Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1696
Peer-review started: March 16, 2021
First decision: May 3, 2021
Revised: May 30, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: November 15, 2021
Processing time: 240 Days and 14.7 Hours
Cancer of the biliary confluence also known as hilar cholangiocarcinoma (HC) or Klatskin tumor, is a rare type of neoplastic disease constituting approximately 40%-60% of intrahepatic malignancies, and 2% of all cancers. The prognosis is extremely poor and the majority of Klatskin tumors are deemed unresectable upon diagnosis. Most patients with unresectable bile duct cancer die within the first year after diagnosis, due to hepatic failure, and/or infectious complications secondary to biliary obstruction. Curative treatments include surgical resection and liver transplantation in highly selected patients. Nevertheless, very few patients are eligible for surgery or transplant at the time of diagnosis. For patients with unresectable HC, radiotherapy, chemotherapy, photodynamic therapy, and liver-directed minimally invasive procedures such as percutaneous image-guided ablation and intra-arterial chemoembolization are recommended treatment options. This review focuses on currently available treatment options for unresectable HC and discusses future perspectives that could optimize outcomes.
Core Tip: Most patients with hilar cholangiocarcinoma (HC) are not candidates for surgery or liver transplant at the time of diagnosis. Recently, several options for the management of unresectable HC have emerged and due to the complexity of this disease, a multi-disciplinary approach with multimodal treatment is recommended, including surgery, medical oncology, radiation oncology, diagnostic radiology, interventional radiology, gastroenterology, and pathology. Recent data suggest an improvement in overall survival, better response rates, and tumor control in patients with unresectable HC can be achieved by combining chemotherapy and minimal invasive ablatives strategies.
- Citation: Inchingolo R, Acquafredda F, Ferraro V, Laera L, Surico G, Surgo A, Fiorentino A, Marini S, de'Angelis N, Memeo R, Spiliopoulos S. Non-surgical treatment of hilar cholangiocarcinoma. World J Gastrointest Oncol 2021; 13(11): 1696-1708
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1696.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1696
Cancer of the biliary confluence also known as hilar cholangiocarcinoma (HC) or Klatskin tumor, is a rare type of neoplastic disease constituting approximately 40%-60% of intrahepatic malignancies, and 2% of all cancers[1]. It mainly affects subjects over 65 years of age and established risk factors are primary sclerosing cholangitis, biliary tract lithiasis, and parasitic liver disease (biliary ascariasis, liver schistosomiasis, and fluke infestation), while other associated risk factors include, chronic pancreatitis, cirrhosis, inflammatory bowel disease, advanced age and male gender[2]. Typical symptoms are painless jaundice, cachexia, fatigue, and abdominal pain, usually reflecting the advanced stage of the disease at presentation, while concomitant cholangitis is present only in up to 10% of the cases. The prognosis is extremely poor as the majority of Klatskin tumors are deemed unresectable upon diagnosis and most patients with unresectable bile duct cancer die within the first year after diagnosis, due to hepatic failure, and/or infectious complications secondary to biliary obstruction[3].
Recommended imaging modalities for the diagnosis and staging of HC include computed tomography, magnetic resonance imaging, and magnetic resonance cholangiopancreatography, which provide detailed information regarding the location and extent of HC (Bismuth–Corlette classification), vessel involvement and metastases. Criteria of unresectability include locally advanced (LA) tumor (mainly vessel involvement), lymph node metastases beyond the hepatoduodenal ligament, distant metastases, and patient’s performance status[4]. Endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC) are mainly reserved for biopsy and/or palliative procedures to relieve obstruction (endoscopic or percutaneous biliary drainage). The most frequent HC histological type is the mucinous adenocarcinoma followed by the papillary type which is correlated with a more favorable prognosis. The only curative treatment remains surgical margin-negative (R0) resection (extended hemi-hepatectomy in most cases) with extrahepatic bile duct resection, hepatectomy, and en-bloc lymphadenectomy and if surgery is not an option, liver transplantation provides acceptable outcomes in highly selected patients. Nevertheless, survival rates for surgical after resection range between 10% and 40% at 5 years, with reported recurrence rates up to 50%-70%, even after R0 resection[5]. However, the percentage of patients eligible for resection remains low, around 25%[4]. For unresectable disease, radiotherapy, chemotherapy, and photodynamic therapy (PDT) are included in the treatment algorithm, while liver-directed, minimally invasive treatments such as percutaneous image-guided ablation options and intra-arterial chemoembolization have been more recently developed[6-8]. Due to the variety of diagnostic and treatment modalities involved in HC management, a multi-disciplinary approach is recommended including hepatobiliary and transplant surgeons, medical and radiation oncologists, diagnostic and interventional radiologists, gastroenterologists, and pathologists[3,5]. This review focuses on available treatment options for unresectable HC and discusses future perspectives that aim in the optimization of current outcomes.
In the case of LA inoperable tumors in patients who are not suitable for liver transplant, locoregional therapies could be considered as a valid alternative to treat such patients. Different ablative therapies have been studied for the treatment of advanced HC, including irreversible electroporation (IRE), PDT, and endobiliary radiofrequency ablation (ERFA) (Table 1).
| Ref. | Study design | Treatment | No. of patients | Outcomes | Complications/Adverse events |
| Hsiao et al[13] | Single-center, single-arm, retrospective | IRE | 9 | Median overall survival: 26 mo; progression-free survival: 18 mo | None reported |
| Martin et al[14] | Single-center, single-arm, retrospective | IRE | 26 | Median survival without biliary drainage: 305 d (range 92–458); disease-free: 11.5% | Complications: 3/26 (11.5%; severe 7.7%) |
| Li et al[18] | Single-center, comparative, retrospective | PDT + stent vs stent-only | 62 (30 vs 32) | Median survival: PDT + stent 14.2 vs stent-only 9.8 mo, P = 0.003 | Adverse events: 24 (38.7%) vs 20 (29.0%), P = 0.239 |
| Mizandari et al[22] | Single-center, single-arm, retrospective | Endobiliary RFA | 39 | Median survival: 89.5 d (range 14-260) | None reported |
| Andrašina et al[27] | Single-center, prospective, multimodal oncological therapy | TACE or IA chemotherapy with or without SC vs IV SC | 40 (17 vs 23) | Median overall survival: 13.5 mo (range, 11.0-18.8 mo). Median overall survival IA: 25.2 mo (range, 15.2-31.3 mo) vs IV SC 11.5 mo (range, 8.5-12.6 mo) in (P < 0.05) | None reported |
IRE is an image-guided ablation technique based on creating short-pulsed high-voltage current fields which applied for local control and progression of the primary LA tumor.
IRE may be considered not only for LA HC but also for patients with late-onset resection-site recurrence (after 6 mo).
As clinical practice and literature reported not all hepatic lesions are suitable for thermal ablation with radiofrequency ablation (RFA) or microwave ablation due to the possibility of damaging adjacent structures such as central bile ducts and gallbladder; moreover ablation close to large vessels can be ineffective because of heat sink effects or can cause vessel thrombosis[9]; IRE may potentially overcome the limitations of other modalities, such as skin phototoxicity in PDT, possible heat-sink effect in thermal ablation and the need for multiple fractions in stereotactic body radiotherapy (SBRT)[10]. As the effect of IRE is confined to the cell membrane and, in contrast to other ablative techniques, no thermal tissue damage occurs, thus avoiding vessels or duct injury.
Depending on the magnitude of the electric field and its exposure time, pulsed electric fields (PEFs) provoke either temporary (reversible) permeabilization of cell membranes and when the PEFs exceed a certain threshold value (w650 V/cm) delivered in 70–80 microseconds, irreversible injury to the membranes or permanent (irreversible) is induced with membrane disruption resulting in massive cell apoptosis[11].
There are no strict size criteria, IRE seems to be most effective for tumors < 3 cm in diameter.
IRE requires to be carried out under general anesthesia with complete neuromus
The neoplastic mass is surrounded by a defined number of needles ranging from two to six. In order to perform a macroscopic complete ablation with a 5 mm margin, the interelectrode distances should range from 10 to 24 mm, with a maximum angulation between electrodes of 15°.
Given the complex anatomy of the liver hilum and the proximity of the hepatic duct, portal vein, and hepatic arteries, IRE may be associated with severe complications.
Dollinger et al[12] analyzed injury to venous structures and bile duct structures within 1 cm of an IRE ablation zone in hepatic tumors[12]. Only 10% of vessels demonstrated lesions, including portal vein thrombosis and vessel narrowing, which resolved in most patients. However, partial portal vein thrombosis is a relative contraindication because of the increased risk of worsening of the thrombus. Severe cardiac arrhythmias or cardiac dysfunction are considered contraindications to IRE procedure[10].
Bile leak and hepatic artery or portal vein thrombosis are possible complications associated with the procedure, necessitating careful monitoring and instruction of patients on discharge.
IRE has advantages of effective local tumor control, safety, fewer complications, and an absence of heat-sink effects. In literature is reported high efficacy in local tumor control with overall survival (OS) of 24.8 ± 6.84 mo and disease progression-free survival (PFS) of 18.5 ± 8.41 mo[13].
In properly selected patients with obstructive jaundice, it safely achieves biliary decompression, therefore IRE can be used to increase catheter-free days and optimize the overall quality of life[14].
However, there are no reports available describing the median or long-term survival of patients with HC following IRE procedure.
Overall, based on current literature, IRE represents a promising technique concerning safety and local control for HPB tumors ineligible for resection or thermal ablation due to their proximity to vital structures.
Preclinical studies have demonstrated that IRE creates a well-defined boundary between ablated and non-ablated tissue; thus, the cells are either destroyed or remain intact. Compared with thermal ablation, perivascular tumor ablation with IRE appears to result in less frequent recurrence, indicating that the effectiveness of IRE is not influenced by the heat sink effect[15].
On the other hand, this technique presents some disadvantages compared to other thermal ablation such as RF and Mowat Wilson syndrome, because IRE needs to be performed under general anesthesia, is more complex and is much more expensive[16].
Although more clinical trials and comparative studies are required to validate the efficacy of ire in comparison with others non surgical treatment for HC.
PDT is a two-step procedure with either percutaneous transhepatic cholangioscopy (PTCS) or ERCP. At the time of ERCP, a bougie catheter choledochoscope is advanced to the level of the malignant stricture and used to deliver the laser fiber. The first step of the procedure involves the intravenous administration of photosensitizing agents that accumulate within cancer cells; subsequently, after an interval required for the drug to accumulate in the cancer, the tumor is exposed to non-thermal laser light of the appropriate photoactivation wavelength. Light activation leads to the formation of singlet oxygen free radicals and the destruction of nearby cells.
There are two major PDT methods for HC, ERCP and PTCS ones. ERCP is the preferred method but requires X-ray fluoroscopy to display the optical fiber marker at the tumor site. On the other side, the major advantage of PTCS is direct viewing of the tumor for more accurate localization and assessment of therapeutic response, while disadvantages include relatively greater trauma due to percutaneous approach.
Common adverse events after PDT include acute cholangitis, pancreatitis, haemobilia, liver abscess, and skin photosensitivity reactions. Severe skin photo
Recent studies have shown that PDT for unresectable cholangiocarcinoma can reduce bile duct stenosis, improve quality of life, and prolong survival[18].
Multiple prospective and retrospective series have demonstrated an increase in survival of 2-3 mo with the addition of PDT to biliary stenting in a palliative setting. A phase II pilot study by Wiedmann et al[19] evaluating PDT as a neoadjuvant modality demonstrated a 1-year survival of 83%[19].
There are few studies about the clinical applicability of RFA for malignant bile duct obstruction. ERFA catheters (Figure 1) were first introduced less than 10 years ago[20], and these catheters are easily used with a standard-sized duodenoscope, therefore RFA procedure can be performed either through endoscopic or percutaneous access. The rationale of those catheters is to destroy locally the malignant biliary stricture; local coagulative necrosis caused by RFA has the potential to delay tumor growth, prolonging the duration of stent patency[21].
ERCP-directed RFA is a novel procedure that induces local coagulative necrosis by delivering thermal energy via a bipolar probe by using high-frequency alternating current over a guidewire to the level of the stricture of interest by using fluoroscopic guidance, during ERCP or through endoscopic ultrasonography[22].
Several studies concerning endoscopic RFA procedures of malignant biliary strictures have been published. However, data are limited, because of small sample sizes, lack of randomization, and study heterogeneity (biliary tumor site). Complications reported after ERFA are sepsis, cholecystitis, and pancreatitis.
Most studies using ERCP-guided RFA in the treatment of HC assessed improve
Reports comparing the beneficial effects of endoscopic RFA therapy for the survival of patients with biliary cancer are rare.
Endoscopic RFA can significantly alleviate jaundice, reduce the thickness of tumor lesions, prolong HC stent patency, improved the quality of life, without increasing complications’ rate.
In his study, Yang et al[25] reported that bilirubin levels at 2 wk were significantly reduced in the RFA + stent group compared with the stent-only group, suggesting that RFA could reduce jaundice more rapidly. Moreover, stent patency of the RFA + stent group was significantly longer than that of the stent-only group. RFA combined with stent placement can prolong biliary tract patency and OS without increasing the incidence of adverse events in patients with cholangiocarcinoma[25].
Also, the percutaneous approach of intrabiliary tract RF ablation, firstly described in 2013 by Mizandari et al[21] in patients with unresectable malignant hilar biliary obstruction is considered a feasible and safe procedure because it can be performed following biliary decompression with minimal discomfort to the patient.
In the last few years, very little scientific literature has been produced about locoregional palliative intra-arterial therapies for unresectable HC.
Even in the last expert consensus statement by Mansour et al[3], there is no mention of intra-arterial therapies, considering systemic chemoradiation with or without intraluminal brachytherapy (ILBT) as the best choice in tumor control rate[3].
A retrospective cohort study conducted at the Liaoning Cancer Hospital by Zheng et al[26] investigates the clinical efficacy of cisplatin-based and gemcitabine transcatheter arterial chemoembolization combined with radiotherapy after biliary drainage or biliary stent implantation in patients with HC, thus obtaining a median survival time of 20 mo, almost doubling that of the control group (10.5 mo). The median patency time of the biliary stent (15.6 mo) was also more than doubled compared with the control group[26].
In 2010, Andrašina et al[27] published a prospective study on multimodal oncological therapy for unresectable cholangiocarcinoma, selecting 43 patients who underwent metallic-stent implantation followed by ILBT; 38 of these (88%) had hilar involvement. Patients have been divided into two arms: The intra-arterial arm consisted of patients treated with a locally intra-arterial infusion via a Port catheter percutaneously inserted into the hepatic artery of Cisplatin and 5-fluorouracil (5-FU) completed by a non-selective embolization with iodized oil (Lipiodol) and/or systemic chemotherapy, while the intravenous arm was treated only with systemic chemotherapy. The median OS from diagnosis was 25.2 mo in the IA arm and 11.5 mo in the IV arm[27].
This was a not randomized study and patients were selected according to the principle of individually tailored multimodal oncological therapy. Highly vascularized tumors, which could be the target of chemoembolizations, have a naturally better prognosis than hypovascular ones.
The role of chemotherapy is sometimes associated with transplantation in the unresectable disease limited-stage; since 2005 some experience is described to investigate the role of liver transplantation after chemoradiation in stage I and II HCs. In this protocol, seventy-one patients were enrolled in the transplant protocol and received neoadjuvant external beam radiotherapy to a target dose of 4500 cGy in 30 fractions. Concomitantly, intravenous fluorouracil (5-FU) was given. Two to three weeks after the completion of external beam radiotherapy, a transluminal boost of radiation was delivered using a transcatheter Iridium-192 brachytherapy wire; authors conclude that liver transplantation with neoadjuvant therapy currently appears to have greater efficacy than resection for selected patients with localized, node-negative HC. Despite differences in the patient groups, transplantation with neoadjuvant therapy achieved better local control and higher patient survival than did conventional resection[28].
Darwish Murad et al[29] analyze data from 12 United States participating centers reported 319 patients; Patients with HC who were treated with neoadjuvant therapy followed by liver transplantation had a 65% recurrence-free survival rate after 5 years, demonstrating this therapy to be highly effective in very selected cases[29]. This was not a randomized controlled trial, so further study are needed.
In the unresectable disease, palliative chemotherapy or chemoradiation is the only treatment that must be attempted. Consistent data suggest the use of first-line gemcitabine and cisplatin chemotherapy in patients with advanced disease; the trial randomly assigned 410 patients with ECOG-PS ≤ 2 to systemic chemotherapy with gemcitabine alone or cisplatin–gemcitabine; the study showed an OS benefit in favor of cisplatin–gemcitabine (hazard ratio 0.64)[30]; in some selected and limited stage cases, oncologists use the combination of gemcitabine and cisplatin as neoadjuvant intent; in cases of stable disease or partial response, it can be considered external beam radiotherapy with concomitant capecitabine oral administration. But there are no randomized trials to confirm the use.
Some trials are ongoing to investigate the role of triple-chemotherapy combinations in the first-line setting, such as cisplatin-gemcitabine combined with nab-paclitaxel or with S1 (tegafur, gimeracil, and oteracil), and FOLFIRINOX (5-FU, oxaliplatin, and irinotecan; AMEBICA study, NCT02591030). Acelarin, (NUC-1031) a first-in-class nucleotide analog, with cisplatin will be compared with gemcitabine and cisplatin combination therapy in a phase III study (NCT04163900). At the progression of first-line chemotherapy, the choice of the second-line chemotherapy is unclear. The ABC-06 trial showed a higher although modest median OS in the FOLFOX arm, differences in survival at 6 mo (35.5% vs 50.6%) and 12 mo (11.4% vs 25.9%) and the treatment is clinically meaningful[31]. FOLFOX can be considered a new standard of care in the second-line setting.
At the moment, the role of chemotherapy is mainly related to the advanced disease with a palliative purpose.
New target therapies have demonstrated a potential role in the intrahepatic cholangiocarcinoma treatment with isocitrate dehydrogenase (IDH) 1-IDH2 mutation and FGFR2 fusion[32]. So some phase III trials with IDH1–IDH2 or FGFR inhibitors as first- and/or second-line treatment are ongoing[33].
Due to the various inter-tumoral and intra-tumoral heterogeneity of cholagiocarcinoma, the hilar and peri-HC are considered different subtypes with different genetic alterations such as the mutations of AT-rich interactive domain (ARID)1B, E74-like factor (ELF)3, protein polybromo-1 (PBRM1), protein kinase cAMP-activated catalytic subunit alpha (PRKACA), and sub unit beta (PRKACB)[32]. At the moment there are not studies to investigate the role of specific drugs in this setting. Other and several functional studies with the PKCACA and PKCACB fusion genes will be mandatory for understanding pathogenesis in perihilar and distal cholangiocarcinoma[34].
The role of immunotherapy in that kind of disease is uncertain and under investigation. The programmed cell death protein 1 (PD-1), the programmed death-ligand 1 (PD-L1) and T lymphocyte-associated antigen 4 are the most known immune check point inhibitors drug targets. Studies are ongoing with monoclonal antibodies such as ipilimumab or tremelimumab (anti- CTL4) or antibodies targeting PD-L1, such as durvalumab, or its receptor PD-1, such as nivolumab or pembrolizumab. Preliminary data suggest a higher response rate in intrahepatic cholangiocarcinoma treatment with the genetic signature of microsatellite instability that can predict the response to the immune check point inhibition[35].
So the immune-modulating therapies could be promising options for the subgroup of patients with cholangiocarcinoma harboring high mutational loads[36].
The future direction of the medical treatment of HC it might be a combination of therapies involving immunotherapy plus chemotherapy, immunotherapy and radiotherapy[37].
For example, it is well established that the sensitivity of the immune system to the tumors is increased during the radiotherapy with a synergistic effect due to the changing of micro environment and apposition of new neo antigens. Some cases are reported of refractory advanced intrahepatic or HC that were treated in a satisfied way with anti-PD-1 antibody following or concurrent with SBRT[38].
Further studies are necessary to validate their efficacy and safety and to become the basis and direction for future researches for the treatment of HC patients.
In patients with inoperable or metastatic disease combined radio-chemotherapy or exclusive chemotherapy may be proposed.
Radiation therapy (external beam RT ± brachytherapy) with or without concomitant chemotherapy (5-FU or gemcitabine) is a potential choice in the treatment of patients with LA disease in good performance status. Since local progression of unresectable cholangiocarcinoma can lead to pain, biliary obstruction with severe hepatic insufficiency, this modality can control tumor-related symptoms and prolong survival.
However, the rarity of cancer associated with the lack of literature in this field of study with few clinical trials available (retrospective and non-randomized) means that the role of RT in this setting of patients is not yet well defined[39].
Some studies have shown improvements in symptoms of HC patients treated with radiotherapy with a median survival rate between 9 and 14 mo[40,41]. Classically the dose used is about 45-50 Gy delivered at 1.8-2 Gy/fraction with or without ILBT boost[42].
In a phase 2 study, 128 patients with intrahepatic malignancies, including 46 patients with cholangiocarcinoma, patients received a median dose of 60.75 Gy delivered in 1.5 Gy/fraction twice daily with conformational 3D technique. An improvement in survival compared to historical controls was observed, with 12 patients (of 33 evaluable patients with cholangiocarcinoma) achieving a complete or partial response to disease[43].
A retrospective analysis of 48 patients with gallbladder carcinoma and cholangiocarcinoma treated between 1998 and 2018 and a median radiotherapy dose of 50.4 Gy, achieved a median OS of 12.0 mo with OS at 2, 3, and 5 years of 33%, 20%, and 7%, respectively. In the univariate analysis, biologically effective dose (BED) > 59.5 Gy 10 was associated with improved PFS and OS and primary tumor size was associated with worsening PFS[44].
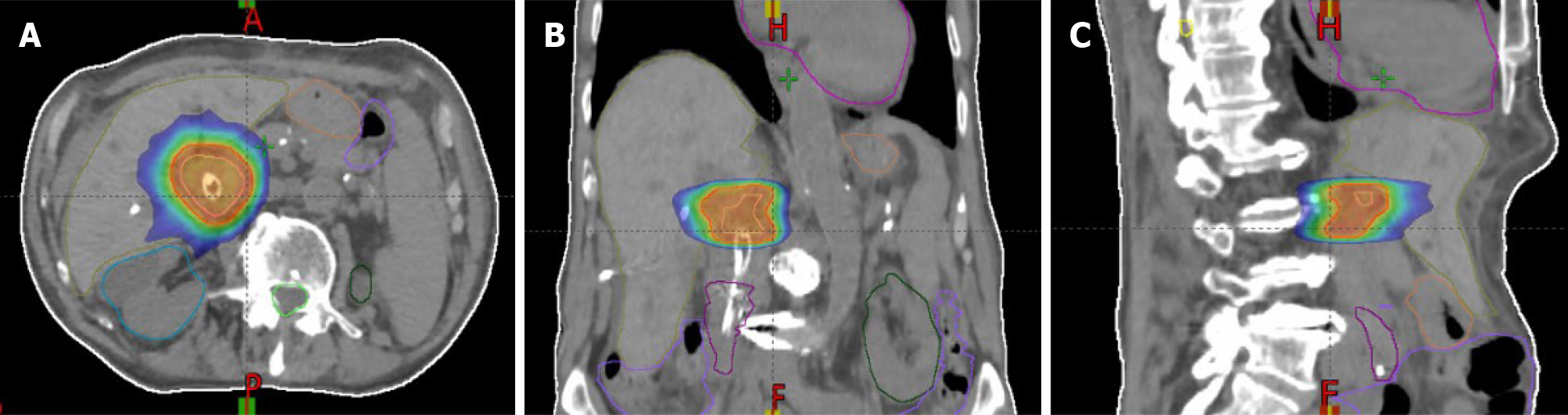
In the last decades, modern technological advances such as intensity-modulated RT, the ability to perform SBRT treatments (Figure 2), respiratory management methods, and imaging guidance during therapy, have enabled potentially ablative doses to be delivered for the treatment of cholangiocarcinoma[45,46].
However, the results of dose escalation studies for the treatment of HC were not clearly as favorable as those for intrahepatic cholangiocacinoma[47]. A multi-center retrospective study of patients with HC reported improved median survival in patients receiving > 40.0 Gy compared with those receiving less. A retrospective analysis of 52 patients with unresectable HC, suggested a possible association between increased radiation dose and improved LC[48].
In a recent study, 80 patients treated with RT for unresectable HC were retrospectively analyzed[49] in which RT was administered at doses of 30-75 Gy for a median BED of 59.5 Gy. The cohort was divided into a conventional dose group (BED ≤ 59.5) and a high dose RT (HDRT) group (> 50.4 Gy in 28 fractions, BED > 59.5) and. The HDRT group did not demonstrate better freedom from local progression or OS. Furthermore, HDRT was associated with the onset of grade 3 or higher lymphopenia[50]. These results suggest that higher doses do not provide elevated LC and OS benefits in HC. The proximity of HC tumors to the duodenum and/or small intestine is the factor limiting the ability to completely cover the tumor with high doses of radiation (tolerance doses < 50 Gy)[46].
Historically, the use of ILBT has shown an advantage in treating HC as a boost after EBRT or as a definitive treatment, given the possibility of limiting high doses to the liver or intestine[50] and studies are supporting its association with improved stent preservation and survival[51].
An Italian pooled analysis collected retrospective data from 3 radiotherapy Centers analyzing, from 1992 to 2017, 73 patients treated with EBRT + ILBT or EBRT alone in combination with chemotherapy or exclusive ILBT (with Ir 192 both HDR and LDR). The results demonstrated excellent local control, especially in patients treated with EBRT + LIRT + CHT or exclusive LIRT, in the absence of a clear impact on OS. Surely, careful selection of patients could allow us to evaluate who could benefit most from treatment with ILBT obtaining greater benefits[52].
SBRT has also been extensively explored as a potentially curative treatment strategy for patients with LA cholangiocarcinoma or in patients with local relapse. The total doses used ranged from 45 to 60 Gy in 3-5 fractions resulting in median survival of 11-29 mo[53]. The SBRT not only has the advantage of limiting doses to surrounding organs but also of limiting treatment times by increasing compliance with therapies and facilitating integration with systemic treatment.
Sandler et al[54] analyzed 31 patients with intrahepatic cholangiocarcinoma (19%) or extrahepatic cholangiocarcinoma (81%) who received SBRT at a median dose of 40 Gy in 5 fractions[54]. The median OS was 15.7 mo, the 2-year OS was 33%, and the 2-year LC was 47%. Serious adverse events occurred in 16% of patients (9% with grade 3-4 duodenal ulceration or bleeding).
A recent systematic review analyzed 10 studies (none of which were randomized) with at least 10 patients enrolled per study, in which SBRT was used for the treatment of intra- and extrahepatic cholangiocarcinoma[55]. Dose prescribing methods and total dose/fraction were highly variable with a median prescribed SBRT dose between 30 and 60 Gy in 3-5 fractions and median BED between 57.6 and 180.0 Gy. The survival results were almost comparable to those of standard chemoradiotherapy and CHT with a median OS of 15.0 mo. Results in terms of LC and toxicity would also demonstrate that SBRT treatment is reasonably effective with acceptable treatment-related toxicities. Overall, treatment-related acute and late toxicities were found to be acceptable and at rates almost comparable to those reported after chemoradiotherapy ± ILRT boost.
However, all the studies conducted so far show that the minimum available evidence in the setting of SBRT for cholangiocarcinoma highlights the need for high-quality studies in this area. In terms of OS, the preliminary results do not appear much different from those of standard chemoradiotherapy. Therefore, SBRT can be considered a therapeutic option in selected patients with cholangiocarcinoma, in association with adjuvant CHT.
A new field of study in this setting of patients is certainly carbon ion radiotherapy (CIRT) which offers a higher relative biological efficacy (RBE) compared to photons and the Bragg peak and limited lateral scattering of the beam offer higher dose delivery than photons, allowing higher dose delivery to the tumor, reducing the dose to healthy tissue[56]. However, very few CIRT studies exist for cholangiocarcinoma, based on a small cohort of patients and a single randomized but retrospective multicenter study[57]. In the latter, 56 patients with cholangiocarcinoma treated with CIRT were analyzed; more than 80% were inoperable. The most commonly prescribed CIRT dose was 76 Gy (RBE) in 20 fractions [effective biological dose (BED) of 105 with α/β = 10]. This study revealed a median MST survival time of 14.8 mo for all 56 patients, 23.8 mo for 27 patients with intrahepatic cholangiocarcinoma, and 12.6 mo for 29 patients with HC after CIRT. Among the serious toxicity events noted, liver failure or sepsis following bile duct stenosis or cholangitis may occur during the natural course of HC, which may have adversely affected tolerance to treatment; moreover, biliary tract stenosis and pre-CIRT cholangitis have been observed in patients with HC and persisting even after CIRT could directly influence the prognosis. The study's OS and MST rates were comparable to those of previous proton or SBRT treatments, however, given the numerous limitations (retrospective study, different fractionations used, numerous cases lost to follow-up, and short median follow-up) and the safety of CIRT for cholangiocarcinoma remains poorly understood, although CIRT may be considered a promising therapy for patients with cholangiocarcinoma non fit surgery.
In conclusion, the role of radiotherapy in its different approaches for the treatment of LA HC is not yet clear in terms of modalities, timing, and doses for which clinical trials would be necessary. Furthermore, intensifying treatment for cholangiocarcinoma with novel systemic agents, in combination with radiation, could broaden therapeutic prospects.
Ongoing research is focused on the concept of personalized therapy and precision medicine, based on the heterogeneity of the molecular profile of HC[58]. Whole exome and transcriptome sequencing has detected that intrahepatic HC demonstrates IDH1/2 and BAP1 mutations and FGFR2 gene fusions and research findings indicate that immune checkpoint inhibitors could be used to patients with a poor prognosis subtype of high mutational load and increased immune activity[32]. The goal of molecular research is to develop a tailored therapy protocol based on molecular profiling, in order to minimize toxicity and optimize efficiency. Only recently, Wang et al[59] published a retrospective study investigating the molecular profile of intrahepatic cholangiocarcinoma in the Chinese population, using next-generation sequencing. The identified genomic alterations were used for personalized therapy and targeted or immunotherapy agents demonstrated superior survival and tumor response outcomes compared to standard chemotherapy[59]. Moreover, genome sequencing and animal model studies suggest that gain-of-function mutations in the IDH gene, could be involved in a subset of cancers with inflammatory signature and trials with IDH inhibitors are ongoing[60].
Nevertheless, prospective randomized control trials investigating precision medicine protocols are still awaited and several issues remain to be resolved as the complexity of HC requires in depth analysis of the biological mechanisms of the disease.
Recent advances in percutaneous minimally invasive treatment options include endoluminal RFA following tumor in growth in the hilum and the use or drug-eluting stents which is been investigated in both experimental animal models and extremely limited human trials[61-64].
Multimodality treatment protocols combining percutaneous minimally invasive therapies with systemic chemotherapy, modern RT, and PDT have been previously described and seem promising, however, large-scale studies are missing[65].
According to current evidence, future research could be focused on the comparison of the efficacy of IRE and other therapeutic modalities, RFA plus stent placement compared to RFA alone or stents alone, and the combination of various percutaneous therapies with individualized drug-therapy based on molecular profiling, in order to provide more solid evidence supporting the efficacy of multidisciplinary approaches.
Over the past two decades, several options for the management of unresectable HC have emerged. Due to the complexity of this disease, a multi-disciplinary approach with multimodal treatment is recommended, including surgery, medical oncology, radiation oncology, diagnostic radiology, interventional radiology, gastroenterology, and pathology. Recent studies suggest an improvement in OS, better response rates, and tumor control in patients with unresectable HC can be achieved by combining chemotherapy and ablatives strategies.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen G S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Jarnagin W, Winston C. Hilar cholangiocarcinoma: diagnosis and staging. HPB (Oxford). 2005;7:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (3)] |
| 2. | Suarez-Munoz MA, Fernandez-Aguilar JL, Sanchez-Perez B, Perez-Daga JA, Garcia-Albiach B, Pulido-Roa Y, Marin-Camero N, Santoyo-Santoyo J. Risk factors and classifications of hilar cholangiocarcinoma. World J Gastrointest Oncol. 2013;5:132-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 3. | Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 4. | Ruys AT, van Haelst S, Busch OR, Rauws EA, Gouma DJ, van Gulik TM. Long-term survival in hilar cholangiocarcinoma also possible in unresectable patients. World J Surg. 2012;36:2179-2186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik TM. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3:18-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 6. | Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Koay EJ, Odisio BC, Javle M, Vauthey JN, Crane CH. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments? Hepatobiliary Surg Nutr. 2017;6:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Boehm LM, Jayakrishnan TT, Miura JT, Zacharias AJ, Johnston FM, Turaga KK, Gamblin TC. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Mafeld S, Wong JJ, Kibriya N, Stenberg B, Manas D, Bassett P, Aslam T, Evans J, Littler P. Percutaneous Irreversible Electroporation (IRE) of Hepatic Malignancy: A Bi-institutional Analysis of Safety and Outcomes. Cardiovasc Intervent Radiol. 2019;42:577-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Coelen RJS, Vogel JA, Vroomen LGPH, Roos E, Busch ORC, van Delden OM, Delft FV, Heger M, van Hooft JE, Kazemier G, Klümpen HJ, van Lienden KP, Rauws EAJ, Scheffer HJ, Verheul HM, Vries J, Wilmink JW, Zonderhuis BM, Besselink MG, van Gulik TM, Meijerink MR. Ablation with irreversible electroporation in patients with advanced perihilar cholangiocarcinoma (ALPACA): a multicentre phase I/II feasibility study protocol. BMJ Open. 2017;7:e015810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Ruarus AH, Vroomen LGPH, Puijk RS, Scheffer HJ, Zonderhuis BM, Kazemier G, van den Tol MP, Berger FH, Meijerink MR. Irreversible Electroporation in Hepatopancreaticobiliary Tumours. Can Assoc Radiol J. 2018;69:38-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Dollinger M, Müller-Wille R, Zeman F, Haimerl M, Niessen C, Beyer LP, Lang SA, Teufel A, Stroszczynski C, Wiggermann P. Irreversible Electroporation of Malignant Hepatic Tumors--Alterations in Venous Structures at Subacute Follow-Up and Evolution at Mid-Term Follow-Up. PLoS One. 2015;10:e0135773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Hsiao CY, Yang PC, Li X, Huang KW. Clinical impact of irreversible electroporation ablation for unresectable hilar cholangiocarcinoma. Sci Rep. 2020;10:10883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Martin EK, Bhutiani N, Egger ME, Philips P, Scoggins CR, McMasters KM, Kelly LR, Vitale GC, Martin RCG. Safety and efficacy of irreversible electroporation in the treatment of obstructive jaundice in advanced hilar cholangiocarcinoma. HPB (Oxford). 2018;20:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Niessen C, Igl J, Pregler B, Beyer L, Noeva E, Dollinger M, Schreyer AG, Jung EM, Stroszczynski C, Wiggermann P. Factors associated with short-term local recurrence of liver cancer after percutaneous ablation using irreversible electroporation: a prospective single-center study. J Vasc Interv Radiol. 2015;26:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Giorgio A, Amendola F, Calvanese A, Ingenito E, Santoro B, Gatti P, Ciracì E, Matteucci P, Giorgio V. Ultrasound-guided percutaneous irreversible electroporation of hepatic and abdominal tumors not eligible for surgery or thermal ablation: a western report on safety and efficacy. J Ultrasound. 2019;22:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Nanashima A, Yamaguchi H, Shibasaki S, Ide N, Sawai T, Tsuji T, Hidaka S, Sumida Y, Nakagoe T, Nagayasu T. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: a preliminary study. J Gastroenterol. 2004;39:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Li Z, Jiang X, Xiao H, Chen S, Zhu W, Lu H, Cao L, Xue P, Li H, Zhang D. Long-term results of ERCP- or PTCS-directed photodynamic therapy for unresectable hilar cholangiocarcinoma. Surg Endosc. 35:5655-5664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Wiedmann M, Caca K, Berr F, Schiefke I, Tannapfel A, Wittekind C, Mössner J, Hauss J, Witzigmann H. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer. 2003;97:2783-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Mizandari M, Pai M, Xi F, Valek V, Tomas A, Quaretti P, Golfieri R, Mosconi C, Guokun A, Kyriakides C, Dickinson R, Nicholls J, Habib N. Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol. 2013;36:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Strand DS, Cosgrove ND, Patrie JT, Cox DG, Bauer TW, Adams RB, Mann JA, Sauer BG, Shami VM, Wang AY. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Buerlein RCD, Wang AY. Endoscopic Retrograde Cholangiopancreatography-Guided Ablation for Cholangiocarcinoma. Gastrointest Endosc Clin N Am. 2019;29:351-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos). Gastrointest Endosc. 2011;74:935-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Yang J, Wang J, Zhou H, Zhou Y, Wang Y, Jin H, Lou Q, Zhang X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Zheng WH, Yu T, Luo YH, Wang Y, Liu YF, Hua XD, Lin J, Ma ZH, Ai FL, Wang TL. Clinical efficacy of gemcitabine and cisplatin-based transcatheter arterial chemoembolization combined with radiotherapy in hilar cholangiocarcinoma. World J Gastrointest Oncol. 2019;11:489-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Andrašina T, Válek V, Pánek J, Kala Z, Kiss I, Tuček S, Slampa P. Multimodal oncological therapy comprising stents, brachytherapy, and regional chemotherapy for cholangiocarcinoma. Gut Liver. 2010;4 Suppl 1:S82-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, Nagorney DM. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-458; discussion 458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 428] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 29. | Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88-98.e3; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 30. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3166] [Article Influence: 211.1] [Reference Citation Analysis (1)] |
| 31. | Lamarca A, Palmer D, Wasan H. A randomised phase III, multi-center, open-label study of Active Symptom Control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients with locally advanced/metastatic biliary tract cancers previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol. 2019;37: 4003. [RCA] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 32. | Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, Hiraoka N, Ojima H, Shimada K, Okusaka T, Kosuge T, Miyagawa S, Shibata T. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 941] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 33. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1546] [Article Influence: 309.2] [Reference Citation Analysis (0)] |
| 34. | Razumilava N, Gores GJ. Building a staircase to precision medicine for biliary tract cancer. Nat Genet. 2015;47:967-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 36. | Massironi S, Pilla L, Elvevi A, Longarini R, Rossi RE, Bidoli P, Invernizzi P. New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Guo X, Shen W. Latest evidence on immunotherapy for cholangiocarcinoma. Oncol Lett. 2020;20:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Expression of concern: Shivapurkar et al., "Differential Inactivation of Caspase-8 in Lung Cancers", Cancer Biol Ther 2002; 1:65-9. Cancer Biol Ther. 2013;14:1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 484] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 40. | Cameron JL, Pitt HA, Zinner MJ, Kaufman SL, Coleman J. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159:91-97; discussion 97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 158] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Ohnishi H, Asada M, Shichijo Y, Iijima N, Itobayashi E, Shimura K, Suzuki T, Yoshida S, Mine T. External radiotherapy for biliary decompression of hilar cholangiocarcinoma. Hepatogastroenterology. 1995;42:265-268. [PubMed] |
| 42. | Mattiucci GC, Autorino R, D'Agostino GR, Deodato F, Macchia G, Perri V, Tringali A, Morganti AG, Mutignani M, Valentini V. Chemoradiation and brachytherapy in extrahepatic bile duct carcinoma. Crit Rev Oncol Hematol. 2014;90:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, Knol J, Dawson LA, Pan C, Lawrence TS. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739-8747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Jethwa KR, Sannapaneni S, Mullikin TC, Harmsen WS, Petersen MM, Antharam P, Laughlin B, Mahipal A, Halfdanarson TR, Merrell KW, Neben-Wittich M, Sio TT, Haddock MG, Hallemeier CL. Chemoradiotherapy for patients with locally advanced or unresectable extra-hepatic biliary cancer. J Gastrointest Oncol. 2020;11:1408-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Crane CH, Koay EJ. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer. 2016;122:1974-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Garibaldi C, Jereczek-Fossa BA, Marvaso G, Dicuonzo S, Rojas DP, Cattani F, Starzyńska A, Ciardo D, Surgo A, Leonardi MC, Ricotti R. Recent advances in radiation oncology. Ecancermedicalscience. 2017;11:785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Avila S, Smani DA, Koay EJ. Radiation dose escalation for locally advanced unresectable intrahepatic and extrahepatic cholangiocarcinoma. Chin Clin Oncol. 2020;9:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Crane CH, Macdonald KO, Vauthey JN, Yehuda P, Brown T, Curley S, Wong A, Delclos M, Charnsangavej C, Janjan NA. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys. 2002;53:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Elganainy D, Holliday EB, Taniguchi CM, Smith GL, Shroff R, Javle M, Raghav K, Kaseb A, Aloia TA, Vauthey JN, Tzeng CD, Herman JM, Koong AC, Krishnan SX, Minsky BD, Crane CH, Das P, Koay EJ. Dose escalation of radiotherapy in unresectable extrahepatic cholangiocarcinoma. Cancer Med. 2018;7:4880-4892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, Brock M, Balmanoukian A, Ye X. Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J Natl Compr Canc Netw. 2015;13:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 51. | Tamada K, Sugano K. Diagnosis and non-surgical treatment of bile duct carcinoma: developments in the past decade. J Gastroenterol. 2000;35:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Autorino R, Bisiello S, Pappalardi B, Privitera V, Buwenge M, Piccolo F, Masciocchi C, Tagliaferri L, Macchia G, Curti CD, Luppatteli M, Cerrotta A, Morganti AG, Valentini V, Mattiucci G. Intraluminal Brachytherapy in Unresectable Extrahepatic Biliary Duct Cancer: An Italian Pooled Analysis. Anticancer Res. 2020;40:3417-3421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Barney BM, Olivier KR, Miller RC, Haddock MG. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol. 2012;7:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Sandler KA, Veruttipong D, Agopian VG, Finn RS, Hong JC, Kaldas FM, Sadeghi S, Busuttil RW, Lee P. Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma. Adv Radiat Oncol. 2016;1:237-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Frakulli R, Buwenge M, Macchia G, Cammelli S, Deodato F, Cilla S, Cellini F, Mattiucci GC, Bisello S, Brandi G, Parisi S, Morganti AG. Stereotactic body radiation therapy in cholangiocarcinoma: a systematic review. Br J Radiol. 2019;92:20180688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Ebner DK, Kamada T. The Emerging Role of Carbon-Ion Radiotherapy. Front Oncol. 2016;6:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Kasuya G, Terashima K, Shibuya K, Toyama S, Ebner DK, Tsuji H, Okimoto T, Ohno T, Shioyama Y, Nakano T, Kamada T; Japan Carbon-Ion Radiation Oncology Study Group. Carbon-ion radiotherapy for cholangiocarcinoma: a multi-institutional study by and the Japan carbon-ion radiation oncology study group (J-CROS). Oncotarget. 2019;10:4369-4379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Abou-Alfa GK, Andersen JB, Chapman W, Choti M, Forbes SJ, Gores GJ, Hong TS, Harding JJ, Vander Heiden MG, Javle M, Kelley RK, Kwong LN, Lowery M, Merrell A, Miyabe K, Rhim A, Saha S, Sia D, Tanasanvimon S, Venook A, Valle JW, Walesky C, Whetstine J, Willenbring H, Zhu AX, Mayer D, Stanger BZ. Advances in cholangiocarcinoma research: report from the third Cholangiocarcinoma Foundation Annual Conference. J Gastrointest Oncol. 2016;7:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Wang L, Zhu H, Zhao Y, Pan Q, Mao A, Zhu W, Zhang N, Lin Z, Zhou J, Wang Y, Zhang Y, Wang M, Feng Y, He X, Xu W, Wang L. Comprehensive molecular profiling of intrahepatic cholangiocarcinoma in the Chinese population and therapeutic experience. J Transl Med. 2020;18:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H, Miltiadous O, Walesky C, Deshpande V, Zhu AX, Hezel AF, Yen KE, Straley KS, Travins J, Popovici-Muller J, Gliser C, Ferrone CR, Apte U, Llovet JM, Wong KK, Ramaswamy S, Bardeesy N. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 372] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 61. | So H, Oh CH, Song TJ, Lee HW, Hwang JS, Ko SW, Oh D, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. Feasibility and Safety of Endoluminal Radiofrequency Ablation as a Rescue Treatment for Bilateral Metal Stent Obstruction Due to Tumor Ingrowth in the Hilum: A Pilot Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Kadayifci A, Atar M, Forcione DG, Casey BW, Kelsey PB, Brugge WR. Radiofrequency ablation for the management of occluded biliary metal stents. Endoscopy. 2016;48:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 63. | Shatzel J, Kim J, Sampath K, Syed S, Saad J, Hussain ZH, Mody K, Pipas JM, Gordon S, Gardner T, Rothstein RI. Drug eluting biliary stents to decrease stent failure rates: A review of the literature. World J Gastrointest Endosc. 2016;8:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Jang SI, Kim JH, Kim M, Yang S, Jo EA, Lee JW, Na K, Kim JM, Jeong S, Lee DH, Lee DK. Porcine feasibility and safety study of a new paclitaxel-eluting biliary stent with a Pluronic-containing membrane. Endoscopy. 2012;44:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |