, Yu H. Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res. 2010;70:7455-7464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in Cancer Immunotherapy. Mol Cancer. 2020;19:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 675] [Article Influence: 135.0] [Reference Citation Analysis (0)] |