Published online Dec 15, 2021. doi: 10.4251/wjgo.v13.i12.2190
Peer-review started: May 26, 2021
First decision: June 24, 2021
Revised: July 5, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: December 15, 2021
Processing time: 202 Days and 13 Hours
The long-term effect of anatomic resection (AR) is better than that of non-anatomic resection (NAR). At present, there is no study on microvascular invasion (MVI) and liver resection types.
To explore whether AR improves long-term survival in patients with hepatocellular carcinoma (HCC) by removing the peritumoral MVI.
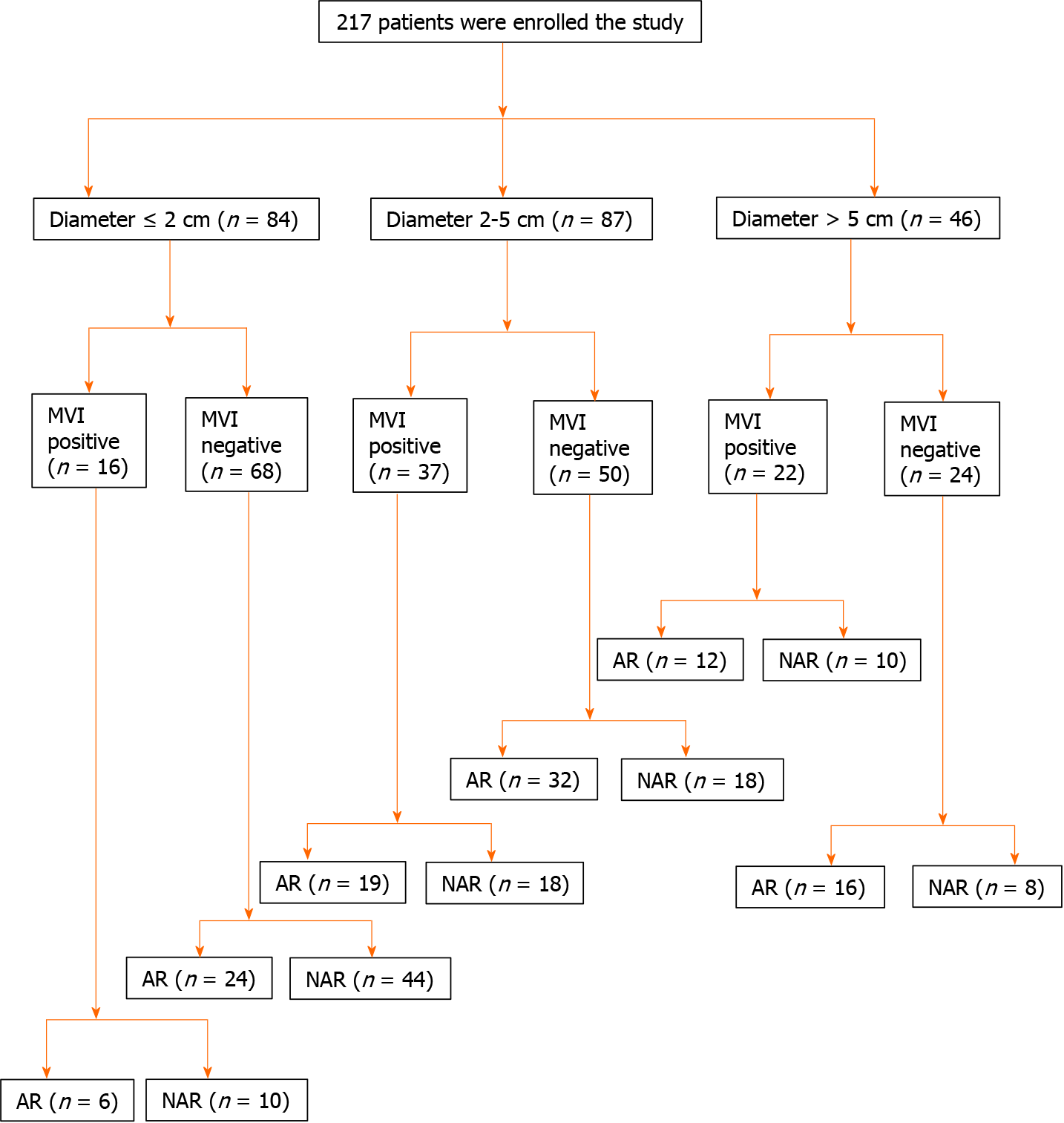
A total of 217 patients diagnosed with HCC were enrolled in the study. The surgical margin was routinely measured. According to the stratification of different tumor diameters, patients were divided into the following groups: ≤ 2 cm group, 2-5 cm group, and > 5 cm group.
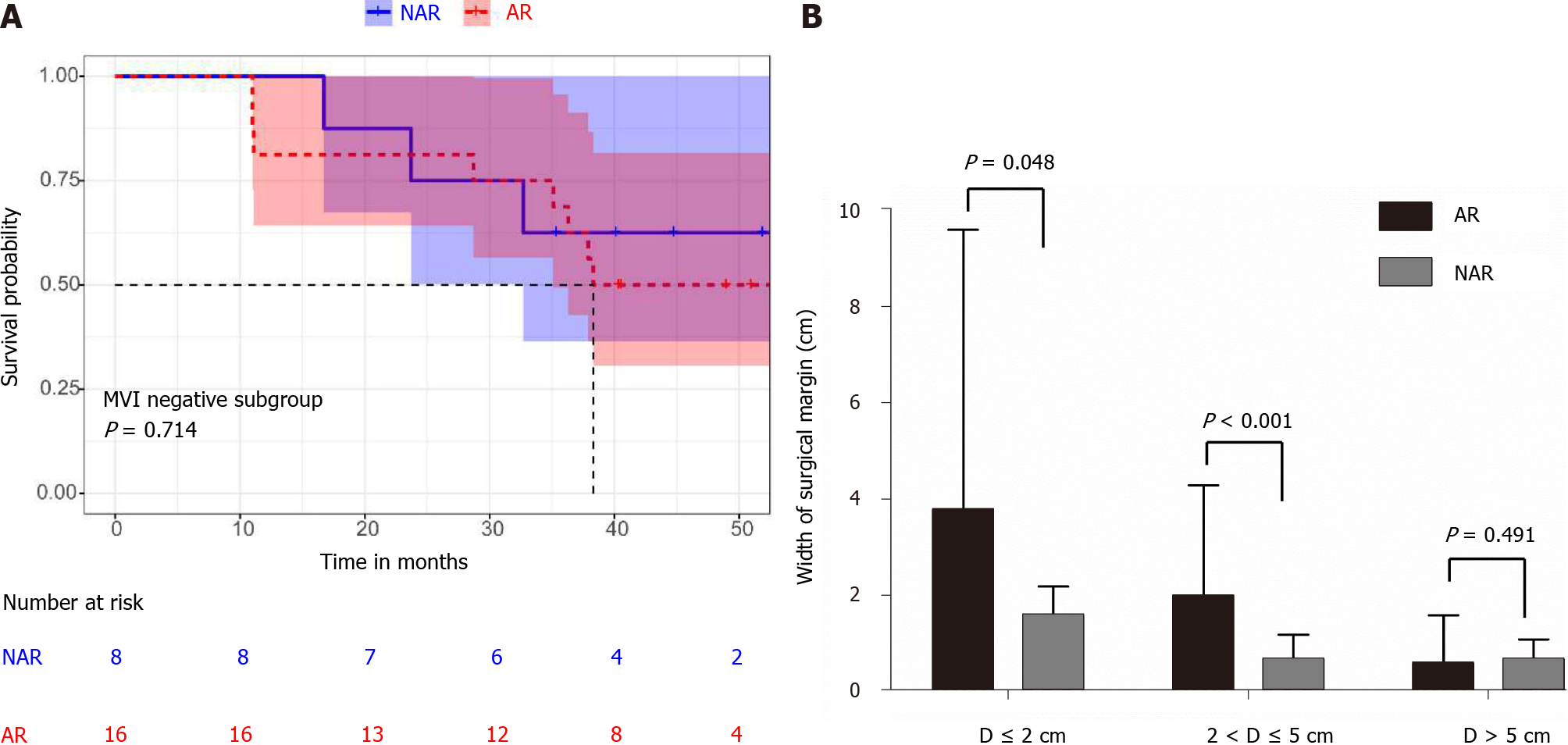
In the 2-5 cm diameter group, the overall survival (OS) of MVI positive patients was significantly better than that of MVI negative patients (P = 0.031). For the MVI positive patients, there was a statistically significant difference between AR and NAR (P = 0.027). AR leads to a wider surgical margin than NAR (2.0 ± 2.3 cm vs 0.7 ± 0.5 cm, P < 0.001). In the groups with tumor diameters < 2 cm, both AR and NAR can obtain a wide surgical margin, and the surgical margins of AR are wider than that of NAR (3.5 ± 5.8 cm vs 1.6 ± 0.5 cm, P = 0.048). In the groups with tumor diameters > 5 cm, both AR and NAR fail to obtain wide surgical margin (0.6 ± 1.0 cm vs 0.7 ± 0.4 cm, P = 0.491).
For patients with a tumor diameter of 2-5 cm, AR can achieve the removal of peritumoral MVI by obtaining a wide incision margin, reduce postoperative recurrence, and improve prognosis.
Core Tip: The prognosis of anatomic resection is better than that of non-anatomic resection with diameters from 2 to 5 cm. For tumor diameters smaller than 2 cm and larger than 5 cm, anatomic resection is not superior to non-anatomic resection. Anatomic resection can achieve the removal of peritumoral microvascular invasion by obtaining a wide incision margin. Both anatomic resection and non-anatomic resection can obtain wide surgical margins in the group with tumor diameters smaller than 2 cm. Both anatomic resection and non-anatomic resection failed to obtain wide surgical margins in the diameter larger than 5 cm group.
- Citation: Zhou JM, Zhou CY, Chen XP, Zhang ZW. Anatomic resection improved the long-term outcome of hepatocellular carcinoma patients with microvascular invasion: A prospective cohort study. World J Gastrointest Oncol 2021; 13(12): 2190-2202
- URL: https://www.wjgnet.com/1948-5204/full/v13/i12/2190.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i12.2190
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, and its high mortality makes it the second leading cause of cancer death[1]. Although the poor prognosis of HCC has improved significantly over the last decade due to increased knowledge of HCC behavior, improvements in staging systems, and multiple therapeutic options compared with other malignancies, HCC still has a high mortality rate[2]. The prognosis of HCC remains very poor due to the high incidence of recurrence and metastasis, and the 5-year recurrence rate after curative treatment remains high (70%), with 15% of HCC patients developing extrahepatic metastasis[3]. One important reason is that tumor cells are able to penetrate the microvasculature, disseminate through the bloodstream to other sites, and form metastatic tumors. Studies have suggested that microvascular invasion (MVI) in HCC is one of the most significant risk factors for recurrence and metastasis in HCC following curative surgical resection[4]. MVI is defined as clusters of cancer cells observed microscopically in vessels located in the tumor capsule and surrounding liver parenchyma[5]. Previous research reported that the incidence of MVI ranged from 15% to 57% in HCC specimens and was associated with tumor size, levels of alpha fetoprotein (AFP), and typical image features[6]. Even for patients with HCC, the presence of MVI increases the risk of recurrence and dramatically shortens long-term survival[7,8]. The main reason for this is that the residual microthrombosis results in early recurrence. A safe surgical margin is a prerequisite for the complete removal of residual microtumor thrombosis. In HCC, invasion of the portal vein and intrahepatic and distant me
We consecutively enrolled 217 patients who underwent AR or NAR from November 2016 to November 2018 at the Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The surgical margin was routinely measured. A pathological specimen of each patient was promptly sent to the pathology department and a detailed pathology report was issued. The pathological characteristics of our data, such as MVI, were derived from the report. The flow diagram of the enrolled patients is displayed in Figure 1. According to the stratification of different tumor diameters, patients were divided into the following groups: ≤ 2 cm group, 2-5 cm group, and > 5 cm group.
The inclusion criteria were: (1) Definitive pathological diagnosis of HCC based on the World Health Organization criteria; (2) Curative resection, defined as complete macroscopic removal of the tumor with negative (R0) margins; (3) No prior anticancer treatment; and (4) Aged between 18 and 80 years. The exclusion criteria were: (1) Distant metastasis; (2) Portal vein tumor thrombosis (PVTT); and (3) Child–Pugh C liver disease. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, and the number of the approval was TJ-IRB20181101. Informed consent was obtained from each patient included in the study.
Completely removing at least one Couinaud segment containing the focus and portal vein in the drainage area of the lesion was defined as an AR. A complete tumor plus a rim of non-neoplastic liver parenchyma was considered an NAR.
All surgeries were accomplished by a team who was able to professionally implement a hepatectomy. Patients were placed in supine position and under general anesthesia. The surgical principles were followed according to the corresponding the Union for International Cancer Control TNM classification. Intraoperative ultrasonography was routinely used in all patients to assess the number and size of the tumors, and their relation to nearby vascular structures. Proper hepatic vascular control techniques, including the selective inflow occlusion (SIO) maneuver and intermittent Pringle maneuvers (IPs), were used to reduce bleeding during liver resection. The SIO maneuver is described by the following procedure: dissecting the portal vein, proper hepatic artery, right and left hepatic arteries, and bile ducts followed by continuously blocking the hepatic artery in the tumor bearing lobe with a bulldog clamp. IPs encircling the hepatoduodenal ligament were performed with cycles of clamping and unclamping times of 15 min and 5 min, respectively.
The patients were surveilled every 1 mo with ultrasonography and AFP during the first 6 mo after surgery and every 3 mo thereafter. Patients were scheduled to have a computerized tomography (CT) scan every 6 mo and a magnetic resonance imaging (MRI) every year. Recurrence was diagnosed by computed tomography scans, magnetic resonance imaging, digital subtraction angiography, and elevated serum AFP level. We reviewed the governmental death registration and performed telephone follow-ups. Patients were excluded if they were not followed up as required, or their governmental data were incomplete. Follow-up was terminated on May 31, 2021. Patients lost to follow-up and with missing data were prematurely excluded. Ul
First, all patients were randomized to receive standard anatomic or non-anatomic resection. After surgery, we measured the surgical margin and identified micro
Data are presented as the mean ± SD. The overall survival (OS) was analyzed using Kaplan-Meier survival curves and a log-rank test. Student’s t-tests were used for comparison between groups where appropriate. A χ2 test was used for comparison between groups where appropriate. P < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 19.0.
The mean follow-up time was 45.2 ± 6.3 mo (median: 46.0 mo; range: 30.6-53.4 mo). The cumulative survival rate for all patients was 90%, 57%, and 39% at 1, 3, and 5 years. All of the category boundaries were defined by the clinical guideline or recognized criterion when continuous variables were categorized. Table 1 demon
| Clinical characteristics | Value |
| Age, yr, (mean ± SD) | 52.6 ± 12.4 |
| Sex, n (%) | |
| Male | 197 (90.8) |
| Female | 20 (9.2) |
| ALT (U/mL) | 30.2 ± 14.3 |
| AST (U/mL) | 38.6 ± 32.0 |
| TBiL (μmol/L) | 15.8 ± 9.6 |
| Child-Pugh score, n (%) | |
| A | 209 (96.3) |
| B | 8 (3.7) |
| Hepatitis virus, n (%) | |
| HBV | 178 (82.0) |
| HCV | 10 (4.6) |
| No | 29 (13.4) |
| Liver cirrhosis, n (%) | |
| No | 43 (19.8) |
| Yes | 174 (80.2) |
| ICG-R15 (%) | 6.6 ± 3.8 |
| AFP (ng/mL), n (%) | |
| ≤ 400 | 144 (66.4) |
| > 400 | 73 (33.6) |
| Tumor diameter (cm) | 4.9 ± 3.5 |
| No. of tumor, n (%) | |
| Singe | 157 (72.4) |
| Multiple | 60 (27.6) |
| Microvascular invasion, n (%) | |
| Yes | 75 (34.6) |
| No | 142 (65.4) |
| BCLC stage, n (%) | |
| 0+A | 92 (42.4) |
| B+C | 125 (57.6) |
| Operation method, n (%) | |
| Open | 56 (25.8) |
| Laparoscopic | 161 (74.2) |
| Operation time (min) | 171 ± 40 |
| Blood loss (mL) | 160 ± 180 |
| Blood transfusion, n (%) | |
| No | 189 (87.1) |
| Yes | 28 (12.9) |
| Hepatic vascular occlusion, n (%) | |
| No | 64 (29.5) |
| Yes | 153 (70.5) |
| Clinical characteristics | AR (n = 103) | NAR (n = 114) | P value |
| Age, yr (mean ± SD) | 54.1 ± 12.5 | 51.3 ± 12.2 | 0.095 |
| Sex, n (%) | 0.812 | ||
| Male | 93 (90.3) | 104 (91.2) | |
| Female | 10 (9.7) | 10 (8.8) | |
| ALT (U/mL) | 30.5 ± 14.5 | 29.8 ± 14.2 | 0.721 |
| AST (U/mL) | 36.3 ± 28.9 | 40.6 ± 34.6 | 0.332 |
| TBiL (μmol/L) | 17.0 ± 10.5 | 15.9 ± 9.0 | 0.098 |
| Child-Pugh score, n (%) | 0.154 | ||
| A | 103 (100) | 106 (93.0) | |
| B | 0 (0) | 2 (7.0) | |
| HBsAg, n (%) | 0.622 | ||
| Positive | 88 (85.4) | 100 (87.7) | |
| Negative | 15 (14.6) | 14 (12.3) | |
| Liver cirrhosis, n (%) | 0.841 | ||
| No | 21 (20.4) | 22 (19.3) | |
| Yes | 82 (79.6) | 92 (80.7) | |
| AFP (ng/mL), n (%) | 0.446 | ||
| < 400 | 71 (68.9) | 73 (64.0) | |
| ≥ 400 | 32 (31.1) | 41 (36.0) | |
| ICG-R15 (%) | 5.9 ± 3.5 | 5.4 ± 3.1 | 0.578 |
| BCLC stage, n (%) | 0.867 | ||
| 0 + A | 46 (44.7) | 46 (40.4) | |
| B + C | 57 (55.3) | 68 (59.6) | |
| Operation method, n (%) | 0.423 | ||
| Open | 24 (23.3) | 32 (28.1) | |
| Laparoscopic | 79 (76.7) | 82 (71.9) | |
| Operation time (min) | 171.4 ± 48.2 | 165.5 ± 45.3 | 0.335 |
| Blood loss (mL) | 210 ± 233 | 170 ± 175 | 0.233 |
| Blood transfusion, n (%) | 0.774 | ||
| No | 89 (86.4) | 100 (87.7) | |
| Yes | 14 (13.6) | 14 (12.3) | |
| Hepatic vascular occlusion, n (%) | 0.314 | ||
| No | 27 (26.2) | 37 (32.5) | |
| Yes | 76 (73.8) | 77 (67.5) | |
| Largest tumor size, (cm) | 5.1 ± 3.6 | 4.7 ± 3.3 | 0.507 |
| No. of tumors, n (%) | 0.884 | ||
| Single | 75 (72.8) | 82 (71.9) | |
| Multiple | 28 (27.2) | 32 (28.1) | |
| Tumor encapsulation, n (%) | 0.051 | ||
| No | 57 (55.3) | 66 (57.9) | |
| Yes | 46 (44.7) | 48 (42.1) | |
| Satellite lesion, n (%) | 0.822 | ||
| Yes | 9 (8.7) | 9 (7.9) | |
| No | 94 (91.3) | 105 (92.1) | |
| Tumor differentiation stage, n (%) | 0.943 | ||
| Edmondson I II | 51 (49.5) | 57 (50.0) | |
| Edmondson III IV | 52 (50.5) | 57 (50.0) | |
| Microvascular invasion, n (%) | 0.845 | ||
| Yes | 37 (35.9) | 38 (33.3) | |
| No | 66 (64.1) | 76 (66.7) |
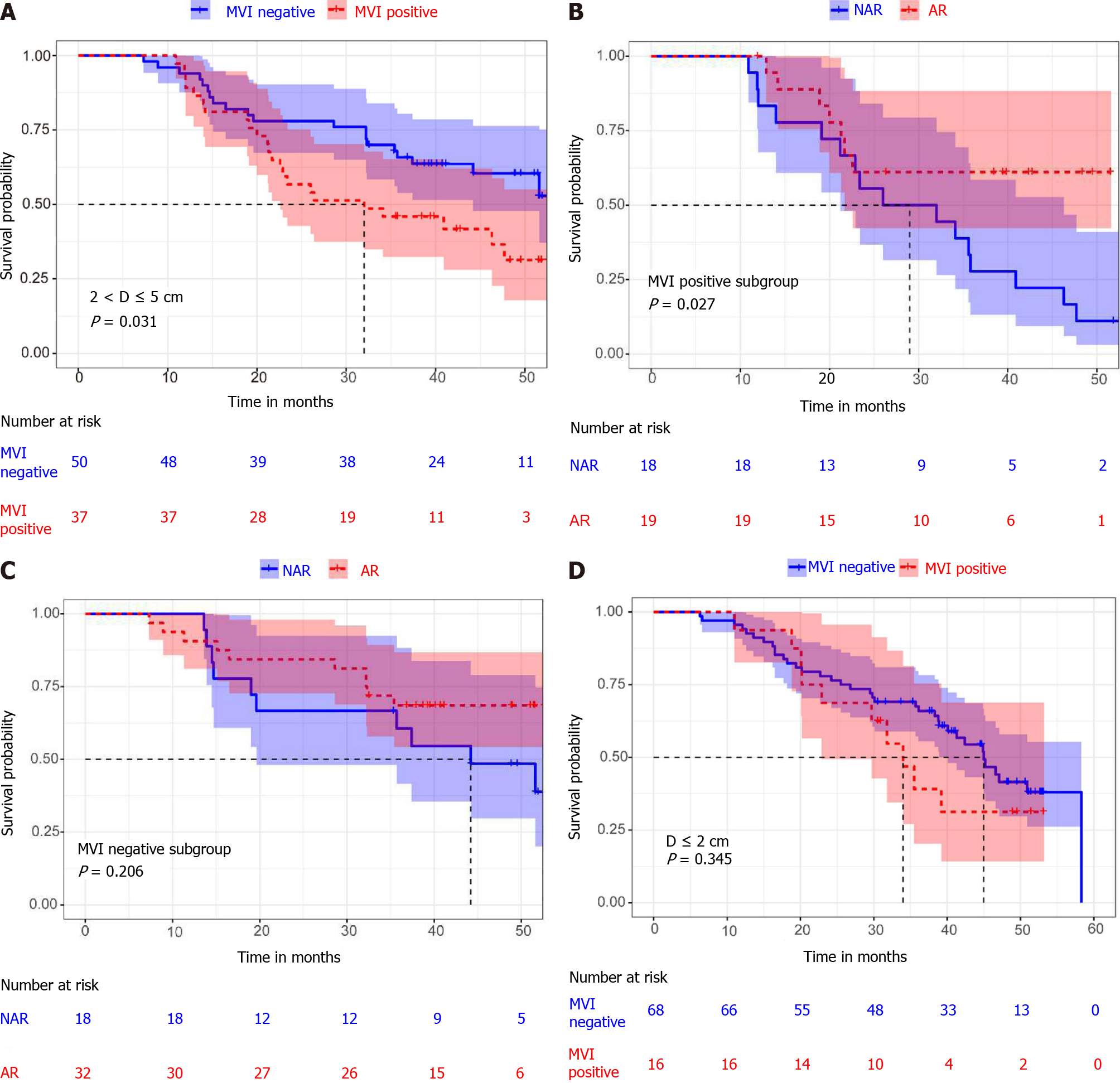
The 217 included patients were divided into three groups: diameter ≤ 2 cm (84/217), 2-5 cm (87/217) and > 5 cm (46/217). In the diameter 2-5 cm group, there was a statistically significant difference between MVI positive and MVI negative patients (median OS 32.0 mo, 95% confidence interval (CI): 13.3-50.8 mo vs not reached, P = 0.031) (Figure 2A). For the MVI positive patients, there was a statistically significant difference between AR and NAR (median OS not reached vs 29.0 mo 95%CI: 8.0-43.9 mo, P = 0.027) (Figure 2B). However, for the MVI negative patients, there were no statistically significant differences between those who underwent AR and NAR (median OS not reached vs 44.2 mo, 95%CI: 27.0-61.5 mo, P = 0.206) (Figure 2C). This suggests that AR improved OS only in MVI positive patients, but not in MVI negative patients in the diameter 2-5 cm group. In addition, we compared the surgical margin of AR and NAR in the 2-5 cm diameter group. We found that AR led to a wider surgical margin than NAR (2.0 ± 2.3 cm vs 0.7 ± 0.5 cm, P < 0.001) (Table 3). We speculated that AR can achieve a wide enough surgical margin, which can remove MVI in advance, reducing the risk of postoperative recurrence and improving the prognosis.
| Tumor diameter | Surgical margin (cm) | ||
| AR | NAR | P value | |
| D ≤ 2 cm | 3.5 ± 5.8 | 1.6 ± 0.5 | 0.048 |
| 2 cm < D ≤ 5 cm | 2.0 ± 2.3 | 0.7 ± 0.5 | < 0.001 |
| D > 5 cm | 0.6 ± 1.0 | 0.7 ± 0.4 | 0.491 |
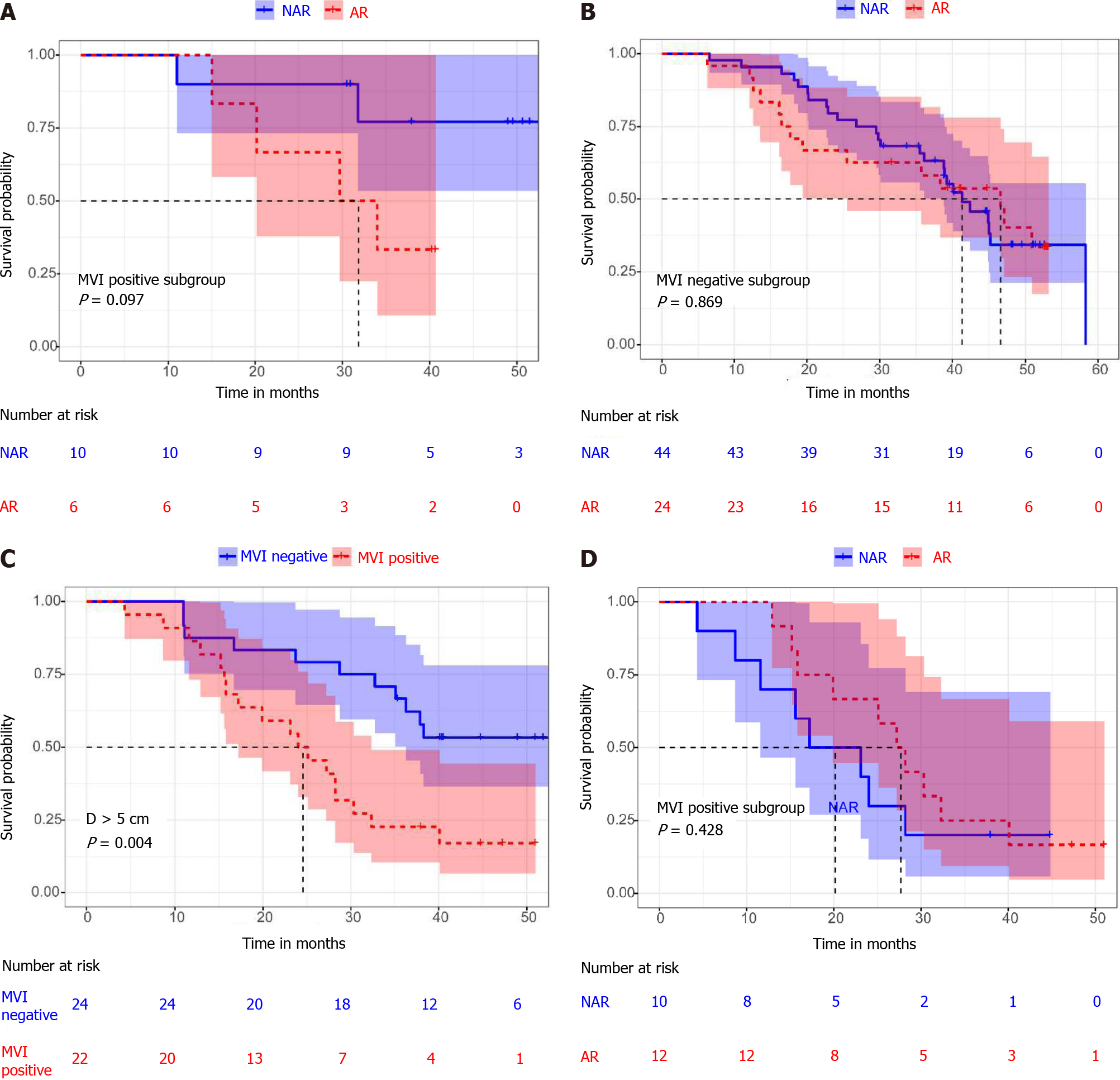
Among patients with a diameter less than 2 cm, there was no statistically significant difference between MVI positive and MVI negative patients (median OS 34 mo 95%CI: 27.6-40.4 mo vs 45.0 mo 95%CI: 39.4-50.6 mo, P = 0.345) (Figure 2D). In addition, there was no statistically significant difference in overall survival between patients who received AR and NAR, whether MVI positive or MVI negative (median OS 32.4 mo 95%CI: 13.0-46.3 mo vs not reached, P = 0.097; median OS 46.6 mo, 95%CI: 32.8-60.4 mo vs 41.3 mo, 95%CI: 34.6-48.1 mo, P = 0.869) (Figure 3A and B). By comparing the surgical margins of AR and NAR patients, we found that although the AR margins were wider than those of NAR patients, the margins of both were greater than 1 cm (3.5 ± 5.8 cm vs 1.6 ± 0.5 cm, P = 0.048) (Table 3; Figure 4B). If a surgical margin of 1 cm is ensured, it can be clinically regarded as a R0 resection. Thus, for patients with a tumor of less than 2 cm in diameter, both AR and NAR can achieve a wide surgical margin to ensure the removal of MVI.
In the group with a diameter > 5 cm, the prognosis of MVI positive patients was significantly worse than that of MVI negative patients (median OS 24.0 mo, 95%CI: 15.7-32.4 mo vs not reached, P = 0.004) (Figure 3C). However, there was no statistically significant difference in overall survival between patients who received AR and NAR, whether MVI positive or MVI negative, (median OS 27.2 mo, 95%CI: 21.9-32.5 mo vs 20.1 mo, 95%CI: 5.6-28.8 mo, P = 0.428; median OS not reached vs 38.3 mo, 95%CI: 19.5-60.5 mo, P = 0.714) (Figures 3D and 4A). In addition, there were no statistically significant differences between AR and NAR in surgical margins and the margins of both were less than 1 cm (0.6 ± 1.0 cm vs 0.7 ± 0.4 cm, P = 0.491) (Table 3; Figure 4B). For patients with tumors larger than 5 cm in diameter, neither AR nor NAR could obtain a wide enough surgical margin to ensure the removal of MVI.
At present, surgeons consider hepatectomy and liver transplantation the optimal therapies to improve prognosis in HCC; however, tumor recurrence is still an important cause of death in patients[16]. Previous research has demonstrated that microvascular invasion is a vital risk factor for the prognosis of HCC patients after curative hepatectomy[17]. As long as a surgical margin of 1 cm is ensured, it can be clinically regarded as an R0 resection[18]. The margin of microvascular invasion is generally no more than 1 cm. Therefore, an R0 resection enables the complete removal of the liver tissue invaded by the microvascular thrombosis. In contrast, positive margins were associated with a worse prognosis[19,20]. Previous studies have shown that a wider surgical margin has been associated with a better prognosis among patients with HCC[21-23]. In addition, a previous study has shown that AR led to a better OS than NAR. In a multivariable analysis, an AR was one of the prognostic factors[9]. AR can reduce the risk of tumor residues and recurrence due to the elimination of venous tumor thrombosis within the resected domain, when at least one complete Couinaud segment and the portal vein in the drainage area of the lesion are removed[24,25]. However, almost all patients with HCC have liver cirrhosis and excessive removal of non-neoplastic liver parenchyma can lead to liver dysfunction and the morbidities of ascites, jaundice, and hypoalbuminemia.
Our data indicated that AR improved OS only in MVI positive patients, but not in MVI negative patients in the 2-5 cm diameter group. We speculated that AR can achieve a wide enough surgical margin, which can remove MVI in advance, reduce the risk of postoperative recurrence, and improve the prognosis. For patients with a tumor diameter of less than 2 cm, both AR and NAR can obtain a wide surgical margin to ensure removal of MVI. Therefore, patients with a diameter less than 2 cm, both AR and NAR, can achieve a good prognosis. In other words, an R0 resection enabled the removal of the liver tissue invaded by the MVI regardless of whether AR or NAR was chosen by the surgeon. This suggests that AR is not necessary for tumors with a diameter of less than 2 cm, as long as sufficient surgical margin is ensured. However, in the > 5 cm group, both AR and NAR cannot guarantee sufficient surgical margin, which is one of the reasons why tumors with a diameter of more than 5 cm have a worse prognosis than tumors with a diameter of less than 2 cm. We believe that the surgeon needs to consider whether the residual liver volume and liver function reserve are sufficient when faced with a very large tumor. Therefore, for tumor diameters larger than 5 cm, the width of the resection margin should be increased appropriately when a sufficient liver volume and a good liver function can be ensured.
The limitations of this study are the relatively small samples, short follow-up time, and a single study center cohort study. A multicenter clinical trial should be designed to further validate the prognostic significance of types of hepatectomy in HCC.
For patients with a tumor diameter of 2-5 cm, AR can achieve the removal of pe
At present, most studies suggest that anatomical resection is more effective than non-anatomical resection in the tumor diameter ranging from 2 cm to 5 cm. However, for tumors smaller than 2 cm and larger than 5 cm in diameter, the advantage of anatomic hepatectomy is not significant. Why is that? Does anatomic resection (AR) have an advantage over non-anatomic resection (NAR) in hepatocellular carcinoma (HCC) patients with microvascular invasion (MVI)?
Our study aimed to determine the effects of AR and NAR in different tumor diameter stratification. Further analysis shows that AR improves patient outcomes by obtaining a wider surgical margin.
This study compared the efficacy of AR and NAR in different tumor diameter subgroups in a prospective cohort study.
First, all patients were randomized to receive standard anatomic or non-anatomic resection. After surgery, we measured the surgical margin and identified micro
When the tumor is enormous and the remaining liver tissue is insufficient, AR may not be appropriate. For patients with a tumor diameter of 2-5 cm, AR can achieve the removal of peritumoral MVI by obtaining a wide incision margin, reducing post
The doctor should ensure sufficient surgical margin on the premise of ensuring the safety of the operation. Therefore, for patients with a tumor diameter of 2-5 cm, AR should be strongly recommended.
The study could guide doctors in their choice of surgical procedures. In general, AR guarantees a wider surgical margin. However, a wider surgical margin means that more healthy liver tissue has to be removed. Almost all patients with HCC have liver cirrhosis, and the excessive removal of non-neoplastic liver parenchyma can lead to liver dysfunction and the morbidities of ascites, jaundice, and hypoalbuminemia. When the tumor is enormous and the remaining liver tissue is insufficient, AR may not be appropriate.
The authors would like to thank Dr. Chang Shu who is a of statistics (Translational Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology) for her technical assistance of statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yagi H S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64596] [Article Influence: 16149.0] [Reference Citation Analysis (176)] |
| 2. | Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 433] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 3. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 658] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 4. | Wang L, Jin YX, Ji YZ, Mu Y, Zhang SC, Pan SY. Development and validation of a prediction model for microvascular invasion in hepatocellular carcinoma. World J Gastroenterol. 2020;26:1647-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Shirabe K, Toshima T, Kimura K, Yamashita Y, Ikeda T, Ikegami T, Yoshizumi T, Abe K, Aishima S, Maehara Y. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int. 2014;34:937-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, Brandi G, Pinna AD, Golfieri R. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma? Radiology. 2016;279:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 292] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 7. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 8. | Erstad DJ, Tanabe KK. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26:1474-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 9. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 503] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 10. | Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, Ikai I, Kudo M, Kojiro M, Makuuchi M, Monden M, Matsuyama Y, Nakanuma Y, Takayasu K; Liver Cancer Study Group of Japan. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Moris D, Tsilimigras DI, Kostakis ID, Ntanasis-Stathopoulos I, Shah KN, Felekouras E, Pawlik TM. Anatomic versus non-anatomic resection for hepatocellular carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol. 2018;44:927-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Couinaud C. Anatomic principles of left and right regulated hepatectomy: technics. J Chir (Paris). 1954;70:933-966. [PubMed] |
| 13. | Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, Felekouras E, Dillhoff M, Schmidt C, Pawlik TM. Parenchymal-Sparing Versus Anatomic Liver Resection for Colorectal Liver Metastases: a Systematic Review. J Gastrointest Surg. 2017;21:1076-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Viganò L, Procopio F, Mimmo A, Donadon M, Terrone A, Cimino M, Fabbro DD, Torzilli G. Oncologic superiority of anatomic resection of hepatocellular carcinoma by ultrasound-guided compression of the portal tributaries compared with nonanatomic resection: An analysis of patients matched for tumor characteristics and liver function. Surgery. 2018;164:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Field WBS, Rostas JW, Philps P, Scoggins CR, McMasters KM, Martin RCG 2nd. Wide versus narrow margins after partial hepatectomy for hepatocellular carcinoma: Balancing recurrence risk and liver function. Am J Surg. 2017;214:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Lee MW, Lim HK. Management of sub-centimeter recurrent hepatocellular carcinoma after curative treatment: Current status and future. World J Gastroenterol. 2018;24:5215-5222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Yamashita YI, Imai K, Yusa T, Nakao Y, Kitano Y, Nakagawa S, Okabe H, Chikamoto A, Ishiko T, Yoshizumi T, Aishima S, Maehara Y, Baba H. Microvascular invasion of single small hepatocellular carcinoma ≤3 cm: Predictors and optimal treatments. Ann Gastroenterol Surg. 2018;2:197-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Cherqui D, Tantawi B, Alon R, Piedbois P, Rahmouni A, Dhumeaux D, Julien M, Fagniez PL. Intrahepatic cholangiocarcinoma. Results of aggressive surgical management. Arch Surg. 1995;130:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, Zöpf T, Trarbach T, Malagó M, Baba HA, Broelsch CE. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP, Yang G, Yan X, Zhang YD, Liu XS. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 494] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 21. | Kabir T, Syn NL, Tan ZZX, Tan HJ, Yen C, Koh YX, Kam JH, Teo JY, Lee SY, Cheow PC, Chow PKH, Chung AYF, Ooi LL, Chan CY, Goh BKP. Predictors of post-operative complications after surgical resection of hepatocellular carcinoma and their prognostic effects on outcome and survival: A propensity-score matched and structural equation modelling study. Eur J Surg Oncol. 2020;46:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Aoki T, Kubota K, Hasegawa K, Kubo S, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, Nakashima O, Matsuyama Y, Murakami T, Kudo M; Liver Cancer Study Group of Japan. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg. 2020;107:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Tsilimigras DI, Sahara K, Moris D, Hyer JM, Paredes AZ, Bagante F, Merath K, Farooq AS, Ratti F, Marques HP, Soubrane O, Azoulay D, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Effect of Surgical Margin Width on Patterns of Recurrence among Patients Undergoing R0 Hepatectomy for T1 Hepatocellular Carcinoma: An International Multi-Institutional Analysis. J Gastrointest Surg. 2020;24:1552-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Wakai T, Shirai Y, Sakata J, Kaneko K, Cruz PV, Akazawa K, Hatakeyama K. Anatomic resection independently improves long-term survival in patients with T1-T2 hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1356-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.5] [Reference Citation Analysis (0)] |