Published online May 15, 2021. doi: 10.4251/wjgo.v13.i5.409
Peer-review started: February 19, 2021
First decision: March 15, 2021
Revised: March 22, 2021
Accepted: April 26, 2021
Article in press: April 26, 2021
Published online: May 15, 2021
Processing time: 76 Days and 13.4 Hours
Plexiform fibromyxoma (PF) is a very rare mesenchymal neoplasm of the stomach that was first described in 2007 and was officially recognized as a subtype of gastric mesenchymal neoplasm by World Health Organization (WHO) in 2010. Histologically, PF is characterized by a plexiform growth of bland spindle to ovoid cells embedded in a myxoid stroma that is rich in small vessels. The lesion is usually paucicellular. While mucosal and vascular invasion have been documented, no metastasis or malignant transformation has been reported. Its pathogenesis is largely unknown and defining molecular alterations are not currently available. There are other mesenchymal tumors arising in the gastrointestinal tract that need to be differentiated from PF given their differing biologic behaviors and malignant potential. Histologic mimics with spindle cells include gastrointestinal stromal tumor, smooth muscle tumor, and nerve sheath tumor. Histologic mimics with myxoid stroma include myxoma and aggressive angiomyxoma. Molecular alterations that have been described in a subset of PF may be seen in gastroblastoma and malignant epithelioid tumor with glioma-associated oncogene homologue 1 (GLI1) rearrangement. The recent increase in publications on PF reflects growing recognition of this entity with expansion of clinical and pathologic findings in these cases. Herein we provide a review of PF in comparison to other mesenchymal tumors with histologic and molecular resemblance to raise the awareness of this enigmatic neoplasm. Also, we highlight the challenges pathologists face when the sample is small, or such rare entity is encountered intraoperatively.
Core Tip: Plexiform fibromyxoma (PF) is a rare mesenchymal tumor of the stomach. Due to its rarity, the pathogenesis and molecular alterations of PF are largely unknown. The incidence of the tumor seems to be increasing probably due to a growing awareness of this entity. Histologic mimics of PF include gastrointestinal stromal tumor, smooth muscle tumor, nerve sheath tumor, myxoma and aggressive angiomyxoma. Molecular mimics of PF include gastroblastoma and malignant epithelioid tumor with glioma-associated oncogene homologue 1 (GLI1) rearrang
- Citation: Arslan ME, Li H, Fu Z, Jennings TA, Lee H. Plexiform fibromyxoma: Review of rare mesenchymal gastric neoplasm and its differential diagnosis. World J Gastrointest Oncol 2021; 13(5): 409-423
- URL: https://www.wjgnet.com/1948-5204/full/v13/i5/409.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i5.409
Plexiform fibromyxoma (PF) is a recently described mesenchymal tumor of the gastrointestinal (GI) tract. Recently our group reported that the incidence of PF relative to GI stromal tumor (GIST), a close mimic of PF, is 1.7 % over a span of 20 years[1]. The tumor is mostly found in the gastric antrum, however PF involving other segments of the GI tract have been reported[1-5]. Due to its rarity, the pathogenesis and molecular alterations of PF are largely unknown[6]. However, its incidence appears to be rapidly increasing in the literature probably due to a growing awareness of this entity[1,7]. While PF behaves in a benign manner, its histologic mimics such as GISTs, smooth muscle tumors and nerve sheath tumors show malignant potential[8,9]. Moreover, its molecular mimics, such as gastroblastoma and malignant epithelioid tumor with glioma-associated oncogene homologue 1 (GLI1) rear
PF was first reported by Takahashi et al[11] in 2007. The authors identified two tumors in the gastric antrum showing similar histomorphology. The morphology of the tumors was not consistent with any known gastric neoplasm. The authors used the term “Plexiform Angiomyxoid Myofibroblastic Tumor” in their report implying its plexiform growth pattern, increased vascularity within the myxoid stroma, and the proliferation of bland spindle cells that were proven to be myofibroblasts by immunohistochemistry and ultrastructural examination. Both tumors lacked mutations in the exons 9, 11, 13 and 17 of C-KIT gene and the exons 12 and 18 of the platelet-derived growth factor receptor alpha (PDGFRA) gene, further supporting that these tumors represent new entity distinct from GIST[11].
In 2008, four additional cases were reported with similar morphology and were referred as “Plexiform Angiomyxoid Tumor”[12-14]. In 2009, Miettinen reported twelve additional PF cases. Also, he re-reviewed previous case studies that had reported tumors with similar morphology but using different terms including “stomach myxoma”, “giant myxoma of the stomach”, “gastric fibromyxoma”, “fibromyxoma of the stomach”, “gastric myxoma”, and “fibromyxoangioma of the stomach” between 1955-2004. However, despite the morphologic similarities between these tumors and PF, these cases were not proven to be PF due to the lack of immunohistochemistry[8]. In 2010, Takahashi et al[7] reported an additional case of PF and provided a review of eighteen PF cases that had been reported in the literature. The tumor was officially recognized as a subtype of gastric mesenchymal tumors and the term PF was endorsed by the 4th edition of World Health Organization (WHO) classification of tumors of the digestive system in 2010[15].
PFs have been reported as either case reports or small case series. To date, a total of 130 PF cases have been reported in the English literature[1,6,16-22]. Given the fact that only nineteen cases had been described by 2010 (approximately three years after the first description of the entity), this remarkable increase in its incidence in a short period of time may be attributable to the increased awareness of PF by clinicians and pathologists[1,7].
PF has been reported roughly equally in males and females in most studies[7,15,23,24] whereas a slight female predominance was found in one study[6]. The median age at presentation is 40 years to 50 years (ranging from 5 years to 81 years), but it can also be seen in pediatric patients[6,15]. The most common site for PF is gastric antrum[6,9]. In addition, it has been reported in the duodenum, jejunum, gallbladder and mediastinum[1,3-6,25,26].
The clinical presentation is usually nonspecific. Most common symptoms are “abdominal symptoms” such as abdominal pain/discomfort, fullness, nausea and vomiting followed by symptoms of blood loss such as bleeding, syncope and anemia. These symptoms may be attributable to the tumor location as well as the hypervas
PF shows a benign behavior[1,6,9]. Even though vascular invasion, lymphatic invasion, mucosal invasion and ulcerations have been reported, neither recurrence nor metastasis has been reported[1,6,8,9,27].
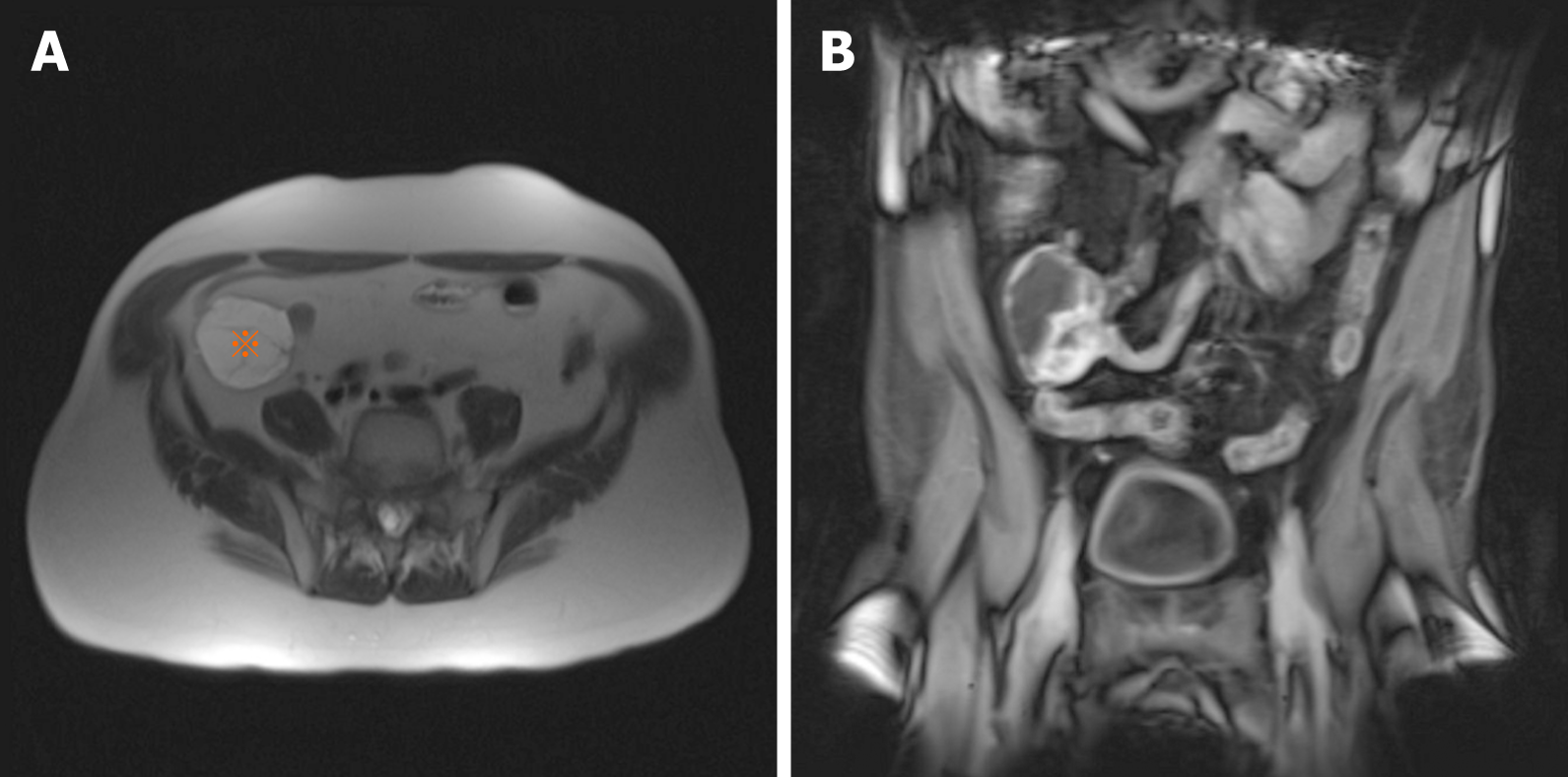
Data regarding the radiologic features of PF are limited in the literature due to its rarity[28]. Imaging findings may be suggestive of another mesenchymal tumor such as GIST[22]. A small tumor can be missed on computed tomography (CT) and may require invasive imaging modality such as capsule endoscopic examination[4]. On CT and magnetic resonance imaging (MRI), PF presents as a solid, cystic or solid/cystic mass with well-defined borders[28] (Figure 1). Due to the increased vascularity of the tumor, CT may show mild enhancement of the solid portion during the arterial phase and strengthened progressive enhancement during the venous and delayed phases[28].
In a recent review, MRI was considered superior to CT for visualizing the extent and components of PF. On MRI, gastric PF consistently showed low signal intensity on the T1-weighted images and high signal intensity on the T2-weighted images. On contrast-enhanced MR images, the solid portion also exhibited heterogeneously gradual enhancement[28]. The gradual enhancement pattern with prominent enhancement in the delayed phase is compatible with the myxoid nature of PF[29].
The reported size of PF ranges from 0.8 cm to 17.0 cm[6]. On endoscopy, hemorrhage and ulceration can be observed due to hypervascularity of the tumor[4,6]. Indeed, tumor surface ulceration is common, and this finding is associated with hemorrhage-related signs or symptoms[6]. PF appears as a tan/pink, rubbery mass, and it usually arises from the submucosa or muscularis propria of the gastric antrum with a lobulated/nodular growth pattern and well-defined borders[6].
PF demonstrates a multinodular, myxoid or gelatinous appearance with or without hemorrhage[8,15].
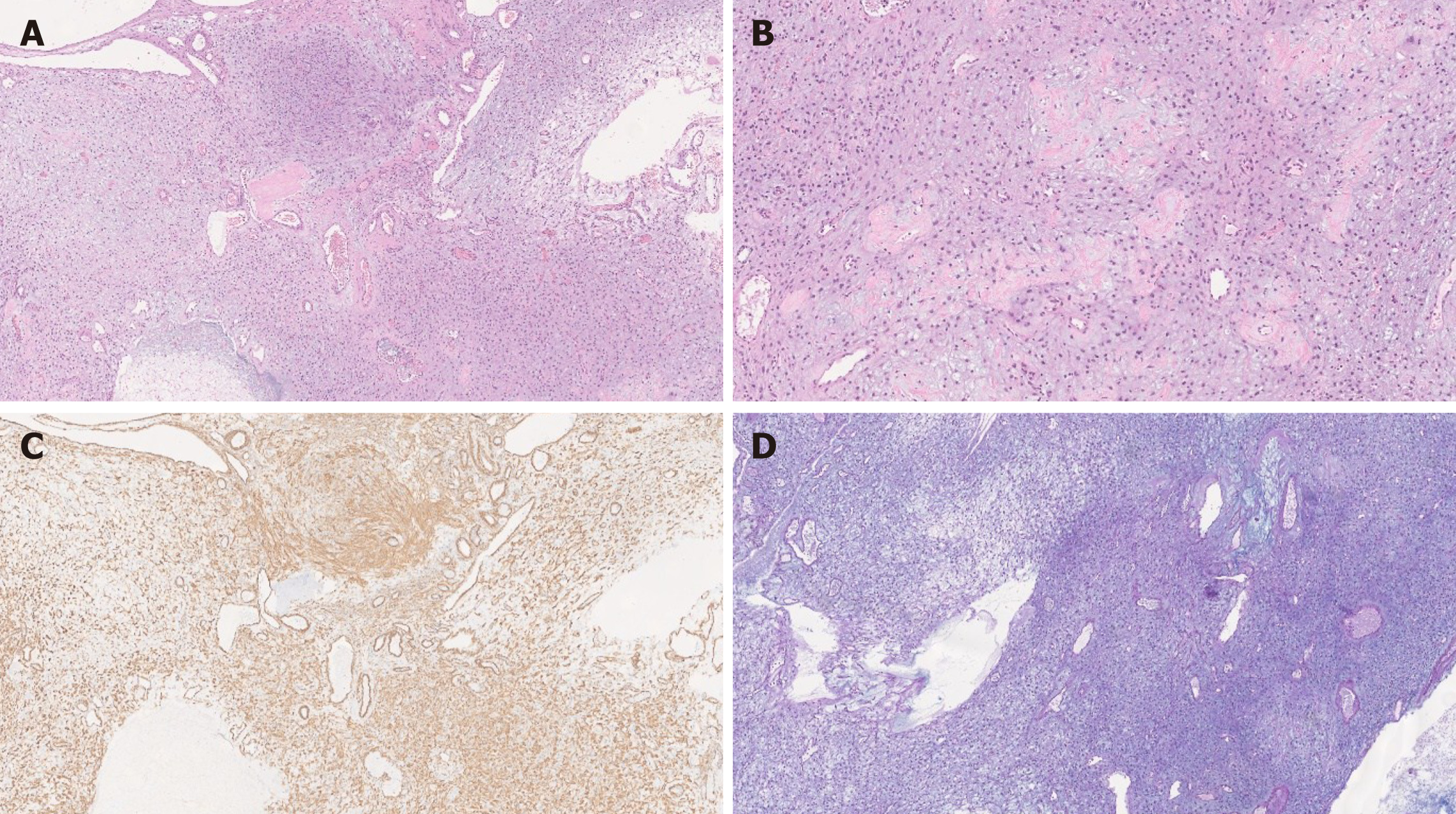
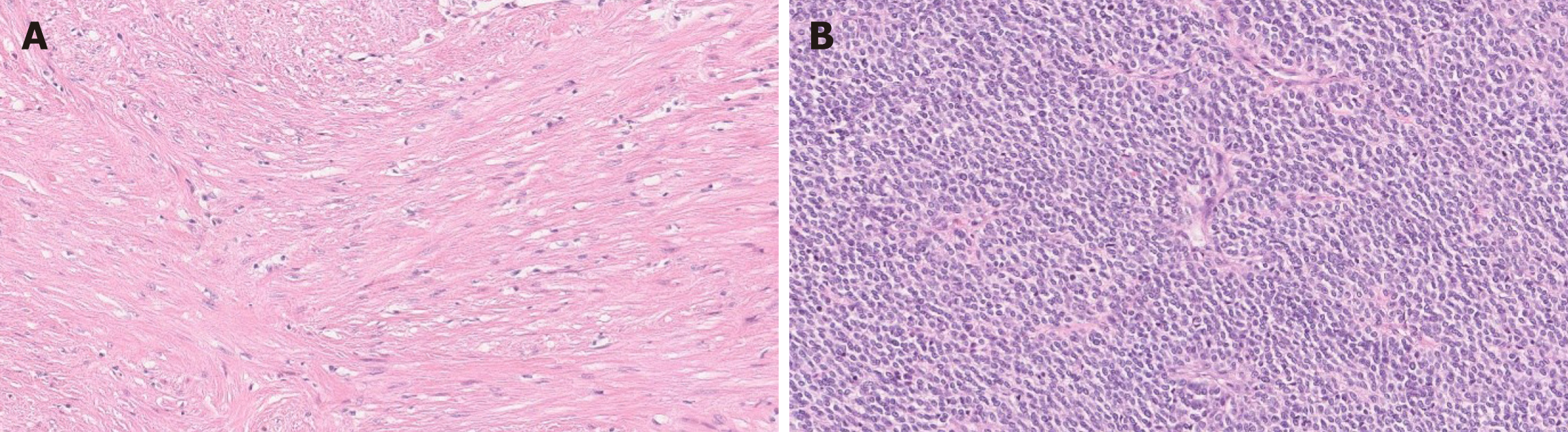
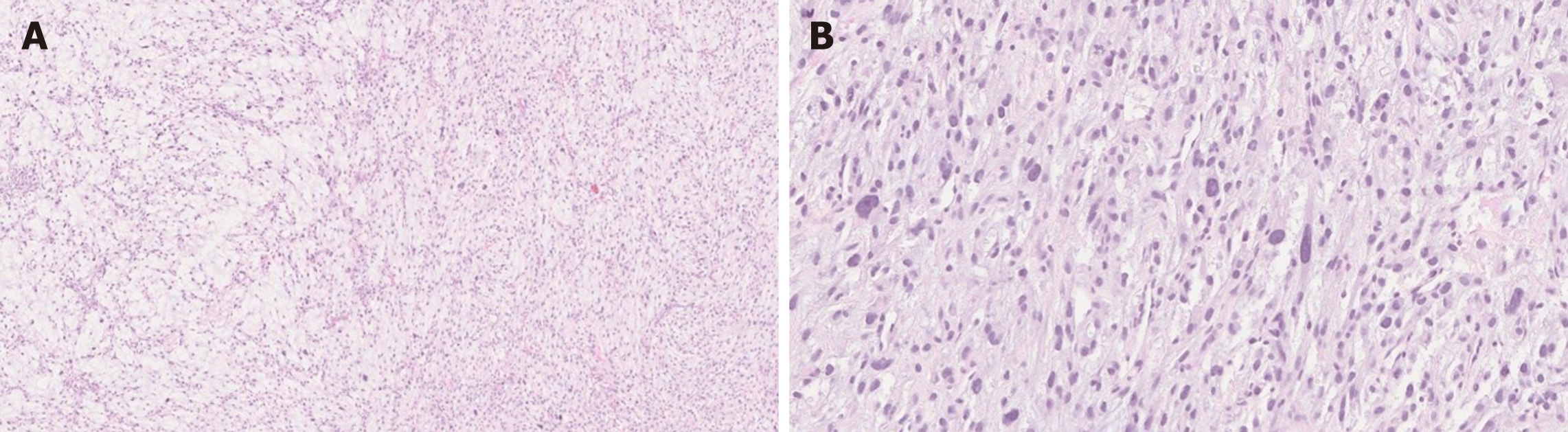
Histologically, PF shows a multinodular, plexiform growth pattern with a proliferation of ovoid to spindle cells within myxoid stroma, and an increased vascularity. The spindle cells are bland, without significant atypia or mitotic activity[6,8,15] (Figure 2). Usually features indicative of aggressive behavior such as vascular and lymphatic invasion are absent; however, these findings have been documented in some cases[4,6,8,27]. There is no tumor necrosis, however ulceration with necrosis has been reported in two cases[4,6,30].
Immunohistochemically, the tumor cells are positive for smooth muscle actin (SMA) and vimentin, and can show focal or partial staining for CD10, desmin and caldesmon. The spindle cells are negative for DOG-1, C-KIT, epithelial membrane antigen (EMA), ALK, S-100 and CD34 with a low Ki-67 proliferation index[6,15]. CD31 and/or CD34 can highlight prominent vascularity. The myxoid stroma is positive for Alcian Blue special stain[6,8,15]. Focal keratin positivity of the spindle cells has been reported in the literature only in two cases[31]. Thus, focal keratin staining does not exclude PF diagnosis.
The diagnosis of PF on pre- or intraoperative biopsy and/or fine-needle aspiration (FNA) specimens can be extremely challenging. Especially, PF can be misdiagnosed as GIST on FNAs without the aid of immunohistochemistry[32]. On FNA, PF shows bland spindle cells without nuclear hyperchromasia or prominent nucleoli[19,32,33].
To date, no specific molecular or genetic alterations have been identified in PF. PF lacks C-KIT and PDGFRA gene mutations that are definitional alterations of GIST[6,8]. In Spans et al[2]’s study, 3 (18%) of 16 cases of PF have been found to harbor MALAT1 (metastasis associated lung adenocarcinoma transcript 1; in 11q12) and GLI1 (in 12q13) translocation, similar to alterations demonstrated in other tumors including gastroblastoma and malignant epithelioid tumor with GLI1 rearrangement. Banerjee et al[34] reported 8 cases of PFs with PTHC1 inactivation. One case also showed partial PTCH1 deletion of exons 15–24 on chromosome 9q, and the other showed bi-allelic chromosome 9q deletions of PTCH1 and FANCC.
GIST is the most common mesenchymal neoplasm of the stomach and GI tract, and is the most important differential diagnosis of PF. Similar to PF, GISTs are frequently found incidentally on CT scan[35]. As opposed to PF, however, GIST can behave as malignant with a metastatic potential[15,36,37]. In symptomatic cases, small tumors usually present with nonspecific GI symptoms whereas large tumors can cause obstruction or bleeding. Imaging studies may show features suggestive of GIST but cannot render a definitive diagnosis of GIST. However, these studies can help to determine the behavior of the tumor based on its size, location, infiltration of nearby structures and the presence or absence of metastasis[38]. In cases without definitive features of malignancy, the risk for malignant transformation can be assessed based on tumor size, location and mitotic activity upon histologic examination of the resected specimen[39].
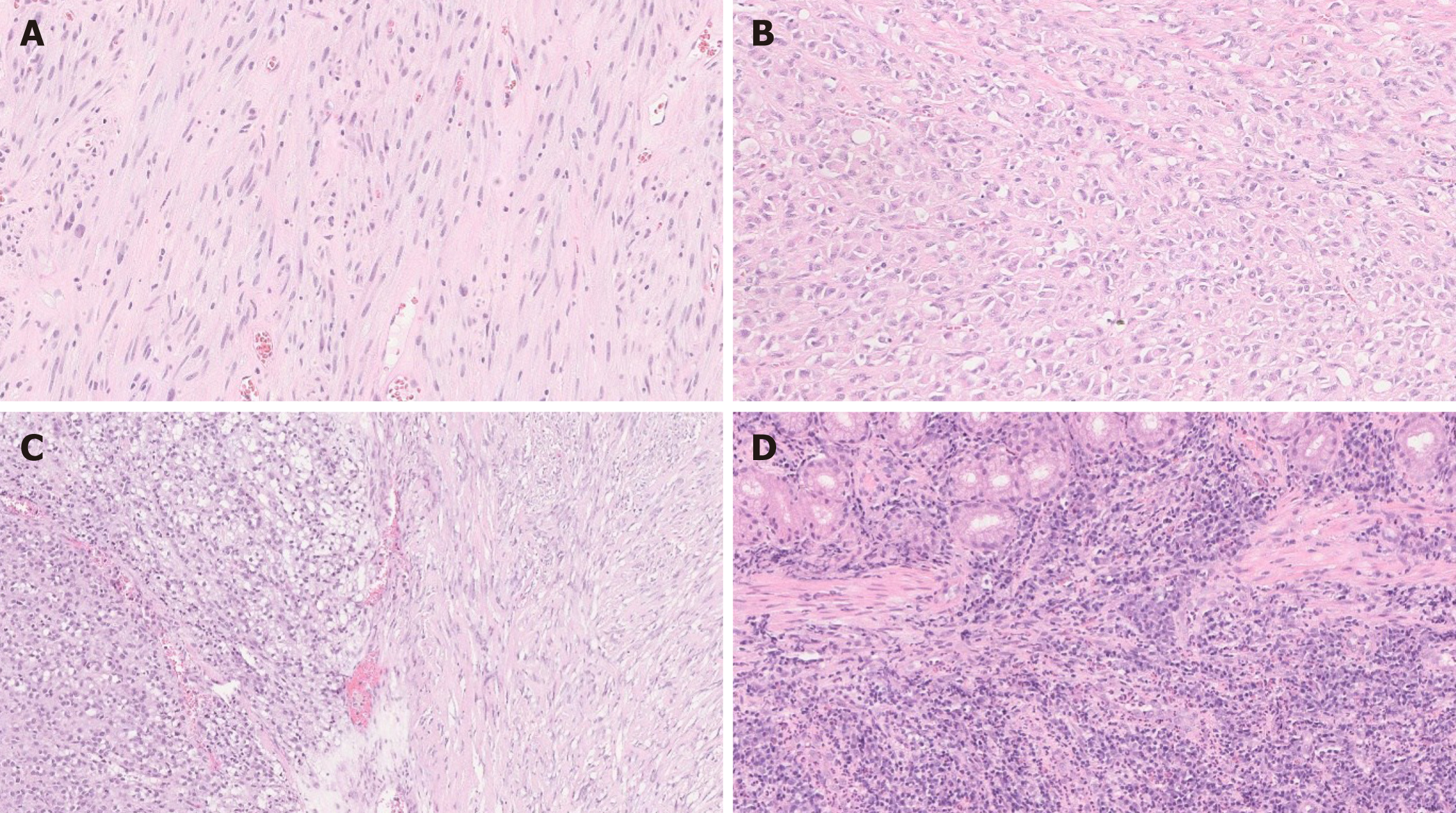
Histologically, GIST is composed of a proliferation of spindle cells and/or epithelioid cells[36,38] (Figure 3). Various cytologic and growth patterns have been described, to include sclerosing spindle cell, palisading and vacuolated spindle cell, hypercellular spindle cell, sarcomatous spindle cell, sclerosing epithelioid GIST with a syncytial pattern, epithelioid GIST with a dyscohesive pattern, hypercellular epithelioid, and sarcomatous epithelioid pattern. Paranuclear vacuoles are frequent findings and can be seen in over 90% of the cases[36]. Immunohistochemically, the tumor cells are diffusely positive for C-KIT and DOG-1. CD34 is positive in most spindle cell GIST and is less commonly expressed in epithelioid variants. Focal desmin, S-100 and keratin positivity can be seen in GIST[15,39].
The morphology and clinical behavior of GIST vary depending on underlying genetic alterations. For example, NF1 and C-KIT mutated GISTs show spindle cell morphology whereas succinate dehydrogenase (SDH)-deficient GISTs show epithelioid cell morphology[40-43]. Most GISTs harbor gain of function KIT or PDGFRA mutations, exon 11 of KIT mutation being the most frequent. Small and large bowel GISTs usually show mutations in KIT while PDGFRA-mutated GISTs usually arise in the stomach[40].
Approximately 10%-15% of GISTs lack KIT or PDGFRA mutations[41]. Among these, SDH-deficient GIST shows multinodular growth pattern, thus closely mimic PF[9]. SDH-deficient GIST is usually encountered in young adults and predominantly found in the stomach. On microscopic examination, it shows a proliferation of epithelioid to spindle cells that shows plexiform involvement of the muscularis propria[41] (Figure 4). Immunohistochemically, SDHB (SDH) stain can be used to confirm SDH deficiency; SDHB expression is lost if any subunit of the enzyme is inactivated. Similar to common spindle cell and epithelioid GISTs, SDH-deficient GIST is strongly and diffusely positive for C-KIT and DOG-1 immunostain even though it lacks KIT mutations and shows a loss of SDHB[40]. Lymphovascular invasion is common in this subtype of GIST and is seen in > 50% of cases, while lymph node metastasis is uncommon[41]. The tumor may recur or present with peritoneal, hepatic and lymph node metastases. However, SDH-deficient GIST usually shows slow progression[37,41].
Myxoid GIST also can show multinodular growth pattern with spindle cells that are in myxoid stroma with thin blood vessels, and can closely mimic PF. However, the tumor cells are diffusely and strongly positive for C-KIT, DOG-1 and CD34 (and are negative for SMA, desmin, cytokeratin and CD10), which separate it from PF[44].
Patients with neurofibromatosis 1 (NF1) can develop GISTs due to biallelic inactivation of NF1 gene. These patients most often present at middle age or later with multiple GISTs in the small intestine. NF1-associated GISTs are usually not aggressive with favorable prognosis, but malignant transformation has been documented[42].
Familial GIST syndrome is associated with rare germline KIT mutations. The patients are predisposed to an early development of GISTs. These patients often have multiple GISTs and show skin findings including maculopapular cutaneous mastocytosis and cutaneous hyperpigmentation. Also, interstitial cells of Cajal hyperplasia is common in these patients[40].
Mesenchymal tumors originating from smooth muscle tissue show a proliferation of spindle cells, similar to PF. Smooth muscle tumors are rare in the GI tract compared to GISTs except in the esophagus and colon, wherein leiomyoma of the muscularis mucosa is the most common primary mesenchymal tumor[45].
Leiomyoma is the most common benign smooth muscle tumor of the GI tract and shows two histologic subtypes: polypoid and intramural. Polypoid leiomyoma is more common and is predominantly found in the esophagus and rectosigmoid colon. The tumors originate from the muscularis mucosa and usually present as small (< 1 cm) mucosal polypoid lesions. Intramural leiomyoma is less common than polypoid counterpart and is found in the muscularis propria of the distal esophagus and proximal stomach[45,46]. Leiomyoma is extremely rare in the duodenum and small bowel[46].
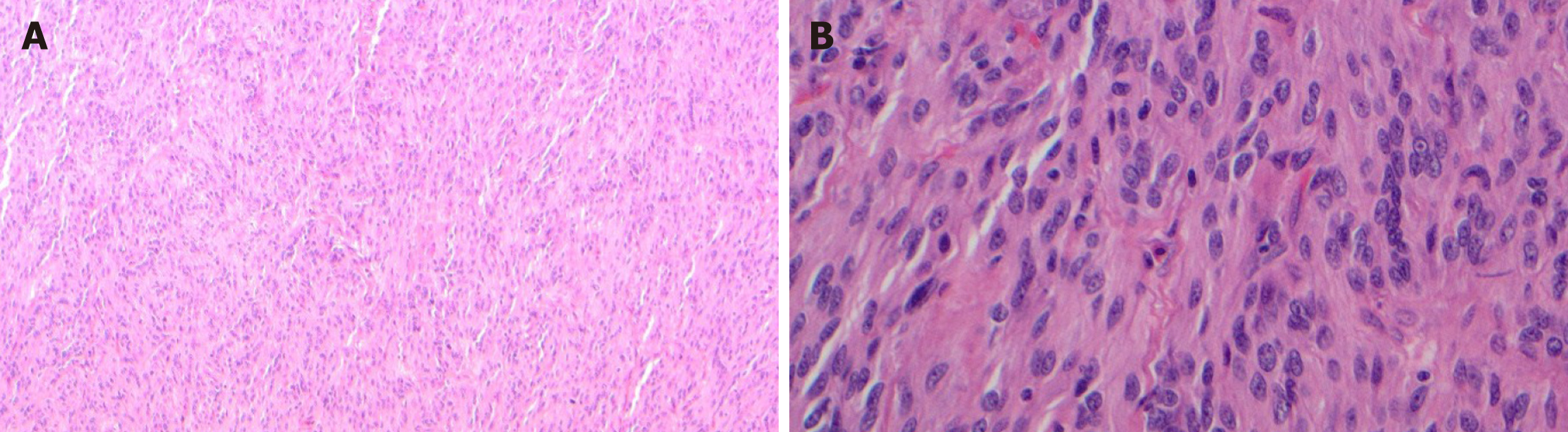
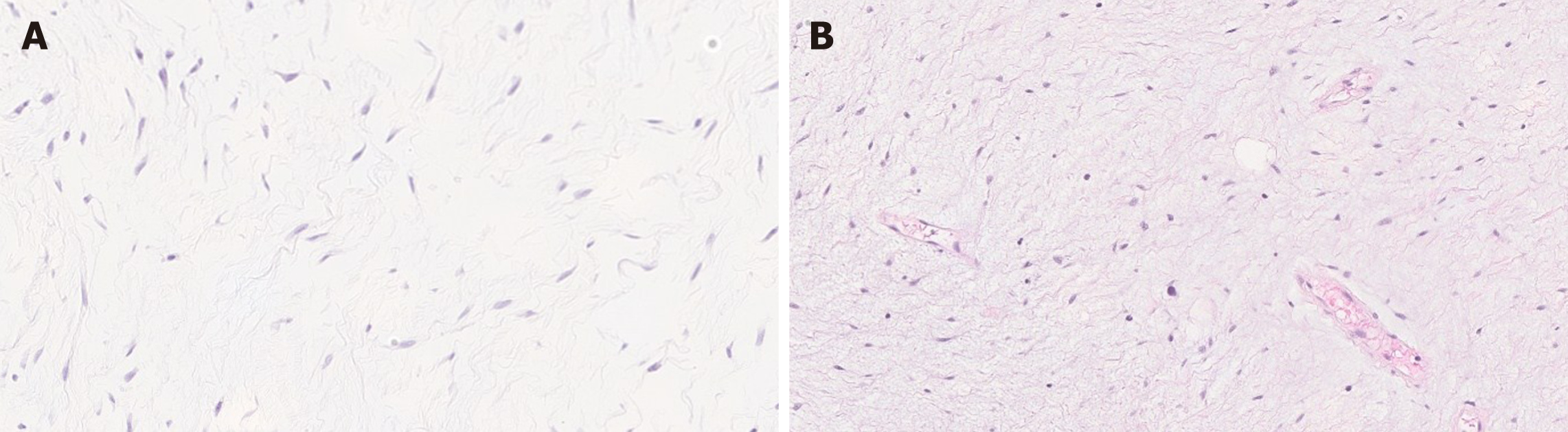
Histologically, leiomyoma exhibits a proliferation of bland spindle cells with cigar shaped nuclei and abundant eosinophilic cytoplasm without atypia, mitosis, or necrosis. Leiomyomas may recur when incompletely excised[45] (Figure 5A).
Leiomyosarcoma is malignant mesenchymal smooth muscle tumor. Although rarer than leiomyoma, leiomyosarcoma can be encountered throughout the GI tract[45]. Similar to leiomyoma, leiomyosarcoma can present as polypoid mucosal lesion, or intra/transmural mass[45,46]. Colorectum is the most common site and the stomach is the least common site for leiomyosarcomas in the GI tract[15,45]. Histologically, leiomyosarcoma is composed of a proliferation of spindle cells with marked atypia, brisk mitotic activity with or without tumor necrosis[45,46] (Figure 5B).
Immunohistochemically, both leiomyoma and leiomyosarcoma are positive for desmin and SMA, and are negative for DOG-1, C-KIT, S-100 and CD34[15,45,46].
Mesenchymal tumors with nerve sheath differentiation may show a proliferation of spindle and/or epithelioid cells and closely resemble PF histologically[40,47,48]. Nerve sheath tumor is the third most common mesenchymal neoplasm of the GI tract. It comprised < 5 % of the GI tract mesenchymal tumors in a single center study. The two most common nerve sheath tumors of the GI tract are granular cell tumor (GCT) and schwannoma[47].
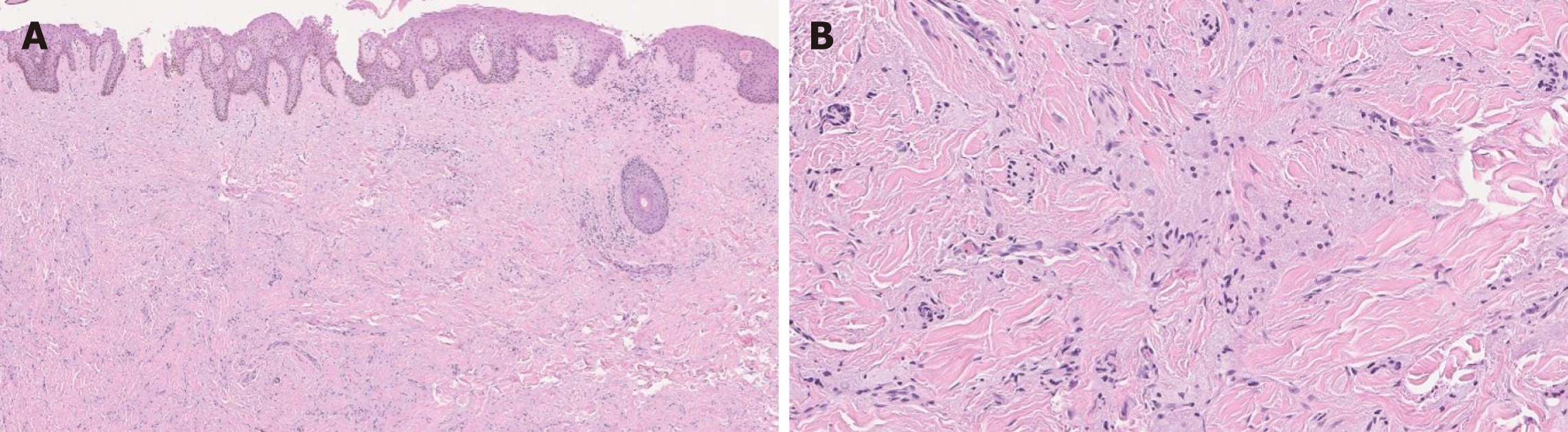
The most common location for GCT in the GI tract is the distal esophagus/ gastroesophageal junction. Histologically, GCT consists of a proliferation of sheets of large polygonal to spindled cells with abundant eosinophilic granular cytoplasm and bland nuclei[40,47,48]. The cells are positive for S-100 and the cytoplasmic granules are positive for PAS. In addition, GCTs in the esophagus are often associated with squamous pseudoepitheliomatous hyperplasia[40,47] and may mimic squamous cell carcinoma especially in a limited, superficial sample (Figure 6).
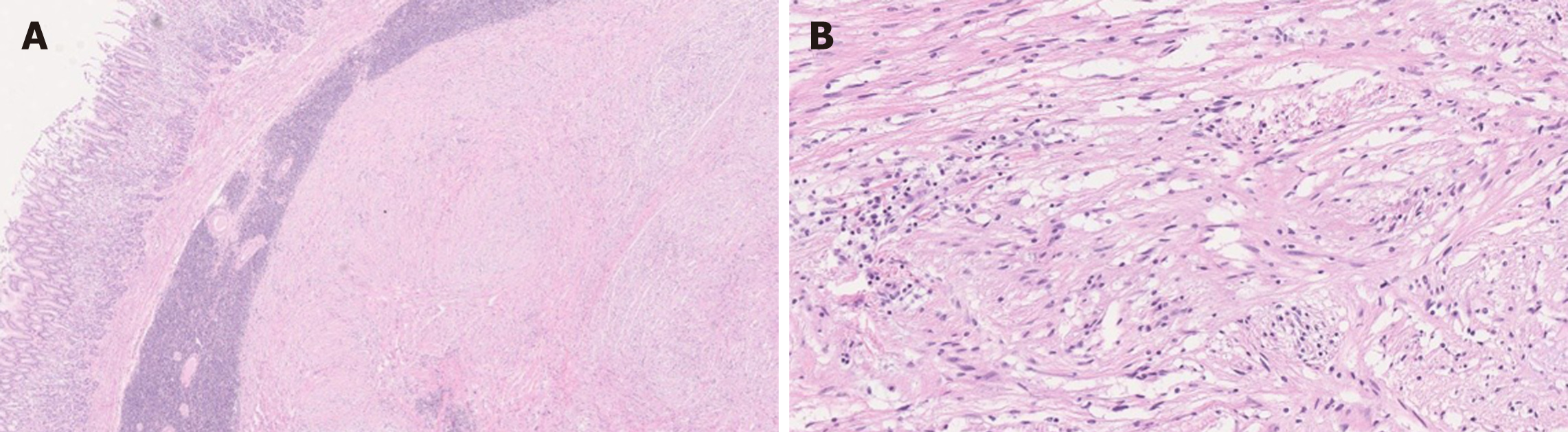
Schwannomas are most commonly seen in the stomach in the GI tract[49]. While schwannomas are characterized by a proliferation of spindle cells, the histomorphology of gastric schwannomas is different from that of peripheral schwannomas. For example, gastric schwannomas are unencapsulated, relatively well-circumscribed and have lymphoid cuffs sometimes with germinal centers at the periphery, whereas peripheral schwannomas are usually encapsulated, and without lymphoid cuffs[15,49]. Gastric schwannomas frequently show a microtrabecular growth pattern in a collagenous background[15,49], and can show an infiltrative spread between the muscularis propria. Nuclear palisading and hyalinized vessels are less common in gastric schwannomas compared to the peripheral counterparts[49] (Figure 7). Immunohistochemically, the lesional spindle cells are strongly and diffusely positive for S-100 and are negative for DOG-1 and C-KIT[47,49]. Gastric schwannoma can also be associated with NF1. Therefore, in a patient with NF1, mesenchymal tumor of the stomach is not necessarily neurofibroma[47]. A rare variant of gastric schwannoma with signet-ring-cell appearance of the tumor cells has been reported[50].
Moreover, NF1 patients can present with variable mesenchymal neoplasms of the GI tract including benign solitary neurofibroma, diffuse or plexiform neurofibroma, malignant peripheral nerve sheath tumor (MPNST), GIST, and neuroendocrine tumors. Neurofibroma exhibits a proliferation of bland spindle Schwann cells admixed with fibroblasts and mast cells in a myxoid or mucinous matrix. Therefore, neurofibroma is also considered a differential diagnosis of PF. Neurofibroma can be solitary or can show a plexiform growth pattern. Most of the cases are asym
Other less common and rare nerve sheath tumors with spindled morphology include NF1-associated peripheral nerve sheath tumor (PNST) and gastric perineuriomas. Even though some PNSTs are associated with NF1 or NF2, a great majority of PNSTs are sporadic[47].
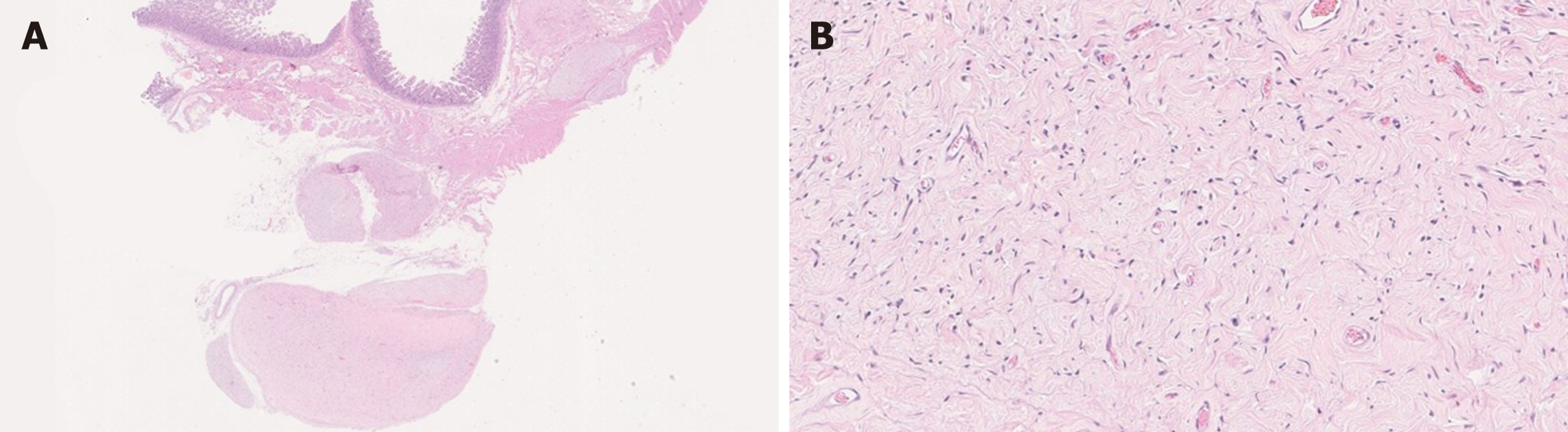
Histologically, PNST is composed of wavy spindle cells, fibroblasts, and strands of collagen[52]. The stroma can be myxoid and the tumor may show a plexiform multinodular growth[51,52] (Figure 8). The tumor cells are diffusely positive for S-100 and CD34 and are negative for C-KIT, DOG-1, and SMA[52]. Perineurioma shows slender cells with wavy nuclei that are diffusely positive for EMA and variably positive for CD34. The lesional cells are negative for S-100, C-KIT, desmin and SMA[47].
MPNST is extremely rare in the GI tract. Only a few cases have been reported in the literature. Histologically, MPNST is composed of alternating hypercellular and hypocellular zones of spindle cells with hyperchromatic and pleomorphic nuclei and frequent mitoses, with fascicular growth pattern (Figure 9). The tumor cells are weakly positive for S-100 and are negative for C-KIT, DOG-1, CD34, desmin, EMA and cytokeratin[53].
These two entities are considered as differential diagnoses of PF given their myxoid stroma.
Myxoma is extremely rare in the stomach. Only one case of gastric myxoma has been reported in the literature to the best of our knowledge[54]. In this report, the tumor was well circumscribed and situated mainly in the submucosa with an extension to the overlying mucosa. The stroma of the tumor was composed of hypocellular myxoid tissue that was poor in collagen and elastic fibers. Scattered small capillaries were noted within the tumor, however the capillaries did not show a plexiform architecture. Although the authors did not document cytomorphology of the lesional cells, the immunostains for AE1/AE3, CAM5.2, CK7, CK20, p53, α-SMA, desmin, S-100, C-KIT and CD34 were negative. Ki-67 proliferation index was 1%[54]. A few cases with similar morphology and immunohistochemistry have been reported in the small and large bowel[55-57] (Figure 10A).
Aggressive angiomyxoma is a rare mesenchymal tumor of the pelvis and perineum of adults. The tumor is infiltrative, and there is a risk of recurrence following resection. The frequent sites of aggressive angiomyxoma include the vulvovaginal, perineal, and groin regions in women and less commonly in inguinoscrotal region and perineum in men[58].
Histologically, aggressive angiomyxoma is hypocellular and shows a proliferation of spindle and stellate-shaped cells in a myxoid background. Blood vessels varying in caliber may show hyalinization[59] (Figure 10B). The tumor cells are positive for estrogen (ER) and/or progesterone receptor (PR)s as well as SMA, desmin, vimentin and CD34. The tumor cells are negative for S-100, C-KIT, and cytokeratin[58,59]. Rearrangements involving HMGA2 gene on chromosome 12 have been implicated in the pathogenesis of this tumor[60,61]. To the best of our knowledge, no primary angiomyxoma of GI tract has been reported in the literature.
Both tumors lack the plexiform architecture of PF. The spindle cells in myxoma are negative for SMA, which is different from PF. Likewise, the spindle cells in angiomyxoma are positive for ER and PR, which is different from PF.
Gastroblastoma is a rare gastric tumor that has been first described in 2009. The tumor has been reported both in pediatric and adult populations[40,62-64]. Histologically, gastroblastoma exhibits a biphasic growth pattern wherein areas of uniform spindled cells forming diffuse sheets are admixed with areas of epithelial cells with glandular/ tubular differentiation forming nests, sheets or cords[40].
By immunohistochemistry, the epithelial cells are positive for AE1:AE3, CK18, and partially for CK7, while they are negative for CK5/6, CK20 and EMA. The spindle cells are positive for vimentin and CD10. Both elements are negative for C-KIT, SMA, desmin, S-100, CD34, CD99, ER, p63, calretinin, chromogranin, synaptophysin, and thyroid transcription factor 1[62].
The tumor is negative for SS18 rearrangement by in-situ hybridization that is usually present in synovial sarcoma, another tumor with a biphasic growth pattern[62].
Gastroblastoma has been recognized as a malignant epithelial tumor by WHO 2019. However, due to its biphasic and nodular growth pattern with spindle and epithelioid cells, as well as recurrent MALAT1–GLI1 fusion, we consider this tumor as part of the differential diagnosis of PF[40,62,64].
Malignant epithelioid tumor with GLI1 rearrangement is a recently described epithelioid neoplasm of the soft tissue that harbors MALAT1–GLI1 fusion with a metastatic potential[65]. In the GI tract, only one case in the jejunum has been reported[10]. Later, Agaram et al[66] reported GLI1 gene amplifications in a subset of soft tissue tumors with similar morphology, even though these tumors showed broader morphologic spectrum and inconsistent immunohistochemistry. Recently, a soft tissue mass with similar morphology without GLI1 rearrangement by FISH testing has been also described. This tumor lacked rearrangement of the GLI1 locus. However, co-amplification GLI1 and DDIT3 that is near GLI1 on chromosome 12, was identified[66]. Subsequently, the term “GLI activated epithelioid cell tumour” has been proposed to redefine the genetic background of this entity[67].
Although the cytomorphology of the lesional cells vary (epithelioid, ovoid, round to spindle), given that a subset of PF harbors MALAT1 (in 11q12) and GLI1 (in 12q13) translocation[66], we include these tumors in the differential diagnosis of PF. The tumor cells show variable positivity for S-100, SMA and cytokeratin[67].
We herein summarize aforementioned findings that may pose diagnostic challenges (Table 1).
| Neoplasm | Morphology | Immunohistochemistry | Molecular alteration | Location | Behavior |
| Plexiform fibromyxoma | Ovoid to spindle | SMA (+), CD10 (+/-), desmin (+/-), caldesmon (+/-), C-KIT (-), DOG-1 (-), S-100 (-), CD34 (-), ALK (-), cytokeratin (usually -) | MALAT1–GLI1, PTHC1 inactivation | Stomach | Benign |
| GIST | Spindle, epithelioid or mixed | C-KIT (+), DOG-1 (+), S-100 (usually -), CD34 (+), desmin (usually -), cytokeratin (-), SDHB (no loss) | KIT, PDGFRA | Stomach, small intestine, entire GI tract | Benign or malignant |
| SDH deficient-GIST | Usually epithelioid | C-KIT (+), DOG-1 (+), S-100 (-), CD34 (+), SDHB (loss), desmin (-), cytokeratin (-) | No KIT or PDGFRA mutation | Stomach | Benign or malignant |
| Smooth muscle tumor | Mostly spindle, rarely epithelioid | Desmin (+), SMA (+), DOG-1 (-), C-KIT (-), S-100 (-), CD34 (-) | Esophagus, colon | Benign or malignant | |
| Nerve sheath tumor | Wavy spindle | GCT: S-100 (+), Schwannoma: S-100 (+); Neurofibroma: CD34 (+), S-100 (+); Perineurioma: EMA (+); MPNST: S-100 (weak +) | NF1, NF2 | Esophagus (GCT), stomach (schwannoma) | Benign or malignant |
| Myxoma | Bland spindle or stellate | Vimentin (+), CD34 (+/-), S-100 (+/-), cytokeratin (-), SMA (-) | Rare in GI tract | Benign | |
| Angiomyxoma | Spindle to stellate | ER (+), PR (+), SMA (+), desmin (+), vimentin (+), CD34 (+/-), C-KIT (-), DOG-1 (-), S-100 (-), cytokeratin (-) | HMGA2 rearrangement | Extremely rare in GI Tract | Benign, locally aggressive |
| Gastroblastoma | Biphasic with epithelial and spindle | Epithelial cells: AE1:AE3 (+), CK18 (+); Spindle cells: vimentin (+), CD10(+); Both: C-KIT (-), SMA (-), desmin (-), S-100 (-), CD34 (-), CD99 (-), ER (-), p63 (-), calretinin (-), chromogranin (-), synaptophysin (-), TTF-1 (-) | MALAT1–GLI1 | Stomach | Malignant |
| Malignant epithelioid tumor with GLI1 rearrangement | Epithelioid, ovoid, round to spindle | S-100 (+/-), SMA (+/-), cytokeratin (+/-) | MALAT1–GLI1, GLI amplification, GLI1 fusions | Soft tissue, jejunum | Malignant |
SDH-deficient and myxoid GIST can show multinodular growth pattern similar to PF, however the tumor cells are reactive with C-KIT and DOG-1 in both[9,40,44]. GIST can show focal desmin positivity similar to PF and leiomyoma[36]. Similarly, C-KIT immunostain can highlight the interstitial cells of Cajal that are entrapped in leiomyoma giving a false impression of GIST in a small sample. However unlike GIST, leiomyoma does not show diffuse C-KIT and DOG-1 staining[9,43]. Pseudoepitheliomatous hyperplasia of the squamous mucosa overlying GCT can mimic squamous cell carcinoma[40]. Differential diagnosis of neurofibromatosis- associated GI mes
The current treatment of choice for PF is surgical excision. No molecular alteration-based targeted therapy is available[6].
PF is an extremely rare entity that is likely under-recognized. Even though histologic features indicative of aggressive behavior such as vascular and lymphatic invasion have been reported, no malignant transformation or metastasis have been reported. Differential diagnosis includes variable mesenchymal tumors of the GI tract with spindled morphology and/or myxoid stroma, among which GIST is the closest mimic. A subset of PF has been shown to harbor certain molecular alterations, however no universal molecular alterations have been identified. Recently described mesenchymal tumors share some molecular alterations described in PF and broaden the differential diagnoses. Notable increase in publications regarding PF appear to reflect growing awareness of this entity, which may aid in the correct diagnosis of PF and help to better understand the biology of this tumor.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin ZD S-Editor: Gao CC L-Editor: A P-Editor: Zhang YL
| 1. | Arslan ME, Li H, Jennings TA, Lee EC, Nigam A, Lee H. Frequency of Plexiform Fibromyxoma relative to gastrointestinal stromal tumor: A single center study. Ann Diagn Pathol. 2020;48:151568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Spans L, Fletcher CD, Antonescu CR, Rouquette A, Coindre JM, Sciot R, Debiec-Rychter M. Recurrent MALAT1-GLI1 oncogenic fusion and GLI1 up-regulation define a subset of plexiform fibromyxoma. J Pathol. 2016;239:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Moris D, Spanou E, Sougioultzis S, Dimitrokallis N, Kalisperati P, Delladetsima I, Felekouras E. Duodenal plexiform fibromyxoma as a cause of obscure upper gastrointestinal bleeding: A case report. Medicine (Baltimore). 2017;96:e5883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Zhang WG, Xu LB, Xiang YN, Duan CH. Plexiform fibromyxoma of the small bowel: A case report. World J Clin Cases. 2018;6:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Banerjee N, Gupta S, Dash S, Ghosh S. Plexiform angiomyxoid myofibroblastic tumour of the duodenum: a rare entity. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Su HA, Yen HH, Chen CJ. An Update on Clinicopathological and Molecular Features of Plexiform Fibromyxoma. Can J Gastroenterol Hepatol. 2019;2019:3960920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Takahashi Y, Suzuki M, Fukusato T. Plexiform angiomyxoid myofibroblastic tumor of the stomach. World J Gastroenterol. 2010;16:2835-2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol. 2009;33:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 9. | Doyle LA, Hornick JL. Mesenchymal Tumors of the Gastrointestinal Tract Other than GIST. Surg Pathol Clin. 2013;6:425-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Prall OWJ, McEvoy CRE, Byrne DJ, Iravani A, Browning J, Choong DY, Yellapu B, O'Haire S, Smith K, Luen SJ, Mitchell PLR, Desai J, Fox SB, Fellowes A, Xu H. A Malignant Neoplasm From the Jejunum With a MALAT1-GLI1 Fusion and 26-Year Survival History. Int J Surg Pathol. 2020;28:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Takahashi Y, Shimizu S, Ishida T, Aita K, Toida S, Fukusato T, Mori S. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol. 2007;31:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Yoshida A, Klimstra DS, Antonescu CR. Plexiform angiomyxoid tumor of the stomach. Am J Surg Pathol. 2008;32:1910-2; author reply 1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Rau TT, Hartmann A, Dietmaier W, Schmitz J, Hohenberger W, Hofstaedter F, Katenkamp K. Plexiform angiomyxoid myofibroblastic tumour: differential diagnosis of gastrointestinal stromal tumour in the stomach. J Clin Pathol. 2008;61:1136-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Galant C, Rousseau E, Ho Minh Duc DK, Pauwels P. Re: Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol. 2008;32:1910; author reply 1912-1910; author reply 1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Miettinen M, Fletcher CD, Kindblom LG, Tsui WM. Mesenchymal tumors of the stomach. In: Bosman FT, Carneiro F, Hruban R, Teise ND. WHO classification of tumors of the digestive system. 4th ed. Lyon: IARC, 2010: 74-79. |
| 16. | Vieites Branco I, Silva JC, Pinto F, Pires F, Almeida A. Rare mesenchymal antral gastric tumors: Case reports of glomus tumor and plexiform fibromyxoma. Radiol Case Rep. 2020;15:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Hong YP, Yu J, Wang CY, Su YR, Chen C, Deng WH, Wang WX. Plexiform Fibromyxoma of the Stomach. J Gastrointest Surg. 2020;24:909-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Tang J, Liu F. Plexiform Fibromyxoma: A Rare Mesenchymal Tumor Found in the Esophagus. Am J Gastroenterol. 2020;115:648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Gan Y, Hammoud G, Esebua M. A rare case of plexiform fibromyxoma in stomach: FNA diagnosis with histological correlation and differential diagnoses. Ann Diagn Pathol. 2020;44:151453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Magadán Álvarez C, Olmos-Martínez JM, Toledo Martínez E, Trugeda Carrera MS, Fernández Díaz MJ, Martín Rivas B, Mazorra Horts R, Mayorga Fernández MM, Arias Pacheco RD. Gastric plexiform fibromyxoma, an uncommon mesenchymal tumor. Rev Esp Enferm Dig. 2021;113:183-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Pei JY, Tan B, Liu P, Cao GH, Wang ZS, Qu LL. Gastric plexiform fibromyxoma: A case report. World J Clin Cases. 2020;8:5639-5644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Nasralla A, Alwabari M, Alsaif O, Amr SS. Gastric Plexiform Fibromyxoma Arising in the Cardia in an Adolescent Male: A Rare Tumor with an Unusual Location. Case Rep Surg. 2020;2020:9037960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Morris MW, Sullivan L, Sawaya DE, Steiner MA, Nowicki MJ. Gastric plexiform fibromyxoma tumor in a child – case report and review of the literature. J Pediatr Surg Case Rep. 2016;4:38-41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Hu G, Chen H, Liu Q, Wei J, Feng Y, Fu W, Zhang M, Wu H, Gu B, Ren J. Plexiform fibromyxoma of the stomach: a clinicopathological study of 10 cases. Int J Clin Exp Pathol. 2017;10:10926-10933. [PubMed] |
| 25. | Duckworth LV, Gonzalez RS, Martelli M, Liu C, Coffin CM, Reith JD. Plexiform fibromyxoma: report of two pediatric cases and review of the literature. Pediatr Dev Pathol. 2014;17:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Fassan M, Salmaso R, Saraggi D, Alaggio R, Guido M, Balsamo L, Carniato S, Gruppo M, Ninfo V, Bardini R, Rugge M. Plexiform fibromyxoma of the gallbladder. Pathologica. 2015;107:181-184. [PubMed] |
| 27. | Kawara F, Tanaka S, Yamasaki T, Morita Y, Ohara Y, Okabe Y, Hoshi N, Toyonaga T, Umegaki E, Yokozaki H, Hirose T, Azuma T. Gastric plexiform fibromyxoma resected by endoscopic submucosal dissection after observation of chronological changes: A case report. World J Gastrointest Oncol. 2017;9:263-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Yang MX, Zhao ZH, Yang JF, Chen B, Shen XZ, Wei JG, Wang BY. Imaging findings of gastric plexiform fibromyxoma with a cystic change: A case report and review of literature. Medicine (Baltimore). 2017;96:e8967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Akai H, Kiryu S, Shinozaki M, Ohta Y, Nakano Y, Yasaka K, Ohtomo K. Computed tomography and magnetic resonance imaging of a plexiform angiomyxoid myofibroblastic tumor: a case report. BMC Med Imaging. 2017;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Lee PW, Yau DT, Lau PP, Chan JK. Plexiform fibromyxoma (plexiform angiomyxoid myofibroblastic tumor) of stomach: an unusual presentation as a fistulating abscess. Int J Surg Pathol. 2014;22:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Quero G, Musarra T, Carrato A, Fici M, Martini M, Dei Tos AP, Alfieri S, Ricci R. Unusual focal keratin expression in plexiform angiomyxoid myofibroblastic tumor: A case report and review of the literature. Medicine (Baltimore). 2016;95:e4207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Lai J, Kresak JL, Cao D, Zhang D, Zhang S, Leon ME, Shenoy A, Liu W, Trevino J, Starostik P, Gonzalo DH, Wang H, Liu X, Fan X. Gastric Plexiform Fibromyxoma: A Great Mimic of Gastrointestinal Stromal Tumor (GIST) and Diagnostic Pitfalls. J Surg Res. 2019;239:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Rohit M, Bhatt A, Cruise M, Wearsch PA, Goldblum JR, Sturgis CD. Endoscopic ultrasound FNA: An illustrated review of spindle cell neoplasms of the upper gastrointestinal tract including a novel case of gastric plexiform fibromyxoma. Diagn Cytopathol. 2018;46:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Banerjee S, Corless CL, Miettinen MM, Noh S, Ustoy R, Davis JL, Tang CM, Yebra M, Burgoyne AM, Sicklick JK. Loss of the PTCH1 tumor suppressor defines a new subset of plexiform fibromyxoma. J Transl Med. 2019;17:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Nakajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 864] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 37. | Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. 2021;14:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 38. | Parab TM, DeRogatis MJ, Boaz AM, Grasso SA, Issack PS, Duarte DA, Urayeneza O, Vahdat S, Qiao JH, Hinika GS. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019;10:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (2)] |
| 39. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1297] [Article Influence: 72.1] [Reference Citation Analysis (33)] |
| 40. | Papke DJ Jr, Hornick JL. Recent developments in gastroesophageal mesenchymal tumours. Histopathology. 2021;78:171-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Miettinen M, Wang ZF, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011;35:1712-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 42. | Ylä-Outinen H, Loponen N, Kallionpää RA, Peltonen S, Peltonen J. Intestinal tumors in neurofibromatosis 1 with special reference to fatal gastrointestinal stromal tumors (GIST). Mol Genet Genomic Med. 2019;7:e927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Wardelmann E, Neidt I, Bierhoff E, Speidel N, Manegold C, Fischer HP, Pfeifer U, Pietsch T. c-kit mutations in gastrointestinal stromal tumors occur preferentially in the spindle rather than in the epithelioid cell variant. Mod Pathol. 2002;15:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Li B, Zhang QF, Han YN, Ouyang L. Plexiform myxoid gastrointestinal stromal tumor: a potential diagnostic pitfall in pathological findings. Int J Clin Exp Pathol. 2015;8:13613-13618. [PubMed] |
| 45. | Miettinen M, Fetsch JF. Evaluation of biological potential of smooth muscle tumours. Histopathology. 2006;48:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Agaimy A, Wünsch PH. True smooth muscle neoplasms of the gastrointestinal tract: morphological spectrum and classification in a series of 85 cases from a single institute. Langenbecks Arch Surg. 2007;392:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Agaimy A, Märkl B, Kitz J, Wünsch PH, Arnholdt H, Füzesi L, Hartmann A, Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Prematilleke IV, Sujendran V, Warren BF, Maynard ND, Piris J. Granular cell tumour of the oesophagus mimicking a gastrointestinal stromal tumour on frozen section. Histopathology. 2004;44:502-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 50. | Tozbikian G, Shen R, Suster S. Signet ring cell gastric schwannoma: report of a new distinctive morphological variant. Ann Diagn Pathol. 2008;12:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Garrouche N, Ben Abdallah A, Arifa N, Hasni I, Ben Cheikh Y, Ben Farhat W, Ben Amor S, Jemni H. Spectrum of gastrointestinal lesions of neurofibromatosis type 1: a pictorial review. Insights Imaging. 2018;9:661-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Ahn S, Chung CS, Kim KM. Neurofibroma of the Colon: A Diagnostic Mimicker of Gastrointestinal Stromal Tumor. Case Rep Gastroenterol. 2016;10:674-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Kim EY, Lee SH, Yoo HM, Song KY, Park CH. Gastric Malignant Peripheral Nerve Sheath Tumor: A Case Report. Int J Surg Pathol. 2015;23:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Tereda T. Gastric mucinous myxoma: a case report. Case Rep Clin Pathol. 2016;3:1-4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Wang Y, Sharkey FE. Myxoma of the small bowel in a 47-year-old woman with a left atrial myxoma. Arch Pathol Lab Med. 2003;127:481-484. [PubMed] |
| 56. | Varsamis N, Tavlaridis T, Lostoridis E, Tziastoudi E, Salveridis N, Chatzipourgani C, Pouggouras C, Pakataridis A, Christodoulidis C. Myxoma of the small intestine complicated by ileo-ileal intussusception: Report of an extremely rare case. Int J Surg Case Rep. 2013;4:609-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Bsirini C, Findeis-Hosey JJ, Huber AR. Cecal Mucosal Myxoma: The First Report of a New Type of Mesenchymal Colon Polyp. Int J Surg Pathol. 2019;27:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Tian Y, Kang S, Li L, Zhao XW. Aggressive Angiomyxoma of the Abdominopelvic Cavity: A Case Report. Ann Clin Case Rep. 2017;2:1263. |
| 59. | Benson JC, Gilles S, Sanghvi T, Boyum J, Niendorf E. Aggressive angiomyxoma: case report and review of the literature. Radiol Case Rep. 2016;11:332-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Nucci MR, Weremowicz S, Neskey DM, Sornberger K, Tallini G, Morton CC, Quade BJ. Chromosomal translocation t(8;12) induces aberrant HMGIC expression in aggressive angiomyxoma of the vulva. Genes Chromosomes Cancer. 2001;32:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Rabban JT, Dal Cin P, Oliva E. HMGA2 rearrangement in a case of vulvar aggressive angiomyxoma. Int J Gynecol Pathol. 2006;25:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Miettinen M, Dow N, Lasota J, Sobin LH. A distinctive novel epitheliomesenchymal biphasic tumor of the stomach in young adults ("gastroblastoma"): a series of 3 cases. Am J Surg Pathol. 2009;33:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Ma Y, Zheng J, Zhu H, Dong K, Zheng S, Xiao X, Chen L. Gastroblastoma in a 12-year-old Chinese boy. Int J Clin Exp Pathol. 2014;7:3380-3384. [PubMed] |
| 64. | Montgomery EA. Gastroblastoma. In: World Health Organization Classification of Tumours Editorial Board. Digestive system tumours. 5th ed. Lyon, France: IARC Press, 2019: 102-103. |
| 65. | Antonescu CR, Agaram NP, Sung YS, Zhang L, Swanson D, Dickson BC. A Distinct Malignant Epithelioid Neoplasm With GLI1 Gene Rearrangements, Frequent S100 Protein Expression, and Metastatic Potential: Expanding the Spectrum of Pathologic Entities With ACTB/MALAT1/PTCH1-GLI1 Fusions. Am J Surg Pathol. 2018;42:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 66. | Agaram NP, Zhang L, Sung YS, Singer S, Stevens T, Prieto-Granada CN, Bishop JA, Wood BA, Swanson D, Dickson BC, Antonescu CR. GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions. Mod Pathol. 2019;32:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 67. | Aivazian K, Mahar A, Jackett LA, Kimble RM, Scolyer RA. GLI activated epithelioid cell tumour: report of a case and proposed new terminology. Pathology. 2021;53:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen's disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012;5:852-862. [PubMed] |