Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1668
Peer-review started: February 22, 2021
First decision: April 19, 2021
Revised: April 20, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: November 15, 2021
Processing time: 263 Days and 2.1 Hours
Pancreatic cancer is a highly lethal malignancy with low resection and survival rates and is not sensitive to radiotherapy and chemotherapy. Ferroptosis is a novel form of nonapoptotic regulated cell death characterized by the accumulation of lipid peroxides and reactive oxygen species involved in iron metabolism. Ferroptosis has a significant role in the occurrence and development of various tumors. Previous studies have shown that regulating ferroptosis-induced cell death inhibited tumor growth in pancreatic cancer and was synergistic with other antitumor drugs to improve treatment sensitivity. Herein, we discuss the mecha
Core Tip: Many studies have confirmed that ferroptosis is closely related to the occur
- Citation: Yang Y, Zhang ZJ, Wen Y, Xiong L, Huang YP, Wang YX, Liu K. Novel perspective in pancreatic cancer therapy: Targeting ferroptosis pathway. World J Gastrointest Oncol 2021; 13(11): 1668-1679
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1668.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1668
Pancreatic ductal adenocarcinoma (PDAC) accounts for approximately 90% of all pancreatic malignancies and is commonly known as pancreatic cancer. PDAC is a highly lethal malignancy, wherein the number of deaths in 2018 was almost the same as the number of new cases (432,242 and 458,918, respectively; GLOBOCAN database)[1]. The clinical features of PDAC include a short course, rapid progression, and high probability of malignancy. Surgery is the only option to cure PDAC; however, most patients are diagnosed at advanced stages because of the absence of distinctive clinical symptoms, they lose the opportunity for radical surgery. The postoperative 5-year survival rate of patients with PDAC is 12%-27%[2]. Currently, adjuvant gemcitabine chemotherapy is commonly performed after surgical resection; however, the 5-year survival rate is only 22.5%-26.0%[3-5]. Moreover, the high resistance of PDAC cells to gemcitabine limits its efficacy. Novel adjuvant chemotherapy drugs, such as modified FOLFIRINOX and a 5-fluorouracil derivative (S-1), have been approved in recent years for patients who have undergone resection of PDAC. A study reported that the 3-year survival rate of patients was 63.4% in the modified FOLFIRINOX group; however, the outcome was associated with an increased risk of toxic effects[6]. The 3-year survival rate of the patients in the S-1 group was 59.0%. However, the data were limited by the fact that all the patients were East-Asian residents of Japan[7]. The global incidence of PDAC has increased over the past few decades, significantly affecting the health of the patients and causing a heavy social burden. Therefore, the need of the hour is to explore new, targeted therapies for PDAC.
Ferroptosis is a novel form of nonapoptotic regulated cell death (RCD)[8] characterized by the accumulation of lipid peroxides and reactive oxygen species (ROS) involved in iron metabolism[9]. ROS react with polyunsaturated fatty acids (PUFAs) in the lipid membrane to generate excessive amounts of lipid peroxides, resulting in cell membrane damage and eventually ferroptosis. Studies have shown that ferroptosis is involved in the occurrence and development of various diseases, such as neuropathy[10], ischemia-reperfusion injury[11], acute renal failure[12], and cancer. A study reported that ferroptosis might be a common and dynamic form of RCD in the treatment of cancer[13].
More than 90% of PDAC patients have mutations in the KRAS gene that promotes proliferation, alters cellular metabolism, and affects invasion and autophagy[14]. Mutations in KRAS lead to a significant increase in intracellular ROS[15]. To avoid cell death, cancer cells must promptly remove intracellular ROS during rapid division. A study reported that PDAC cells transport a large amount of cystine/cysteine to synthesize glutathione (GSH) as a compensatory mechanism, thereby eliminating excess intracellular ROS[16]. Ferroptosis is closely related to the production of cys
In 2003, Dolma et al[17] discovered an antitumor drug named erastin that induced cell death without causing changes in nuclear morphology, DNA fragmentation, and caspase 3 activation. Moreover, caspase inhibitors did not reverse the process. Subsequently, the group identified RAS-selective lethal small molecule 3 (RSL3), which induced cell death similar to that caused by erastin[18]. In 2012, Dixon et al[8] found that erastin inhibited the cystine/glutamate antiporter (system XC-), causing excessive accumulation of lipid ROS, ultimately leading to an iron-dependent oxida
Compared with other RCDs such as necrosis, apoptosis, and autophagy[19] (Table 1), ferroptosis is characterized by the maintenance of an intact nucleus, nonaggregation of chromatin, nonrupture and foaming of the protoplast membrane, condensed mi
| Ferroptosis | Necrosis | Apoptosis | Autophagy | |
| Morphological features | Condensed mitochondrial membrane densities, reduction or vanishing of mitochondria crista, and outer mitochondrial membrane rupture | Organelle swelling, plasma membrane damage, cell disruption | Cell membrane foaming, cell shrinkage and the formation of apoptotic bodies | Cytoplasm vacuolization, formation of autophagosomes and removal of substances through lysosomes |
| Biochemical features | Iron accumulation; lipid peroxidation; glutaminolysis | Activation of RIPK1, RIPK3, and MLKL; activation of inflammasome and release of pro-inflammatory cytokines | DNA fragmentation; Caspases cascade activation; Ca2+/mg2+ -dependent endogenous nuclease and calpain activation | MAP1LC3B-I to MAP1LC3B-II conversion; increased autophagic flux and lysosomal |
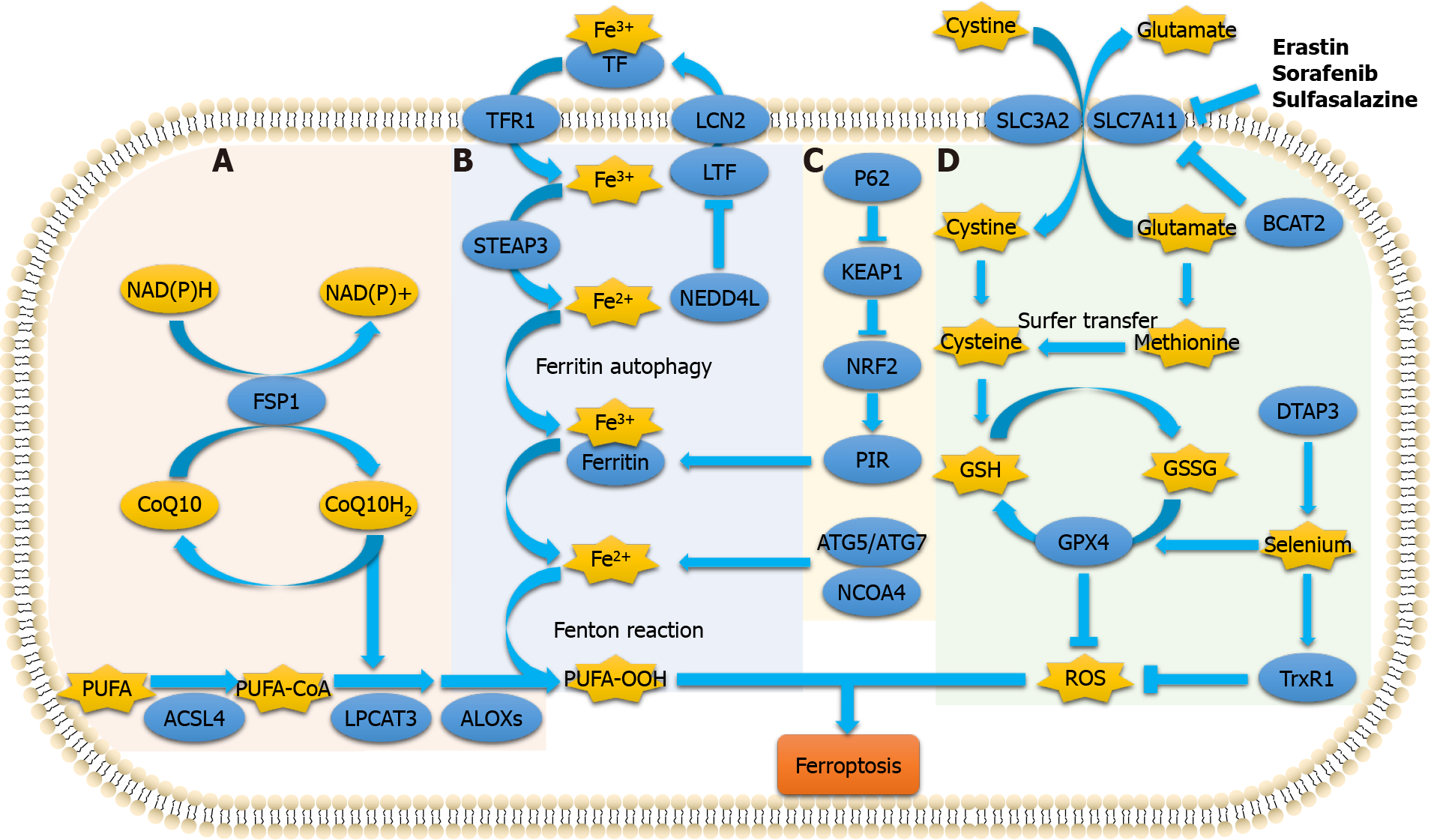
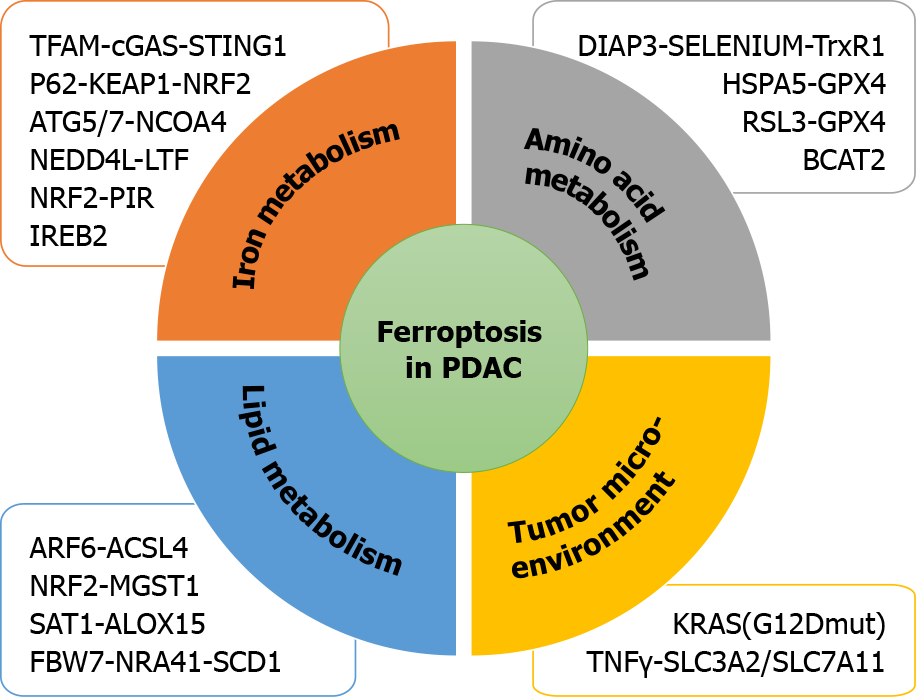
Currently, the metabolic mechanism of ferroptosis is known to include three processes. (1) Iron metabolism includes participation of iron ions in the formation of ROS through enzymatic or non-enzymatic reactions to mediate ferroptosis; (2) Amino acid metabolism includes GSH, which is a substrate of GPX4. GSH is the most important intracellular antilipid oxidation molecule. Cysteine is the raw material required for its synthesis, and an abundance of intracellular cysteine determines the synthesis of GSH and the process of cellular resistance to lipid oxidation, ultimately affecting ferroptosis[21,22]; and (3) Lipid metabolism is involved. The accumulation of lipid peroxides, especially phospholipid peroxides, is considered a landmark of ferroptosis[23]. A recent study reported that ferroptosis suppressor protein 1 exists as an independent parallel system that cooperates with GPX4 and GSH to suppress phospholipid peroxidation and ferroptosis[24]. Furthermore, induction of ferroptosis occurs by regulating the tumor microenvironment (Figure 1).
In recent years, systemic treatment of PDAC has mainly relied on 5-fluorouracil and gemcitabine-based therapy. However, because of rapid and widespread development of chemical resistance, the prognosis remains poor. Recent studies have demonstrated that ferroptosis is associated with PDAC (Figure 2). Therefore, inducing ferroptosis is a new strategy to combat PDAC.
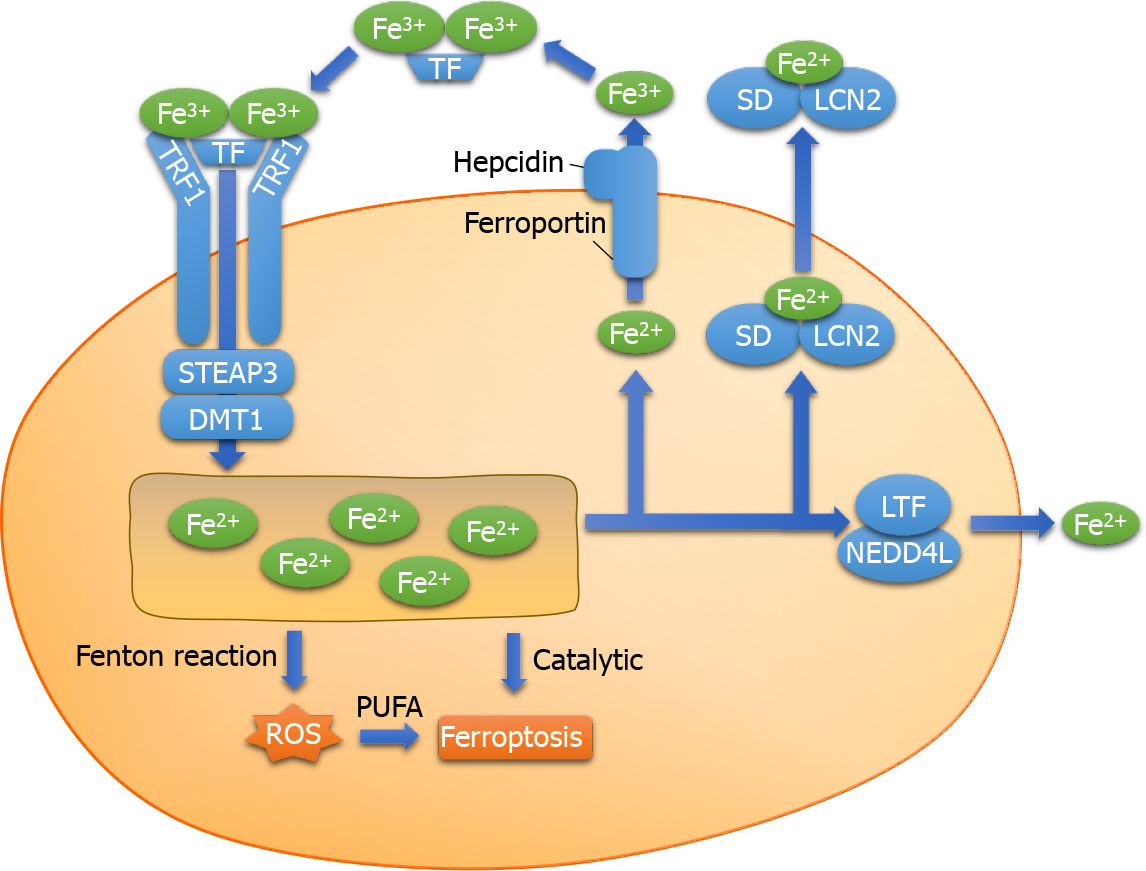
Extracellular ferric ions (Fe3+) form a conjugate with transferrin and are transported via the transferrin receptor 1 on the surface of the cell membrane. First, the conjugate enters the cell by endocytosis. Subsequently, Fe3+ are reduced by the six-transmem
Excess intracellular Fe2+ catalyzes the Fenton reaction, in which Fe2+ reacts with H2O2 to produce Fe3+ and hydroxyl radicals. The hydroxyl radical is a type of ROS that can damage proteins, lipids, and DNA, affect the function of cell membranes, and lead to cell death[26]. From the perspective of chemical reactions of intracellular Fe2+, the Fenton reaction may be considered one of the important processes involved in ferroptosis. The reaction between H2O2 and Fe2+ generates Fe3+ along with OH− and hydroxyl radicals (Formula 1). The hydroxyl radical is one of the most active ROS. In addition, the Fenton reaction can generate peroxy free radicals[27] (Formulas 2 and 3). The series of reactions suggest that iron ions act as a catalyst to promote the pro
Ferritin is composed of two subunits, namely ferritin heavy chain (FHC) and ferritin light chain (FLC). A study reported that iron-responsive element-binding protein 2 increased the expression of FHC and FLC to inhibit ferroptosis[29]. Lactotransferrin (LTF) is a member of the transferrin family that is associated with increased in
The autophagic degradation of ferritin to release Fe2+ is known as ferritinophagy, which is mediated by nuclear receptor coactivator 4 (NCOA4)[32]. Ferritinophagy is closely associated with the physiological and pathological processes of cell growth, proliferation, differentiation, apoptosis, and carcinogenesis. Under physiological conditions, ferritinophagy is tightly regulated by the iron-dependent protein network to maintain the balance of iron in cells and perform its functions. However, excessive activation of ferritinophagy leads to intracellular iron overload and accumulation of a large amount of ROS in a short period, resulting in ferroptosis. Therefore, it has been proposed that ferroptosis is a type of autophagy-dependent cell death[33].
Overexpression of NCOA4 enhances the degradation of ferritin, increases intra
| Inducers | Target | Inhibited by | Ref. |
| Erastin | System XC-/GPX4 | CPX | Yang et al[18] |
| Sulfasalazine | System XC- | β-ME, CHX, DFO, Fer-1, NAC, or Trolox | Kim et al[38,68] |
| Sorafenib | System XC- | DFO, Fer-1, Trolox, or VE | Lachaier et al[37] |
| Artesunate | System XC- | DFO or Fer-1 | Xie et al[9] |
| RSL3 | GPX4 | CPX, DFO, Ebs, Fer-1, Lip-1, Trolox, or U0126 | Yang et al[18] |
| Rapamycin | GPX4 | Lip-1 | Liu et al[60] |
| FIN56 | GPX4 | DFO, BSO and α-Toc | Liang et al[68] |
| FINO2 | GPX4/Iron | β-ME or Fer-1 | Liang et al[68] |
| Piperlongumine | GPX4 | Fer-1, lip-1, CPX and DFO | Yamaguchi et al[63] |
| Ruscogenin | Iron | DFO, FAC | Song et al[59] |
| Irisin | Iron, ROS, and glutathione depletion | Not mentioned | Yang et al[64] |
Cellular entry and exit of cysteine and glutamic acid require a specific transporter, system XC-, which is a heterodimer formed by the glycosylated heavy chain CD98hc, which is also called solute carrier family 3 member 2 (SLC3A2), and nonglycosylated xCT (SLC7A11) joined by disulfide bonds[42]. Cystine is reduced to cysteine to synthesize GSH and regulate downstream lipid peroxidation. As an electron donor, GSH converts toxic phospholipid peroxides into nontoxic phospholipid alcohols and oxidized glutathione under the action of GPX4[43]. In addition to system XC-, cysteine can be transported directly into the cell by the alanine-serine-cysteine system, which is also known to inhibit ferroptosis[44]. Furthermore, cystine can be synthesized from methionine via the transsulfuration pathway.
Many cells rely on system XC- for cystine uptake, which is the rate-limiting step for cysteine synthesis. Blocking or inhibiting this step leads to a decrease in intracellular cysteine, inhibits the lipid repair function of GPX4, and ultimately induces ferroptosis. It has been reported that erastin and its analogs (e.g., sulfasalazine and sorafenib) can block the transport function of system XC- and induce ferroptosis. Wang et al[39] studied the effect of system XC- on ferroptosis and found that branched-chain amino acid transaminase 2 (BCAT2) was the key enzyme mediating the metabolism of sulfur amino acids. BCAT2 was found to regulate intracellular glutamate concentration and its activation by ectopic expression specifically antagonized the inhibition of system XC- and protected PDAC cells from ferroptosis in vitro and in vivo. Furthermore, BCAT2 participates in the synergistic mechanisms of sulfasalazine and sorafenib to induce ferroptosis. Therefore, BCAT2 may be considered a suppressor of ferroptosis, and inhibiting intracellular glutamate synthesis might be effective in inducing ferrop
Another small molecule, RSL3 directly inhibits GPX4, leading to the accumulation of lipid ROS and ferroptosis. Selenium increases the antiferroptotic activity of GPX4 through a selenocysteine residue at 46. In addition, selenium is incorporated during the synthesis of selenoproteins such as thioredoxin reductase 1 (TrxR1; direct re
Fatty acids are substrates of lipid peroxidation reactions, and are esterified to form membrane phospholipids. PUFAs are more prone to oxidation than either saturated or monounsaturated fatty acids (MUFAs). Membrane phospholipids react with oxygen and adjacent lipids to generate phospholipid hydroperoxide (PL-OOH). The reaction product of Fe2+ and PL-OOH continues to react with lipids to generate phospholipid radicals for a new round of lipid peroxidation[46]. The degradation products of PL-OOH damage the cell membrane. Extensive lipid peroxidation affects the fluidity and structure of the cell membrane, increases its permeability, and leads to cell death. Lipid peroxidation is catalyzed by long-chain acyl-CoA synthetase 4 (ACSL4), lyso
Lipid peroxides cause cellular damage through several mechanisms. The first is by the decomposition of lipid peroxides into ROS, which further amplifies the lipid peroxidation process. Second, lipid peroxides alter the physical structure of the membrane, with changes in thickness, the degree of curvature, and pore formation that results in the release of harmful substances and disrupting intracellular me
The tumor microenvironment, including the tumor cells, vascular system, extracellular matrix, and immune cells, is an important factor affecting the outcomes of therapy. It has been reported that nano-inducers of ferroptosis attract iron from the extracellular environment to increase the intracellular content. It has also been shown that simultaneous upregulation of FHC and downregulation of GSH that increased the levels of intracellular ROS led to ferroptosis in tumor cells[55]. Another inducer is a near-infrared photosensitizer, IR780, which can be loaded into perfluorocarbon nanodroplets. The function of the inducer functions depends on differences of the microenvironments of normal and tumor tissue such as oxygen level, pH, and the immune system, among other factors. Photodynamic therapy activated oxygen en
Ferroptosis has an important role in tumor cell death and inhibition of tumor growth; therefore, inducing ferroptosis in PDAC is expected to become a new therapeutic strategy. Inducers of ferroptosis can be divided into several categories based on the regulatory mechanism.
Song et al[59] reported that ruscogenin induced ferroptosis by regulating the levels of transferrin and ferroportin. Ruscogenin increased the concentration of intracellular Fe2+ and the production of ROS, which was inhibited by deferoxamine.
Liu et al[60] observed that rapamycin caused autophagy-dependent ferroptosis by inducing the degradation of GPX4 protein but did not inhibit GPX4 gene transcription. In animal studies, the researchers observed that GPX4 depletion in PDAC cells enhanced the anticancer activity of rapamycin in vivo. Li et al[61] proposed a new model of cell death, wherein mitochondrial DNA stress triggered autophagy-dependent ferroptosis. Degradation of zalcitabine-induced transcription factor A, mitochondrial triggered oxidative DNA damage, the release of mitochondrial DNA into the cytosol, and subsequent activation of the cyclic GMP-AMP synthase-stimulator of interferon genes pathway. Zalcitabine suppressed pancreatic tumor growth via the autophagy-dependent ferroptosis.
Several key enzymes (ACSL4, LPCAT3, and ALOXs) are involved in lipid oxidation and can be regulated to induce ferroptosis. Studies have reported that erastin and RSL3 induced ferroptosis in PDAC[62], and ALOXs enhanced the sensitivity of RAS-mutated tumor cells to erastin and RSL3[50].
Piperlongumine (PL) is a natural product with cytotoxic properties restricted to cancer cells. PL acts by significantly increasing ROS levels in an iron-dependent manner. Yamaguchi et al[63] found that PL rapidly induced the death of human PDAC cells chiefly through the inhibition of GPX4, and sulfasalazine enhanced cell death. Moreover, sulfasalazine enhanced the cancer cell-killing ability of the combination of PL and cotylenin A, which is a plant growth regulator with potent antitumor activity.
Bao et al[64] investigated the effects of irisin on the expression of the ROS-related protein NRF2 and the autophagy-related protein, microtubule-associated protein 1A/1B-light chain 3 during ferroptosis. They observed that irisin promoted the up
Ferroptosis is a new model of cell death induced by small molecules such as erastin and RSL3, which are regulated at multiple levels. In this review, we briefly described the mechanism of ferroptosis, which includes iron, amino acid, and lipid metabolism, and summarized the regulatory pathways of ferroptosis in PDAC. The occurrence and development of ferroptosis are accompanied by the accumulation of ROS, resulting in lipid peroxidation of the cell membrane. Inducing ferroptosis can cause the death of PDAC cells, and can have a synergistic role with anticancer drugs to improve the sensitivity of PDAC to the existing treatment modalities. In addition, the level of ferroptosis inducer is associated with the prognosis of the disease. Therefore, in
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sato H S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Daamen LA, Groot VP, Intven MPW, Besselink MG, Busch OR, Koerkamp BG, Mohammad NH, Hermans JJ, van Laarhoven HWM, Nuyttens JJ, Wilmink JW, van Santvoort HC, Molenaar IQ, Stommel MWJ; Dutch Pancreatic Cancer Group. Postoperative surveillance of pancreatic cancer patients. Eur J Surg Oncol. 2019;45:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Skau Rasmussen L, Vittrup B, Ladekarl M, Pfeiffer P, Karen Yilmaz M, Østergaard Poulsen L, Østerlind K, Palnæs Hansen C, Bau Mortensen M, Viborg Mortensen F, Sall M, Detlefsen S, Bøgsted M, Wilki Fristrup C. The effect of postoperative gemcitabine on overall survival in patients with resected pancreatic cancer: A nationwide population-based Danish register study. Acta Oncol. 2019;58:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1763] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 5. | Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Ohge H, Sueda T. Impact of adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for adenocarcinoma of the body or tail of the pancreas. J Gastrointest Surg. 2009;13:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1942] [Article Influence: 277.4] [Reference Citation Analysis (0)] |
| 7. | Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y; JASPAC 01 Study Group. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 784] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 8. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11690] [Article Influence: 899.2] [Reference Citation Analysis (1)] |
| 9. | Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 2617] [Article Influence: 290.8] [Reference Citation Analysis (0)] |
| 10. | Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol Med. 2019;133:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 11. | Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11:3052-3059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 408] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 12. | Belavgeni A, Meyer C, Stumpf J, Hugo C, Linkermann A. Ferroptosis and Necroptosis in the Kidney. Cell Chem Biol. 2020;27:448-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 13. | Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 1238] [Article Influence: 206.3] [Reference Citation Analysis (0)] |
| 14. | Mann KM, Ying H, Juan J, Jenkins NA, Copeland NG. KRAS-related proteins in pancreatic cancer. Pharmacol Ther. 2016;168:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Storz P. KRas, ROS and the initiation of pancreatic cancer. Small GTPases. 2017;8:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, Decker AR, Sastra SA, Palermo CF, Andrade LR, Sajjakulnukit P, Zhang L, Tolstyka ZP, Hirschhorn T, Lamb C, Liu T, Gu W, Seeley ES, Stone E, Georgiou G, Manor U, Iuga A, Wahl GM, Stockwell BR, Lyssiotis CA, Olive KP. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 869] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 17. | Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 1053] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 18. | Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1491] [Cited by in RCA: 1394] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 19. | Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1493] [Cited by in RCA: 1749] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 20. | Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3672] [Cited by in RCA: 4507] [Article Influence: 643.9] [Reference Citation Analysis (0)] |
| 21. | Maiorino M, Conrad M, Ursini F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid Redox Signal. 2018;29:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 471] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 22. | Doll S, Conrad M. Iron and ferroptosis: A still ill-defined liaison. IUBMB Life. 2017;69:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 23. | D'Herde K, Krysko DV. Ferroptosis: Oxidized PEs trigger death. Nat Chem Biol. 2017;13:4-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 24. | Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O'Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1492] [Cited by in RCA: 2125] [Article Influence: 354.2] [Reference Citation Analysis (0)] |
| 25. | Graham RM, Chua AC, Herbison CE, Olynyk JK, Trinder D. Liver iron transport. World J Gastroenterol. 2007;13:4725-4736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (3)] |
| 26. | Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1488] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 27. | Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82-83:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 931] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 28. | Thévenod F. Iron and Its Role in Cancer Defense: A Double-Edged Sword. Met Ions Life Sci. 2018;18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Song J, Liu T, Yin Y, Zhao W, Lin Z, Lu D, You F. The deubiquitinase OTUD1 enhances iron transport and potentiates host antitumor immunity. EMBO Rep. 2021;22:e51162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 30. | Wang Y, Liu Y, Liu J, Kang R, Tang D. NEDD4L-mediated LTF protein degradation limits ferroptosis. Biochem Biophys Res Commun. 2020;531:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 31. | Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 32. | Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1649] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 33. | Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 715] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 34. | Zhu S, Zhang Q, Sun X, Zeh HJ 3rd, Lotze MT, Kang R, Tang D. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017;77:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 423] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 35. | Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1891] [Cited by in RCA: 2423] [Article Influence: 605.8] [Reference Citation Analysis (0)] |
| 36. | Hu N, Bai L, Dai E, Han L, Kang R, Li H, Tang D. Pirin is a nuclear redox-sensitive modulator of autophagy-dependent ferroptosis. Biochem Biophys Res Commun. 2021;536:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Lachaier E, Louandre C, Godin C, Saidak Z, Baert M, Diouf M, Chauffert B, Galmiche A. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res. 2014;34:6417-6422. [PubMed] |
| 38. | Kim EH, Shin D, Lee J, Jung AR, Roh JL. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 2018;432:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (1)] |
| 39. | Wang K, Zhang Z, Tsai HI, Liu Y, Gao J, Wang M, Song L, Cao X, Xu Z, Chen H, Gong A, Wang D, Cheng F, Zhu H. Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ. 2021;28:1222-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 40. | Siu LL, Awada A, Takimoto CH, Piccart M, Schwartz B, Giannaris T, Lathia C, Petrenciuc O, Moore MJ. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res. 2006;12:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Sinn M, Liersch T, Riess H, Gellert K, Stübs P, Waldschmidt D, Lammert F, Maschmeyer G, Bechstein W, Bitzer M, Denzlinger C, Hofheinz R, Lindig U, Ghadimi M, Hinke A, Striefler JK, Pelzer U, Bischoff S, Bahra M, Oettle H. CONKO-006: A randomised double-blinded phase IIb-study of additive therapy with gemcitabine + sorafenib/placebo in patients with R1 resection of pancreatic cancer - Final results. Eur J Cancer. 2020;138:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Liu MR, Zhu WT, Pei DS. System Xc-: a key regulatory target of ferroptosis in cancer. Invest New Drugs. 2021;39:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 148] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 43. | Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 5173] [Article Influence: 470.3] [Reference Citation Analysis (0)] |
| 44. | Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 366] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 45. | Rong Y, Gao J, Kuang T, Chen J, Li JA, Huang Y, Xin H, Fang Y, Han X, Sun LQ, Deng YZ, Li Z, Lou W. DIAPH3 promotes pancreatic cancer progression by activating selenoprotein TrxR1-mediated antioxidant effects. J Cell Mol Med. 2021;25:2163-2175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35:830-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 1620] [Article Influence: 270.0] [Reference Citation Analysis (0)] |
| 47. | Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, Stockwell BR. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol. 2015;10:1604-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 654] [Cited by in RCA: 766] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 48. | Feng H, Stockwell BR. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:e2006203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 563] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 49. | Ye Z, Hu Q, Zhuo Q, Zhu Y, Fan G, Liu M, Sun Q, Zhang Z, Liu W, Xu W, Ji S, Yu X, Xu X, Qin Y. Abrogation of ARF6 promotes RSL3-induced ferroptosis and mitigates gemcitabine resistance in pancreatic cancer cells. Am J Cancer Res. 2020;10:1182-1193. [PubMed] |
| 50. | Sun QY, Zhou HH, Mao XY. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid Med Cell Longev. 2019;2019:2749173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 51. | Kuang F, Liu J, Xie Y, Tang D, Kang R. MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chem Biol. 2021;28:765-775.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 52. | Fischer NW, Prodeus A, Malkin D, Gariépy J. p53 oligomerization status modulates cell fate decisions between growth, arrest and apoptosis. Cell Cycle. 2016;15:3210-3219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016;113:E6806-E6812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 591] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 54. | Ye Z, Zhuo Q, Hu Q, Xu X, Mengqi Liu, Zhang Z, Xu W, Liu W, Fan G, Qin Y, Yu X, Ji S. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 2021;38:101807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 55. | Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ, Gao M, Jiang X, Monette S, Pauliah M, Gonen M, Zanzonico P, Quinn T, Wiesner U, Bradbury MS, Overholtzer M. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11:977-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 460] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 56. | Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46:3830-3852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 657] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 57. | Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 769] [Cited by in RCA: 1907] [Article Influence: 317.8] [Reference Citation Analysis (0)] |
| 58. | Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 435] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 59. | Song Z, Xiang X, Li J, Deng J, Fang Z, Zhang L, Xiong J. Ruscogenin induces ferroptosis in pancreatic cancer cells. Oncol Rep. 2020;43:516-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Liu Y, Wang Y, Liu J, Kang R, Tang D. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 2021;28:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 61. | Li C, Zhang Y, Liu J, Kang R, Klionsky DJ, Tang D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17:948-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 307] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 62. | Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, Koshiishi I, Torii S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187-2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 340] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 63. | Yamaguchi Y, Kasukabe T, Kumakura S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol. 2018;52:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 64. | Yang BC, Leung PS. Irisin Is a Positive Regulator for Ferroptosis in Pancreatic Cancer. Mol Ther Oncolytics. 2020;18:457-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 65. | Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2:517-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 407] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 66. | Wang K, Zhang Z, Wang M, Cao X, Qi J, Wang D, Gong A, Zhu H. Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des Devel Ther. 2019;13:2135-2144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 67. | Muri J, Thut H, Bornkamm GW, Kopf M. B1 and Marginal Zone B Cells but Not Follicular B2 Cells Require Gpx4 to Prevent Lipid Peroxidation and Ferroptosis. Cell Rep. 2019;29:2731-2744.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 68. | Liang C, Zhang X, Yang M, Dong X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater. 2019;31:e1904197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 1045] [Article Influence: 174.2] [Reference Citation Analysis (0)] |