Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1648
Peer-review started: April 29, 2021
First decision: June 16, 2021
Revised: June 28, 2021
Accepted: August 18, 2021
Article in press: August 18, 2021
Published online: November 15, 2021
Processing time: 196 Days and 20.4 Hours
Gastrointestinal (GI) cancer, including esophageal, gastric, and colorectal cancer, is one of the most prevalent types of malignant carcinoma and the leading cause of cancer-related deaths. Despite significant advances in therapeutic strategies for GI cancers in recent decades, drug resistance with various mechanisms remains the prevailing cause of therapy failure in GI cancers. Accumulating evidence has demonstrated that the transforming growth factor (TGF)-β signaling pathway has crucial, complex roles in many cellular functions related to drug resistance. This review summarizes current knowledge regarding the role of the TGF-β signaling pathway in the resistance of GI cancers to conventional chemotherapy, targeted therapy, immunotherapy, and traditional medicine. Various processes, including epithelial-mesenchymal transition, cancer stem cell development, tumor microenvironment alteration, and microRNA biogenesis, are proposed as the main mechanisms of TGF-β-mediated drug resistance in GI cancers. Several studies have already indicated the benefit of combining antitumor drugs with agents that suppress the TGF-β signaling pathway, but this approach needs to be verified in additional clinical studies. Moreover, the identification of potential biological markers that can be used to predict the response to TGF-β signaling pathway inhibitors during anticancer treatments will have important clinical implications in the future.
Core Tip: The transforming growth factor (TGF)-β signaling pathway is involved in the drug resistance of gastrointestinal (GI) cancers. This review summarizes the current understanding of the roles played by the TGF-β signaling pathway in resistance to conventional chemotherapy, targeted therapy, immunotherapy, and traditional medicine in GI cancers as well as the various processes by which this occurs, including epithelial-mesenchymal transition, cancer stem cell development, tumor microenvironment alteration, and microRNA biogenesis.
- Citation: Lv X, Xu G. Regulatory role of the transforming growth factor-β signaling pathway in the drug resistance of gastrointestinal cancers. World J Gastrointest Oncol 2021; 13(11): 1648-1667
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1648.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1648
Gastrointestinal (GI) cancer, including esophageal cancer (EC), gastric cancer (GC), and colorectal cancer (CRC), is one of the most prevalent types of malignant carcinoma, falling within the top six in mortality according to global cancer statistics in 2018. In both sexes, CRC is the second leading cause of cancer death (9.2% of total cancer deaths), closely followed by GC (8.2%), and EC as the sixth leading cause of mortality (5.3%)[1]. CRC is also the second most common cause of cancer death in the United States[2]. Despite improvements in current therapeutic strategies, including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy, clinical prognoses and therapeutic responses of GI cancer patients are far from satisfactory because of delayed diagnosis, recurrence, poor clinical response, high cost, and medication side effects[3,4].
Chemotherapy is the most commonly used treatment for patients with advanced GI cancer. The most widely used chemotherapeutic regimens for GI cancer are fluorouracil and platinum[5-7]. Despite the continual development of new chemotherapeutic strategies, resistance to anticancer drugs remains a significant problem that is responsible for unfavorable clinical outcomes and treatment failures. Chemoresistance, including intrinsic and acquired drug resistance, is defined as the resistance of cancer cells to various structurally and functionally unrelated anti-cancer drugs[8]. The mechanisms of drug resistance are complex and closely related to various signaling pathways that are activated by many stimuli to promote chemoresistance[9].
The transforming growth factor (TGF)-β signaling pathway is deregulated in cancer and can have tumor-suppressive or tumor-promoting roles, depending on the molecular and cellular context[10,11]. In the GI tract, TGF-β has crucial and complex roles in many cellular functions related to drug resistance, such as maintaining stem cell homeostasis, regulating epithelial to mesenchymal transition, modulating immunity, and promoting fibrosis[12,13]. In this review, we discuss the role of the TGF-β signaling pathway in regulating chemoresistance in GI cancers.
Molecular investigations have revealed several mechanisms underlying chemoresistance, including the epithelial-mesenchymal transition (EMT), the efflux of intracellular chemotherapeutic drugs, noncoding RNAs, stem cell development, and the tumor microenvironment[14-17]. EMT is a complex and important cellular program in which epithelial cells shed their differentiated characteristics and acquire mesenchymal phenotypes, including motility, invasiveness, and resistance to apoptosis. Cells undergoing EMT become more invasive and exhibit increased resistance to anticancer drugs[18,19]. In addition, EMT has been found to result in stem cell-like characteristics and is positively correlated with the expression of ATP-binding cassette (ABC) transporters[18,20,21]. Different stimulus-induced EMT may contribute to chemoresistance via the upregulation of distinct transcription factors[19].
Failure of cancer chemotherapy can also be caused by changes in the expression or activity of membrane transporters, primarily those belonging to the ABC transporter family. ABC transporters can export chemotherapeutic agents out of the cell, thereby reducing intracellular drug levels and drug sensitivity and ultimately contributing to cancer chemoresistance[22,23]. In addition, ABC proteins transport signaling molecules that contribute to tumorigenesis[24].
Increasing evidence shows that non-coding RNAs, especially microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), can affect chemoresistance by forming a competing endogenous RNA regulatory network with mRNAs[25]. MiRNAs can play roles in drug resistance by targeting hundreds of tumor-related gene transcripts and affecting complex molecular pathways[14,26]. Specific miRNAs may be used as potential predictive biomarkers to guide individualized chemotherapy by reversing drug resistance[14].
Cancer stem cells (CSCs), which make up a distinct population within the tumor mass, possess unique self-renewal, multilineage differentiation, and potent tumorigenic abilities[27,28]. These cells acquire chemoresistance through various pathways involving apoptosis and DNA repair mechanisms[29]. In addition, upon exposure to cytotoxic therapies, CSCs can convert non-CSCs to CSC-like cells that may persist after treatment and serve as a mechanism for relapse. In GI malignancies, CSCs are abundant and contribute to chemotherapeutic resistance[15].
Interactions of tumor cells with alterations of the microenvironment, such as energy deprivation, hypoxia, and inflammation, give rise to heterogeneity and chemoresistance. Most tumor cells display deviations from the normal energy metabolism, allowing them to survive in hypoxic and low nutrient microenvironments[30,31]. Mitochondrial dysfunction and fatty acid (FA) metabolism are associated with chemotherapeutic resistance[31,32]. Hypoxia can also drive tumor resistance to chemotherapy by upregulating hypoxia-inducible factor-1 (HIF-1) and its downstream genes[33]. Inflammation and inflammatory mediators, including TGF-β, have been shown to contribute to the development, progression, metastasis, and chemoresistance of cancer[34,35]. In addition, the gut microbiota, which is linked to chronic inflammation and carcinogenesis[36], has an important role in the modulation of the host response to antitumor treatments, especially chemotherapy and immunotherapy[37]. Moreover, emerging evidence has demonstrated that cancer-associated fibroblasts (CAFs), one of the critical components of the tumor microenvironment, confer substantial resistance to chemotherapy and influence tumor cell responsiveness to immune checkpoint inhibitors[38].
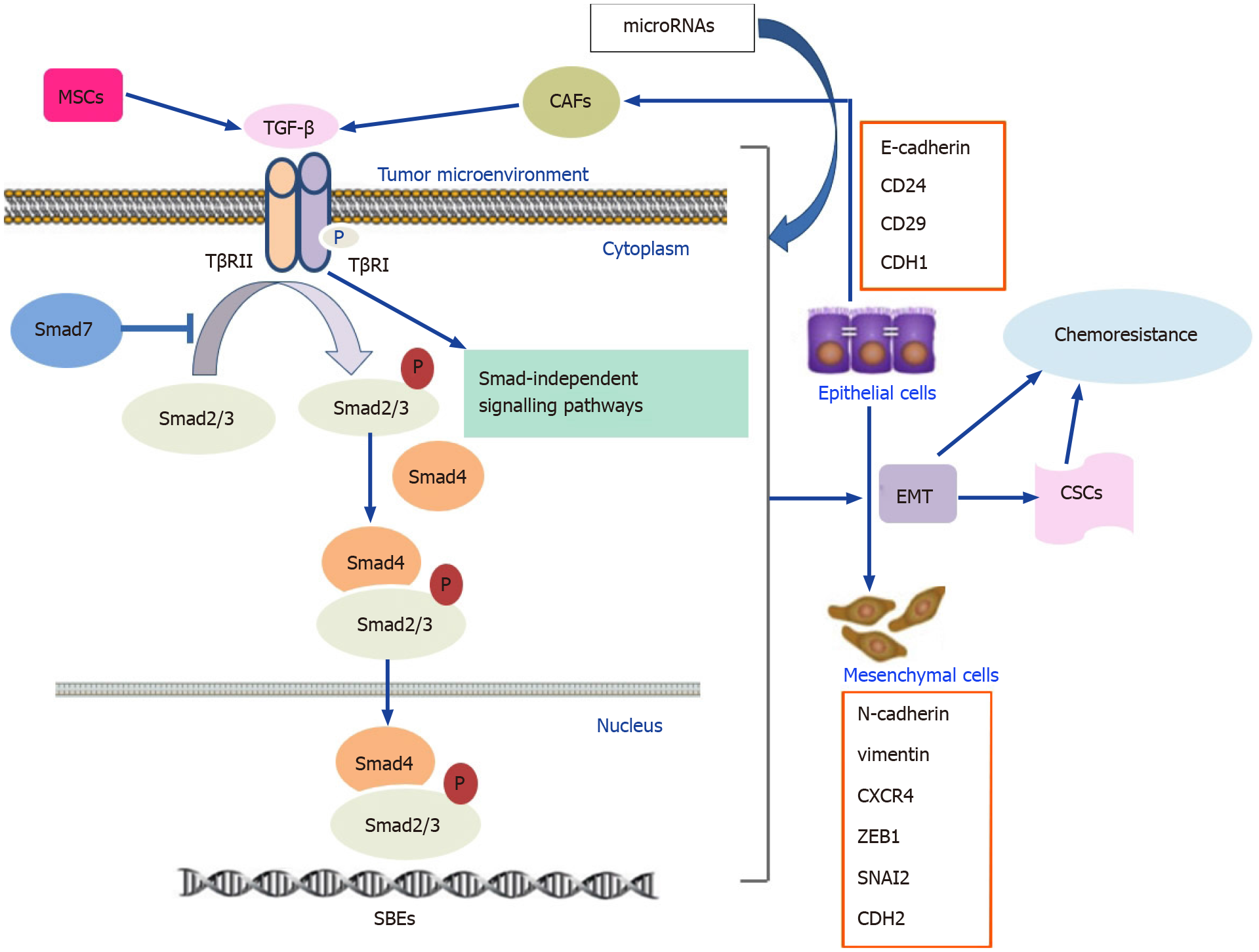
The TGF-β signaling pathway can be subdivided into canonical Smad-dependent and noncanonical Smad-independent pathways. In the canonical pathway, TGF-β initially binds to the TGF-β type 2 receptor (TβRII), which recruits and phosphorylates the kinase domain of TGF-β type 1 receptor (TβRI), leading to the activation and phosphorylation of Smad2 and Smad3. Then, phosphorylated Smad2 and Smad3 bind to Smad4, allowing the entire complex to translocate into the nucleus. In the nucleus, the Smad complex regulates transcriptional activity by interacting with Smad binding elements within downstream target genes[39-41]. Smad7 negatively regulates the TGF-β signaling pathway by blocking the interaction between Smads and receptors and inhibiting the phosphorylation of Smad2 and Smad3[42,43]. In addition to the Smad-dependent pathway, the binding of the TGF-β ligand to its receptors also activates several Smad-independent signaling pathways, including the mitogen-activated protein kinase, phosphoinositide 3-kinase (PI3K)/AKT, and Rho-associated protein kinase pathways[44,45].
The TGF-β signaling pathway has an important role in controlling tissue development, proliferation, apoptosis, differentiation, and homeostasis[46]. Disruption of this signaling pathway leads to various diseases, including some cancers. In cancer cells, TGF-β signaling causes EMT and CSC-like traits, resulting in an aggressive phenotype and a poor prognosis[47,48]. In addition to its direct effect on epithelial tumor cells, TGF-β controls tumor development by regulating the tumor microenvironment and growth factors from the surrounding stroma[13,49]. Furthermore, TGF-β signaling activation in the tumor microenvironment suppresses antitumor immune responses and supports cancer cell survival[50]. TGF-β has been found to inhibit multiple components of the immune system, including natural killer cells, CD8+ cytotoxic T lymphocytes, B-cell proliferation, and immunoglobulin A secretion[51]. Therefore, the TGF-β signaling pathway is associated with drug resistance and immune system escape.
In CRC, TGF-β1 expression is markedly increased and is correlated with poor clinical outcomes and a high risk of relapse[52,53]. TGF-β1 expression is also increased in GC mucosa and precancerous gastric cells[54,55]. However, active TGF-β1 is expressed most highly in smooth muscle actin-positive fibroblasts rather than in the malignant epithelial cells of gastric tumors[56]. In GC patients, high serum and tissue TGF-β1 levels are associated with lymph node involvement and poor prognosis[57]. Moreover, increased expression of TGF-β is found in EC[58]. In sum, serum and tissue TGF-β levels are upregulated in GI cancers and are associated with metastases and poor prognoses. Alterations in the TGF-β signaling pathway, especially receptor and Smad gene mutations, are commonly observed in GI cancers where they lead to tumor formation and metastasis[13]. Mutations in the TGF-β signaling pathway are found in 80% of CRC cell lines and approximately one-third of CRC tumors[46]. A decreased or complete loss of TGF-β receptor expression is common in patients with esophageal adenocarcinoma, primary gastric tumors, and CRC[49]. TβRII mutations frequently occur in the advanced stages of the colon[59] and gastric tumors along with progressive microsatellite instability (MSI-H)[49,60]. The overall incidence of TβRII mutations is approximately 30% in CRC, while frameshift mutations can be found in approximately 80% of MSI-H CRC[60,61]. TβRII mutations in CRC cells can contribute to the malignant phenotype via multiple pathways, regulate the components secreted by cancer cells, and directly promote inflammation in the tumor microenvironment[50]. Compared with TβRII, mutations in its counterpart TβRI are less frequent in both CRC and GC[13,60].
A study of over 700 cases of sporadic CRCs reveals that the prevalence of Smad4, Smad2, and Smad3 mutations was 8.6%, 3.4%, and 4.3%, respectively, with a combined prevalence of 14.8%[62]. Both Smad2 and Smad4 are located on chromosome 18q, which is commonly deleted in CRC[63]. However, Smad2 and Smad4 mutations tend to occur in the early and advanced stages of CRC, respectively[13,61,64]. Loss of Smad4 contributes to colorectal carcinogenesis[46] and may be a predictive biomarker of the response to 5-fluorouracil (5-FU)-based chemotherapy[65]. In GC, the expression of Smad3 is low or even undetectable in 40% of tissues, so mutations in Smad2 and Smad3 have not been described[13].
Accumulating evidence suggests that the expression levels of components of the TGF-β signaling pathway are closely associated with response to chemotherapy. Immunohistochemical analysis of 78 patient biopsies reveals that p-Smad2/3 expression was elevated in C-type CRC tumors, which benefit the least from chemotherapy[66]. Mediator Complex Subunit 12 (MED12) negatively regulates TβRII through physical interactions; therefore, its suppression induces the activation of TGF-β signaling[67]. In CRC cells, both MED12 knockdown and recombinant TGF-β treatment result in resistance to cisplatin, oxaliplatin (OXA), and 5-FU[66,67]. However, another study shows that TGF-β2 suppression was associated with recurrence in patients with colorectal adenocarcinomas. In addition, disease-free survival (DFS) and overall survival (OS) are significantly longer in patients with tumors expressing TGF-β2[68]. Additionally, in esophageal squamous cell carcinoma (ESCC) patients, TGF-β1–509C/T polymorphisms benefit from radiochemotherapy and therefore might be useful genetic markers for predicting radiochemotherapy response[69]. In GI cancers, the TGF-β pathway is correlated with resistance to antitumor agents, including conventional chemotherapy, targeted therapy, immunotherapy, and traditional medicine. In Table 1, we provide a summary of the relationships between the TGF-β signaling pathway and drug resistance in GI cancers.
| Cancer type | In vivo/In vitro | Upstream regulator | Alteration of TGF-β signaling | Effect | Downstream antitumor drug | Ref. |
| CRC | SK-CO-1 cells | MED12 knockdown | The activation of TGF-β signaling or TGF-β treatment | Resistance | DDP, OXA, and 5-FU | Brunen et al[66], 2013 |
| CRC | HCT116/HCT116p53KO chemoresistant cell lines | - | TGF-β1 treatment/TβRI inhibition | Resistance/sensitivity | 5-FU | Romano et al[82], 2016 |
| CRC | HCT116 cells | - | Smad4 knockdown | Sensitivity | Dox | Li et al[103], 2015 |
| CRC | in vivo, CRC animal model, stable OXA-resistant cell line HCT116/OXA | Curcumin | Inhibition of p-Smad2 and p-Smad3 | Sensitivity | OXA | Yin et al[90], 2019 |
| CRC | The resistant cell model HCT-8/5-FU cell line | Hedyotis diffusa Willd | Inhibition of TGF-β signaling | Antimetastasis in 5-FU-resistant cells | 5-FU | Lai et al[116], 2017 |
| CRC | HCT116 and DLD1 CRC cell lines | - | siRNA-mediated knockdown of SMAD2/3, TGF-β inhibitor SB431542 | Sensitivity | OXA | Kim et al[89], 2019 |
| CRC | RKO cells | - | Silencing of TβRII expression, TβRI inhibitor LY2157299 | Sensitivity | BETi | Shi et al[112], 2016 |
| CRC | HCT116 cells | - | TGF-β inhibitor LY2157299 | Sensitivity | 5-FU | Quan et al[81], 2019 |
| CRC | CT26 cells | Chemokine C-C motif ligand-1 secreted by Snail-expression fibroblasts | Phosphorylated Smad2 | Resistance | 5-FU or paclitaxel | Li et al[143], 2018 |
| CRC | 5-FU resistant cell line(HCT-8/5-FU) | Pien Tze Huang (PZH) | Suppression of TGF-β and Smad4 | Overcome MDR and inhibit EMT | - | Shen et al[117], 2014 |
| CRC | Patients | - | P-Smad3 overexpression | Resistance | 5-FU and leucovorin, capecitabine | Huang et al[78], 2015 |
| CRC | HCT116 Smad4+/+ and Smad4-/- cell lines | - | Smad4 defect | Resistance | 5-FU | Papageorgis et al[76], 2011 |
| CRC | in vivo, colorectal tumor biopsies | - | Normal SMAD4 diploidy | Sensitivity | 5-FU and mitomycin | Boulay et al[73], 2002 |
| CRC | Dukes CRC patients | - | Low SMAD4 mRNA and protein levels | Resistance | 5-FU-based adjuvant chemotherapy | Alhopuro et al[75], 2005 |
| CRC | Colorectal tumor biopsies | - | The amplification of STRAP, an inhibitor of TGF-β signaling | Resistance | 5-FU /mitomycin C adjuvant chemotherapy | Buess et al[74], 2004 |
| CRC | Colo205 and RKO cells | - | TGF-β1 treatment | Resistance | 5-FU, etoposide | Moon et al[80], 2019 |
| CRC | Mouse models | - | Blockade of TGF-β signaling | Sensitivity | Anti-PD-1-PD-L1 checkpoint therapy | Tauriello et al[115], 2018 |
| CRC | Mice models of MC38-derived tumors | - | 1D11 antibody anti-TGF-β mAb | Sensitivity | Anti-PD1 plus anti-CD137 mAb | Rodríguez-Ruiz et al[114], 2019 |
| CRC | SNU-C5/5-FU -resistant cells. | (1S,2S,3E,7E,11E)-3,7,11,15-cembratetraen-17,2-olide (LS-1) from Lobophytum sp | The increase of Smad-3 phosphorylation and the nuclear localization of p-Smad3 and Smad4 | Sensitivity | 5-FU | Kim et al[118], 2015 |
| CRC | The early stages of colorectal carcinogenesis in rats | 5-FU/thymoquinone (TQ) combination therapy | Upregulation of the TGF-β1, TβRII, Smad4 | Sensitivity | 5-FU | Kensara et al[122], 2016 |
| CRC | Azoxymethane (AOM) rat model | Vitamin D3/5-FU co-therapy | Upregulation of the TGF-β1, TβRII, smad4 | Sensitivity | 5-FU | Refaat et al[77], 2015 |
| CRC | RKO cells | Oxymatrine | Inhibition of the Smad2 phosphorylation and the formation of Smad2/3/4 | Sensitivity | - | Wang et al[119], 2017 |
| EC | Paclitaxel-resistant EC109 cells | - | BMP-4 and p-Smad1/5 overexpression | Resistance | Paclitaxel | Zhou et al[100], 2017 |
| ESCC | KYSE-150 and KYSE-180 cells, xenograft tumors in nude mice | - | TβRI inhibitor LY2157299 | Sensitivity | DDP and taxol | Zhang et al[142], 2017 |
| ESCC | Xenotransplanted tumor mice model | - | Dual PD-1/PD-L1 and TGF-β blockades | Sensitivity | PD-1/PD-L1 blockade | Chen et al[139], 2018 |
| EC and GC | EC cells T.T, GC cells MKN28 and MKN45 | - | Pretreatment with TGF-β | Sensitivity | Adriamycin | Izutani et al[104], 2002 |
| EAC | EAC cells, EAC patient-derived xenograft tumors | - | TβR inhibitor and trastuzumab, pertuzumab | Sensitivity | Trastuzumab and Pertuzumab | Ebbing et al[106], 2017 |
| EC | KYSE150 andKYSE450 cells | Garcinol | Inhibition of the p300/CBP and p-Smad2/3 expression | Sensitivity | - | Wang et al[120], 2020 |
| ESCC | Patients | - | High serum levels of VEGF-A and TGF-β1 | Resistance | Taxane-based/5-FU -based chemotherapy | Cheng et al[79], 2014 |
| ESCC | TE1 | - | Anti-TGF-β2 neutralizing mAb and SB-431542 | Sensitivity | Trastuzumab | Mimura et al[110], 2005 |
| ESCC | TE1/TE5 | - | Anti-TGF-β2 neutralizing mAb/exogenous addition of TGF-β2 | Sensitivity/resistance | Cetuximab | Kawaguchi et al[109], 2007 |
| ESCC | ECA109 and TE1 cells | Overexpression of LEF1 | Upregulation of p-Smad2, p-Smad3, and TGF-β | Resistance | DDP | Zhao et al[130], 2019 |
| GC | AGS cells | Glycoprotein from the Capsosiphon fulvescens | Inhibition of TGF-β1-activated FAK/PI3K/AKT pathways | Sensitivity | - | Kim et al[121], 2013 |
| GC | SGC7901 and BGC823 cells | HMMR | Upregulation of p-Smad2 level and the nuclearaccumulation of Smad2 | Resistance | 5-FU | Zhang et al[84], 2019 |
| GC | A peritoneal-metastatic cell line, 60As6 | - | TGF-β treatment | Sensitivity | Docetaxel | Fujita et al[99], 2015 |
| GC | MKN-45 cells | Eribulin | Inhibition of the TGF-β/Smad pathway | Sensitivity | - | Kurata et al[126], 2018 |
| GC | Peritoneal mesothelial cells (HPMCs) | Paclitaxel | Inhibition of phosphorylation of Smad2 | Reduce stromal fibrosis | - | Tsukada et al[98], 2013 |
| GC | NCI-N87 cells | - | TGF-β treatment | Resistance | Trastuzumab | Zhou et al[107], 2018 |
| AGS and MKN45 cells | MSCs | Activated TGF-β signaling | Resistance | 5-FU and OXA | He et al[146], 2019 | |
| CRC | Patients | - | TGF-β2 expression | Sensitivity | Fluoropyrimidine | Kim et al[68], 2009 |
Fluorouracil: 5-FU belongs to the antimetabolite family[70] and is a commonly used chemotherapeutic regimen for CRC and GC. 5-FU, a pyrimidine analog and an inhibitor of thymidylate synthase, is incorporated into RNA or DNA in the place of uracil or thymine and leads to the prevention of DNA replication and cell death[71]. Unfortunately, the treatment effectiveness of 5-FU is reduced, and its clinical application is limited by the emergence of drug resistance. The response rate to 5-FU is limited to 10%–15% in CRC. Various strategies have been used to improve the efficacy of 5-FU, resulting in the extension of the median survival to 30 mo[72].
A study of colorectal tumor biopsies shows that CRC patients with normal Smad4 diploidy experienced a threefold higher benefit from postoperative 5-FU-based adjuvant chemotherapy than those with Smad4 deficiency[73]. Another study of the same collection of tumor specimens reveals that serine-threonine receptor-associated protein, a TGF-β-signaling inhibitor that acts at the receptor level, was a predictor of unfavorable responses to 5-FU-based adjuvant chemotherapy[74]. Similarly, CRC patients treated with surgery and 5-FU-based adjuvant therapy and followed for over 6 years to evaluate the prognostic value of Smad4 expression, demonstrate that patients with a low level of Smad4 expression had shortened DFS and OS compared with those with a high level of Smad4 expression[75]. In HCT116 colon cancer cells, Smad4 deficiency is found to be responsible for 5-FU resistance[76]. Moreover, in an azoxymethane rat model of colon cancer, vitamin D3 supplementation promotes the efficacy of 5-FU through multiple mechanisms including increased expression of TGF-β1, TβRII, and Smad4[77]. In brief, the results indicate that the effectiveness of adjuvant 5-FU-based chemotherapy might depend on TGF-β signaling in CRC.
As the TGF-β signaling pathway appears to have both suppressive and promoting effects in cancer, other studies have suggested that activation of the TGF-β signaling pathway might induce resistance to 5-FU in GI cancers. Immunohistochemical staining in patients with stage II-III advanced rectal cancer showed that p-Smad3 overexpression was associated with poor preoperative responses to fluoropyrimidine-based chemoradiotherapy. Therefore, p-Smad3 could be a potential predictor of a poor response to radiochemotherapy[78]. Moreover, pre-CCRT serum TGF-β1 levels were found to be negatively correlated with DFS in patients with ESCC receiving concurrent neoadjuvant chemoradiotherapy with taxane-based/5-FU-based chemotherapy followed by esophagectomy[79]. In CRC cells, TGF-β1 treatment was found to increase apoptotic resistance in cells exposed to therapeutics including 5-FU and etoposide[80]. TGF-β inhibition was found to sensitize HCT116 cells to 5-FU treatment and suppress cell migration[81]. Likewise, TβRI inhibition reduced proliferation and increased cell death in chemoresistant cancer cells[82]. Furthermore, Moon et al[83] found that Smad3/4 acted as a drug sensitivity regulator in TGF-β-mediated chemoresistant CRC cells, and knockdown of Smad3/4 significantly decreased tumor propagation and migration in the presence of 5-FU[83]. In GC, hyaluronan-mediated motility receptor is a key regulator of chemoresistance, and its upregulation was found to promote EMT and CSC properties by activating the TGF-β/Smad2 signaling pathway, ultimately leading to 5-FU resistance[84].
Platinum compounds: Platinum compounds are used as single agents or in combinatorial regimens for the treatment of GI cancers. The molecular mechanism of platinum compound-induced apoptosis involves the inhibition of DNA synthesis and repair, resulting in cell cycle arrest. This effect is mediated by the activation of various signal transduction pathways[85]. OXA is an important platinum-based option for the treatment of CRC[86]. In two multicenter trials in which single-agent OXA was administered as first-line treatment of advanced CRC, response rates were 12% and 24%, progression-free survival was 4 mo, and median survival was 14.5 mo and 13 mo, respectively[87].
In CRC cells, TGF-β1 contributes to OXA resistance primarily through EMT, which leads to antiapoptotic effects and the attenuation of DNA damage[88]. Furthermore, both siRNA-mediated knockdown of Smad2/3 and treatment with the potent TGF-β inhibitor SB43154225 suppress migration and invasion and increase therapeutic sensitivity to OXA in HCT116 and DLD1 CRC cell lines[89]. Curcumin, a naturally occurring polyphenolic substance extracted from the Curcaceae plant Curcuma longa, sensitizes CRC to OXA treatment by inhibiting the TGF-β/Smad2/3 pathway in the OXA-resistant cell line HCT116/OXA and in an in vivo animal model of CRC[90]. In EC, TGF-β secreted from CAF-like fibroblasts induces chemoresistance to cisplatin, which is reversed after administration of TGF-β neutralizing antibodies[91].
Taxoid compounds: Paclitaxel (PTX) is an antineoplastic agent derived from the bark of the Pacific yew Taxus brevifolia[92]. Docetaxel is a semi-synthetic taxane that primarily acts to promote microtubule assembly and prevents the depolymerization of assembled microtubules[93]. Both PTX and docetaxel exert potent antitumor effects by stabilizing microtubules, resulting in cell cycle arrest and apoptosis[94]. The results of a multicenter trial in patients with advanced or recurrent GC showed that the response rate to PTX as a second-line monotherapy was 17.5%[95]. The median duration of response to PTX monotherapy was 2.8 mo in patients with advanced gastric or gastroesophageal junction adenocarcinoma, and the patients eventually developed resistance to PTX[96]. The results of a phase II study in previously-untreated GC patients reported overall response rates to single-agent docetaxel in the range of 17% to 24%[97].
Peritoneal dissemination is the most common mode of metastasis in GC. Low-dose PTX can significantly inhibit Smad2 phosphorylation in human peritoneal mesothelial cells, leading to a decrease in stromal fibrosis[98]. The results of a microarray analysis showed that C-X-C chemokine receptor type 4 (CXCR4) was a novel marker for highly metastatic CSCs. Treatment with TGF-β enhanced the anticancer effect of docetaxel via the induction of cell differentiation/asymmetric cell division within the CXCR4-positive gastric CSC population, even when the cells were in a dormant state[99]. Bone morphogenetic protein 4 (BMP-4), which is involved in TGF-β signaling, is upregulated in PTX-resistant human esophageal carcinoma EC109 cells and docetaxel-resistant human GC MGC803 cells. p-Smad1/5, which is also involved in the TGF-β/Smad pathway, is also overexpressed in EC109/Taxol cells[100].
Doxorubicin: Doxorubicin (Dox), a chemotherapeutic agent extensively used to treat a wide range of cancers, exerts cytotoxic and DNA damaging effects through interference with nucleoside metabolism, but is less efficacious in GI cancers relative to other cancer types[101]. The antineoplastic activity of Dox is attributed to its intercalation into the DNA helix and its ability to generate free radicals[102]. In HCT116 colon cancer cells, long-term administration of low concentrations of Dox may promote resistance partly via the activation of TGF-β signaling. Moreover, knockdown of Smad4 significantly increases the sensitivity of HCT116 cells to Dox, in part via the inhibition of multidrug-resistant plasma membrane glycoprotein expression and reversal of the EMT process[103]. Therefore, the combination of Dox treatment and TGF-β downregulation might be a potential therapeutic strategy to overcome chemoresistance.
Adriamycin: Adriamycin (ADM) generates superoxide radicals that kill tumor cells by damaging DNA, directly intercalating into DNA, and preventing DNA replication. In human EC cells (T.T) and GC cells (MKN28 and MKN45), pretreatment with TGF-β1 results in increased sensitivity to ADM. In vivo, the combined administration of TGF-β1 and ADM delayed tumor growth better than either treatment alone and further exhibited synergistic antitumor effects[104].
Knockdown of MED12 in the CRC cell lines SK-CO-1 (KRASV12) and SW1417 (BRAFV600E) resulted in the activation of MEK/ERK and induced resistance to the MEK inhibitor AZD6244 (selumetinib). Moreover, TGF-β-induced resistance to AZD6244 and the BRAF inhibitor PLX4032 (vemurafenib) have also been observed in CRC cells[67]. However, another study demonstrated that vemurafenib downregulated the expression of TGF-β and p-Smad3 in HT29 CRC cells[105]. Trastuzumab, a human epidermal growth factor receptor (HER)2-targeting antibody, is the only available targeted agent for first-line palliative systemic treatment of HER2-positive esophagogastric adenocarcinoma (EAC). EAC cells become resistant to trastuzumab and the HER2-HER3 signaling inhibitor pertuzumab by activating TGF-β signaling, which subsequently induces EMT. TGF-β receptor inhibitors were shown to increase the antitumor efficacy of trastuzumab and pertuzumab in EAC cells and EAC patient-derived xenograft tumors[106]. Sensitivity of the GC cell line NCI-N87 to trastuzumab was significantly decreased after treatment with TGF-β. Moreover, TGF-β was upregulated in trastuzumab-resistant NCI-N87/TR cells[107]. Cetuximab and trastuzumab, humanized antibodies against the HER family, exert antitumor effects by directly inhibiting epidermal growth factor receptor (EGFR) tyrosine kinase activity, inhibiting cell cycle progression, and activating proapoptotic molecules[108]. In addition, an anti-TGF-β2 neutralizing mAb enhances cetuximab-mediated and trastuzumab-mediated antibody-dependent cellular cytotoxicity (ADCC) in TE1 TGF-β-producing ESCC cells. The TGF-β signaling inhibitor SB-431542 was found to enhance trastuzumab-mediated ADCC of TE1 cells. Furthermore, the exogenous addition of TGF-β2 significantly decreased cetuximab-mediated ADCC in non-TGF-β2-producing TE5 cells, and TGF-β2 inhibited the activity of trastuzumab-mediated ADCC in TE1 cells[109,110]. TGF-β expression is upregulated in three FGFR2-amplified SNU-16 GC cell lines that are resistant to AZD4547, BGJ398, and PD173074. However, parental SNU-16 cells treated with TGF-β1 did not undergo EMT, and inhibition of TβRI was not sufficient to reverse EMT in the resistant cells[111]. Bromodomain and extraterminal domain protein inhibitors (BETis) are in clinical trials as a novel class of cancer therapeutics. Both TβRII knockdown and treatment with the small-molecule TβRI inhibitor LY2157299 (galunisertib) were reported to increase the sensitivity of RKO colon carcinoma cells to BETis[112].
Treatment with the TGF-β inhibitors P144 and P17 may be able to enhance the efficacy of immunotherapies by increasing antitumor immune responses[113]. Moreover, treatment with the TGF-β-neutralizing mAb 1D11 enhanced the abscopal effect of radiotherapy as well as overall treatment efficacy in subcutaneous large MC38 colorectal tumors in conjunction with anti-programmed cell death protein 1 (PD-1) plus anti-CD137 mAb[114]. In mice with progressive metastatic liver disease, enabling immune infiltration using TGF-β inhibitors render tumors susceptible to anti-PD-1/Programmed cell death ligand 1 (PD-L1) checkpoint-based therapies[115]. Immunotherapies directed against TGF-β signaling may have broad applications in treating patients with advanced CRC.
Traditional herbal medicine has an important role in reversing the resistance of CRC cells to 5-FU. Hedyotis diffusa Willd, a traditional Chinese herbal medicine in the family of Rubiaceae, may exert its antimetastatic activity by suppressing TGF-β/Smad4 signaling pathway-mediated EMT in 5-FU-resistant CRC cells[116]. Similarly, the traditional Chinese medicine formula Pien Tze Huang can effectively overcome multidrug resistance and inhibit EMT via suppression of the TGF-β pathway in the 5-FU-resistant CRC cell line HCT-8/5-FU[117]. Moreover, (1S,2S,3E,7E,11E)-3,7,11,15-Cembratetraen-17,2-olide (LS-1), a marine cembrenolide diterpene from Lobophytum
Various other Chinese herbs have been reported to exert antitumor or synergistic antitumor effects via TGF-β signaling pathway-mediated mechanisms. Oxymatrine, an alkaloid extracted from the Chinese herb Sophora flavescens Ait, can exert antimetastatic and anti-invasive effects through the inhibition of Smad2 phosphorylation and the formation of Smad2/3/4 in colorectal carcinoma RKO cells[119]. Garcinol, a natural compound extracted from Gambogic genera, can inhibit EC metastasis in vitro and in vivo by dose-dependent suppression of p-Smad2/3 expression in the nucleus[120]. In addition, a glycoprotein from the green alga Capsosiphon fulvescens was shown to suppress the proliferation and migration of AGS GC cells by downregulating integrin expression via inhibition of the TGF-β1-activated FAK/PI3K/AKT pathways[121]. However, combination therapy with 5-FU and thymoquinone, which is the main bioactive compound derived from Nigella sativa, enhanced antitumor effects in a preclinical rat model of colorectal tumorigenesis partly by upregulating the expression of TGF-β1, TβRII, and Smad4[122].
TGF-β secreted from tumor cells is involved in paracrine signaling cascades that promote EMT and activate CAFs. CAFs, in turn, secrete more TGF-β that further drives EMT. Extracellular TGF-β binds to its receptor, resulting in the expression of key EMT genes. Furthermore, TGF-β can promote non-Smad pathways to accelerate EMT progression[16]. It has been reported that fibronectin, a marker of EMT progression, induced EMT through Smad3/4-mediated TGF-β signaling[123]. Therefore, TGF-β is an important inducer of EMT. SW837 rectal cancer cells treated with a TβR inhibitor or transfected with TβRII siRNA exhibited downregulation of mesenchymal markers, such as N-cadherin and vimentin and EMT regulators, including Snail, Twist, Slug, and Zeb1[124]. Ginsenoside Rb2, the bioactive component in ginseng, inhibited EMT in CRC cells by inhibiting the expression of Smad4 and p-Smad2/3[125]. Similarly, eribulin significantly inhibited EMT by downregulating the TGF-β/Smad pathway in GC[126]. The EMT phenotype has been observed in GC cell lines resistant to 5-FU and AZD4547 and CRC cell lines resistant to BGJ398, PD173074, and OXA[84,111,127]. Anticancer drugs can activate the TGF-β signaling pathway and further induce EMT, which is closely associated with chemotherapy resistance and evasion of immune surveillance[10,128]. Dox treatment of HCT116 colon cancer cells was found to increase TGF-β1 and p-Smad2/3 expression and induce an EMT phenotype, exemplified by a reduction in E-cadherin and the upregulation of vimentin and N-cadherin. The changes ultimately resulted in the acquisition of Dox resistance. Furthermore, silencing Smad4 by stable RNA interference reversed the EMT process and increased the sensitivity of HCT116 cells to Dox[103]. In EAC cells, EMT has been identified as a chemoradiation resistance mechanism in which EMT is mediated by the autocrine production of TGF-β in response to chemoradiation. Neutralization of TGF-β ligands effectively counteracted chemoradiation-induced EMT by reversing the mesenchymal phenotype[129]. EAC cells incubated with trastuzumab and pertuzumab can secrete ligands for the TGF-β receptor and induce EMT-related changes, including reduced expression of epithelial markers (CD24, CD29, and CDH1) and increased expression of mesenchymal markers (CXCR4, VIM, ZEB1, SNAI2, and CDH2), resulting in drug resistance. However, combining the drugs with a TGF-β receptor inhibitor caused the cells to regain an epithelial phenotype[106].
Emerging evidence indicates that CSCs are the main factor underlying therapeutic failure, and chemotherapeutic resistance. The TGF-β pathway has been identified as a major stem cell-associated signaling pathway. ESCC has been found to arise from CSCs. Zhao et al[130] showed that the TGF-β signaling pathway contributed to the lymphoid enhancer-binding factor 1-mediated CSC-like phenotype in ESCC cells. In EC, the TGF-β1 inhibitor SB525334 significantly suppressed the migration and invasion of sphere-forming stem-like cells, which possess key traits of CSCs, including chemoresistance[131]. EMT is a critical process for the generation and maintenance of CSCs and the invasive front of ESCC. Moreover, the EGFR inhibitors erlotinib and cetuximab can both markedly suppress CSCs enrichments via TGF-β1-mediated EMT in ESCC[132]. In mouse GC cells, activation of the TGF-β pathway downregulated the expression of Sca-1, which has been identified as a potential CSC enrichment marker. High expression of Sca-1 was related to increased resistance to cisplatin/fluorouracil-based chemotherapy[133]. In addition, TGF-β enhanced the anticancer effect of docetaxel by inducing the differentiation of gastric CSCs[99].
TGF-β is a pleiotropic cytokine with potent immunosuppressive effects. TGF-β downregulates CD8+ and CD4+ T cell activation and stimulates the differentiation of immune-suppressive regulatory T (Treg) cells[10,114]. CRC cells secrete anti-inflammatory cytokines, including TGF-β, which can affect the dendritic cell (DC) phenotype and support tumor escape from immune surveillance[134]. However, the TGF-β receptor inhibitor SB-431542 can induce potent phenotypic and functional maturation of DCs and trigger an antitumor immune response[135]. In ESCC, TGF-β1 was shown to partially contribute to the downregulation of CD16 on natural killer (NK) cells, resulting in NK cell dysfunction[136].
TGF-β signaling pathway activation plays an important role in immune evasion and contributes to immune checkpoint therapy failure[137,138]. Enabling immune infiltration by blocking TGF-β signaling renders tumors susceptible to anti-PD-1-PD-L1 checkpoint-based therapy[115]. Moreover, the TGF-β neutralizing monoclonal antibody 1D11 markedly enhanced the abscopal effects and the overall treatment efficacy in conjunction with an anti-PD-1 plus anti-CD137 mAb combination in large MC38 colorectal tumors[114]. In ESCC, myeloid-derived suppressor cell-derived TGF-β increased PD-1 expression on CD8+ T cells, which led to resistance to PD-1/PD-L1 blockade in the tumor microenvironment. Dual PD-1/PD-L1 and TGF-β pathway blockades restored the function and antitumor ability of CD8+ T cells[139]. Furthermore, combined treatment with cyclophosphamide and interleukin (IL)-12-expressing adenovirus, which might be a valid immunotherapeutic strategy for advanced GI cancer, was shown to revert the Treg immunosuppressive phenotype by blocking the secretion of IL-10 and TGF-β, resulting in loss of their DC inhibitory activity[140].
CAFs are the most abundant cell type in the tumor microenvironment. One of the main sources of CAFs is endothelial cells undergoing EMT, which is mainly promoted by TGF-β[141]. CAFs can confer TGF-β1-mediated ESCC cell resistance to several chemotherapeutic drugs, including cisplatin, taxol, irinotecan, 5-FU, carboplatin, docetaxel, pharmorubicin, and vincristine. Inhibition of CAF-secreted TGF-β1 signaling via treatment with the TβRI inhibitor LY2157299 significantly enhanced chemosensitivity[142]. Moreover, TGF-β secreted by miR-27-induced CAFs induced chemoresistance to cisplatin in EC[91]. In CRC, Snail-expressing 3T3 fibroblasts exhibit CAF properties that support 5-FU and PTX chemoresistance via TGF-β/NF-κB-mediated CCL1 secretion[143]. Tang et al[144] found that, in CRC, hypoxia-inducible factor 1α (HIF-1α) and CAF-secreted TGF-β2 synergistically induced the expression of GLI2, which promoted chemoresistance.
Mesenchymal stem cells (MSCs), an important part of the tumor environment, contribute to the development of drug resistance[145]. In GC cells, TGF-β1 secretion by MSCs activated Smad2/3 and induced expression of the lncRNA MACC1-AS1 that promoted FA oxidation-dependent stemness and chemoresistance to 5-FU and OXA[146].
Emerging evidence indicates that some miRNAs can regulate the resistance of GI cancers to a variety of chemotherapeutic drugs through the TGF-β signaling pathway, as summarized in Table 2. In HT-29 colon cancer cells, overexpression of miR-146a was found to be associated with various processes in the cancer microenvironment, including enhancement of 5-FU and irinotecan resistance and promotion of TGF-β secretion[147]. MiR-21 was shown to increase both stemness and the overall proportion of CSCs in colon cancer cells by downregulating TβRII, a direct target of miR-21, and by activating the Wnt/β-catenin pathway[148]. MiR-34a was found to mediate OXA resistance in CRC cells by inhibiting macroautophagy via regulation of the TGF-β/Smad4 pathway[149]. However, the expression levels of miR-552 were negatively correlated with resistance to 5-FU-based chemotherapy in CRC cells. Mechanically, miR-552 directly targeted the 3'-UTR of Smad2, and stable knockdown of Smad2 reversed miR-552 deficiency-induced 5-FU resistance[150]. Overexpression of miR-455–3p conferred resistance to cisplatin and docetaxel in ESCC cells, whereas miR-455–3p antagonism reversed chemoresistance and reduced the number of CD90+ and CD271+ tumor-initiating cells via the suppression of multiple stemness-associated pathways, including TGF-β signaling[151]. Moreover, miR-27 has shown to play a role in cisplatin resistance in EC through the transformation of normal fibroblasts into CAFs and the induction of TGF-β secretion from the CAFs[91]. In GC, overexpression of miR-577 contributed to TGF-β-mediated EMT and stemness by forming a positive feedback loop, resulting in chemoresistance to OXA[152]. However, overexpression of miR-187 in GC cells alleviated cisplatin resistance by inhibiting the TGF-β/Smad signaling pathway[153]. Furthermore, overexpression of miR-204 was found to sensitize 5-FU-resistant GC cells through the suppression of TβRII-mediated EMT[154].
| miRNA | Tumor type | Target | Effect on drug resistance | Ref. |
| miR-21 | CRC cell line HCT-116 | Downregulation of TβRII | Induction of stemness | Yu et al[148], 2012 |
| miR-552 | CRC tissues of patients, CRC cell lines SW-480 and SW-620 | The 3′-UTR of Smad2 | Reduction 5-FU resistance | Zhao et al[150], 2019 |
| miR-34a | CRC cell line HT29 | Downregulation of the TGF-β/Smad4 signaling pathway | Acquired chemoresistance to oxaliplatin | Sun et al[149], 2017 |
| miR-455-3p | ESCC cell lines Eca109 and Kyse30 | Enhanced expression level of p-Smad2 | Resistance to DDP and docetaxel | Liu et al[151], 2017 |
| miR-27 | ESCC cell line TE10 | TGF-β secreted from CAF-like fibroblasts | Resistance to DDP | Tanaka et al[91], 2015 |
| miR-187 | DDP-resistant GC cells SGC7901/DDP | Downregulated TGF-β1 and p-Smad4 | Alleviates DDP-resistance | Zhu et al[153], 2019 |
| miR-204 | GC cell lines AGS and SGC-7901 | Target TβRII | Sensitizes GC cells to 5-FU | Li et al[154], 2018 |
Drug resistance, which leads to unfavorable clinical outcomes and treatment failure, remains a considerable challenge in the treatment of GI cancers. The TGF-β signaling pathway plays an important role in the regulation of the drug responses to conventional chemotherapy, targeted therapy, immunotherapy, and traditional medicine. Furthermore, TGF-β-mediated drug resistance in GI cancers is closely associated with several processes, including EMT, CSC development, alteration of the tumor microenvironment, and miRNA biogenesis (Figure 1).
Despite improvements in treatment strategies, EC, GC, and metastatic CRC have a poor prognosis, with 5-year OS rates of 15%–25%, 29.3%, and 14%, respectively[2,155,156]. The key obstacle to therapeutic success is the development of drug resistance, highlighting the urgency driving the development of alternative treatments for GI cancers. Many reports indicate the benefits of combining antitumor agents with agents that suppress TGF-β signaling. However, the findings require further verification by additional clinical studies. The use of some small-molecule inhibitors of TGF-β signaling is currently being investigated in both preclinical and clinical trials[60,157]. As TGF-β possesses paradoxical activities, the identification of potential biological markers related to the response to TGF-β inhibitors would have important clinical implications and would help select patients most likely to benefit from their use.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong LT S-Editor: Fan JR L-Editor: Filipodia P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55586] [Article Influence: 7940.9] [Reference Citation Analysis (131)] |
| 2. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3239] [Article Influence: 647.8] [Reference Citation Analysis (2)] |
| 3. | Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019;125:4139-4147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 316] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 4. | Chu JN, Choi J, Ostvar S, Torchia JA, Reynolds KL, Tramontano A, Gainor JF, Chung DC, Clark JW, Hur C. Cost-effectiveness of immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Cancer. 2019;125:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Adlard JW, Richman SD, Seymour MT, Quirke P. Prediction of the response of colorectal cancer to systemic therapy. Lancet Oncol. 2002;3:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Roselló S, Papaccio F, Roda D, Tarazona N, Cervantes A. The role of chemotherapy in localized and locally advanced rectal cancer: A systematic revision. Cancer Treat Rev. 2018;63:156-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, Park SR, Fujii M, Kang YK, Chen LT. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 8. | Wei L, Wang X, Lv L, Zheng Y, Zhang N, Yang M. The emerging role of noncoding RNAs in colorectal cancer chemoresistance. Cell Oncol (Dordr). 2019;42:757-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950-59964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 462] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 10. | Colak S, Ten Dijke P. Targeting TGF-β Signaling in Cancer. Trends Cancer. 2017;3:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 744] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 11. | Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 895] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 12. | Chruścik A, Gopalan V, Lam AK. The clinical and biological roles of transforming growth factor beta in colon cancer stem cells: A systematic review. Eur J Cell Biol. 2018;97:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Katz LH, Likhter M, Jogunoori W, Belkin M, Ohshiro K, Mishra L. TGF-β signaling in liver and gastrointestinal cancers. Cancer Lett. 2016;379:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Hong L, Han Y, Yang J, Zhang H, Zhao Q, Wu K, Fan D. MicroRNAs in gastrointestinal cancer: prognostic significance and potential role in chemoresistance. Expert Opin Biol Ther. 2014;14:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Sonbol MB, Ahn DH, Bekaii-Saab T. Therapeutic Targeting Strategies of Cancer Stem Cells in Gastrointestinal Malignancies. Biomedicines. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Yeldag G, Rice A, Del Río Hernández A. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1357] [Cited by in RCA: 1630] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 18. | Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741-4751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2076] [Cited by in RCA: 2067] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 19. | Zheng H, Liu Z, Liu T, Cai Y, Wang Y, Lin S, Chen J, Wang J, Wang Z, Jiang B. Fas signaling promotes chemoresistance in gastrointestinal cancer by up-regulating P-glycoprotein. Oncotarget. 2014;5:10763-10777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2456] [Cited by in RCA: 2566] [Article Influence: 160.4] [Reference Citation Analysis (0)] |
| 21. | Saxena M, Stephens MA, Pathak H, Rangarajan A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2:e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 22. | Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 798] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 23. | Fletcher JI, Williams RT, Henderson MJ, Norris MD, Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist Updat. 2016;26:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 312] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 24. | Das M, Law S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int J Biochem Cell Biol. 2018;103:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Raziq K, Cai M, Dong K, Wang P, Afrifa J, Fu S. Competitive endogenous network of lncRNA, miRNA, and mRNA in the chemoresistance of gastrointestinal tract adenocarcinomas. Biomed Pharmacother. 2020;130:110570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Aguda BD. Modeling microRNA-transcription factor networks in cancer. Adv Exp Med Biol. 2013;774:149-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Qiu H, Fang X, Luo Q, Ouyang G. Cancer stem cells: a potential target for cancer therapy. Cell Mol Life Sci. 2015;72:3411-3424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Stem J, Flickinger JC Jr, Merlino D, Caparosa EM, Snook AE, Waldman SA. Therapeutic targeting of gastrointestinal cancer stem cells. Regen Med. 2019;14:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Bekaii-Saab T, El-Rayes B. Identifying and targeting cancer stem cells in the treatment of gastric cancer. Cancer. 2017;123:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 1099] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 31. | Guerra F, Arbini AA, Moro L. Mitochondria and cancer chemoresistance. Biochim Biophys Acta Bioenerg. 2017;1858:686-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Lu JH, Wang F, Wang YN, He MM, Wu QN, Lu YX, Yu HE, Chen ZH, Zhao Q, Liu J, Chen YX, Wang DS, Sheng H, Liu ZX, Zeng ZL, Xu RH, Ju HQ. Inhibition of fatty acid catabolism augments the efficacy of oxaliplatin-based chemotherapy in gastrointestinal cancers. Cancer Lett. 2020;473:74-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 33. | Manoochehri Khoshinani H, Afshar S, Najafi R. Hypoxia: A Double-Edged Sword in Cancer Therapy. Cancer Invest. 2016;34:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 34. | Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1033] [Cited by in RCA: 1223] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 35. | Savant SS, Sriramkumar S, O'Hagan HM. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 36. | Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ, Kurilshikov A, Fu J, Joosten LAB, Zhernakova A, Huttenhower C, Wijmenga C, Netea MG, Xavier RJ. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016;167:1125-1136.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 712] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 37. | Garajová I, Balsano R, Wang H, Leonardi F, Giovannetti E, Deng D, Peters GJ. The role of the microbiome in drug resistance in gastrointestinal cancers. Expert Rev Anticancer Ther. 2021;21:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16:282-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 394] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 39. | Massagué J. TGFbeta in Cancer. Cell. 2008;134:215-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2725] [Cited by in RCA: 3120] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 40. | Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 41. | Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4404] [Cited by in RCA: 4575] [Article Influence: 208.0] [Reference Citation Analysis (0)] |
| 42. | Kamiya Y, Miyazono K, Miyazawa K. Smad7 inhibits transforming growth factor-beta family type i receptors through two distinct modes of interaction. J Biol Chem. 2010;285:30804-30813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1433] [Cited by in RCA: 1474] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 44. | Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 310] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 45. | Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1451] [Cited by in RCA: 1424] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 46. | Jung B, Staudacher JJ, Beauchamp D. Transforming Growth Factor β Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology. 2017;152:36-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 47. | Hao Y, Baker D, Ten Dijke P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 782] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 48. | Peñuelas S, Anido J, Prieto-Sánchez RM, Folch G, Barba I, Cuartas I, García-Dorado D, Poca MA, Sahuquillo J, Baselga J, Seoane J. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 425] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 49. | Achyut BR, Yang L. Transforming growth factor-β in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 50. | Itatani Y, Kawada K, Sakai Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 51. | Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 782] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 52. | Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massagué J, Sancho E, Batlle E. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 890] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 53. | Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, Imai Y, Nagashima R, Misawa H, Takeda H, Matsuzawa Y, Kawata S. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258-1262. [PubMed] |
| 54. | Ebert MP, Yu J, Miehlke S, Fei G, Lendeckel U, Ridwelski K, Stolte M, Bayerdörffer E, Malfertheiner P. Expression of transforming growth factor beta-1 in gastric cancer and in the gastric mucosa of first-degree relatives of patients with gastric cancer. Br J Cancer. 2000;82:1795-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Ma GF, Miao Q, Zeng XQ, Luo TC, Ma LL, Liu YM, Lian JJ, Gao H, Chen SY. Transforming growth factor-β1 and -β2 in gastric precancer and cancer and roles in tumor-cell interactions with peripheral blood mononuclear cells in vitro. PLoS One. 2013;8:e54249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Hawinkels LJ, Verspaget HW, van Duijn W, van der Zon JM, Zuidwijk K, Kubben FJ, Verheijen JH, Hommes DW, Lamers CB, Sier CF. Tissue level, activation and cellular localisation of TGF-beta1 and association with survival in gastric cancer patients. Br J Cancer. 2007;97:398-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Hu WQ, Wang LW, Yuan JP, Yan SG, Li JD, Zhao HL, Peng CW, Yang GF, Li Y. High expression of transform growth factor beta 1 in gastric cancer confers worse outcome: results of a cohort study on 184 patients. Hepatogastroenterology. 2014;61:245-250. [PubMed] |
| 58. | Gholamin M, Moaven O, Memar B, Farshchian M, Naseh H, Malekzadeh R, Sotoudeh M, Rajabi-Mashhadi MT, Forghani MN, Farrokhi F, Abbaszadegan MR. Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World J Surg. 2009;33:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Ramamoorthi G, Sivalingam N. Molecular mechanism of TGF-β signaling pathway in colon carcinogenesis and status of curcumin as chemopreventive strategy. Tumour Biol. 2014;35:7295-7305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Villalba M, Evans SR, Vidal-Vanaclocha F, Calvo A. Role of TGF-β in metastatic colon cancer: it is finally time for targeted therapy. Cell Tissue Res. 2017;370:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 61. | Lampropoulos P, Zizi-Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. TGF-beta signalling in colon carcinogenesis. Cancer Lett. 2012;314:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Fleming NI, Jorissen RN, Mouradov D, Christie M, Sakthianandeswaren A, Palmieri M, Day F, Li S, Tsui C, Lipton L, Desai J, Jones IT, McLaughlin S, Ward RL, Hawkins NJ, Ruszkiewicz AR, Moore J, Zhu HJ, Mariadason JM, Burgess AW, Busam D, Zhao Q, Strausberg RL, Gibbs P, Sieber OM. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res. 2013;73:725-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 63. | Bellam N, Pasche B. Tgf-beta signaling alterations and colon cancer. Cancer Treat Res. 2010;155:85-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Takagi Y, Koumura H, Futamura M, Aoki S, Ymaguchi K, Kida H, Tanemura H, Shimokawa K, Saji S. Somatic alterations of the SMAD-2 gene in human colorectal cancers. Br J Cancer. 1998;78:1152-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Zhang B, Zhang B, Chen X, Bae S, Singh K, Washington MK, Datta PK. Loss of Smad4 in colorectal cancer induces resistance to 5-fluorouracil through activating Akt pathway. Br J Cancer. 2014;110:946-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 66. | Brunen D, Willems SM, Kellner U, Midgley R, Simon I, Bernards R. TGF-β: an emerging player in drug resistance. Cell Cycle. 2013;12:2960-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Huang S, Hölzel M, Knijnenburg T, Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C, Prahallad A, Groenendijk FH, Mittempergher L, Nijkamp W, Neefjes J, Salazar R, Ten Dijke P, Uramoto H, Tanaka F, Beijersbergen RL, Wessels LF, Bernards R. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell. 2012;151:937-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 360] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 68. | Kim JC, Roh SA, Cho DH, Kim TW, Yoon SN, Kim CW, Yu CS, Kim SY, Kim YS. Chemoresponsiveness associated with canonical molecular changes in colorectal adenocarcinomas. Anticancer Res. 2009;29:3115-3123. [PubMed] |
| 69. | Zhou YL, Zhang WC, Chen XB, Xiao ZF, Qiao Y, Yu DK, Lin DX, Tan W. [The association between polymorphism of transforming growth factor-β1 and radiochemotherapy response and survival in esophageal squamous cell carcinoma patients]. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45:583-587. [PubMed] |
| 70. | Peters GJ. Novel developments in the use of antimetabolites. Nucleosides Nucleotides Nucleic Acids. 2014;33:358-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 507] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 72. | Chen J, Han M, Saif MW. TAS-102 an Emerging Oral Fluoropyrimidine. Anticancer Res. 2016;36:21-26. [PubMed] |
| 73. | Boulay JL, Mild G, Lowy A, Reuter J, Lagrange M, Terracciano L, Laffer U, Herrmann R, Rochlitz C. SMAD4 is a predictive marker for 5-fluorouracil-based chemotherapy in patients with colorectal cancer. Br J Cancer. 2002;87:630-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Buess M, Terracciano L, Reuter J, Ballabeni P, Boulay JL, Laffer U, Metzger U, Herrmann R, Rochlitz C. STRAP is a strong predictive marker of adjuvant chemotherapy benefit in colorectal cancer. Neoplasia. 2004;6:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Alhopuro P, Alazzouzi H, Sammalkorpi H, Dávalos V, Salovaara R, Hemminki A, Järvinen H, Mecklin JP, Schwartz S Jr, Aaltonen LA, Arango D. SMAD4 levels and response to 5-fluorouracil in colorectal cancer. Clin Cancer Res. 2005;11:6311-6316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Papageorgis P, Cheng K, Ozturk S, Gong Y, Lambert AW, Abdolmaleky HM, Zhou JR, Thiagalingam S. Smad4 inactivation promotes malignancy and drug resistance of colon cancer. Cancer Res. 2011;71:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 77. | Refaat B, El-Shemi AG, Kensara OA, Mohamed AM, Idris S, Ahmad J, Khojah A. Vitamin D3 enhances the tumouricidal effects of 5-Fluorouracil through multipathway mechanisms in azoxymethane rat model of colon cancer. J Exp Clin Cancer Res. 2015;34:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Huang MY, Lin CH, Huang CM, Tsai HL, Huang CW, Yeh YS, Chai CY, Wang JY. Relationships between SMAD3 expression and preoperative fluoropyrimidine-based chemoradiotherapy response in locally advanced rectal cancer patients. World J Surg. 2015;39:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Cheng JC, Graber MS, Hsu FM, Tsai CL, Castaneda L, Lee JM, Chang DT, Koong AC. High serum levels of vascular endothelial growth factor-A and transforming growth factor-β1 before neoadjuvant chemoradiotherapy predict poor outcomes in patients with esophageal squamous cell carcinoma receiving combined modality therapy. Ann Surg Oncol. 2014;21:2361-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Moon JR, Oh SJ, Lee CK, Chi SG, Kim HJ. TGF-β1 protects colon tumor cells from apoptosis through XAF1 suppression. Int J Oncol. 2019;54:2117-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Quan Q, Zhong F, Wang X, Chen K, Guo L. PAR2 Inhibition Enhanced the Sensitivity of Colorectal Cancer Cells to 5-FU and Reduced EMT Signaling. Oncol Res. 2019;27:779-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Romano G, Santi L, Bianco MR, Giuffrè MR, Pettinato M, Bugarin C, Garanzini C, Savarese L, Leoni S, Cerrito MG, Leone BE, Gaipa G, Grassilli E, Papa M, Lavitrano M, Giovannoni R. The TGF-β pathway is activated by 5-fluorouracil treatment in drug resistant colorectal carcinoma cells. Oncotarget. 2016;7:22077-22091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Moon SU, Kang MH, Sung JH, Kim JW, Lee JO, Kim YJ, Lee KW, Bang SM, Lee JS, Kim JH. Effect of Smad3/4 on chemotherapeutic drug sensitivity in colorectal cancer cells. Oncol Rep. 2015;33:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Zhang H, Ren L, Ding Y, Li F, Chen X, Ouyang Y, Zhang Y, Zhang D. Hyaluronan-mediated motility receptor confers resistance to chemotherapy via TGFβ/Smad2-induced epithelial-mesenchymal transition in gastric cancer. FASEB J. 2019;33:6365-6377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 85. | Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel). 2011;3:1351-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1005] [Cited by in RCA: 1236] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 86. | Haller DG. Recent updates in the clinical use of platinum compounds for the treatment of gastrointestinal cancers. Semin Oncol. 2004;31:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Carrato A, Gallego J, Díaz-Rubio E. Oxaliplatin: results in colorectal carcinoma. Crit Rev Oncol Hematol. 2002;44:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Mao L, Li Y, Zhao J, Li Q, Yang B, Wang Y, Zhu Z, Sun H, Zhai Z. Transforming growth factor-β1 contributes to oxaliplatin resistance in colorectal cancer via epithelial to mesenchymal transition. Oncol Lett. 2017;14:647-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Kim YH, Lee SB, Shim S, Kim A, Park JH, Jang WS, Lee SJ, Myung JK, Park S, Kim MJ. Hyaluronic acid synthase 2 promotes malignant phenotypes of colorectal cancer cells through transforming growth factor beta signaling. Cancer Sci. 2019;110:2226-2236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Yin J, Wang L, Wang Y, Shen H, Wang X, Wu L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-β/Smad2/3 signaling pathway. Onco Targets Ther. 2019;12:3893-3903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 91. | Tanaka K, Miyata H, Sugimura K, Fukuda S, Kanemura T, Yamashita K, Miyazaki Y, Takahashi T, Kurokawa Y, Yamasaki M, Wada H, Nakajima K, Takiguchi S, Mori M, Doki Y. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis. 2015;36:894-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 92. | Gelmon K. The taxoids: paclitaxel and docetaxel. Lancet. 1994;344:1267-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 93. | Bekaii-Saab TS, Villalona-Calero MA. Preclinical experience with docetaxel in gastrointestinal cancers. Semin Oncol. 2005;32:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Ojima I, Das M. Recent advances in the chemistry and biology of new generation taxoids. J Nat Prod. 2009;72:554-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 95. | Koizumi W, Akiya T, Sato A, Yamaguchi K, Sakuyama T, Nakayama N, Tanabe S, Higuchi K, Sasaki T, Sekikawa T; Tokyo Cooperative Oncology Group (TCOG GI Group). Second-line chemotherapy with biweekly paclitaxel after failure of fluoropyrimidine-based treatment in patients with advanced or recurrent gastric cancer: a report from the gastrointestinal oncology group of the Tokyo cooperative oncology group, TCOG GC-0501 trial. Jpn J Clin Oncol. 2009;39:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1756] [Article Influence: 159.6] [Reference Citation Analysis (0)] |
| 97. | Ajani JA. Docetaxel for gastric and esophageal carcinomas. Oncology (Williston Park). 2002;16:89-96. [PubMed] |
| 98. | Tsukada T, Fushida S, Harada S, Terai S, Yagi Y, Kinoshita J, Oyama K, Tajima H, Ninomiya I, Fujimura T, Ohta T. Low-dose paclitaxel modulates tumour fibrosis in gastric cancer. Int J Oncol. 2013;42:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 99. | Fujita T, Chiwaki F, Takahashi RU, Aoyagi K, Yanagihara K, Nishimura T, Tamaoki M, Komatsu M, Komatsuzaki R, Matsusaki K, Ichikawa H, Sakamoto H, Yamada Y, Fukagawa T, Katai H, Konno H, Ochiya T, Yoshida T, Sasaki H. Identification and Characterization of CXCR4-Positive Gastric Cancer Stem Cells. PLoS One. 2015;10:e0130808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Zhou K, Shi X, Huo J, Liu W, Yang D, Yang T, Qin T, Wang C. Bone morphogenetic protein 4 is overexpressed in and promotes migration and invasion of drug-resistant cancer cells. Int J Biol Macromol. 2017;101:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Matsunaga T, Kezuka C, Morikawa Y, Suzuki A, Endo S, Iguchi K, Miura T, Nishinaka T, Terada T, El-Kabbani O, Hara A, Ikari A. Up-Regulation of Carbonyl Reductase 1 Renders Development of Doxorubicin Resistance in Human Gastrointestinal Cancers. Biol Pharm Bull. 2015;38:1309-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 474] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 103. | Li J, Liu H, Yu J, Yu H. Chemoresistance to doxorubicin induces epithelial-mesenchymal transition via upregulation of transforming growth factor β signaling in HCT116 colon cancer cells. Mol Med Rep. 2015;12:192-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 104. | Izutani R, Kato M, Asano S, Imano M, Ohyanagi H. Expression of manganese superoxide disumutase influences chemosensitivity in esophageal and gastric cancers. Cancer Detect Prev. 2002;26:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Cordeiro HG, de Sousa Faria AV, Ferreira-Halder CV. Vemurafenib downmodulates aggressiveness mediators of colorectal cancer (CRC): Low Molecular Weight Protein Tyrosine Phosphatase (LMWPTP), Protein Tyrosine Phosphatase 1B (PTP1B) and Transforming Growth Factor β (TGFβ). Biol Chem. 2020;401:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Ebbing EA, Steins A, Fessler E, Stathi P, Lesterhuis WJ, Krishnadath KK, Vermeulen L, Medema JP, Bijlsma MF, van Laarhoven HWM. Esophageal Adenocarcinoma Cells and Xenograft Tumors Exposed to Erb-b2 Receptor Tyrosine Kinase 2 and 3 Inhibitors Activate Transforming Growth Factor Beta Signaling, Which Induces Epithelial to Mesenchymal Transition. Gastroenterology. 2017;153:63-76.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Zhou X, Men X, Zhao R, Han J, Fan Z, Wang Y, Lv Y, Zuo J, Zhao L, Sang M, Liu XD, Shan B. miR-200c inhibits TGF-β-induced-EMT to restore trastuzumab sensitivity by targeting ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 2018;25:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 108. | Kawaguchi Y, Kono K, Mimura K, Mitsui F, Sugai H, Akaike H, Fujii H. Targeting EGFR and HER-2 with cetuximab- and trastuzumab-mediated immunotherapy in oesophageal squamous cell carcinoma. Br J Cancer. 2007;97:494-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 109. | Kawaguchi Y, Kono K, Mimura K, Sugai H, Akaike H, Fujii H. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer. 2007;120:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 110. | Mimura K, Kono K, Hanawa M, Kanzaki M, Nakao A, Ooi A, Fujii H. Trastuzumab-mediated antibody-dependent cellular cytotoxicity against esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:4898-4904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 111. | Grygielewicz P, Dymek B, Bujak A, Gunerka P, Stanczak A, Lamparska-Przybysz M, Wieczorek M, Dzwonek K, Zdzalik D. Epithelial-mesenchymal transition confers resistance to selective FGFR inhibitors in SNU-16 gastric cancer cells. Gastric Cancer. 2016;19:53-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 112. | Shi X, Mihaylova VT, Kuruvilla L, Chen F, Viviano S, Baldassarre M, Sperandio D, Martinez R, Yue P, Bates JG, Breckenridge DG, Schlessinger J, Turk BE, Calderwood DA. Loss of TRIM33 causes resistance to BET bromodomain inhibitors through MYC- and TGF-β-dependent mechanisms. Proc Natl Acad Sci U S A. 2016;113:E4558-E4566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Llopiz D, Dotor J, Casares N, Bezunartea J, Díaz-Valdés N, Ruiz M, Aranda F, Berraondo P, Prieto J, Lasarte JJ, Borrás-Cuesta F, Sarobe P. Peptide inhibitors of transforming growth factor-beta enhance the efficacy of antitumor immunotherapy. Int J Cancer. 2009;125:2614-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Rodríguez-Ruiz ME, Rodríguez I, Mayorga L, Labiano T, Barbes B, Etxeberria I, Ponz-Sarvise M, Azpilikueta A, Bolaños E, Sanmamed MF, Berraondo P, Calvo FA, Barcelos-Hoff MH, Perez-Gracia JL, Melero I. TGFβ Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol Cancer Ther. 2019;18:621-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 115. | Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1312] [Article Influence: 187.4] [Reference Citation Analysis (0)] |
| 116. | Lai Z, Yan Z, Chen W, Peng J, Feng J, Li Q, Jin Y, Lin J. Hedyotis diffusa Willd suppresses metastasis in 5fluorouracilresistant colorectal cancer cells by regulating the TGFβ signaling pathway. Mol Med Rep. 2017;16:7752-7758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Shen A, Chen H, Chen Y, Lin J, Lin W, Liu L, Sferra TJ, Peng J. Pien Tze Huang Overcomes Multidrug Resistance and Epithelial-Mesenchymal Transition in Human Colorectal Carcinoma Cells via Suppression of TGF-β Pathway. Evid Based Complement Alternat Med. 2014;2014:679436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Kim EJ, Kang JI, Kwak JW, Jeon CH, Tung NH, Kim YH, Choi CH, Hyun JW, Koh YS, Yoo ES, Kang HK. The anticancer effect of (1S,2S,3E,7E,11E)-3,7,11, 15-cembratetraen-17,2-olide(LS-1) through the activation of TGF-β signaling in SNU-C5/5-FU, fluorouracil-resistant human colon cancer cells. Mar Drugs. 2015;13:1340-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Wang X, Liu C, Wang J, Fan Y, Wang Z, Wang Y. Oxymatrine inhibits the migration of human colorectal carcinoma RKO cells via inhibition of PAI-1 and the TGF-β1/Smad signaling pathway. Oncol Rep. 2017;37:747-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 120. | Wang J, Wu M, Zheng D, Zhang H, Lv Y, Zhang L, Tan HS, Zhou H, Lao YZ, Xu HX. Garcinol inhibits esophageal cancer metastasis by suppressing the p300 and TGF-β1 signaling pathways. Acta Pharmacol Sin. 2020;41:82-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 121. | Kim YM, Kim IH, Nam TJ. Capsosiphon fulvescens glycoprotein reduces AGS gastric cancer cell migration by downregulating transforming growth factor-β1 and integrin expression. Int J Oncol. 2013;43:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 122. | Kensara OA, El-Shemi AG, Mohamed AM, Refaat B, Idris S, Ahmad J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des Devel Ther. 2016;10:2239-2253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 123. | Câmara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis Tissue Repair. 2010;3:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 124. | Park JH, Kim YH, Park EH, Lee SJ, Kim H, Kim A, Lee SB, Shim S, Jang H, Myung JK, Park S, Kim MJ. Effects of metformin and phenformin on apoptosis and epithelial-mesenchymal transition in chemoresistant rectal cancer. Cancer Sci. 2019;110:2834-2845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 125. | Dai G, Sun B, Gong T, Pan Z, Meng Q, Ju W. Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of colorectal cancer cells by suppressing TGF-β/Smad signaling. Phytomedicine. 2019;56:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 126. | Kurata T, Fushida S, Kinoshita J, Oyama K, Yamaguchi T, Okazaki M, Miyashita T, Tajima H, Ninomiya I, Ohta T. Low-dose eribulin mesylate exerts antitumor effects in gastric cancer by inhibiting fibrosis via the suppression of epithelial-mesenchymal transition and acts synergistically with 5-fluorouracil. Cancer Manag Res. 2018;10:2729-2742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, Gray MJ, Cheng H, Hoff PM, Ellis LM. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12:4147-4153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 432] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 128. | Moustakas A, Heldin P. TGFβ and matrix-regulated epithelial to mesenchymal transition. Biochim Biophys Acta. 2014;1840:2621-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 129. | Steins A, Ebbing EA, Creemers A, van der Zalm AP, Jibodh RA, Waasdorp C, Meijer SL, van Delden OM, Krishnadath KK, Hulshof MCCM, Bennink RJ, Punt CJA, Medema JP, Bijlsma MF, van Laarhoven HWM. Chemoradiation induces epithelial-to-mesenchymal transition in esophageal adenocarcinoma. Int J Cancer. 2019;145:2792-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Zhao Y, Zhu J, Shi B, Wang X, Lu Q, Li C, Chen H. The transcription factor LEF1 promotes tumorigenicity and activates the TGF-β signaling pathway in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2019;38:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Yue D, Zhang Z, Li J, Chen X, Ping Y, Liu S, Shi X, Li L, Wang L, Huang L, Zhang B, Sun Y, Zhang Y. Transforming growth factor-beta1 promotes the migration and invasion of sphere-forming stem-like cell subpopulations in esophageal cancer. Exp Cell Res. 2015;336:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 132. | Sato F, Kubota Y, Natsuizaka M, Maehara O, Hatanaka Y, Marukawa K, Terashita K, Suda G, Ohnishi S, Shimizu Y, Komatsu Y, Ohashi S, Kagawa S, Kinugasa H, Whelan KA, Nakagawa H, Sakamoto N. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biol Ther. 2015;16:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Park JW, Park JM, Park DM, Kim DY, Kim HK. Stem Cells Antigen-1 Enriches for a Cancer Stem Cell-Like Subpopulation in Mouse Gastric Cancer. Stem Cells. 2016;34:1177-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 134. | Chistiakov DA, Orekhov AN, Bobryshev YV. Dendritic Cells in Colorectal Cancer and a Potential for their Use in Therapeutic Approaches. Curr Pharm Des. 2016;22:2431-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 135. | Tanaka H, Shinto O, Yashiro M, Yamazoe S, Iwauchi T, Muguruma K, Kubo N, Ohira M, Hirakawa K. Transforming growth factor β signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol Rep. 2010;24:1637-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 136. | Watanabe M, Kono K, Kawaguchi Y, Mizukami Y, Mimura K, Maruyama T, Izawa S, Fujii H. NK cell dysfunction with down-regulated CD16 and up-regulated CD56 molecules in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 137. | Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, Bin J, Liao Y, Rao J, Liao W. Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures. Cancer Immunol Res. 2019;7:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 662] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 138. | Zhou YJ, Zhu GQ, Lu XF, Zheng KI, Wang QW, Chen JN, Zhang QW, Yan FR, Li XB. Identification and validation of tumour microenvironment-based immune molecular subgroups for gastric cancer: immunotherapeutic implications. Cancer Immunol Immunother. 2020;69:1057-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 139. | Chen X, Wang L, Li P, Song M, Qin G, Gao Q, Zhang Z, Yue D, Wang D, Nan S, Qi Y, Li F, Yang L, Huang L, Zhang M, Zhang B, Gao Y, Zhang Y. Dual TGF-β and PD-1 blockade synergistically enhances MAGE-A3-specific CD8+ T cell response in esophageal squamous cell carcinoma. Int J Cancer. 2018;143:2561-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 140. | Malvicini M, Ingolotti M, Piccioni F, Garcia M, Bayo J, Atorrasagasti C, Alaniz L, Aquino JB, Espinoza JA, Gidekel M, Scharovsky OG, Matar P, Mazzolini G. Reversal of gastrointestinal carcinoma-induced immunosuppression and induction of antitumoural immunity by a combination of cyclophosphamide and gene transfer of IL-12. Mol Oncol. 2011;5:242-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 141. | Ciszewski WM, Sobierajska K, Wawro ME, Klopocka W, Chefczyńska N, Muzyczuk A, Siekacz K, Wujkowska A, Niewiarowska J. The ILK-MMP9-MRTF axis is crucial for EndMT differentiation of endothelial cells in a tumor microenvironment. Biochim Biophys Acta Mol Cell Res. 2017;1864:2283-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 142. | Zhang H, Xie C, Yue J, Jiang Z, Zhou R, Xie R, Wang Y, Wu S. Cancer-associated fibroblasts mediated chemoresistance by a FOXO1/TGFβ1 signaling loop in esophageal squamous cell carcinoma. Mol Carcinog. 2017;56:1150-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 143. | Li Z, Chan K, Qi Y, Lu L, Ning F, Wu M, Wang H, Wang Y, Cai S, Du J. Participation of CCL1 in Snail-Positive Fibroblasts in Colorectal Cancer Contribute to 5-Fluorouracil/Paclitaxel Chemoresistance. Cancer Res Treat. 2018;50:894-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 144. | Tang YA, Chen YF, Bao Y, Mahara S, Yatim SMJM, Oguz G, Lee PL, Feng M, Cai Y, Tan EY, Fong SS, Yang ZH, Lan P, Wu XJ, Yu Q. Hypoxic tumor microenvironment activates GLI2 via HIF-1α and TGF-β2 to promote chemoresistance in colorectal cancer. Proc Natl Acad Sci U S A. 2018;115:E5990-E5999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 145. | Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 2012;106:1901-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 146. | He W, Liang B, Wang C, Li S, Zhao Y, Huang Q, Liu Z, Yao Z, Wu Q, Liao W, Zhang S, Liu Y, Xiang Y, Liu J, Shi M. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38:4637-4654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 147. | Khorrami S, Zavaran Hosseini A, Mowla SJ, Soleimani M, Rakhshani N, Malekzadeh R. MicroRNA-146a induces immune suppression and drug-resistant colorectal cancer cells. Tumour Biol. 2017;39:1010428317698365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 148. | Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal J, Sarkar FH, Majumdar AP. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFβR2) in colon cancer cells. Carcinogenesis. 2012;33:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 149. | Sun C, Wang FJ, Zhang HG, Xu XZ, Jia RC, Yao L, Qiao PF. miR-34a mediates oxaliplatin resistance of colorectal cancer cells by inhibiting macroautophagy via transforming growth factor-β/Smad4 pathway. World J Gastroenterol. 2017;23:1816-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 150. | Zhao P, Ma YG, Zhao Y, Liu D, Dai ZJ, Yan CY, Guan HT. MicroRNA-552 deficiency mediates 5-fluorouracil resistance by targeting SMAD2 signaling in DNA-mismatch-repair-deficient colorectal cancer. Cancer Chemother Pharmacol. 2019;84:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 151. | Liu A, Zhu J, Wu G, Cao L, Tan Z, Zhang S, Jiang L, Wu J, Li M, Song L, Li J. Antagonizing miR-455-3p inhibits chemoresistance and aggressiveness in esophageal squamous cell carcinoma. Mol Cancer. 2017;16:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 152. | Luo Y, Wu J, Wu Q, Li X, Zhang J, Rong X, Rao J, Liao Y, Bin J, Huang N, Liao W. miR-577 Regulates TGF-β Induced Cancer Progression through a SDPR-Modulated Positive-Feedback Loop with ERK-NF-κB in Gastric Cancer. Mol Ther. 2019;27:1166-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 153. | Zhu QL, Li Z, Lv CM, Wang W. MiR-187 influences cisplatin-resistance of gastric cancer cells through regulating the TGF-β/Smad signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:9907-9914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 154. | Li LQ, Pan D, Chen Q, Zhang SW, Xie DY, Zheng XL, Chen H. Sensitization of Gastric Cancer Cells to 5-FU by MicroRNA-204 Through Targeting the TGFBR2-Mediated Epithelial to Mesenchymal Transition. Cell Physiol Biochem. 2018;47:1533-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 155. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1957] [Article Influence: 163.1] [Reference Citation Analysis (5)] |
| 156. | Liu N, Wu Y, Cheng W, Wang L, Zhuang L. Identification of novel prognostic biomarkers by integrating multi-omics data in gastric cancer. BMC Cancer. 2021;21:460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |