Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1813
Peer-review started: March 12, 2021
First decision: May 3, 2021
Revised: May 11, 2021
Accepted: August 25, 2021
Article in press: August 25, 2021
Published online: November 15, 2021
Processing time: 245 Days and 11.2 Hours
Despite the use of current standard therapy, the prognosis of patients with unresectable hepatocellular carcinoma (HCC) is poor, with median survival times of 40 mo for intermediate HCC (Barcelona Clinic Liver Cancer [BCLC] stage B) and 6–8 mo for advanced HCC (BCLC stage C). Although patients with early-stage HCC are usually suitable for therapies with curative intention, up to 70% of patients experience relapse within 5 years. In the past decade, the United States Food and Drug Administration has approved different immunogenic treatment options for advanced HCC, the most common type of liver cancer among adults. Nevertheless, no treatment is useful in the adjuvant setting. Since 2007, the multi-kinase inhibitor sorafenib has been used as a first-line targeted drug to address the increased mortality and incidence rates of HCC. However, in 2020, the IMbrave150 trial demonstrated that combination therapy of atezolizumab (anti-programmed death-ligand 1 [PD-L1]) and bevacizumab (anti-vascular endothelial growth factor [VEGF]) is superior to sorafenib, a single anti-programmed death 1/PD-L1 antibody inhibitor used as an anti-cancer monotherapy for HCC treatment.
To conduct a systematic literature review to evaluate the evidence supporting the efficacy and safety of atezolizumab/bevacizumab as preferred first-line drug therapy over the conventional sorafenib or atezolizumab monotherapies, which are used to improve survival outcomes and reduce disease progression in patients with unresectable HCC and non-decompensated liver disease.
A comprehensive literature review was conducted using the PubMed, Scopus, ScienceDirect, clinicaltrials.gov, PubMed Central, Embase, EuropePMC, and CINAHL databases to identify studies that met the inclusion criteria using relevant MeSH terms. This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and risk of bias (RoB) were assessed using the Cochrane RoB 2 tool and Sevis.
In the atezolizumab/bevacizumab group, an improvement in overall tumor response, reduction of disease progression, and longer progression-free survival were observed compared to monotherapy with either sorafenib or atezolizumab. Hypertension and proteinuria were the most common adverse events, and the rates of adverse events were comparable to those with the monotherapy. Of the studies, there were two completed trials and two ongoing trials analyzed using high quality and low bias. A more thorough analysis was only performed on the completed trials.
Treatment of HCC with atezolizumab/bevacizumab combination therapy was confirmed to be an effective first-line treatment to improve survival in patients with unresectable HCC and non-decompensated liver disease compared to monotherapy with either sorafenib or atezolizumab.
Core Tip: Hepatocellular carcinoma (HCC), the most common primary malignancy of the liver, is a leading cause of cancer-related deaths. Combination immunotherapy for the treatment of advanced HCC is attracting increasing attention because of the superiority of clinical results compared to sorafenib, the standard of care. Combination therapy with atezolizumab/bevacizumab has been compared to sorafenib and atezolizumab monotherapies. Current findings indicate that combination therapy is as effective as first-line therapeutic options for improving survival rates in patients with unresectable HCC and non-decompensated liver disease.
- Citation: Ahmed F, Onwumeh-Okwundu J, Yukselen Z, Endaya Coronel MK, Zaidi M, Guntipalli P, Garimella V, Gudapati S, Mezidor MD, Andrews K, Mouchli M, Shahini E. Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma: A systematic review. World J Gastrointest Oncol 2021; 13(11): 1813-1832
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1813.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1813
Hepatocellular carcinoma (HCC) accounts for 75%-85% of primary liver cancers, and is the sixth most common cancer and fourth leading cause of cancer-related deaths worldwide[1]. Surgical resection, thermal ablation, and liver transplantation represent the conventional approaches used for patients with early-stage HCC (Barcelona Clinic Liver Cancer [BCLC] stage A). Moreover, for patients who are not surgical candidates, systemic chemotherapy can be alternatively employed. Patients with early-stage HCC are usually suitable for curative treatments. However, the prognosis of patients with unresectable HCC is usually poor, with median survival times of 40 mo for inter
Tumor cells can activate different immune checkpoint pathways that modify immunosuppressive functions. Specifically, in the last several decades, the emergence of immune checkpoint inhibitors (ICIs) that target the human programmed death-ligand 1 (PD-L1)/programmed death 1 (PD-1) pathway has led to the high potential to treat a wide spectrum of solid tumors including HCC[4].
Sorafenib (BAY 43-9006) is a multi-kinase inhibitor that blocks the activity of Raf serine/threonine kinase, as well as other receptor tyrosine kinases such as VEGFR-2 and VEGFR-3, platelet-derived growth factor receptor-β, c-KIT, fms-like tyrosine kinase 3, and RET. Its ability to block these pathways leads to the inhibition of tumor angiogenesis, tumor cell proliferation, and migration while increasing the rate of apoptosis[5,6].
In 2007, the United States Food and Drug Administration (FDA) approved sorafenib for the treatment of metastatic HCC due to its anti-angiogenic properties[6]. It has shown survival benefits by extending the median survival time of patients with unresectable HCC (10.7 mo compared to 7.9 mo in the placebo group)[2].
Atezolizumab, another ICI of interest, is a monoclonal antibody that targets PD-L1[7] and prevents the interaction between the PD-1 and A7-1 receptors, resulting in the reversal of T-cell suppression[8]. Bevacizumab is an anti-angiogenic antibody that inhibits angiogenesis and neoplasm growth by targeting VEGF[9]. Anti-VEGF treatments can decrease VEGF-mediated immunosuppression and also improve anti-PD-1/PD-L1 functions[10,11].
Given the nature of these immunotherapies, it has been postulated that a combination of atezolizumab with bevacizumab should have safe and synergistic anti-tumor effects on HCC. The phase III IMbrave150 trial showed that bevacizumab combined with atezolizumab leads to better overall survival (OS) and progression-free survival (PFS) outcomes over sorafenib therapy in patients with unresectable HCC[12]. Moreover, atezolizumab/bevacizumab combination therapy was also demonstra
The synergistic effects of atezolizumab-bevacizumab therapy compared to the sorafenib monotherapy are remarkable enough to warrant further study. Hence, this systematic review analyzed the documented evidence comparing the efficacy and safety of atezolizumab/bevacizumab combination therapy with monotherapy regimens, such as sorafenib or atezolizumab, in patients with unresectable HCC (Supplementary Figure 1).
This study included a data collection of randomized controlled trials (RCTs), which evaluated adult patients (aged 18 and older) with unresectable HCC to receive combination therapy of intravenous atezolizumab (1200 mg) plus bevacizumab (15 mg/kg) every 3 wk (or periodically). The study dataset was further divided into a control segment consisting of sorafenib monotherapy, atezolizumab monotherapy, or placebo.
The RCTs incorporated primary efficacy outcomes of mortality, measured as a median number of deaths and stratified hazard ratios (HRs). Moreover, the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (Supplementary Table 1) measured secondary outcomes of OS, median OS, and median PFS as tumor response proportions or percentages. An example of the aforementioned was demonstrated in the overall confirmed objective response, confirmed complete and partial responses, stable disease, progressive disease, and disease control rate.
RCTs safety measurements evaluated included patients with adverse events (AEs) from causes that included serious treatment-related AEs and treatment-related mortality events. Additionally, AEs that resulted from drug dosing modifications and/or interruptions were evaluated along with drug withdrawal trials that included participants with Grade 3-5 AEs. Any unfavorable clinical or laboratory result associated with an investigational intervention during the clinical trial was considered an AE; hence, this included any unfavorable and life-threatening medical outcome that required inpatient hospitalization or prolongation of hospitalization.
All RCTs evaluated in the study had documented hepatitis virological status as well as a Child-Pugh classification of A or B (Supplementary Table 2). However, the data excluded trials involving patients who received treatments for medical conditions other than HCC, as well as participants with autoimmune liver disease or any autoimmune conditions and participants in Child-Pugh class C. Non-human studies, non-English and unpublished articles were also excluded.
A comprehensive and extensive literature review of published articles was conducted to identify RCTs that met the inclusion and exclusion criteria using appropriate MeSH terms. This systematic review was performed following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The search was conducted using the Cochrane, Cochrane Central, PubMed Central, Scopus, ScienceDirect, WHO trials, clinicaltrials.gov, Google Scholar, Embase, CINAHL, and MedLine databases. The following terms and Boolean operators were employed in MeSH and free-text searches to identify relevant articles: “Hepatocellular carcinoma,” “liver tumor,” “liver cancer,” “atezolizumab and bevacizumab,” and “sorafenib.”
The data search was conducted until December 27, 2020. The search criteria were broadened by identifying additional studies from the reference lists of selected articles, as well as by the “related articles” function of PubMed. Additionally, the systematic review was registered in PROSPERO, the international prospective registry of systematic reviews of the National Institute for Health Research (CRD42021237736). For transparency, the study was pre-registered on the open science framework (URL: https://osf.io/esvk9), and in PROSPERO.
Selection of studies: All articles used in this document were screened for eligibility via their titles and abstracts. Thereafter, the full-text of all chosen studies was examined in detail. Two independent reviewers were chosen to perform the screening and examination process according to predefined eligibility criteria for the qualitative review.
Two review authors (MKC and MZ) independently extracted the data and summarized the trial characteristics in each table. They were also involved in extracting the baseline characteristics of the participants, study design, geography, settings, methods, types of interventions (dosage, route of administration, regimen protocol), efficacy, and safety outcomes.
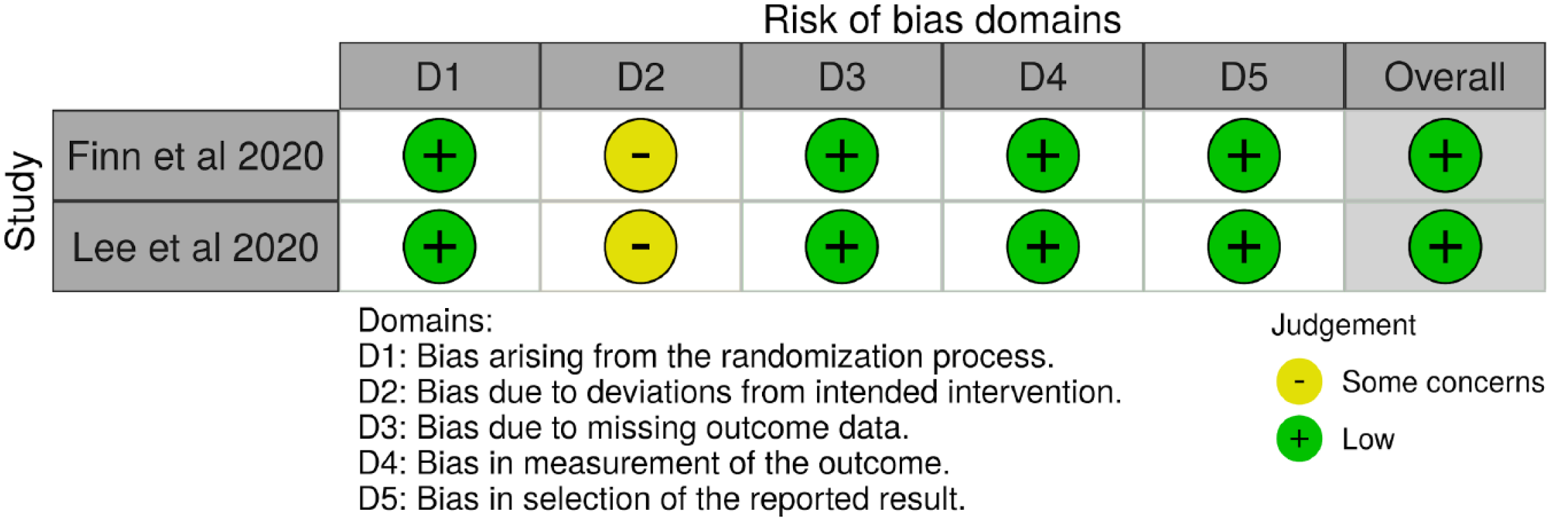
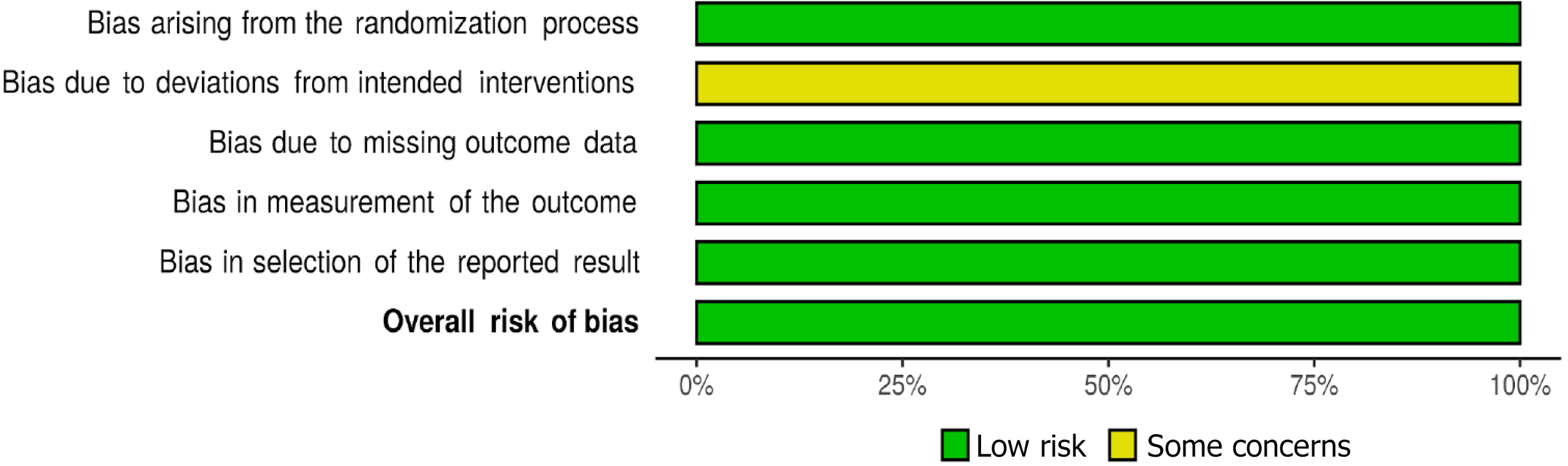
This study assessed the risk of bias (RoB) in the included studies by using the revised Cochrane RoB 2 tool for randomized trials. This tool was used to assess the RoB across the following five domains: Bias arising from the randomization process, bias due to deviation from the intended intervention, bias due to missing outcome, bias in the measurement of outcome, and bias in the selection of the reported results. Moreover, the Robvis data software was used to create a RoB traffic light plot (Figure 1) and a RoB summary plot (Figure 2).
The studies that measured relevant objective data outcomes (e.g., mean survival rates) according to their study protocols, but included non-retrievable online data, missing content or unclear data, were cleared up by reaching out to the original authors of the published reports. However, if no response was obtained from the original authors, the selected study was excluded from the analyses.
A descriptive analysis of all study results was performed. All continuous variables such as mean and median were analyzed, and all dichotomous outcomes were investigated as proportions and percentages. Furthermore, epidemiological variables (e.g., risk ratio, attributable risk, and numbers needed to treat) to measure certain effects of intervention such as mortality were estimated as deemed necessary.
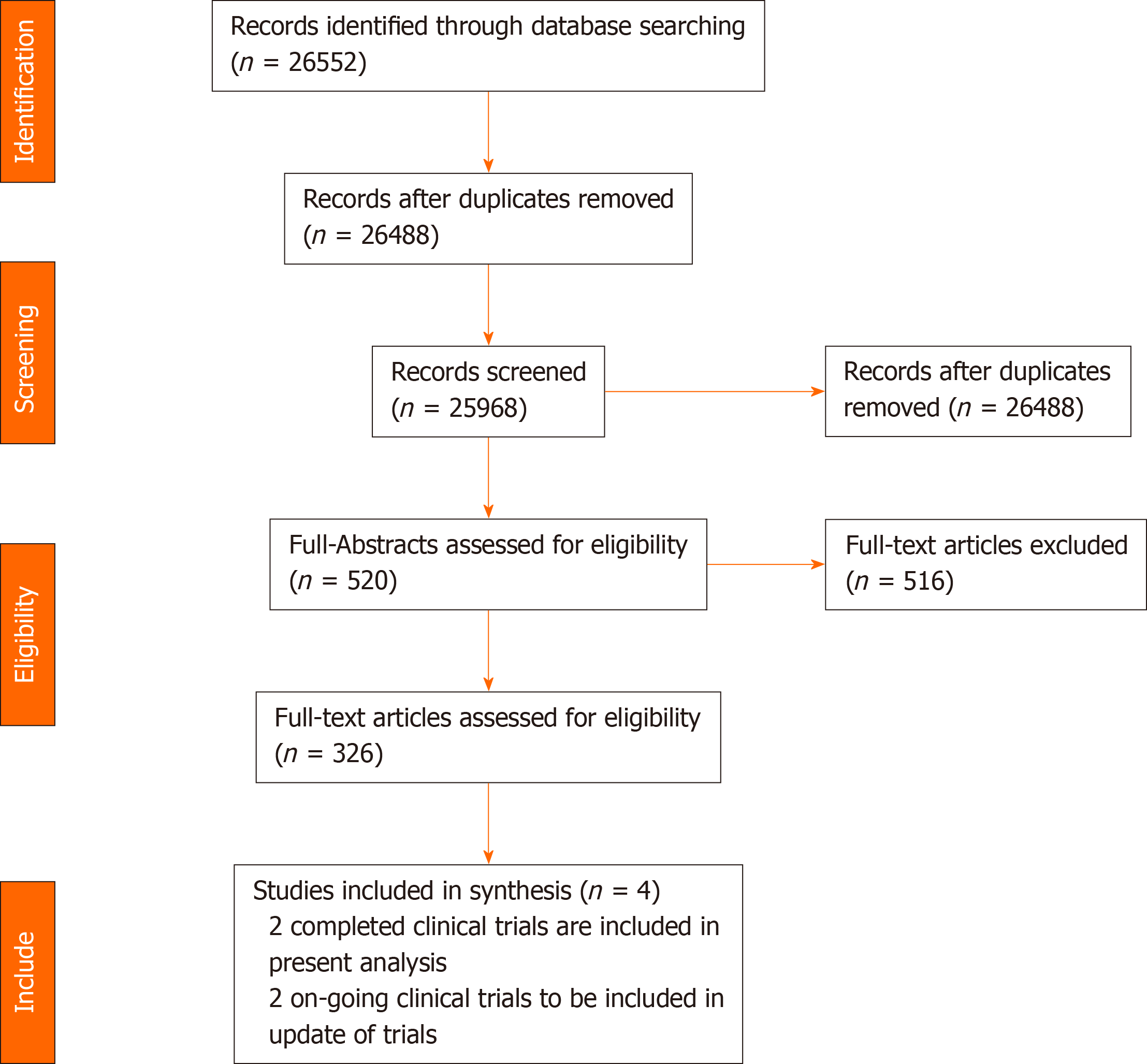
The results of the literature search are summarized in Figure 3. Initially, 520 potentially eligible articles were considered. However, 326 full-text articles that were predominantly cohort studies and a few RCTs were evaluated after screening the abstracts. Subsequently, four RCTs were included in the literature search after excluding 516 articles according to the eligibility criteria. Of the four trials included, two were ongoing (La Roche[14] and Hack et al[15]), and two have been concluded (Finn et al[12], 2020, and Lee et al[16], 2020). Data from the ongoing clinical trials and completed studies are illustrated in Table 1. Hence, the two completed trials were included in the final analyses.
| Ongoing clinical trials | La Roche[14], 2020 | Hack et al[15], 2020 |
| Country of enrollment | Italy | 170 sites in 25 countries (Asia) |
| Study design | Single-arm, multi-Center, randomized clinical control trial | Multi-center randomized open-label, clinical control trial |
| Study phase | IIIb | III |
| Study quality | NA (study is still ongoing) | NA (study is still ongoing) |
| Intervention | Atezolizumab plus bevacizumab | Atezolizumab plus bevacizumab |
| Dose: atezolizumab 1200 mg IV infusion q3w + bevacizumab 15 mg/kg IV Q3W | Dose: atezolizumab 1200 mg every 3 wk + bevacizumab 15 mg/every 3 wk | |
| Control | Standard of care | Active surveillance |
| No specifications for control arm reported | ||
| Number of patients | 150 | 662 |
| Intervention/control | Intervention not specified | Intervention 501 |
| Control: Not specified | Control: 119 | |
| Median age (range) | Not reported | Not reported |
| Intervention/control | Study included individuals > 18 yr | Study included individuals > 18 yr |
| -Duration of follow-up in mo | Not reported | Intervention: 8.6 mo |
| Intervention/control | Control: 6.5 mo | |
| Types of outcomes reported | Overall survival | Overall survival |
| Median progression-free survival | Median progression-free survival | |
| Grade 3-5 adverse events | Grade 3 or 4 adverse events | |
| Disease control | Disease control | |
| Objective response rate | ||
| Time to progression | ||
| Duration of response | ||
| Post-progression survival | ||
| Data that could not be evaluated/data missing | NA (study is still ongoing) | NA (study is still ongoing) |
| Completed studies | Finn et al[12], 2020 | Lee et al[16], 2020 |
| Country/ies of Enrollment | 111 sites in 17 countries, which include the United States, China, Japan, Germany, France, South Korea, Russia, Canada, and Taiwan | 26 sites in 7 countries, which include the United States, Japan, South Korea, Taiwan, Australia, and New Zealand |
| Study design | Open-label, randomized clinical trial | Multi-arm study with five cohorts |
| However, only the two cohorts focusing on hepatocellular carcinoma, Groups A and F, are described here in this study | ||
| Phase | III | Ib |
| Study Quality | Low risk of bias | Low risk of bias |
| Intervention | Atezolizumab plus bevacizumab | Atezolizumab plus bevacizumab |
| Dose: 1200 mg atezolizumab + 15 mg/kg of bevacizumab IV q3w | Dose: 1200 mg atezolizumab + 15 mg/kg of bevacizumab IV q3w | |
| Control | Sorafenib monotherapy | Atezolizumab monotherapy |
| Dose: 400 mg sorafenib PO BID | Dose: 1200 mg atezolizumab | |
| Number of patients | 501 | 403 |
| Intervention/control | Intervention: 336 | Group A1: 104 |
| Control: 165 | Group F+: 60 | |
| Control: | ||
| 59 included in efficacy analysis1 | ||
| 58 included in safety analysis | ||
| 1 discontinued before receiving any treatment due to elevated alkaline phosphatase concentrations1 | ||
| Median duration of follow-up (mo, [IQR]) | Overall: 8.6 mo | Overall follow-up not given, see stratified data below |
| Intervention/control | Group A1: 12.4 (IQR 8.0-16.2) | |
| Intervention: 8.9 | Group F+: 6.6 (IQR 5.5-8.5) | |
| Control: 6.7 (IQR 4.2-8.2) | ||
| Control: 8.1 | ||
| Primary outcomes reported | Mortality rates | Mortality rates |
| Hazard ratio for death | Hazard ratio for death | |
| Secondary Outcomes reported | Overall survival | Overall survival |
| Median progression free survival | Median progression free survival | |
| Grade 3-5 adverse events | Grade 3-4 adverse events | |
| Disease control | Disease control | |
| Objective response rate | Objective response rate | |
| Time to progression | Time to progression | |
| Duration of response | Duration of response | |
| Post-progression survival | Post-progression survival |
This review included the two concluded trials in the present analyses (Finn et al[12] and Lee et al[16]) as well as the results of two currently ongoing trials of La Roche[14] and Hack et al[15] that will be used in future updates.
La Roche[14] is a Phase IIIb, single-component, multicenter study of atezolizumab/bevacizumab, which is currently ongoing. Hack et al[15] is also currently ongoing, and is evaluating randomized patients included in an intervention dataset (atezolizumab/bevacizumab) and patients assigned to the control portion of the dataset undergoing active surveillance. Patients included in the control were allowed to crossover to the intervention dataset (atezolizumab/bevacizumab) after confirmed recurrence.
Lee et al[16] is part of an open-label, multicenter, multi-segmental, phase 1b study also known as GO30140 study, which enrolled patients at 26 academic centers and community oncology practices in seven countries worldwide. The study included five cohorts, but only the results of the two HCC cohorts, Groups A (atezolizumab monotherapy) and F, are described within this review article. Finn et al[12] compared the intervention dataset (atezolizumab/bevacizumab) with the control dataset (sorafenib monotherapy) and compared patients from 111 sites in 17 countries. Hence, details of the trials and participants are shown in Table 1.
As La Roche[14] and Hack et al[15] are currently ongoing, information for primary and secondary objectives are incomplete. It should be noted that while incomplete data were not assessed in this document, the authors of this manuscript fully intend to obtain updated data concerning related objectives in the future.
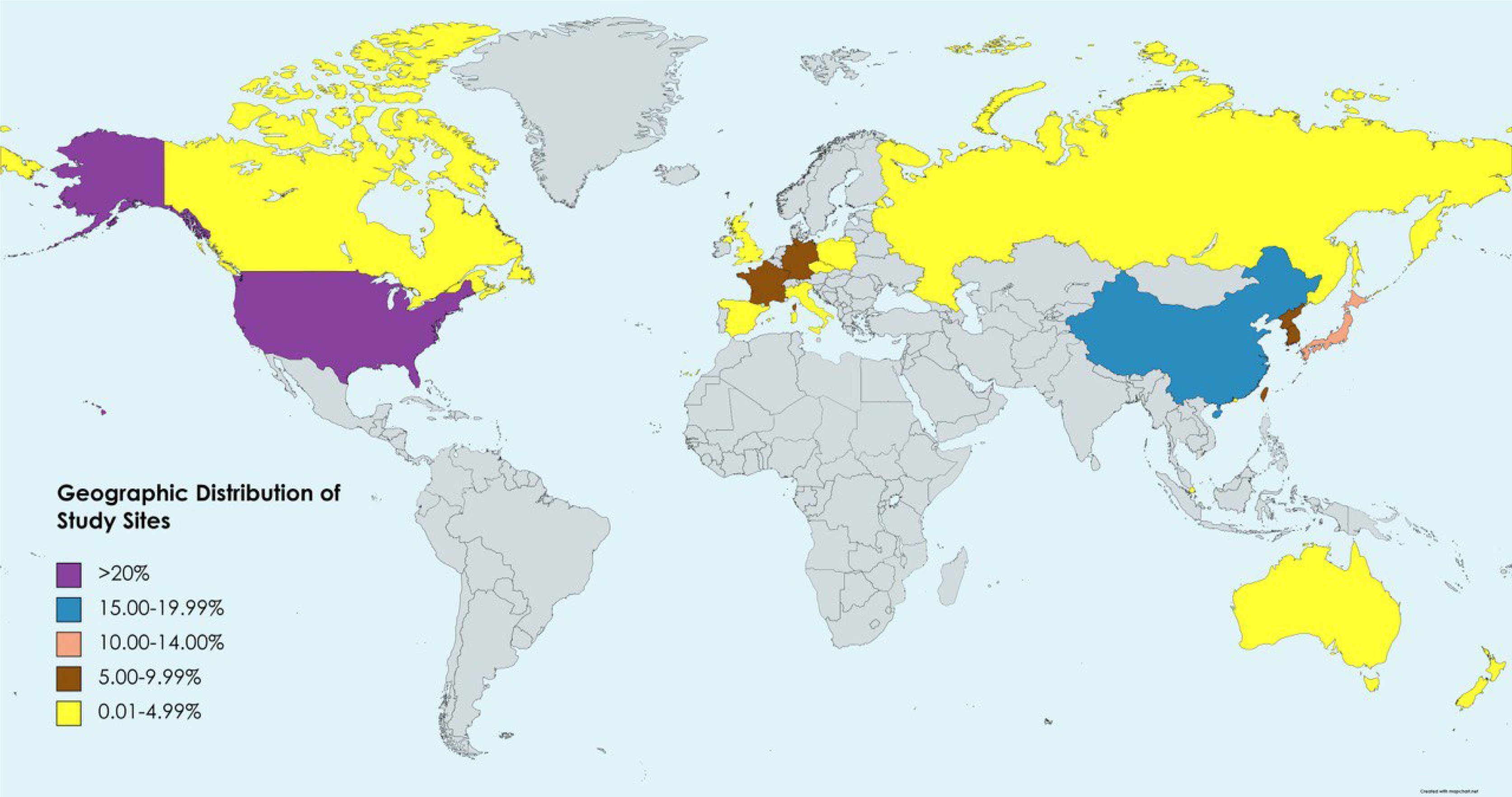
The Finn et al[12] and Lee et al[16] studies encompass a total of 724 patients and have been evaluated as follows. All clinical trials comprised a large sample of patients recruited from more than 310 sites across more than 20 countries. The countries included sites in North America (United States, Canada), Europe (United Kingdom, Germany, France, Italy, Poland, Spain, Russia, Czech Republic), and Asia-Pacific (China mainland, Japan, Republic of Korea, Taiwan, Australia, Hong Kong, Russia, Singapore, and New Zealand). The distribution of sites is shown in Figure 4. The specific patient profiles of La Roche[14] and Hack et al[15] have not yet been published (Table 1).
The clinical trials recruited adult volunteers of both genders, with locally advanced metastatic or unresectable HCC (or both). All trials used the American Association for the Study of Liver Disease criteria for histologic, cytologic, and clinical diagnostic confirmation. A documented hepatitis virological status was also required and a history of autoimmune disease was considered an exclusion criterion.
Eligible patients in the trials, who had not previously received systemic therapy for HCC, had an Eastern Cooperative Oncology Group (ECOG) performance score of either 0 or 1 and a Child-Pugh class of A or B (Supplementary Table 2). The studies attributed their exclusion of Child-Pugh class C to the increased risk of patient death due to related underlying cirrhosis. The patients with underlying cirrhosis were excluded from the study to avoid potentially confounding impact on the assessment of treatment-related antitumor efficacy. La Roche[14], Finn et al[12], and Lee et al[16] included patients who had HCC that was measurable as per the RECIST 1.1 (Supplementary Table 1). However, there was no specific mention of RECIST 1.1 in Hack et al[15].
Moreover, Hack et al[15], Finn et al[12], and Lee et al[16] employed BCLC staging (Supplementary Table 3). There was no specific mention of BCLC staging in La Roche[14].
Baseline characteristics of patients across the two completed trials and treatment modalities were adequately balanced (Table 2). Both studies had a median age range of about 63-years-old. Specifically, Finn et al[12] had a median age of 64 (interquartile range, [IQR], 56-71) and 66 (IQR, 59-71) years for its interventional (atezolizumab/bevacizumab) and control (sorafenib monotherapy), respectively. Whereas Lee et al[16] had a median age of 62 (IQR, 23-82) for Group A (atezolizumab/bevacizumab), 60 (IQR, 22-82) for Group F (atezolizumab/bevacizumab) and 63 (23-85) for the control (atezolizumab monotherapy), respectively. Both studies predominantly included the male sex (83%), Asian (62%) and Caucasian (30%) ethnicities, Child-Pugh class A (99%), and advanced BCLC (stage C) (84%) in the treatment and control groups. Both studies reported a higher prevalence of patients with extrahepatic spread, positivity for hepatitis B, and a history of alcohol use. Finn et al[12] included mostly ECOG 0 patients than ECOG 1, while the opposite was observed for Lee et al[16] Only about 35% of patients across the studies had alpha-fetoprotein > 400 ng per milliliter. Regarding PD-L1 status, more patients had tumor cell or immune cell ≥ 1% than any other classification, across treatment and control for both studies. Finn et al[12] showed the number of patients who previously experienced local therapy for HCC, and almost half of the patients had at least one treatment on both the atezolizumab/bevacizumab (48%, 161/336 patients) and sorafenib (52%, 85/165 patients) arms. Whereas Lee et al[16] did not show those patients who had prior local therapy for HCC.
| Finn et al[12], 2020 | Lee et al[16], 2020 | ||||
| Interventional arm | Control arm | Interventional arm | Control arm | ||
| Atezolizumab-bevacizumab combination therapy | Sorafenib monotherapy | Atezolizumab-bevacizumab combination therapy given in both Arms A and F | Atezolizumab monotherapy | ||
| Group A | Group F+ | ||||
| n = 336 | n = 165 | n = 104 | n = 60 | n = 59 | |
| Median age (IQR), yr | 64 (56-71) | 66 (59-71) | 62 (23-82) | 60 (22-82) | 63 (23-85) |
| Gender, n (%) | |||||
| Male | 277 (82) | 137 (83) | 84 (81) | 54 (90) | 49 (83) |
| Female | 59 (18) | 28 (17) | 20 (19) | 6 (10) | 10 (17) |
| Race, n (%) | |||||
| White | 123 (37) | 52 (32) | 20 (19) | 14 (23) | 9 (15) |
| Asian | 188 (56) | 96 (58) | 75 (72) | 45 (75) | 47 (80) |
| Black or African American | 6 (1.8) | 4 (2.4) | 7 (7) | 1 (2) | 2 (3) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Unknown | 19 (6) | 12 (7) | 2 (2) | 0 (0) | 0 (0) |
| Geographic region, n (%) | |||||
| Asian excluding Japan | 133 (40) | 68 (41) | 59 (57) | 39 (65) | 39 (66) |
| Rest of the world (United States, Australia, New Zealand, and Japan) | 203 (60) | 97 (59) | 45 (43) | 21 (35) | 20 (34) |
| Child Pugh, n (%) | |||||
| Child Pugh A5 | 239 (72) | 121 (73) | 77 (74) | 43 (72) | 42 (71) |
| Child Pugh A6 | 94 (28) | 44 (27) | 21 (20) | 17 (28) | 17 (29) |
| Child Pugh A7 | 0 (0) | 0 | 6 (6) | 17 (28) | 17 (29) |
| Child Pugh B | 1 (< 1) | 0 (0) | NA | NA | NA |
| ECOG performance status, n (%) | |||||
| ECOG 0 | 209 (62) | 103 (62) | 52 (50) | 27 (45) | 25 (42) |
| ECOG 1 | 127 (38) | 62 (38) | 52 (50) | 33 (55) | 34 (58) |
| BCLC, n (%) | |||||
| BCLC stage A (early) | 8 (2) | 6 (4) | 0 (0) | 0 (0) | 2 (3) |
| BCLC stage B (intermediate) | 52 (15) | 26 (16) | 10 (10) | 6 (10) | 4 (7) |
| BCLC stage C (advanced) | 276 (82) | 133 (81) | 94 (90) | 54 (90) | 53 (90) |
| Alpha-fetoprotein > 400 ng per milliliter | 126 (38%) | 61 (37%) | 37 (36%) | 18 (30%) | 19 (32%) |
| Macrovascular invasion | 129 (38%) | 71 (43%) | 55 (53%) | 20 (33%) | 25 (42%) |
| Extrahepatic spread | 212 (63%) | 93 (56%) | 91 (88%) | 47 (78%) | 50 (85%) |
| Hepatitis B | 164 (49%) | 76 (46%) | 51 (49%) | 34 (57%) | 32 (54%) |
| Hepatitis C | 72 (21%) | 36 (22%) | 31 (30%) | 11 (18%) | 10 (17%) |
| Non-viral | 100 (30%) | 85 (52%) | 22 (21%) | 15 (25%) | 17 (29%) |
| Alcohol use, n (%) | |||||
| Previous | 166 (50) | 79 (48) | 58 (56) | 39 (65) | 32 (54) |
| Never | 121 (36) | 61 (37) | 32 (31) | 14 (23) | 21 (36) |
| Current | 48 (14) | 25 (15) | 14 (13) | 7 (12) | 6 (10) |
| Varices at baseline | 88 (26%) | 43 (26%) | NA | NA | NA |
| Varices treated at baseline | 36 (11%) | 23 (14%) | NA | NA | NA |
| PD-L1 status, n (%) | 124 | 58 | NA | NA | NA |
| TC and IC < 1% | 45 (36) | 25 (43) | 25 (24) | 15 (25) | 18 (31) |
| TC or IC ≥ 1% | 79 (64) | 33 (57) | 61 (59) | 28 (47) | 34 (58) |
| TC ≥ 5% or IC ≥ 5% | 46 (37) | 17 (29) | 37 (36) | 8 (13) | 16 (27) |
| TC ≥ 10% or IC ≥ 10% | 12 (10) | 5 (9) | 30 (29) | 5 (8) | 6 (10) |
| Data missing | NA | NA | 18 (17) | 17 (28) | 8 (14) |
| Prior local therapy for HCC, n (%) | |||||
| At least one treatment | 161 (48) | 85 (52) | NA | NA | NA |
| Transarterial chemoembolization | 130 (39) | 70 (42) | NA | NA | NA |
| Radiofrequency ablation | 47 (14) | 24 (15) | NA | NA | NA |
| Prior radiotherapy | 34 (10) | 17 (10) | NA | NA | NA |
There were no significant differences that were explicitly stated between interventional and control in the published manuscripts of Finn et al[12] and Lee et al[16]. The following baseline characteristics were evaluated for differences: Median age, sex, race, geographic region, Child-Pugh class, ECOG stage, BCLC stage, alpha-fetoprotein levels, macrovascular invasion, extrahepatic spread, hepatitis status, alcohol use, PD-L1 status, and prior local therapy for HCC. Moreover, regarding gastroesophageal varices (current/treated), there were no specific indications of statistically significant differences between the interventional and control groups in Finn et al[12] as well. On the other hand, Lee et al[16] did not report the prevalence of varices but stated that varices were managed when present, according to the standard of care.
Mortality rates: According to Finn et al[12], mortality occurred in 28.6% of patients (96/336) in the atezolizumab/bevacizumab group during a follow-up duration of 8.9 mo at the clinical data cut-off, and was significantly lower than that reported in the sorafenib group (39.4%; 65/165 patients) during a similar surveillance time of 8.1 mo (P < 0.001 by χ2) (Table 3).
| Ref. | Finn et al[12], 2020 | Lee et al[16], 2020 | |||
| Schemes | Atezolizumab-bevacizumab combination therapy | Sorafenib monotherapy | Atezolizumab-bevacizumab combination therapy given in both Arm A and F | Atezolizumab monotherapy | |
| Total patients | Group A | Group F+ | |||
| n = 336 | n = 165 | n = 104 | n = 60 | n = 59 | |
| Primary efficacy outcomes | |||||
| Mortality | |||||
| n (%) | 96 (28.6) | 65 (39.4) | 16 (27) | 0 (0) | 18 (31) |
| Two-tail P value | P = 0.0033 | P = 0.0033 | No P value reported | No P value reported | No P value reported |
| HR for disease progression, CI | 0.59, 95%CI: 0.47-0.76 | Not applicable | NA | NA | NA |
| Two-tail P value | P < 0.001 | ||||
| HR for death, CI | 0.58, 95%CI: 0.42-0.79 | NA | NA | NA | NA |
| Two-tail P value | P < 0.001 | ||||
| HR for progression-free survival, CI | NA | NA | 0.55, 80%CI: 0.40-0.74 | NA | |
| Two-tail P value | P = 0.011 | ||||
| Secondary efficacy outcomes tumor survival and progression of disease | |||||
| Overall/survival rate, n (%) | n not explicitly reported | n not explicitly reported | 57 (55) | 16 (27) | 18 (31) |
| n (%) | -67.2 | -54.6 | |||
| 95%CI | CI not reported | CI not reported | CI not reported | ||
| 61.3-73.1 | 45.2-64 | ||||
| Median overall survival in mo | Not estimable | 13.2 mo | 17.1 mo | Median overall survival was not reached | Median overall survival was not reached |
| 95%CI | |||||
| (10.4 to not estimable) | (13.8 to not estimable) | (8.3 to not estimable) | (8.2 to not estimable) | ||
| 6 mo overall survival rates | NA | NA | NA | ||
| 95%CI | 84.80% | 72.20% | |||
| 80.9-88.7 | 80.9-88.7 | ||||
| 12 mo overall survival rates | 67.20% | 54.60% | NA | NA | NA |
| 95%CI | 61.3-73.1 | 45.2-64 | |||
| Median progression-free survival (mo), (95%CI) | 6.8 mo | 4.3 mo | 7.3 mo | 5.6 mo | 3.4 mo |
| (5.7-8.3) | (4.0-5.6) | (5.4-9.9) | (3.6-7.4) | (1.9-5.2) | |
| Overall confirmed objective response | n not explicitly reported (20%) | n not explicitly reported (17%) | |||
| n (%) as per RECIST 1.1 | (11-32) | (8-29) | |||
| 95%CI | 89 (27.3%) | 19 (11.9%) | 37 (36%) | ||
| (22.5-32.5) | (7.4-18) | (26-46) | |||
| Confirmed objective response-complete response as per RECIST 1.1, n (%) | 18 (5.5) | 0 (0) | 12 (12) | 1 (2) | 3 (5) |
| Confirmed objective response-Partial response as per RECIST 1.1, n (%) | 71 (21.8) | 19 (11.9) | 25 (24) | 11 (18) | 7 (12) |
| Stable disease n (%) as per RECIST 1.1 | 151 (46.3) | 69 (43.4) | 37 (36) | 28 (47) | 19 (32) |
| Progressive disease | |||||
| n (%) as per RECIST 1.1 | 64 (19.6) | 39 (24.5) | 25 (24) | 17 (28) | 25 (42) |
| Disease control rate, n (%) | 240 (73.6) | 88 (55.3) | 74 (71) | 40 (67) | 29 (49) |
| Ongoing objective response at data cut off, n (%) | 77/89 (86.5) | 13/19 (68.4) | NA | NA | NA |
| Safety outcomes (adverse events) | |||||
| Overall patients with an adverse event from any cause, n (%) | 323 (98.2) | 154 (98.7) | 100 (96) | 57 (95) | 52 (90) |
| Treatment-related serious adverse events, n (%) | 125 (38) | 48 (30.8) | 25 (24) | 7 (12) | 2 (3) |
| Treatment-related mortality | 161 deaths (%) | 3 (3%) | 0 (%) | NA | |
| It was not explicitly stated how many deaths there were in relation to treatment in either intervention or control arm1 | |||||
| Adverse events leading to dose modifications, n (%) | 163 (49.5) | 95 (60.9) | 50 (48) | 9 (15) | 5 (9) |
| Adverse events leading to withdrawal from any trial drug, n (%) | 51 (15.5) | 16 (10.3) | 18 (17) | 6 (10) | 0 (0) |
| Number of participants with Grade 3 and above, n (%) | 5-15 (4.6) | 9 (5.8) | 55 (53) | 22 (37) | 8 (14) |
| Types of Grade 3-4 adverse events | |||||
| Adverse events | Note: All stratified data reported below are Grade 3 or 4 | Note: All stratified data reported below are Grade 3, except increased aspartate aminotransferase (note stratification) | |||
| Hypertension, n (%) | 50 (15.2) | 19 (12.2) | 15 (14) | 3 (5) | 1 (1) |
| Decreased appetite, n (%) | 4 (1.2) | 6 (3.8) | 1 (1) | 0 (0) | 0 (0) |
| Fatigue, n (%) | 8 (2.4) | 5 (3.2) | 1 (1) | 0 (0) | 0 (0) |
| Pyrexia, n (%) | 4 (1.2) | 2 (1.3) | 2 (2) | 0 (0) | 0 (0) |
| Rash, n (%) | 0 (0) | 4 (2.6) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea, n (%) | 6 (1.8) | 8 (5.1) | 3 (3) | 1 (2) | 0 (0) |
| Abdominal pain, n (%) | 4 (1.2) | 4 (2.6) | 4 (4) | 0 (0) | 0 (0) |
| Constipation, n (%) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Cough, n (%) | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Nausea, n (%) | 1 (0.3) | 1 (0.6) | NA | NA | NA |
| Weight decrease, n (%) | 0 (0) | 1 (0.6) | NA | NA | NA |
| Epistaxis, n (%) | 0 (0) | 1 (0.6) | NA | NA | NA |
| Asthenia, n (%) | 1 (0.3) | 4 (2.6) | NA | NA | NA |
| Infusion-related reaction, n (%) | 8 (2.4) | 0 (0) | NA | NA | NA |
| Palmar-Plantar erythrodysesthesia syndrome, n (%) | 0 (0) | 13 (8.3) | NA | NA | NA |
| Proteinuria, n (%) | 10 (3) | 1 (0.6) | 7 (7) | 3 (5) | 0 (0) |
| Increased aspartate aminotransferase, n (%) | 23 (7.0) | 8 (5.1) | Grade 3: 3 (3) | 2 (3) | 2 (3) |
| Grade 4: 2 (2) | |||||
| Increased alanine aminotransferase, n (%) | 12 (3.6) | 2 (1.3) | NA | NA | NA |
| Blood bilirubin increase, n (%) | 8 (2.4) | 10 (6.4) | NA | NA | NA |
| Decreased platelet count, n (%) | 11 (3.3) | 2 (1.3) | 5 (5) | 0 (0) | 0 (0) |
| Pancreatitis, n (%) | 1 (0.3) | 2 (1.3) | NA | NA | NA |
| Hepatic Encephalopathy, n (%) | 2 (0.6) | 2 (1.3) | NA | NA | NA |
| Pulmonary Embolism, n (%) | 3 (0.9) | 2 (1.3) | NA | NA | NA |
| Cholangitis, n (%) | 4 (1.2) | 1 (0.6) | NA | NA | NA |
| Acute kidney failure, n (%) | 1 (0.3) | 3 (1.9) | NA | NA | NA |
| Gastrointestinal hemorrhage, n (%) | 4 (1.2) | 3 (1.9) | NA | NA | NA |
| Esophageal varices hemorrhage, n (%) | 6 (1.8) | 1 (0.6) | NA | NA | NA |
| Upper gastrointestinal hemorrhage, n (%) | 2 (0.6) | 2 (1.3) | NA | NA | NA |
| Asthenia, n (%) | 1 (0.3) | 4 (2.6) | NA | NA | NA |
| Types of Grade 5 adverse events | |||||
| Grade 5 adverse events, n (%) | 15 (4.6) | 9 (5.8) | 0 (0) | 0 (0) | 0 (0) |
| Not stratified1 | Not stratified1 | ||||
| Not evaluable/data missing | |||||
| Not evaluable, n (%) | 8 (2.5) | 14 (8.8) | NA | NA | NA |
| Data missing, n (%) | 14 (4.3) | 18 (11.3) | NA | NA | NA |
The overall mortality reported by Lee et al[16] in the atezolizumab/bevacizumab group was significantly different (higher in Group A, 27% [16/104 patients] and zero in Group F, [0/60 patients]; P < 0.0001 by χ2), and was significantly lower than that in the atezolizumab group (31%; 18/59 patients) at a median follow-up of 12.4 mo (P < 0.0001 by χ2). Lee et al[16] also showed a 7% mortality (7/10 patients) in Group A and did not report deaths related to AEs in Group F.
Using epidemiological analyses, this review estimated the relative risk (RR) of death from the combination therapy vs the monotherapy to be 0.72 (Finn et al[12]) and 0.87 (Lee et al[16]), respectively. The calculated RR reduction was 0.28 and 0.13, respectively, for both studies. The attributable risk was 0.108 (Finn et al[12]) and 0.04 (Lee et al[16]), and the number needed to treat (NNT) 9.2 for atezolizumab/bevaci
Hazard ratio for deaths and PFS: According to Finn et al[12], the stratified hazard ratio (HR) for death was 0.58 (95% confidence interval [CI]: 0.42-0.79; P < 0.001), whereas Lee et al[16] did not report on HR for death but rather estimated the HR for PFS stratified HR 0.55 (80%CI: 0.40-0.74; P = 0.011) in Group F (Table 3).
Overall/median survival: According to Finn et al[12], the estimated rates of OS at 6 and 12 mo were 84.8% (95%CI: 80.9-88.7) and 67.2% (95%CI: 61.3-73.1) in the atezolizumab/bevacizumab group, respectively. These results were significantly higher compared to 72.2% (95%CI: 65.1-79.4) and 54.6% (95%CI: 45.2-64.0) in the sorafenib group, respectively (P < 0.001) (Table 3). For Lee et al[16], median OS in Group A atezolizumab/bevacizumab was 17.1 mo (95%CI: 13.8 to not estimable), with only 55% (57 patients) still alive at the end of the surveillance. Median OS was not reached in atezolizumab/bevacizumab Group F. Additionally, neither Group F nor the atezolizumab group had estimable results as follows: (atezolizumab/bevacizumab: 95%CI: 8.3 mo to not estimable; atezolizumab group: 8.2 mo to not estimable).
Median PFS: Both studies reached significantly longer PFS in the atezolizumab/ bevacizumab dataset vs their respective monotherapy data set. In detail, median PFS was 6.8 mo (95%CI: 5.7-8.3) for patients treated with atezolizumab/bevacizumab compared to 4.3 mo (95%CI: 4.0-5.6] for patients treated with sorafenib alone in Finn et al[12] study (P < 0.001). On the other hand, for Lee et al[16], median PFS was 7.3 mo (95%CI: 5.4-9.9) and 5.6 mo (95%CI: 3.6-7.4) in Group A and Group F (atezolizumab/ bevacizumab), respectively, vs 3.4 mo (95%CI: 1.9-5.2) for atezolizumab monotherapy (P < 0.001) (Table 3).
Disease progression or death: At baseline, Finn et al[12] reported higher extrahepatic spread in 212/336 patients (63%) in the atezolizumab/bevacizumab group compared to 93/165 patients (56%) in the sorafenib group (P = 0.0005, by χ2) (Table 2). These percentages were significantly lower than those observed by Lee et al[16], where extrahepatic spread was shown in 91/104 (88%), 47/50 (78%), and 50/69 (85%) patients, for Groups A, F (atezolizumab/bevacizumab), and atezolizumab monotherapy, respectively (P = 0.004 by χ2). Moreover, in the study of Finn et al[12] atezolizumab/bevacizumab and sorafenib groups experienced similar disease progression (58.6% [97/336 patients] vs 66.1% [109/165 patients]; P = 0.10 by χ2) (Table 3). The stratified HR for progression or death was estimated to be 0.58 (95%CI: 0.42-0.79; P < 0.001).
Tumor response rate: A better overall tumor-confirmed objective response with combination therapy compared to the respective monotherapies in the control groups was reported. For Finn et al[12], a significantly higher overall tumor response was observed in 89/336 patients (27.3%; 95%CI: 22.5-32.5) with atezolizumab/ bevacizumab combination therapy than the one obtained with sorafenib in 19/165 patients (11.9%; 95%CI: 7.4-18.0) (P < 0.001 by χ2). Whereas Lee et al[16] detected a better overall tumor response in Group A of the combination therapy compared to Group F and the atezolizumab group, which were similar (36% [95%CI: 26-46] vs 20% [95%CI: 11-32] vs 17% [95%CI: 8-29]; P = 0.011). Further details concerning the other indices of tumor response, including the confirmed partial/complete/ongoing objective responses are shown in Table 3.
Disease control rate: The disease control rate was significantly higher in both trials for atezolizumab/bevacizumab combination therapy than sorafenib or atezolizumab monotherapies (Table 3). The estimates were 73.6% (240/336 patients) vs 55.3% (88/165 patients) for atezolizumab/bevacizumab and sorafenib (P < 0.001 by χ2), and 71% (74/104 patients), 67% (40/60 patients), and 49% (29/58 patients) for Group A, Group F and atezolizumab (P = 0.016 by χ2), respectively.
Overall AEs: Finn et al[12] estimated similar AEs that were contributed from any cause in 98.2% (323/336 patients) and 98.7% (154/165 patients) for the atezolizumab/ bevacizumab vs sorafenib monotherapy groups (P = 0.17 by χ2), respectively. Likewise, according to Lee et al[16], 96% (100/104 patients), 95% (57/60 patients), and 90% (52/58 patients) for Groups A/F (atezolizumab/bevacizumab) and atezolizumab (P = 0.13 by χ2), respectively (Table 3).
Treatment-related serious AEs: Finn et al[12] reported higher treatment-related AEs with atezolizumab/bevacizumab compared to sorafenib monotherapy (38% [125/336 patients] vs 30.8% [48/165 patients]; P < 0.0001 by χ2). Lee et al[16] recorded 24% (25/104 patients), 12% (7/60 patients), and 3% (2/58 patients) for Groups A/F (atezolizumab/bevacizumab) and atezolizumab (P < 0.001 by Fisher’s Exact Test), respectively (Table 3).
Grade 3-5 AEs: Details of the treatment-related deaths and severe AEs as well as other indices of safety are shown in Table 3. Finn et al[12] and Lee et al[16] reported Grade 3-5 AEs in 10% and 15% of participants, respectively. In the Finn et al[12] study, hypertension occurred in 15.2% and 12.2% for the combination therapy and sorafenib monotherapy groups, and 14%, 5%, and 1% in Groups A/F (atezolizumab/ bevacizumab) and atezolizumab, respectively (Lee et al[16]). Furthermore, Proteinuria occurred in 3% and 0.6% in the combination therapy and sorafenib monotherapy groups, respectively, whereas in 7%, 5%, and 0% for Groups A/F (atezolizumab/ bevacizumab) and atezolizumab groups, respectively. Other Grade 3-5 AEs that were reported included abdominal pain, fatigue, rashes, pyrexia, and palmar-plantar erythrodysesthesia syndrome (Table 3). Finn et al[12] registered fewer Grade 5 AEs in the combination therapy compared to sorafenib monotherapy (4.6% vs 5.8%), whereas Lee et al[16] study registered 0% of such events.
The RoB assessment for Finn et al[12] and Lee et al[16] trials resulted in high quality with a low RoB. However, in the two ongoing studies (La Roche[14] and Hack et al[15]), the RoB was not evaluated given that incomplete information existed (Figures 1 and 2).
Both completed trials (Finn et al[12] and Lee et al[16]) showed adequate randomization with a low RoB arising from the randomization process that was performed through an interactive voice-response or Web-response system in permuted blocks. There was also fair allocation concealment. For Lee et al[16], an independent statistician was responsible for generating the randomization sequence, which was subsequently stored within the interactive voice systems.
In both completed studies (Finn et al[12] and Lee et al[16]), open-label trials were implemented, and consequently had neither blinding nor masking of interventions. Lee et al[16] described 26 participants who deviated from the initially assigned atezolizumab/bevacizumab to atezolizumab monotherapy without describing post-crossover efficacy and safety results. Moreover, Finn et al[12] found it less cumbersome to not administer intravenous placebo infusions. Hence, due to the lack of blinding or masking and because of deviations from intended interventions, the two completed studies were estimated to have some RoB concerns.
All RCT results showed a low risk of attrition bias from the missing outcome data. Both studies also had appropriate measurements of survival outcomes (Finn et al[12]). For example, both used PFS and objective tumor response with the RECIST 1.1 (Supplementary Table 1) as well as the HCC-specific mRECIST by investigator assessment and independent faculty review. Thus, they displayed a low risk of measurement bias as well as lower selective reporting bias.
In the past, early-stage HCC has been treated with surgical treatment and/or thermal ablation. These procedures have been associated with high recurrence rates and therefore considered to have poor prognosis. The development of immunotherapy has led to new alternatives in treating HCC patients with advanced stages of the disease, who are considered unresectable with the standard surgery. Different treatments have been used for unresectable cases of HCC, including ICIs and VEGF inhibitors such as sorafenib, atezolizumab, and bevacizumab (Supplementary Figure 2).
In this systematic review, the results of Finn et al[12] and Lee et al[16] studies were summarized. The purpose was to combine with their studies the ongoing results of the two additional trials of La Roche[14] and Hack et al[15] to determine which of the therapeutic regimens could support a stellar efficacy and safety profile. Two clinical trials included 724 participants in about 137 sites in over 19 different countries were identified (Figure 4). The completed trials of Finn et al[12] and Lee et al[16] recruited participants mostly from the United States, Canada, the United Kingdom, Germany, France, Italy, Poland, Spain, Russia, Czech Republic, China mainland, Japan, Republic of South Korea, Taiwan, Australia, Hong Kong, Russia, Singapore, and New Zealand, which are mostly high and middle-income countries[17]. Both trials were assessed as having an overall low RoB outcome.
The results of this review demonstrate that the combination of atezolizumab/ bevacizumab had a beneficial effect on improving the overall efficacy and safety in treating patients with early-stage HCC compared to sorafenib or atezolizumab monotherapy. The combination therapy resulted in the prevention of mortality, increased OS and PFS rates, as well as better disease control and response rate. However, the proportion of participants with AEs from any cause were similar in both trials (Table 3).
Specifically, Finn et al[12] showed a higher OS rate with atezolizumab/bevacizumab than sorafenib groups along with a 42% reduced hazard of death at 6 and 12 mo of surveillance[12]. PFS rate and time were significantly higher in the atezolizumab/ bevacizumab group when compared to sorafenib (54.5% vs 37.2% and 6.8 mo vs 4.3 mo, respectively)[12]. Finn et al[12] reported a significantly lower mortality rate in the atezolizumab/bevacizumab than the sorafenib group at a median follow-up of 8.6 mo (28.6% vs 39.4%). The overall confirmed objective response, disease control rate, and median time to deterioration of quality of life were better in the atezolizumab/ bevacizumab group compared to the sorafenib group[12]. The objective response in the Finn et al[12] and Lee et al[16] studies were more than two times higher using the combination therapy than the monotherapy (27.3% vs 11.9% and 36% vs 17%, respectively).
In the Lee et al[16] study, the atezolizumab/bevacizumab combination was given in both Groups A and F as specified in the study protocol. Median OS was estimated to be 17.1 mo in the atezolizumab/bevacizumab group but was not estimated for the atezolizumab monotherapy group. In both Groups A and F, objective response was confirmed with the primary endpoint according to RECIST 1.1. Secondary efficacy outcomes were also achieved that included objective response (based on RECIST 1.1 assessment) and independent review assessment (HCC specific mRECIST) showing PFS, duration of response, and time to radiographic progression. Irrespective of PD-L1 status, there was a progressive survival benefit with atezolizumab/bevacizumab compared to the atezolizumab monotherapy. This progressive survival benefit was observed despite the difficulty experienced comparing efficacy within Groups A and F due to the varied follow-up periods[16]. Moreover, Lee et al[16] reported that responses with long surveillance were expected to change in Group F. Lee et al[16] also recorded a 27% and 0% mortality in the combination therapy Groups A and F, which was significantly lower compared to the 31% in the monotherapy group at the median 12.4 mo follow-up duration. The overall response rate was statistically significant in the combination therapy group (atezolizumab/bevacizumab) compared to the monotherapy group, especially with sorafenib[16]. Thus, in this study[16] the primary endpoint was PFS as per RECIST 1.1 and OS. IMbrave 150 results demonstrated a significantly better PFS, OS, and response rate with atezolizumab/bevacizumab combination therapy than with sorafenib[18].
Ongoing trials by La Roche[14] and Hack et al[15]are phase III randomized trials with atezolizumab/bevacizumab. Although the trials have not yet been finalized, the results to date are considered meaningful and support the objective of their study. In Finn et al[12], and Lee et al[16] studies, the profile of safety outcomes were rather comparable with the exception concerning the AEs related to dose modifications which were lower in the combination (atezolizumab/bevacizumab) therapy group than monotherapy (sorafenib) group (Finn et al[12]). Moreover, Finn et al[12] also showed a slightly higher rate of AEs with Grade 3 and above, especially regarding objective response in the atezolizumab/bevacizumab group compared to the sorafenib group (86.5% vs 68.4%, respectively). The rate of AEs leading to withdrawal from any drug trials in the atezolizumab/bevacizumab therapy group was significantly greater compared to the sorafenib group (15.5% vs 10.3%, respectively). The rate of AEs more than Grade 3 was less in the atezolizumab/bevacizumab group than the sorafenib group (4.6% vs 5.8%, respectively).
Overall, combination therapy demonstrated a better safety profile in both studies. There was a difference in the types of Grade 3-5 AEs reported in Finn et al[12] such as palmar-plantar erythrodysesthesia syndrome and increased bilirubin as well as infusion-related reactions, which were not reported in the study by Lee et al[16]. In Finn et al[12], hypertension occurred at a slightly higher rate in the atezolizumab/ bevacizumab group than in the sorafenib monotherapy group (15% vs 12%, respectively). Infusion-related reaction only occurred in the atezolizumab/ bevacizumab group (2.4% of cases) and additionally, palmar-plantar erythrodysesthesia syndrome was detected in the sorafenib group (8.3%). Furthermore, the occurrence of abdominal pain and asthenia was low in the atezolizumab/bevacizumab group (1.2% and 0.3%, respectively) compared to the sorafenib group (2.6% and 2.6%, respectively). There was also a mild increase in proteinuria (3%), aspartate aminotransferase (7%), alanine aminotransferase (3.6%) in those treated with atezolizumab/ bevacizumab combination therapy compared to those treated with sorafenib alone (proteinuria [0.6%], aspartate aminotransferase [5.1%], alanine aminotransferase [1.3%]). There was also a slight decrease in platelet count in the atezolizumab/ bevacizumab group (3.3%) compared to sorafenib monotherapy (1.3%)[12].
In Lee et al[16], AEs leading to withdrawal from the trial were reported only in Groups A and F (17% and 10%, respectively). The rate of AEs Grade 3 and above was greater in combination therapy (53% for Group A and 37% for Group F) than atezolizumab monotherapy (14%). Some differences in the Grade 3-5 types of AEs especially were identified with Group A when compared to atezolizumab alone. For instance, when considering the prevalence of hypertension as one of the most commonly occurring AEs, it was present in 14% of Group A and 5% of patients in Group F. Thus, these figures were slightly higher than the atezolizumab group (1%). Additionally, fatigue, abdominal pain, and asthenia occurred only in 1% and 4% of patients in Group A, when compared to Group F or atezolizumab monotherapy. Proteinuria in combination therapy was similar in Groups A and F (7% vs 5%)[16].
A major limitation of this review was that supportive evidence was based on a limited number of completed clinical trials used in the treatment of HCC. Moreover, there was inadequate applicability in low-income countries or developing countries where these novel immune therapies may not be available. Despite the limitations, the analysis of the studies reviewed in this document was considered overall satisfactory.
Additional study limitations included that both Lee et al[16] and Finn et al[12] studies were open-label trials that held a higher risk for bias. Although independent faculty reviewers were used to reduce the potential biases, there were no blinding or masking of the investigators and participants, thus further sustaining the potential for bias. In Lee et al[16], it was challenging to compare the efficacy among Groups A and F due to their different follow-up periods. Also, Lee et al[16] reported crossover participants from monotherapy to combination therapy; however, post-crossover results were not mentioned. The aforementioned could have further created some kind of reporting bias, decreasing the quality of the study. Although both RCTs were satisfactory in terms of quality, they did not present robust evidence. Additionally, in-depth cost-effectiveness analysis, which could have provided further support, was not performed.
It is of great importance to consider cost-effectiveness for combination therapies to be effectively administered worldwide. A study (Hou and Wu[19], 2020) conducted in China, stated that in the base-case analysis, atezolizumab/bevacizumab gained a marginal 0.811 quality-adjusted life-year (QALY) and 1.297 overall life-years with an augmented cost of $49994 as compared with sorafenib, which led to an incremental cost-effectiveness ratio (ICER) of $61613/QALY[16]. The study by Wang et al[20] (2021), conducted using the IMIbrave 150 trial evaluation, reported that atezolizumab/bevacizumab treatment resulted in an increase of 0.623 Life-years, 0.484 QALYs, and $158781 per patient at the base case analysis[20]. The ICER was $322500 per QALY (95%CI: 136275-801509 per QALY)[20]. The negative incremental net benefit of -0.810 QALY reported by Hou et al[19] (2020) as well as the ICER of $322500 per QALY reported by Wang et al[20] (2021) was considered to be rather expensive for combination therapy implementation.
Conversely, both completed trials[12,16] demonstrated promising results in terms of better combination therapy efficacy with atezolizumab/bevacizumab compared to monotherapy (either sorafenib or atezolizumab). PD-1 and PD-L1 play key roles by escaping tumor immune surveillance in tumor progression and survival[15,21]. The PD-1 and PD-L1 antibody inhibitors act against PD-1 or PD-L1, and stimulate T-cell mediated immunity. Although PD-1 is mostly expressed on T cells, they also activate PD-L1 on cancer cells and antigen-presenting cells[15,21]. Therefore, PD-L1 inhibitors cause the resurrection of T-cell mediated anti-tumor immune effects unless other T-cell regulatory proteins are blocked such as cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), thus resulting in improved cancer immunotherapy[15,21].
The CTLA-4 antibody inhibitor (ipilimumab) and PD-1 inhibitors (pembrolizumab, nivolumab) have both been approved for treating various solid tumors including lung cancer, renal cell cancer, and ovarian cancer. Other anti-tumor agents such as kinase inhibitors, checkpoint inhibitors, and targeting agents are used in combination with PD-1 antibody inhibitors[11,22,23]. Even though clinical data show monotherapy as a successful immunotherapy regimen when focusing on safety and efficacy the clinical data shows that novel combination therapies are superior to monotherapy[7].
In this review, findings confirm that atezolizumab/bevacizumab combination therapy can be an effective first-line treatment option to either sorafenib or atezolizumab monotherapy in patients with advanced HCC and non-decompensated liver disease. However, due to the small number of RCTs included, this systematic review may be considered insufficiently robust to provide strong recommendations. Consequently, further research and larger RCTs with cost-effectiveness analysis are necessary to validate our observations and identify the most efficacious and safe therapeutic regimen.
Despite the use of the current standard therapy, the prognosis of unresectable hepatocellular carcinoma (HCC) patients is poor, with median survival times of 40 mo in intermediate HCC (Barcelona Clinic Liver Cancer [BCLC] stage B) and 6–8 mo in advanced HCC (BCLC stage C). Although patients with early-stage HCC are usually suitable for therapies with curative intention, up to 70% of patients may manifest disease relapses at 5-year surveillance. Moreover, no treatment has been demonstrated to be useful in the adjuvant setting.
This systematic review described the evidence for atezolizumab/bevacizumab combination therapy vs sorafenib or atezolizumab monotherapies in improving survival outcomes and reducing disease progression in patients with unresectable HCC.
To evaluate the efficacy and safety of atezolizumab/bevacizumab vs sorafenib or atezolizumab alone, in patients with unresectable HCC with non-decompensated liver disease.
A comprehensive literature review of published articles was conducted to identify studies that met our inclusion criteria using relevant mesh terms. This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and we assessed for risk of bias using the Cochrane ROB tool and Sevis tool to create the traffic light plots and summary plots.
There was an improvement in overall tumor response, reduction of disease progression, and longer progression-free survival in the atezolizumab/bevacizumab group compared to the monotherapy of either sorafenib or atezolizumab.
This study confirms that combination treatment of atezolizumab plus bevacizumab could be a promising alternative to the standard of care sorafenib as a first-line treatment in patients with unresectable HCC and non-decompensated liver disease.
Given the scarcity of randomized controlled trials specifically focusing on this therapeutic strategy, further research is needed to strengthen the current evidence. Two completed clinical trials were analyzed in this research; however, this review will be updated upon the completion of two ongoing trials. Moreover, further evaluation regarding the cost-effectiveness of combination therapy vs monotherapy is still needed valuable information.
We sincerely acknowledge Dr. Karthik Mohan, MD and Dr. Jack Michel, MD for their guidance and support throughout the completion of this project.
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: European Society of Gastrointestinal Endoscopy, No. 66289622.
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akarsu M, Katada K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6048] [Article Influence: 864.0] [Reference Citation Analysis (3)] |
| 3. | Brown ZJ, Greten TF, Heinrich B. Adjuvant Treatment of Hepatocellular Carcinoma: Prospect of Immunotherapy. Hepatology. 2019;70:1437-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 4. | Liu X, Qin S. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges. Oncologist. 2019;24:S3-S10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2966] [Cited by in RCA: 3146] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 6. | Ranieri G, Gadaleta-Caldarola G, Goffredo V, Patruno R, Mangia A, Rizzo A, Sciorsci RL, Gadaleta CD. Sorafenib (BAY 43-9006) in hepatocellular carcinoma patients: from discovery to clinical development. Curr Med Chem. 2012;19:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1367] [Cited by in RCA: 1239] [Article Influence: 154.9] [Reference Citation Analysis (0)] |
| 8. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4180] [Article Influence: 380.0] [Reference Citation Analysis (0)] |
| 9. | Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 703] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 10. | Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, Hernandez G, Mier J, He X, Hodi FS, Denker M, Leveque V, Cañamero M, Babitski G, Koeppen H, Ziai J, Sharma N, Gaire F, Chen DS, Waterkamp D, Hegde PS, McDermott DF. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 559] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 11. | Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 334] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 12. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4682] [Article Influence: 936.4] [Reference Citation Analysis (2)] |
| 13. | Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen YL, Xu Y, Liu J, Zhao H, Pierce WF, Mehta S, Goldberg KB, Theoret MR, Kluetz PG, Pazdur R, Lemery SJ. FDA Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable or Metastatic Hepatocellular Carcinoma. Clin Cancer Res. 2021;27:1836-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 14. | La Roche H. A Phase IIIB, Single Arm, Multicenter Study of Atezolizumab (Tecentriq) in Combination With Bevacizumab to Investigate Safety and Efficacy in Patients With Unresectable Hepatocellular Carcinoma Not Previously Treated With Systemic Therapy-Amethista. 2020. [cited 5 March 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04487067. |
| 15. | Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, Lee HC, Yopp A, Chow P, Qin S. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16:975-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 16. | Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack SP, Spahn J, Liu B, Abdullah H, Wang Y, He AR, Lee KH; GO30140 investigators. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 410] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 17. | World Economic Situation and Prospects, 2018. United Nations. [cited 5 March 2021]. Available from: https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/WESP2020_Annex.pdf. |
| 18. | Cheng AL, Qin S, Ikeda M, Galle P, Ducreux M, Zhu A, Kim TY, Kudo M, Breder V, Merle P. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30:ix186. [RCA] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 19. | Hou Y, Wu B. Atezolizumab plus bevacizumab vs sorafenib as first-line treatment for unresectable hepatocellular carcinoma: a cost-effectiveness analysis. Cancer Commun (Lond). 2020;40:743-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Wang W, Wang J, Zhang X, Wang Y, Shi J, Jia X, Dang S. Atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma: A cost-effectiveness analysis. J Clin Oncol. 2021;39:295-295. [DOI] [Full Text] |
| 21. | Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front Oncol. 2014;4:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 22. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10338] [Article Influence: 795.2] [Reference Citation Analysis (34)] |
| 23. | Chen DS, Hurwitz H. Combinations of Bevacizumab With Cancer Immunotherapy. Cancer J. 2018;24:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |