INTRODUCTION
Pancreatic cancer (PC) is the 7th leading cause of death due to cancer in industrialized countries and the 11th most common cancer globally with 458918 new cases (2.5% of all cancers) and 432242 deaths (4.5% of all cancer deaths) in 2018[1]. Its incidence is variable among regions (age-standardized incidence highest in Europe: 7.7 per 100000 people and lowest in Africa: 2.2 per 100000 people) and is expected to increase[1,2]. Both incidence and mortality of the disease correlate with increasing age and male gender[2].
Modifiable risk factors include alcohol (increased risk high alcohol consumption > three drinks per day, but no association with low-to-moderate alcohol intake), smoking (relative risk 1.74 for current and 1.2 for former smokers; risk persists for at least 10 years after cessation), obesity [body mass index (BMI): 25.0-29.9 kg/m2 or BMI ≥ 30 kg/m2 in early adulthood], dietary factors (red meats, processed meats, cholesterol, foods containing nitrosamines) and exposure to metalworking, pesticides, cadmium, and arsenic[2,3]. Non-modifiable risk factors are gender (global incidence 5.5/100000 for men and 4.0/100000 for women), age (> 50 years), ethnicity (significantly higher in black people than any other racial group), diabetes mellitus (1.8-fold risk increase), family history (double risk when at least two first-degree relatives have PC and 32-fold higher in kindreds with three or more first-degree relatives) genetic factors (10% of patients have some kind of germ-line mutation), chronic infections (Helicobacter pylori), non-O blood group and chronic pancreatitis (1.8% patients longstanding pre-existing chronic pancreatitis will develop PC within 10 years from diagnosis and 4% after 20 years)[2,4].
PC symptomatology is frequently non-specific (abdominal pain, jaundice, pruritus, dark urine, and acholic stools, anorexia, early satiety, dyspepsia, nausea, and eventually weight loss), while the early-stage disease remains frequently asymptomatic and most patients present at an advanced stage with poor prognosis[5,6]. Early detection could reduce mortality and screening of selected sub-groups such as those with a family history), using blood markers could be deemed beneficial in the future. Nevertheless, screening of the general population is not currently recommended[7,8]. Diagnosis and staging include imaging modalities such as contrast-enhanced, triphasic pancreatic computed tomography (CT) protocol, magnetic resonance imaging. Endoscopic ultrasound-guided fine-needle aspiration is indicated for cytological confirmation with a sensitivity of approximately 80%), while cancer antigen 19-9 assists in the confirmation of diagnosis, prediction of prognosis, and recurrence following surgery[7].
Surgical resection remains the only curative therapy for PC. However, 80% to 90% of the patients present with unresectable tumors and the reported 5-year survival rates are low, ranging between 10% and 25%, even after successful resection with tumor-free margins[2,9]. Over the past years, alongside with systemic chemotherapy and radiotherapy (RT), minimally invasive image-guided procedures have emerged for the management of non-operable or recurrent locally advanced PC. These include ablative modalities such as radiofrequency ablation (RFA), microwave ablation (MWA), laser ablation, cryoablation (CA), reversible electrochemotherapy (ECT) and irreversible electroporation (IRE), and high intensity focused ultrasound, while trans-arterial embolization procedures have also been suggested and investigated[10-12]. Following the initially non-satisfactory results, which were correlated with unacceptable complication rates, outcomes of minimally-invasive image-guided procedures were significantly improved, mainly due to the cumulative experience, and technological advances of the devices used, and their incorporation in the treatment algorithm for PC has been gradually accepted in specialized centers[10].
ABLATIVE TECHNIQUE
Patients with stable or partial response RECIST (response evaluation criteria in solid tumors) disease following neoadjuvant therapy, and not eligible for surgery should be considered as potential candidates for ablative therapies[13-16]. Ablative treatment can be divided into thermal (RFA, CA, MWA) and non-thermal (RE, IRE).
Thermal ablation
RFA uses needle electrodes to apply high-frequency alternating current to solid tumors. This process generates high temperatures, resulting in thermal coagulation, necrosis, and protein denaturation within the tumor[15]. It can be performed during laparotomy, percutaneously, or using endoscopic ultrasound. Usually, RFA is adopted for tumor debulking rather than complete ablation, as a safety margin of at least 5 mm from the ablation zone is necessary to avoid thermal damage to vital structures. This treatment is also contraindicated in small tumors with a perivascular growth pattern[17]. Early studies investigating the efficacy and safety of RFA in the treatment of LAPC reported high rates of morbidity (4%-37%) and mortality (0%-25%) due to thermal injury to bile ducts, pancreatic duct, duodenum, vital vessels, and heat-sink effect, resulting in incomplete tumor ablation. Recent literature shows an improved overall survival (OS) ranging between 19.0 and 25.6 mo when combining RFA with chemotherapy[17] by optimizing ideal parameters for temperature range (< 90 degrees), treatment time, and probe placement[15].
CA induces rapid argon-gas-based freezing and thawing of target lesions. The freeze-thaw cycles, based on the Joule-Thompson effect, cause cellular destruction by vascular-mediated cytotoxicity, endothelial damage, and cell death. CA with and without immunotherapy for LAPC treatment in term of OS was studied in a retrospective study: Median OS was higher in the cryoimmunotherapy (13 mo) and cryotherapy groups (7 mo) than in the chemotherapy group (3.5 mo; both P < 0.001) and was higher in the cryoimmunotherapy group than in the cryotherapy (P < 0.05) and immunotherapy groups (5 mo; P < 0.001)[15].
MWA promotes tissue coagulation by the oscillation of water molecules, ultimately generating tissue necrosis. This technique has advantages over RFA in the ease of setup and larger ablation zones in a shorter period but recent literature on the use of MW ablation in PC with survival data is very limited[15].
Non-thermal ablation
IRE uses ultrashort high voltage direct current pulses to create an electric field across the cell membrane. This process disrupts membrane homeostasis and irreversibly alters transmembrane potential, which activates the apoptotic pathway and leads to cell death (Figure 1). IRE has the unique ability to preserve the extracellular matrix, critical vessel structures, bile ducts, intestines and minimize heat-sink effects resulting in potentially incomplete ablation. The technology is commercially available (NanoKnife; Angiodynamics Latham, NY, United States) with 510(k) clearance by the Food and Drug Administration (FDA) for ablation of soft tissue tumors[15,18]. In addition to its cytoreductive abilities, the evidence is emerging on IRE’s capability to induce systemic immunomodulation through active in vivo vaccination against PC cells. IRE induces a systemic immune response following apoptosis and necrosis of tumor cells with the release of antigens and damage-associated molecular pattern molecules (DAMPs). These DAMPs promote the maturation of dendritic cells and other antigen-presenting cells that can subsequently take up the activation of lymph nodal T-cells potentially inducing a durable antitumor T-cell response. This response could then lead to regression in distant metastases, a process known as the “abscopal effect”. These effects in combination with immunotherapy may offer a new treatment paradigm for tumors with low immunogenic potential, like pancreatic ductal adenocarcinoma[16]. IRE can be performed during open or laparoscopic surgical exploration or percutaneous procedure with CT guidance. For IRE, 2-6 electrodes are typically placed around the tumor, with a maximum spacing of 2.0-2.5 cm, using image guidance and an upper limit of 5 cm in tumor diameter is highly recommended[17]. Given the high-risk profile of pancreatic IRE procedures, it is important to consider absolute and relative contraindications and to carefully assessed patients for treatment: it is advised to exclude patients with irreversible bleeding disorders, epilepsy, or any other unstable condition that precludes general anesthesia like patients with a past medical history of cardiac disease (i.e., cardiac arrhythmia, implantable cardioverter defibrillator, pacemaker) given the risk of inducing cardiac arrhythmias when applying the electrical pulses[18]; pervasive involvement of the duodenum is considered an absolute contra-indication; if the patient is affected by biliary obstruction, adequate biliary drainage must be guaranteed prior to treatment and if the tumor is closed to the common bile duct it is highly recommended to ensure biliary protection prior to treatment, as post-IRE swelling can impede passage through the central bile ducts; if a patient suffers from a partially occluded portal vein prior to IRE, portal vein stenting should be performed to prevent acute complete occlusion due to postprocedural swelling. Most frequent adverse events comprise GI-related symptoms including pain, diarrhea, nausea, vomiting, loss of appetite, and delayed gastric emptying[16]. Previous cohort studies report heterogeneous outcomes, with median OS rates varying between 15-32 mo when combining IRE with systemic treatment. A recent systematic review by Moris et al[16] shows an overview of all studies that have utilized IRE for LAPC, with a median OS following IRE between 7 and 27 mo. This variance may be due to selection bias, the utilized IRE approach, the diverse LAPC tumor biology, personalized (neo-) adjuvant chemo- and/or RT protocols, performance status including comorbidities, and other interpersonal patient differences. In general, major complications (e.g., portal vein thrombosis, bleeding, duodenal perforation) are reported in 0%-30% of patients, with mortality rates ranging between 0%-11%[17]. The average cumulative morbidity for surgical and percutaneous IRE was 36% vs 24%, with an average periprocedural mortality rate of 2% vs 0%, respectively[16].
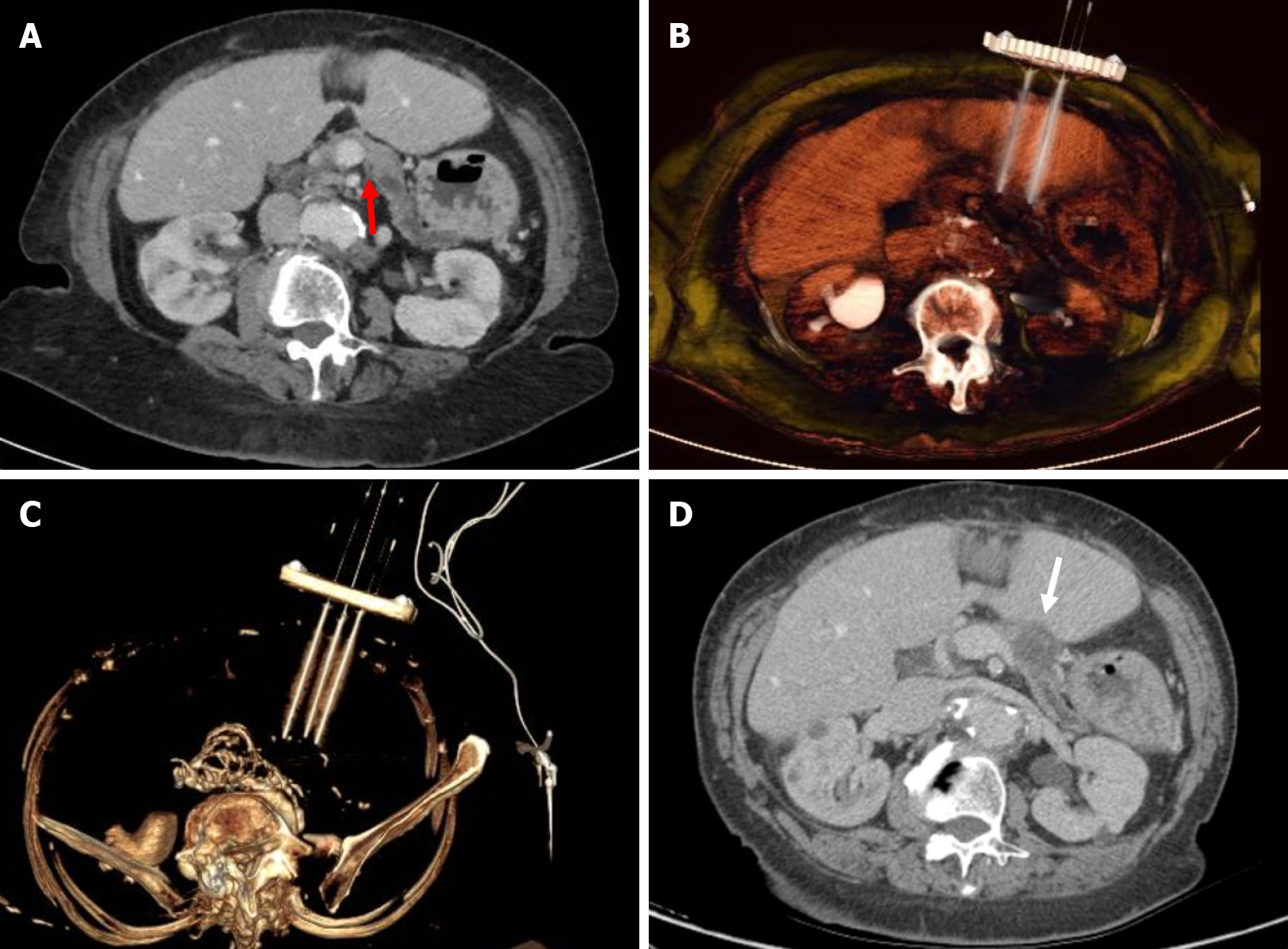
Figure 1 Pancreatic cancer percutaneous irreversible electroporation.
A: Axial computed tomography (CT) showing 2 cm lesion (red arrow) in the body of the pancreas in keeping with ductal adenocarcinoma; B and C: 3D volume rendering CT with parallel needles positioning within the lesion; D: 1-mo CT follow up showing complete ablation of the tumor (white arrow).
Reversible ECT is a new non-thermal ablation technique that avoids possible thermal injury to the peripancreatic vessels like portal mesenteric vein combining the use of chemotherapeutic drugs (bleomycin) with electric pulses for cell membrane electroporation. A transient cell membrane improve permeability is determined by electric pulses, permitting the exposure of the cell to chemotherapeutic drugs[19]. The procedure is divided into four steps: Laparotomy or laparoscopic or percutaneous approach and intraoperative ultrasound to confirm that the pancreatic tumor was unresectable and to exclude distant metastases, needle insertion, bleomycin infusion and electroporation. Eight minutes after the bleomycin infusion, electric pulses were applied and delivered using four single long needle electrodes having 1.2 mm in diameter, and 3 or 4 cm active part. ECT was performed mostly in young patients (mean age, 63 years), with a good performance status and normal BMI. ECT was safe, according to the absence of acute intraoperative adverse effects related to electroporation and effects related to the bleomycin[19]. Nevertheless there is few studies regarding ECT in literatures[20,21] and additional studies should be carried out.
INTRA-ARTERIAL THERAPIES
Since the 1950s, regional intra-arterial chemotherapy (RIAC) was introduced in an attempt to increase cancer survival rates. Intra-arterial chemotherapy generates high drug concentrations in the target areas while maintaining low systemic drug levels. Clinical trials demonstrated that regional intraarterial infusion with Gemcitabine (GEM) improved the response and resectability rates for advanced PC and was well tolerated by patients[22,23]. In 2012, a systematic review and meta-analysis of six randomized controlled trials, comparing systemic chemotherapy, with RIAC, reported that the latter resulted in higher partial remission, clinical benefits and response rates with fewer complications including myelosuppression[24].
With regards to efficacy, it is known that the effect of chemotherapy is concentration-dependent, therefore intra-arterial local infusion which generates higher drug concentrations within targeted regions, could be proven more efficient. In fact, according to the results of two RCTs, RIAC improved the 1-year OS (41.2%-28.6%) compared with systemic chemotherapy[25,26].
Other Authors considered the use of RIAC as neoadjuvant regional chemotherapy with continuous infusion of GEM intending to improve resectability rates in case of locally advanced PC[27]. A study was carried out to investigate the prognostic factors in patients who received GEM-based intra-arterial infusion for advanced PC; young age, pretreatment CA19-9 value < 1000 U/mL, and tumor located at the head of the pancreas indicated better response to RIAC and improved survival[28].
A recent retrospective cohort study of 454 patients with advanced PC compared RIAC via angiographically placed celiac axis catheters vs isolated upper abdominal perfusion (upper abdominal perfusion with stop flow balloon catheters in the aorta and vena cava)[29]. The isolated perfusion group demonstrated superior survival compared to intra- arterial infusion (median survival rates: 12 and 8 mo in stage III; 8.5 and 7 mo in stage IV; respectively)[29].
Future perspectives in local chemotherapy include novel infusion techniques such as Trans-Arterial Micro-Perfusion (TAMP) using the RenovoCath catheter (RenovoRx, Inc.) which was recently approved by the FDA. The TAMP procedure involves arterial segment isolation using proximal and distal occlusion balloons generating increased intra-arterial luminal pressure above the interstitial pressure and forcing the drug across the arterial wall within the tumoral tissue. The specific catheter has the potential to deliver higher drug concentrations within the tumor, while limiting systemic exposure. The TIGeR-PaC Phase III randomized clinical trial is currently enrolling patients with unresectable LAPC to investigate TAMP vs systemic chemotherapy. The goal of the trial is to prove extended median survival and improved quality of life through targeted delivery of therapy. In Phase, I/II studies, TAMP resulted in over two years survival in more than half the patients. The TIGeR-PaC trial, currently includes approximately 30 active clinical sites, and is expected to involve 200 participants in 40 centers in the United States and Europe (clinicaltrials.gov NCT03257033).
ONCOLOGY
LAPC is in general considered incurable and the management remains unclear and controversial. Treatment includes chemotherapy, which can have a potential role as neoadjuvant treatment or in combination with RT on a case-based approach. The aim is to control disease progression, palliation, and improvement of OS. Patients with LAPC have a poor prognosis, with a median OS of 12-14 mo following systemic therapies[14]. The first step before choosing the appropriate treatment plan is the assessment of the patient’s performance status. Patients with locally advanced disease received therapies based on their performance. Patients with a poor performance status are candidates to single-agent chemotherapy or palliative radiation therapy or best supportive care; patients with a good performance status can be considered for a more intensive oncological strategy as chemotherapy or chemoradiation[30].
Formerly, standard treatment included GEM for six months. However, in 2011, the Eastern cooperative Oncology group reported improved OS with the addition of radiation therapy (for a total of 50.4 Gy) to GEM[31].
In 2016, Suker et al[32] conducted a patient-level meta-analysis of 11 studies (315 patients) indicating that LAPC patients treated with FOLFIRINOX demonstrated a median OS of 24.2 mo, which was significantly higher compared to that achieved by GEM therapy (6-13 mo).
While systematic chemotherapy has become the standard for patients with LAPC, surgery remains the only potentially curative therapy. Therefore, the development of a new neoadjuvant treatment that improves survival rate is of the utmost importance. Neoadjuvant chemotherapy should aim in a possible resection, or radiation therapy or IRE. Clinicians and researchers should investigate cancer biology and invest to define predictive and prognostic factors, patients-related features, and biological criteria to identify patients that would benefit from aggressive surgery or chemotherapy and chemoradiation[33].
When surgery is not possible after induction chemotherapy, chemoradiotherapy (intensity modulated radiation therapy or stereotactic body radiation therapy), is a possible option to incite tumor shrinkage and enable secondary resection in a small percentage of patients, while palliation of cancer-related pain is also a significant goal in unresectable tumors. The LAP07 phase III randomized controlled trial was designed to investigate the effect of chemoradiotherapy and erlotinib in the OS of patients with LAPC controlled after 4 mo of GEM-based induction chemotherapy. Unfortunately, both chemoradiotherapy and erlotinib did not provide any benefit in patients with LAPC[34].
On the other hand, in an updated 2018 review and meta-analysis of 41 studies (1018 patients receiving consolidation chemoradiation after induction chemotherapy and 954 patients receiving chemotherapy alone) the authors noted a significant survival benefit for chemoradiation after induction chemotherapy in cases in which chemotherapy lasted for a period of at least 3 mo[35].
Currently, according to the results of the SCALOP multicenter, open-label, randomized, two-arm, phase 2 trial, a capecitabine-based regimen should be preferred over a GEM-based regimen in the context of consolidation chemoradiotherapy after a course of induction chemotherapy for locally advanced PC[36].
Only recently, novel minimally invasive approaches, such as IRE, have been evaluated for treatment of LAPC after induction chemotherapy. The PANFIRE-2 multicenter, prospective, single-arm study investigated the safety and efficacy of percutaneous CT-guided IRE alone or after induction chemotherapy with GEM alone or FOLFIRINOX. In total 50 patients were enrolled (40 with LAPC and 10 with local recurrence) and target median OS was exceeded in patients with LAPC (17 mo) and those with local recurrence (16 mo). Notably, 14 minor and 21 major complications occurred, while one probable IRE-related death was reported[14]. Some data suggest the potential role of IRE after FOLFIRINOX chemotherapy as better results have been noted in specific subgroups of patients. IRE could be considered as a less invasive potentially curative approach for LAPC[37]. Nevertheless, randomized clinical trials are necessary to provide comparative data regarding the efficacy of these three methods.
RT
About 80%-90% of the patients with PC, present with locally advanced disease at diagnosis and consequently a very poor prognosis of less than 5% OS at 5-years[38,39]. In this group of patients, RT associated with systemic therapy plays a crucial role in achieving satisfactory local control (LC). As noted above, the LAP 07 phase III study failed to demonstrate any significant advantage on OS, but a substantially decreased local progression rate (32% vs 46%, P = 0.03) for chemoradiotherapy was noted, without increase in grade 3 to 4 toxicity (except for nausea)[34]. In this context, for patients demonstrating good performance status, the aim of combining RT with systemic therapy is to provide satisfactory LC and avoid or delay local disease progression. However, it is crucial that during RT a higher radiation dose should be delivered to the tumor, limiting doses to healthy tissue.
Conventionally, the standard RT dose has been set around of 50-54 Gy administered over 6 wk using the 3D conformal radiation technique (3D-CRT), a dose initially established on the tolerance of large-field radiation to organ at risks. However, these doses showed a limited LC of the disease, while higher doses often induce treatment-related toxicity that can significantly affect the patient’s quality of life[40].
Over the past decade, modern technological advances such as intensity-modulated RT (IMRT), the introduction of respiratory management methods and improved imaging guidance during therapy, have enabled dose-escalation therapy and facilitated the possibility to reduce treatment volumes, to improve ablative doses to the tumor and consequently clinical outcomes, concurrently reducing treatment toxicity[41]. In fact, like other mobile tumors, pancreatic tumors require assessment of respiratory movements, while RT dosimetry is additionally complicated by the presence of nearby hollow digestive organs.
In a 2015 systematic review, 13 IMRT studies were analyzed and compared with 7 3D-CRT series, in order to compare toxicity and tumor outcomes between these two different RT-techniques for the management of LAPC. Even though no differences in terms of OS and PFS were obtained, IMRT was correlated with reduced risk of side effects[42]. Surely, the absence of OS advantage could be explained by the conventionally delivered dose, which is not sufficient to efficiently control the disease[43].
In a study published by Krishnan et al[44], patients who received an intensified dose with a biological effective dose (BED) > 70 Gy demonstrated higher OS compared to those who received a standard dose BED ≤ 70 Gy. In this scenario, in which the main limitation remains the risk of severe intestinal toxicity, stereotactic body RT (SBRT) has also been largely explored as a potentially effective treatment for patients with LAPC (Figure 2)[45-61]. Of note, poor prognosis related to these tumors has motivated physicians to limit the number of RT sessions and concentrate treatment within one to two weeks, thereby reducing the period of chemotherapy interruption and increasing patient’s compliance.
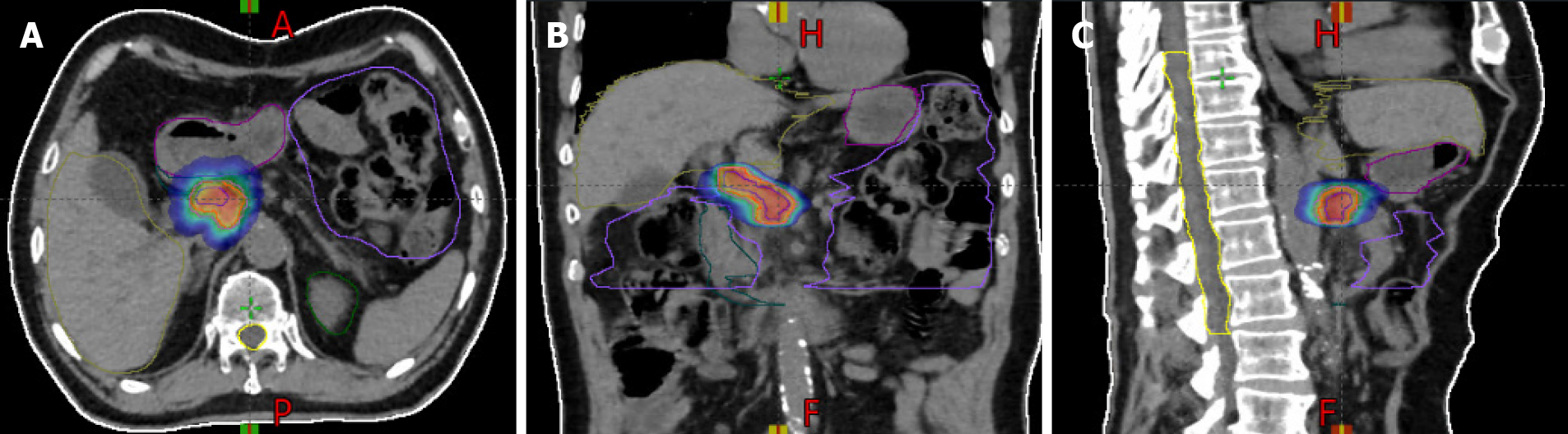
Figure 2 Radiation treatment plan for a patient treated with stereotactic body radiotherapy for local advanced pancreatic cancer.
A-C: The plans show isodose levels in the axial plane (A), coronal plane (B), and sagittal plane (C).
According to recent data on SBRT treatment for LAPC, a large heterogeneity in total dose prescription and fractionations was reported. Generally, the total doses used ranged from 30 Gy to 45 Gy in 3-5 fractions resulting in effective biological dose (BED) superior to 60 Gy (assuming an α/β = 10) in most of the cases[46-60]. This higher dose/fraction can theoretically produce enhanced cell destruction than conventional fractionation[62]. Due to the aggressiveness of the disease which causes a rapid evolution of the metastatic sites, OS is not greatly decreased in SBRT series.
Despite satisfactory LC and median freedom from local progression rates of approx. Eighty percent at 1 year, and very low acute toxicity obtained by SBRT single fraction treatments (25 Gy/1 fr)[45,47,48,51,52], high rates of late toxicity have limited the use of this approach. Therefore, multi-fraction treatment schemes are preferably used. In a retrospective cohort of 167 LAPC patients, 45% of whom were treated in single fraction SBRT and 54% in five-fraction SBRT, five-fraction protocol showed significantly lower gastrointestinal toxicity compared to the single fraction treatment. However, good LC rate was noted in both groups[54].
Similar conclusions were drawn following several retrospective studies that acknowledged the benefit in terms of reduced toxicity when multi-fractionated (3-5 fractions) RT regimens were applied compared to single fraction treatment, while obtaining the same efficacy in terms of LC[49,50,52,53,55-57]. All patients investigated in these studies, received systemic therapy (mainly GEM) during different time points of SBRT treatment (before, after or both before and after SBRT) and therapeutic schemes were variable based on the patients’ clinical status and the grade of the disease. Notably, the indication for intensified local treatment using SBRT in LAPC patients may be abruptly compromised due to the high metastatic potential of the disease.
Only few, non-randomized, retrospective studies compared SBRT and conventional fractionated RT[63] in the local advanced PC curative or neo-adjuvant setting. SBRT was associated with significantly improved OS compared to conventional fractionated RT. Additionally, SBRT was associated with significantly increased rates of pathological complete response and margin-negative resection in neo-adjuvant setting. These are promising results and provide the basis for consideration for prospective validation.
However, the indication for SBRT in order to intensify local treatment may be affected by the high metastatic potential of the disease.
CONCLUSION
The prognosis of LAPC remains poor although recent advancements in multimodality treatment seem to provide some improvement in clinical outcomes. Combined modality treatment using systemic chemotherapy, minimally invasive image-guided procedures and RT are currently under investigation. Neoadjuvant chemotherapy could be used to enable curative surgical resection, radiation therapy, or ablation. SBRT may be employed to obtain satisfactory LC and reduce side effects. Mounting experience with percutaneous thermal ablation and IRE provides superior outcomes with less complications. Considering the currently unsatisfactory results in terms of OS improvement, different treatment options should be investigated to optimize therapy. As the pathogenesis of LAPD is multifactorial and has been associated with genetic factors (mainly germ-line BRCA2 gene mutations, but also various syndromes such as the Lynch syndrome, hereditary breast and ovarian cancer syndrome, familial adenomatous polyposis and Li-Fraumeni syndrome) environmental factors (obesity, smoking habit, diabetes, alcohol consumption, dietary factors such as red meat consumption, and occupational exposure to nickel cadmium and arsenic, and the human microbiome), future treatment directions should focus on the investigation of these factors to provide personalized therapeutic schemes and improve survival[2,64,65].
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carloni R, Ogino S S-Editor: Gao CC L-Editor: A P-Editor: Gao CC