Published online Dec 15, 2021. doi: 10.4251/wjgo.v13.i12.2149
Peer-review started: April 16, 2021
First decision: June 27, 2021
Revised: July 13, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: December 15, 2021
Processing time: 242 Days and 21 Hours
While clinical guidelines recommend hepatocellular carcinoma (HCC) surveil
To quantify HCC surveillance in an Australian cohort, and assess for factors asso
All patients undergoing HCC surveillance liver ultrasounds between January 1, 2018 to June 30, 2018 at a tertiary hospital in Melbourne, Australia, were followed until July 31, 2020, or when surveillance was no longer required. The primary outcome was the percentage of time up-to-date with HCC surveillance (PTUDS). Quantile regression was performed to determine the impact of factors associated with HCC surveillance underutilisation.
Among 775 at-risk patients followed up for a median of 27.5 months, the median PTUDS was 84.2% (IQR: 66.3%-96.3%). 85.0% of patients were followed up by specialist gastroenterologists. Amongst those receiving specialist care, quantile regression demonstrated differential associations at various quantile levels of PTUDS for several factors. Older age at the 25th quantile (estimate 0.002 per percent, P = 0.03), and cirrhotic status at the 75th quantile (estimate 0.021, P = 0.017), were significantly associated with greater percentage of time up-to-date. African ethnicity (estimate -0.089, P = 0.048) and a culturally and linguistically diverse (CALD) background (estimate -0.063, P = 0.01) were significantly associated with lower PTUDS at the 50th quantile, and again for CALD at the 75th quantile (estimate -0.026, P = 0.045).
While median PTUDS in this Australian cohort study was 84.2%, awareness of the impact of specific factors across PTUDS quantiles can aid targeted interventions towards improved HCC surveillance.
Core Tip: This study evaluated the uptake of liver cancer screening in a cohort of high-risk Australians, and found that on average, patients were up-to-date with their surveillance for 84.2% of the study time period. Certain factors, such as absence of cirrhosis, younger age, African ethnicity and a non-English speaking background were associated to varying degrees with lower time up-to-date with hepatocellular carcinoma screening.
- Citation: Low ES, Apostolov R, Wong D, Lin S, Kutaiba N, Grace JA, Sinclair M. Hepatocellular carcinoma surveillance and quantile regression for determinants of underutilisation in at-risk Australian patients. World J Gastrointest Oncol 2021; 13(12): 2149-2160
- URL: https://www.wjgnet.com/1948-5204/full/v13/i12/2149.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i12.2149
Globally, hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and is the fourth leading cause of cancer-related mortality[1-3]. Over the last three decades in Australia, there has been a 306% increase in age-standardised incidence of liver cancer, and a corresponding 184% increase in attributable mortality rates[4]. These concerning escalations are mirrored in recent reports from the United States, and the age-adjusted worldwide incidence of HCC stands at 10.1 cases /100,000 person-years[5,6].
While curative treatment options offer 5-year survival rates in excess of 70%, these are only feasible at early disease stages, often when HCC is clinically silent[1,7].A meta-analysis of cohort studies demonstrated improved early-stage detection and associated survival with HCC screening in at-risk populations[8]. This is particularly accepted in the non-cirrhotic population, for whom surgical resection is often a viable and life-saving intervention for early HCC[9]. As such, international professional societies recommend that at-risk patients undergo HCC surveillance with liver ultrasound (USS) every 6 months, with or without concurrent alpha fetoprotein (AFP) measurements[10,11].
The effectiveness of HCC surveillance relies on consistent clinical uptake of screening, particularly as sensitivity rates of ultrasonography for the detection of HCC are reported to be as low as 60%, thus requiring serial scans to ensure maximum yield[6]. However, several cohort studies have supported longstanding concerns of HCC surveillance underutilisation in clinical practice, with two recent meta-analyses reporting overall pooled adherence rates of 24% and 52%[3,12]. Research into adherence to surveillance is limited by the marked heterogeneity in the definitions of adherence in the literature[3]; studies vary from requiring perfect 6-monthly adherence, to allowing leeway periods of up to 12 mo between imaging, raising the possibility of lost interim early-stage HCC detection[3].
To better incorporate both frequency of screening and quantity of imaging performed, the concept of ‘percentage of time up-to-date with surveillance’ (PTUDS) has been proposed as a more robust, continuous metric to standardise the measurement of adherence to HCC surveillance[3,7]. This retrospective cohort study aimed to quantify the percentage of time up-to-date with HCC screening in an Australian cohort of ‘at-risk’ patients, and to identify determinants associated with higher adherence to surveillance.
This retrospective cohort study at a large tertiary hospital in Melbourne, Australia captured all at-risk patients identified by radiology records as undergoing a liver ultrasound scan (USS) for HCC surveillance within the Austin Hospital radiology department between 1 January 2018 to 30 June 2018. All USS were requested by gastroenterologists, infectious disease physicians and nurse practitioners who manage patients deemed at-risk of HCC in outpatient specialty clinics. Most at-risk patients at our centre were managed in physician-led specialty clinics. A proportion of consenting patients, who were felt to be at low risk of progressive liver disease, were primarily managed in a nurse-led, HCC screening clinic. Eligible patients for the nurse-led clinic were patients not requiring treatment for chronic hepatitis B, and patients with well-compensated cirrhosis from a disease aetiology that was considered adequately treated. All patients referred to the nurse-led clinic were previously seen by physicians and physician oversight was available if needed.
At-risk patients for HCC development were defined according to AASLD guidelines, and included those with cirrhosis irrespective of aetiology, and patients with chronic hepatitis B and additional HCC risk factors (i.e. family history of HCC, African descent, Asian males over 40 years old, Asian females over 50 years old)[10]. Our institution also performs surveillance for patients with chronic hepatitis C and advanced fibrosis (F3 and above), as HCC incidence in this demographic may surpass the threshold for cost-effective screening[6]. Patients who did not meet this criteria or had a documented history of HCC within the last 2 years were excluded.
Patients were followed-up from the index USS until July 31, 2020, or when surveillance was no longer required. Reasons for this included HCC diagnosis (after which patients were diverted into our liver cancer clinic), death, receipt of an orthotopic liver transplant, or when surveillance was no longer deemed appropriate, such as cases of elderly multi-comorbid patients with limited life expectancy. Follow-up periods were also truncated for patients who were discharged to other medical services. In these cases, follow-up end-date was documented as date of the formal discharge letter written by attending clinicians to the patient and/or their primary care physician. This acknowledged the possibility of subsequent external imaging and follow-up.
This study was approved by the Austin Health Research Ethics Committee and carried out in line with the National Statement on Ethical Conduct in Human Research (2007).
Patient demographics, clinical history, laboratory investigations and imaging results were extracted from their electronic medical records at the time of inclusion. Diagnoses of chronic hepatitis B were verified by record review of positive hepatitis B surface antigen or HBV DNA tests at least 6 mo apart. Confirmation of chronic hepatitis C occurred if there was evidence of positive hepatitis C antibody or viral RNA. All cirrhosis diagnoses were verified by review of clinical records by a consultant gastroenterologist, where cirrhosis was confirmed using a combination of clinical, biochemical, radiological and histological findings. Scanned medical records were also reviewed to retrieve externally performed liver imaging.
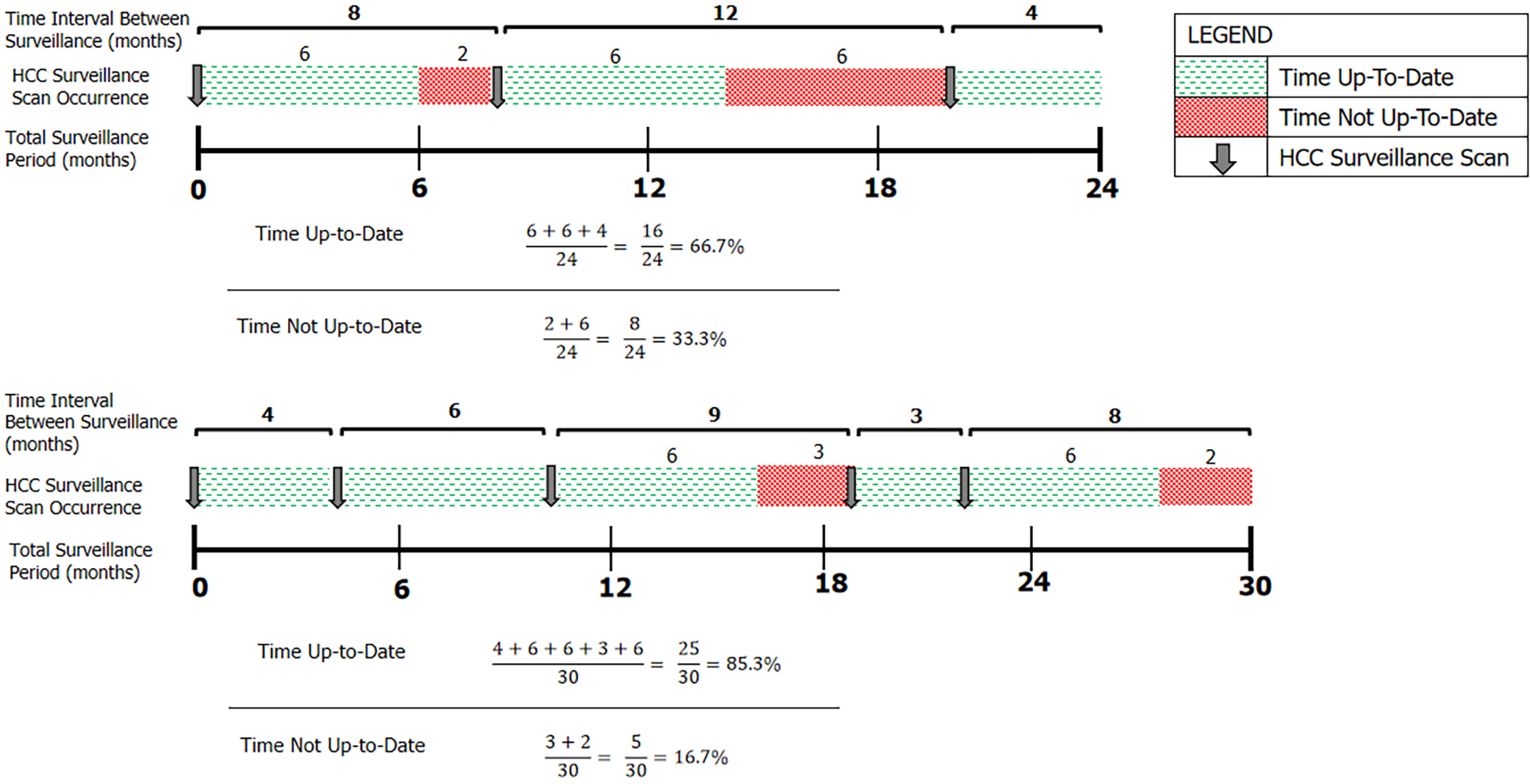
The primary outcome was adherence to HCC surveillance imaging. Accepted imaging modalities included targeted liver USS, or contrast-enhanced CT or MRI, given their routine use in clinical practice when USS images are inadequate. Adherence was defined as the percentage of time up-to-date with screening (PTUDS, %), calculated as the percentage of an individual’s total follow-up time in which they were within 6 mo of an accepted HCC surveillance test. This endpoint was chosen as it accounted for both number and timing of surveillance imaging performed during the screening period. Following surveillance imaging, patients were credited as having 6 mo of ‘time up-to-date’. Any subsequent imaging that occurred within that 6-month period resulted in a re-set of the 6-month interval clock from the date that the new imaging was performed (Figure 1). AFP levels were recorded, but were not included in the primary outcome, given AFP testing is not considered a stand-alone surveillance test.
Demographic variables, based on literature-reported factors, were also extracted from the database and assessed for potential associations with uptake of HCC surveillance. These variables of interest included age, gender, ethnicity, primary language spoken, aetiology of liver disease, cirrhosis status and MELD score.
Descriptive statistical analysis was reported as proportions (%) for categorical variables, and means (with standard deviations) or medians (with IQRs) for continuous variables, depending on the distribution of values. Comparative analysis was conducted using the Student t-test and Wilcoxon rank-sum tests. Univariable and multivariable quantile regression analysis was then performed to assess for factors associated with PTUDS, particularly variables with significant associations at different PTUDS quantiles. Quantile regression modelling was chosen given the non-parametric distribution of the outcome variable relative to covariates, its flexibility in assessing the relationship between determinants of surveillance and PTUDS at the upper and lower tails, and the lack of stringent model assumptions required for valid inference. The median (50th), 25th, and 75th quantiles were selected to provide a description of covariate associations across the range of the distribution of PTUDS, whilst avoiding the effect of extreme outliers. Bootstrapping was used to calculate 95% confidence intervals around the estimates. Both univariate and multivariate models were constructed separately for each of the selected PTUDS quantiles to determine if there were differential covariates associations across the distribution.
Data analysis was performed using Statistical Analysis Software (SAS) v.9.4 and R Software v.4.0.3 using the quantreg package[13,14].
We identified 838 patients who underwent liver USS with radiology record requests for HCC surveillance within the 6-month recruitment period. On further review, 22 patients were excluded due to prior liver transplantation, 1 due to recent history (< 2 years) of HCC, 9 who did not meet criteria for screening (incorrectly classified as cirrhotic) and 31 with chronic hepatitis B who did not meet guideline criteria for surveillance. 775 patients were included in the final analysis. Baseline characteristics are detailed in Table 1.
| Characteristic | n = 775 (%) | mean ± SD | Median (IQR) | |
| Age (years) | 60.0 ± 12.2 | |||
| Sex | Male | 457 (59.0) | ||
| Female | 318 (41.0) | |||
| Ethnicity | Caucasian | 442 (57.0) | ||
| Asian | 249 (32.1) | |||
| African/Middle-Eastern | 68 (8.8) | |||
| Unreported | 16 (2.1) | |||
| Primary language spoken | English | 573 (73.9) | ||
| Non-English | 202 (26.1) | |||
| Indication for hepatocellular carcinoma surveillance | Cirrhosis | 429 (55.3) | ||
| Chronic HBV | 343 (44.3) | |||
| Chronic HCV with Advanced Fibrosis | 3 (0.4) | |||
| Aetiology of Cirrhosis, n = 429 | HBV hepatitis | 58 (13.5) | ||
| HCV hepatitis | 136 (31.7) | |||
| Alcoholic hepatitis | 103 (24.0) | |||
| NASH | 64 (14.9) | |||
| Non-viral, non-alcoholic, non-NASH cirrhosis1 | 68 (15.9) | |||
| HBV anti-viral use, n = 385 | Yes | 206 (53.5) | ||
| No | 179 (46.5) | |||
| Specialty care clinic | General hepatology2 | 453 (58.5) | ||
| Pre-transplant | 126 (16.3) | |||
| Nurse-led surveillance | 80 (10.3) | |||
| Non-liver3 | 76 (9.8) | |||
| Unspecified | 40 (5.2) | |||
| MELD score, n = 429 | 9 (7-13) | |||
The mean age of patients was 60.0 (standard deviation 12.2) years. The majority were male (59.0%), Caucasian (57.0%) and English-speaking (73.9%), with cirrhosis the most common indication for HCC surveillance (55.3%). Of those with cirrhosis, the median MELD score at inclusion was 9. The majority of patients were followed up in hepatology clinics (58.5% general liver, 16.3% pre-transplant, 10.3% nurse-led). The median follow-up time was 27.5 mo (IQR: 26.0-29.0 months). The most commonly used screening modality was ultrasound (86.9%), followed by contrast-enhanced CT liver (9.4%) and contrast-enhanced MRI (3.7%). AFP was performed in conjunction to imaging at least once in 91.9% of patients.
Twenty patients had an interim orthotopic liver transplant, 22 died and 41 were discharged from clinic surveillance, either due to service nonattendance (36%), transfer of care to other institutions (50%), or when surveillance was deemed no longer clinically appropriate (14%). HCCs developed in 22 patients (2.8%) over the course of the study. Of these 22 HCCs, 14 (64%) were detected at an early stage.
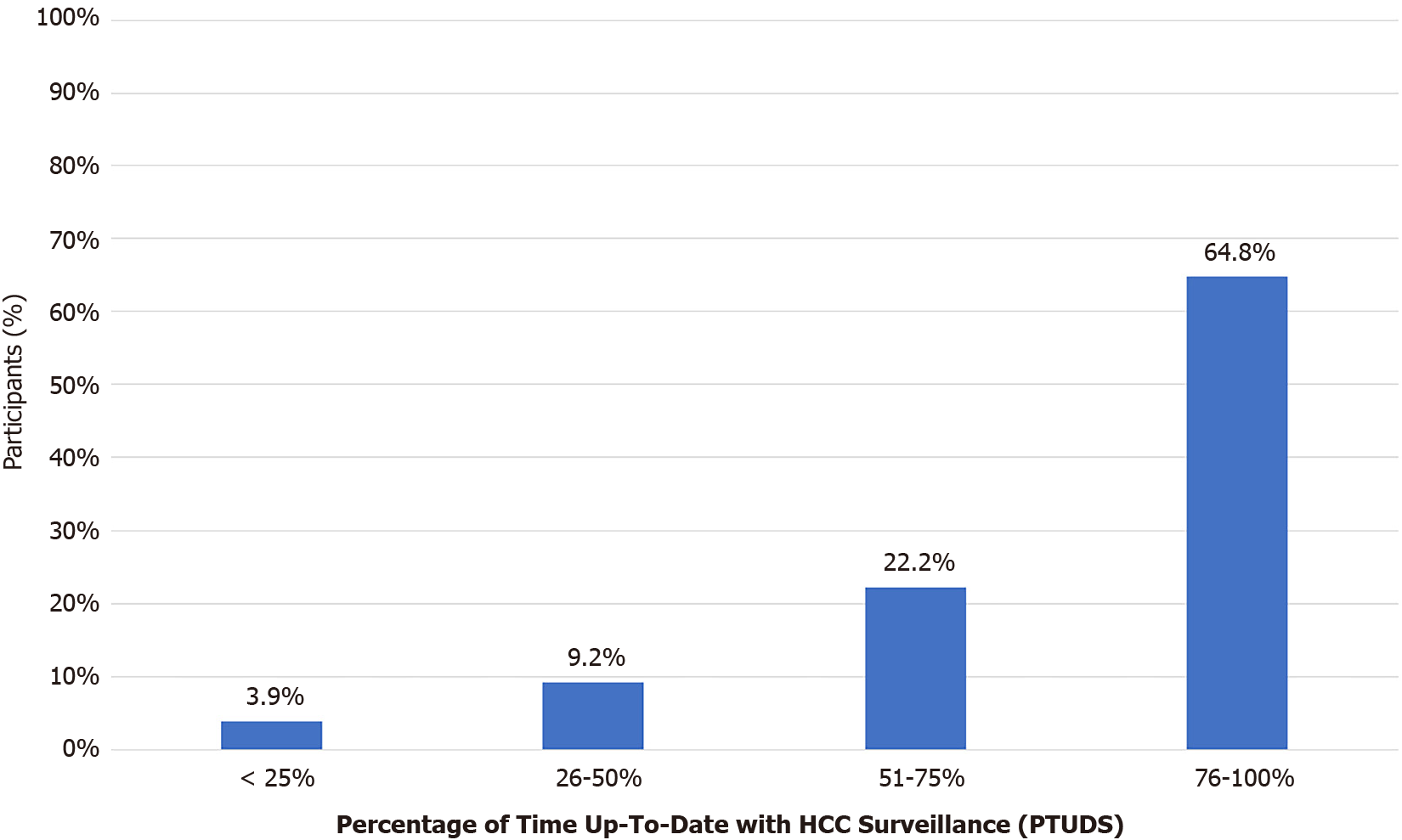
The median PTUDS overall was 84.2% (IQR: 66.3%-96.3%). 13.2% of patients were up-to-date for less than 50% of their surveillance time period, representing average screening intervals of less frequently than 12-monthly (see Figure 2). Only 40% of patients spent at least 90% of their follow-up period up-to-date with surveillance.
Older age (≥ 60 years old), cirrhotic status, Non-Asian ethnicity and English-speaking as the primary language correlated with higher continuous adherence to surveillance (see Table 2). Sex and the receipt of anti-viral therapy in chronic hepatitis B patients did not correlate with differences in surveillance adherence, nor did MELD score in cirrhotics.
| Factor | Hepatocellular carcinoma surveillance adherence (PTUDS) | ||
| < 60 yr | 60 yr | P value | |
| Age | 80.6% | 86.8% | < 0.001 |
| Non-cirrhotic | Cirrhotic | ||
| Cirrhosis status | 80.1% | 87.3% | < 0.001 |
| Non-Asian | Asian | ||
| Ethnicity | 85.1% | 81.2% | 0.04 |
| English | CALD | ||
| Primary language spoken | 85.7% | 80.2% | 0.03 |
| Male | Female | ||
| Sex | 83.3% | 85.6% | 0.58 |
| Treatment naïve | Anti-viral therapy | ||
| Hepatitis B treatment status | 79.4% | 82.1% | 0.07 |
Continuous adherence to HCC screening varied by aetiology of liver disease. Patients with non-alcoholic steatohepatitis (NASH) had higher median PTUDS (90.9%) compared to those with viral hepatitis (82.3%, P = 0.003) and alcohol-induced liver disease (83.8%, P = 0.07). Those with non-viral, non-alcoholic and non-NASH disease also had statistically higher continuous surveillance adherence (median PTUDS 90.8%) than their counterparts whose aetiologies were viral hepatitis (P = 0.0008), or alcohol (P = 0.07). There were no significant differences in median PTUDS between the patients whose HCCs were detected earlier (n = 14), than in those with late HCC detection (n = 8) (100% vs 95.9%, P = 0.73).
Analysis demonstrated significant differences in surveillance adherence based on clinic setting, with highest PTUDS for patients attending liver transplant clinics (median PTUDS 97.4%). This was significantly greater than general hepatology clinics (median PTUDS 82.4%, P < 0.001). Of note, patients attending nurse-led screening clinics achieved continuous surveillance adherence rates that surpassed general hepatology clinics (median PTUDS 90.0%, P = 0.0001). This was not reflected in the performance of non-hepatology clinics, which reported the lowest continuous surveillance adherence rates (median PTUDS 62.3%).
Multiple quantile regression analysis demonstrated that age and clinic type (subspecialty hepatology care vs other) had differential effects across different PTUDS quantiles. Older age (≥ 60 years) increased PTUDS across all quantiles, but this increase was only significant at lower quantiles (25th quantile parameter estimate (PE) = 0.003, P = 0.01; 50th quantile PE = 0.002, P < 0.001). Non-liver-specific clinic care, compared with subspecialty hepatology care, was associated with lower PTUDS across all quantiles (P < 0.001).
The results from subgroup quantile regression analysis of patients attending liver-specific specialty clinics (85.0% of all patients) are demonstrated in Table 3. Primary language spoken, ethnicity, and cirrhosis status were shown to variably affect PTUDS across different quantiles. The association between older age and increased PTUDS was maintained only in the 25th quantile (P = 0.03). Patients with cirrhosis had increased PTUDS compared to their non-cirrhotic counterparts at the 75th quantile (P = 0.02). However, African ethnicity and culturally and linguistically diverse (CALD) backgrounds were both significantly associated with lower PTUDS at the 50th quantile (P = 0.048 and P = 0.01). CALD background was also a strong predictor of lower PTUDS at the 75th quantile (P = 0.045).
| QR Estimates (95%CI) | ||||||
| 25th | P value | 50th | P value | 75th | P value | |
| Variables | ||||||
| Age | 0.002 (0.000, 0.004) | 0.03 | ||||
| African ethnicity | -0.089 (-0.177, -0.001) | 0.048 | ||||
| CALD | -0.063 (-0.110, -0.016) | 0.01 | -0.026 (-0.052, -0.001) | 0.045 | ||
| Cirrhotic status | 0.021 (0.004, 0.039) | 0.02 | ||||
In this Australian retrospective cohort study of HCC surveillance utilisation in 775 at-risk patients, we found that average time up-to-date with 6 monthly screening was 84.2% over a median follow-up of over 2 years. However, less than half (40.8%) of patients spent at least 90% of their surveillance period up-to-date with screening, which represents a concerning proportion of patients at risk of delayed diagnosis of HCC. Younger age, non-cirrhotic status, African ethnicity and CALD background were variably associated across PTUDS quantiles with significantly decreased time up-to-date with HCC surveillance on quantile regression analysis.
Our study is one of the few that presents adherence to HCC surveillance using a continuous outcome measure, contrasting with versions of the binary categorisation of ‘adherent’ and ‘non-adherent’ of other studies[7,15-19]. Depending on definitions used, these all-or-nothing classifications potentially enforce too stringent or too lax restrictions on surveillance intervals, and distort true interpretation of HCC surveillance application in real-time clinical practice. This study has described more accurate quantification of adherence and thus better identifies patients at risk.
Given heterogeneity in the published definitions of adherence, comparisons of surveillance uptake are limited. However, Goldberg et al[7] reported an analysis of patients with cirrhosis, applying the PTUDS measure to characterise adherence using United States Veterans Health Administration data. This national study found a median PTUDS for surveillance by USS only as 10% (IQR: 0%-29%)[7]. The vast discrepancy in median PTUDS compared with our finding of 84.2% is likely multifactorial. First, Goldberg’s study encompassed patients followed-up in both community or specialist practices, and found that number of specialist visits was the strongest predictor for greater surveillance participation[7]. Adherence rates for those in non-specialist care have historically been low, with international and limited local data showing optimal surveillance uptake of only 8.8%-27%[3,20,21]. Specialist input may serve as a surrogate marker for greater frequency of reminders for test follow-up, may select for a group of patients more engaged with healthcare, or indicate more unwell patients undergoing imaging for reasons other than surveillance[22]. In particular, hepatology care as compared to other subspecialty providers may better identify the at-risk cohort eligible for HCC screening, therefore having 85% of patients in this study engaged in hepatology clinics may explain our comparatively high PTUDS. Furthermore, our study recruited patients based on attendance at an initial HCC screening scan, which likely also represents a more informed and engaged subset of all patients requiring HCC surveillance. Our findings are, however, similar to pooled adherence surveillance estimates of 73.7% for patients enrolled in specialist care from a recent meta-analysis, associating specialist care with higher likelihood of HCC surveillance[3].
In addition to specialist gastroenterologist/hepatologist care, this study provided insight into other factors correlated with utilisation of HCC surveillance. The high continuous surveillance adherence found in nurse-led clinic-directed care lends support to the effectiveness of this model of care shown in two cohort studies[23,24]. In keeping with several prior studies, we found lower rates of surveillance in younger patients[7,19,25,26]. Studies assessing adherence by cirrhosis status echoed our finding of screening under-utilisation in patients without cirrhosis. This is potentially attributable to a higher perceived HCC risk with cirrhosis stage by patients and providers, suggesting under-recognition of screening eligibility in this group[12,22,27]. Targeted education of providers, particularly primary care physicians who follow most high-risk patients nationally, on screening guidelines that include non-cirrhotic chronic hepatitis B patients, may improve surveillance rates. Receipt of anti-viral therapy, revealed in other studies to increase the likelihood of surveillance adherence, was not significant on multivariable analysis in our cohort of chronic hepatitis B patients[16,22].
Several studies have reported lower surveillance rates in non-Caucasians, with African American/Black patients significantly less likely to receive surveillance[7,28]. African ethnicity in our cohort was similarly associated with lower PTUDS at the 50th quantile, a concerning finding given higher rates of HCC and younger age at risk within this population[21,29]. This may correlate with our lower screening uptake in patients whose primary language was not English, although to our knowledge, no studies have yet reviewed the impact on HCC screening uptake in patients whose care is provided in a primary language that is not their native tongue. However, other cancer surveillance studies have also identified language as a key barrier in CALD patients’ understanding of screening rationale and participation[30,31]. An Australian qualitative study into colorectal screening uptake within culturally and linguistically diverse groups suggested that language barriers hindered otherwise willing participation[30]. A fatalistic view on cancer diagnoses, or a fear of bad luck due to cultural beliefs, were also identified as potentially modifiable barriers to screening adherence, and suggest that culturally-tailored and language-appropriate resources may need to be employed to target diverse populations[30].
In our study, a total of 22 HCCs were detected, of which 14 were early stage and amenable to curative treatment. The annual incidence rate of HCC in our cohort was 1.3 per 100 person-years. This low incidence of HCC may be due to a high proportion in our cohort of people with treated HCV, and HBV without cirrhosis, representing patients with a relatively lower incidence rate of HCC[32]. We did not find a significant difference in overall adherence in patients with HCCs detected at an earlier compared to a later stage. While our study was not powered to answer this question, it provides interesting insight into the effect of surveillance in a cohort with adherence rates closer to the ideal, and highlights the need for quality assessment of surveillance imaging.
Strengths of this study included use of the continuous variable ‘percentage of time up-to-date with surveillance’ to quantify adherence to HCC screening, and quantile regression modelling to describe covariate effects across the spectrum of PTUDS[3,28]. Our data also reflects contemporary clinical practice, with confirmation of the ‘at-risk’ history of our surveyed population. This is also the first Australian study of a large cohort, and offers a perspective on surveillance uptake in a population not hindered by costs of screening tests. Prior observational studies have raised concerns that patient-level barriers, such as costs of surveillance investigations, may contribute to poorer surveillance receipt[22,33]. While we were unable to assess socioeconomic factors, as this data was unavailable, concerns regarding financial burden of surveillance measures are likely alleviated by their cost-free nature in Australia’s healthcare system. Finally, we accounted for patient receipt of alternate, non-ultrasound imaging. This reflects a more realistic practice of clinicians accepting contrast-enhanced CT or MRI liver imaging as appropriate surveillance studies, thus obviating the performance of additional ultrasonography.
Findings from our study should be interpreted within its limitations. As with retrospective study designs, our study is limited by missing data, unmeasured confounders and selection bias. Our analysis involved patients at a single healthcare centre, and may not be generalised to other practice environments, particularly primary care settings. Secondly, patient enrolment based on an initial HCC surveillance USS attendance in a 6 mo period does not incorporate eligible but non-adherent patients, nor those qualifying for screening but who have not been identified by clinicians as at risk of HCC. As such, our adherence rates may be overestimated towards a well-informed, more inherently adherent and correctly identified cohort. However, this large study provides valuable current insight into maintenance of HCC surveillance in patients already identified to be at risk.
In conclusion, average time-up-to-date with HCC surveillance in an at-risk Australian cohort was 84.2% following an index screening test performed for the purpose of surveillance. Subspecialty care was associated with higher subsequent adherence to surveillance imaging. Conversely, younger age, non-cirrhotic status, African ethnicity, and CALD background, were variably associated with significantly reduced PTUDS across different PTUDS quantiles. Further research into patient and system barriers towards HCC screening will provide further information regarding provider and patient factors to better guide development of appropriate and targeted interventions to increase adherence to HCC surveillance.
Hepatocellular carcinoma (HCC) surveillance rates reported from the United States and Europe remain low, despite published clinical guideline recommendations. Surveillance patterns in Australia, which has the benefit a universal healthcare program, have not been clearly delineated.
Patients, and evaluate factors associated with greater uptake of HCC cancer screening. In incorporating both frequency of screening and quantity of imaging performed, we aimed to have a more continuous way of standardising ‘adherence’. Identification of determinants associated with higher HCC screening adherence aims to guide further areas for intervention.
As stated above, the objectives were characterising continuous HCC surveillance adherence. This method provides a way of standardising ‘adherence’, and thus allows for equal comparison between different studies evaluating the concept of HCC screening adherence.
This was a retrospective cohort study that incorporated data electronic medical records to obtain patient demographics, clinical history, lab investigations and radiological imaging results. Data analysis was both on the univariate and multi
Follow-up of 775 at-risk patients demonstrated that median time-up-to-date with HCC surveillance was 84.2%. However, different patient factors, affected HCC surveillance adherence variably across different ranges of the outcome variable percentage of time up-to-date with HCC surveillance (PTUDS). At the 25th quantile/percentile for PTUDS, older age was associated with greater HCC surveillance. At the 50th quantile, African ethnicity had lower HCC surveillance. At the 75th quantile, cirrhotic status was associated with greater adherence to surveillance. Those of culturally and linguistically diverse backgrounds had lower continuous HCC surveillance rates at both the 50th and 75th quantiles. The ramifications of these findings and identified determinants affecting HCC surveillance participation in other settings, including the primary care setting, are less clear. However, they remain very important areas for further research. In particular, addressing the impact of ethnicity and cultural and linguistic backgrounds on screening uptake may well have beneficial consequent effects in other areas of healthcare.
The study suggests specific patient and systemic factors that contribute to partici
Future research should be directed at determining interventions aimed at the factors identified in this study to be associated with reduced HCC screening adherence. Those that improve participation in HCC surveillance may well benefit from widespread implementation to improve earlier diagnosis of HCCs.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barabino M, Parente A, Tolunay HE S-Editor: Wang LL L-Editor: A P-Editor: Guo X
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3165] [Article Influence: 527.5] [Reference Citation Analysis (37)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 3. | Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021;73:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 215] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 4. | Cocker F, Chien Yee K, Palmer AJ, de Graaff B. Increasing incidence and mortality related to liver cancer in Australia: time to turn the tide. Aust N Z J Public Health. 2019;43:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15305] [Article Influence: 3061.0] [Reference Citation Analysis (4)] |
| 6. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4097] [Article Influence: 585.3] [Reference Citation Analysis (6)] |
| 7. | Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, Baytarian M, Fox R, Hunt K, Pedrosa M, Pocha C, Valderrama A, Kaplan DE. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65:864-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 603] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 9. | Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 10. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3237] [Article Influence: 462.4] [Reference Citation Analysis (1)] |
| 11. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Zhao C, Jin M, Le RH, Le MH, Chen VL, Wong GL, Wong VW, Lim YS, Chuang WL, Yu ML, Nguyen MH. Poor adherence to hepatocellular carcinoma surveillance: A systematic review and meta-analysis of a complex issue. Liver Int. 2018;38:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | SAS Institute Inc. SAS OnDemand for Academics. Cary, NC, USA, 2021. |
| 14. | R Development Core Team. Quantreg: Quantile Regression. R package version 5.85 edition. Vienna, Austria: R Foundation for Statistical Computing, 2020. |
| 15. | Edenvik P, Davidsdottir L, Oksanen A, Isaksson B, Hultcrantz R, Stål P. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int. 2015;35:1862-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Nam JY, Lee JH, Kim HY, Kim JE, Lee DH, Chang Y, Cho H, Yoo JJ, Lee M, Cho YY, Cho Y, Cho E, Yu SJ, Kim YJ, Yoon JH. Oral Medications Enhance Adherence to Surveillance for Hepatocellular Carcinoma and Survival in Chronic Hepatitis B Patients. PLoS One. 2017;12:e0166188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Singal AG, Tiro J, Li X, Adams-Huet B, Chubak J. Hepatocellular Carcinoma Surveillance Among Patients With Cirrhosis in a Population-based Integrated Health Care Delivery System. J Clin Gastroenterol. 2017;51:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Tavakoli H, Robinson A, Liu B, Bhuket T, Younossi Z, Saab S, Ahmed A, Wong RJ. Cirrhosis Patients with Nonalcoholic Steatohepatitis Are Significantly Less Likely to Receive Surveillance for Hepatocellular Carcinoma. Dig Dis Sci. 2017;62:2174-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Tran SA, Le A, Zhao C, Hoang J, Yasukawa LA, Weber S, Henry L, Nguyen MH. Rate of hepatocellular carcinoma surveillance remains low for a large, real-life cohort of patients with hepatitis C cirrhosis. BMJ Open Gastroenterol. 2018;5:e000192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Allard N, Cabrie T, Wheeler E, Richmond J, MacLachlan J, Emery J, Furler J, Cowie B. The challenge of liver cancer surveillance in general practice: Do recall and reminder systems hold the answer? Aust Fam Physician. 2017;46:859-864. [PubMed] |
| 21. | Singal AG, Yopp A, S Skinner C, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Wang C, Chen V, Vu V, Le A, Nguyen L, Zhao C, Wong CR, Nguyen N, Li J, Zhang J, Trinh H, Nguyen MH. Poor adherence and low persistency rates for hepatocellular carcinoma surveillance in patients with chronic hepatitis B. Medicine (Baltimore). 2016;95:e4744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Aberra FB, Essenmacher M, Fisher N, Volk ML. Quality improvement measures lead to higher surveillance rates for hepatocellular carcinoma in patients with cirrhosis. Dig Dis Sci. 2013;58:1157-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Nazareth S, Leembruggen N, Tuma R, Chen SL, Rao S, Kontorinis N, Cheng W. Nurse-led hepatocellular carcinoma surveillance clinic provides an effective method of monitoring patients with cirrhosis. Int J Nurs Pract. 2016;22 Suppl 2:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 26. | Park B, Choi KS, Suh M, Shin JY, Jun JK. Factors associated with compliance with recommendations for liver cancer screening in Korea: a nationwide survey in Korea. PLoS One. 2013;8:e68315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Wong CR, Garcia RT, Trinh HN, Lam KD, Ha NB, Nguyen HA, Nguyen KK, Levitt BS, Nguyen MH. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54:2712-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Singal AG, Li X, Tiro J, Kandunoori P, Adams-Huet B, Nehra MS, Yopp A. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med. 2015;128:90.e1-90.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Kew MC. Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Ann Hepatol. 2013;12:173-182. [PubMed] |
| 30. | Javanparast S, Ward PR, Carter SM, Wilson CJ. Barriers to and facilitators of colorectal cancer screening in different population subgroups in Adelaide, South Australia. Med J Aust. 2012;196:521-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Ferdous M, Goopy S, Yang H, Rumana N, Abedin T, Turin TC. Barriers to Breast Cancer Screening Among Immigrant Populations in Canada. J Immigr Minor Health. 2020;22:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019;11:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (7)] |
| 33. | Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, Yopp AC, Parikh ND, Marrero JA, Singal AG. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |