Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1632
Peer-review started: February 25, 2021
First decision: April 19, 2021
Revised: April 30, 2021
Accepted: August 16, 2021
Article in press: August 16, 2021
Published online: November 15, 2021
Processing time: 259 Days and 18 Hours
The mammalian target of rapamycin (mTOR) acts in two structurally and functionally distinct protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Upon deregulation, activated mTOR signaling is associated with multiple processes involved in tumor growth and metastasis. Compared with mTORC1, much less is known about mTORC2 in cancer, mainly because of the unavailability of a selective inhibitor. However, existing data suggest that mTORC2 with its two distinct subunits Rictor and mSin1 might play a more important role than assumed so far. It is one of the key effectors of the PI3K/AKT/mTOR pathway and stimulates cell growth, cell survival, metabolism, and cytoskeletal organization. It is not only implicated in tumor progression, metastasis, and the tumor microenvironment but also in resistance to therapy. Rictor, the central subunit of mTORC2, was found to be upregulated in different kinds of cancers and is associated with advanced tumor stages and a bad prognosis. Moreover, AKT, the main downstream regulator of mTORC2/Rictor, is one of the most highly activated proteins in cancer. Primary and secondary liver cancer are major problems for current cancer therapy due to the lack of specific medical treatment, emphasizing the need for further therapeutic options. This review, therefore, summarizes the role of mTORC2/Rictor in cancer, with special focus on primary liver cancer but also on liver metastases.
Core Tip: Mammalian target of rapamycin complex 2 (mTORC2) has recently gained importance in cancer research, as it is one of the key effectors of the PI3K/AKT/mTOR pathway and stimulates cell growth, cell survival, metabolism, and cytoskeletal organization. Rictor, the central subunit of mTORC2, was found to be upregulated in different kinds of cancers and is associated with a bad prognosis. We herein discuss the implications of mTORC2 in primary and secondary liver cancer.
- Citation: Joechle K, Guenzle J, Hellerbrand C, Strnad P, Cramer T, Neumann UP, Lang SA. Role of mammalian target of rapamycin complex 2 in primary and secondary liver cancer. World J Gastrointest Oncol 2021; 13(11): 1632-1647
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1632.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1632
The mammalian target of rapamycin (mTOR) is an atypical serine/threonine kinase that controls cell survival, proliferation, and metabolism through phosphorylation of its downstream targets. It acts in two structurally and functionally distinct protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), and can be activated by several stimulating factors as hypoxia, insulin, growth factors, or dysregulation of PI3K/Akt signaling[1,2]. However, upon deregulation, activated mTOR signaling is implicated in the hallmarks of cancer and associated with increased cell survival, uncontrolled cell proliferation, metabolic reprogramming, and aberrant angiogenesis[3], which makes it a promising target in anticancer therapy.
The incidence of primary liver cancer, such as hepatocellular carcinoma (HCC) and intrahepatic cholangiocellular carcinoma (iCCC), is increasing worldwide. Systemic therapy options are limited for both cancer entities. Surgery offers the only chance for cure, although only a minority of patients with HCC or iCCC are eligible for resection. Moreover, the liver is one of the most frequent sites of metastases development. Indeed, in most cancer entities liver metastasis is associated with a dramatic decline of patient’s prognosis, further emphasizing the fundamental impact of this site. A deeper understanding of processes involved in either primary or secondary liver cancer is therefore urgently needed[4-6].
Due to the availability of (more or less) selective mTORC1 targeting agents such as rapamycin, the role of mTORC1 in cancer has been extensively studied for decades. In contrast, mTORC2 was less intensively analyzed, mainly because of the lack of selective pharmacologic inhibitors. However, the last decade has brought up several studies suggesting a role for mTORC2 in cancer. For instance, Rictor (rapamycin insensitive companion of TOR), the central subunit of mTORC2, was found to be upregulated in different kinds of cancer and is associated with impaired prognosis[7-10]. Evidence from primary and secondary liver cancer is summarized in Table 1. In addition, involvement of mTORC2/Rictor in a plethora of processes implicated in tumor growth and also metastasis have been reported[11]. Therefore, the present review summarizes the current knowledge regarding the role of mTORC2 in cancer with focus on primary and secondary liver cancer.
| mTORC2 mediator | Associated with | Measured by | Ref. | ||
| Primary liver cancer | HCC | p-AKTSer473 overexpression | poor outcome (P < 0.02) | IHC | Hu et al[59] |
| Rictor overexpression | Reduced OS (P = 0.0029) | mRNA expression | Xu et al[60] | ||
| Rictor overexpression | Reduced RFS (P = 0.016) | IHC, mRNA expression | Kaibori et al[61] | ||
| iCCC | p-AKT1 overexpression | Improved OS (P = 0.0137) | IHC | Lee et al[76] | |
| CRLM | Data only available for primary CRC | ||||
| Rictor expression | Increasing tumor stage | mRNA expression | Gulhati et al[49] | ||
| Rictor expression | Increasing tumor stage | IHC, mRNA expression | Shuhua et al[81] | ||
| Rictor expression | Reduced OS (P = 0.0004) | IHC | Wang et al[10] | ||
| Secondary liver cancer | Breast cancer liver metastases | Data only available for invasive ductal breast carcinoma | |||
| Rictor expression | Lymph node metastasis | IHC | Zhang et al[90] | ||
| Melanoma liver metastases | Rictor positivity (primary tumor) | Reduced OS (P = 0.018) | IHC | Liang et al[100] | |
| Rictor expression | Tumor stage/metastatic disease | mRNA expression | Schmidt et al[101] | ||
| Renal cancer liver metastases | No data available | ||||
| Gastric cancer liver metastases | Data only available for gastric cancer | ||||
| Rictor, p-AKTSer437 expression | Tumor stage, reduced RFS and OS | IHC | Bian et al[7] | ||
| Rictor expression | Tumor stage, reduced RFS and OS (P = 0.012, P = 0.014) | IHC | Bian et al[110] | ||
| Pancreatic cancer liver metastases | Data only available for pancreatic cancer | ||||
| Rictor expression | Reduced OS | IHC | Schmidt et al[9] | ||
Although mTOR acts through the different complexes mTORC1 and mTORC2, both have several subunits in common: the catalytic kinase mTOR, the scaffolding protein mLST8, the regulatory subunit DEPTOR, and the stabilizing complex Tti1/Tel2. The distinct subunits of mTORC1 are Raptor and PRAS40. While Raptor is important for mTORC1 substrate specificity, stability, and regulation, PRAS40 acts as negative regulator of mTORC1. Activation of mTORC1 depends on nutrient (e.g., amino acids) and growth factor (e.g., insulin) signaling through the PI3K/AKT and Ras-MAPK cascades. Phosphorylated AKT (via the PI3K/AKT pathway) or ERK and RSK (via the Ras-MAPK cascade) inhibit the TSC1/2 complex, which in turn triggers RHEB-mediated activation of mTORC1 leading to phosphorylation of its substrates 4EBP1 and S6K1. The downstream effectors act as key regulators of cap-dependent and cap-independent mTORC1 translation[3] and regulate translation and transcription of different target genes (e.g., HIF1α, etc.) thereby being implicated in cell growth, proliferation, and metabolism[12].
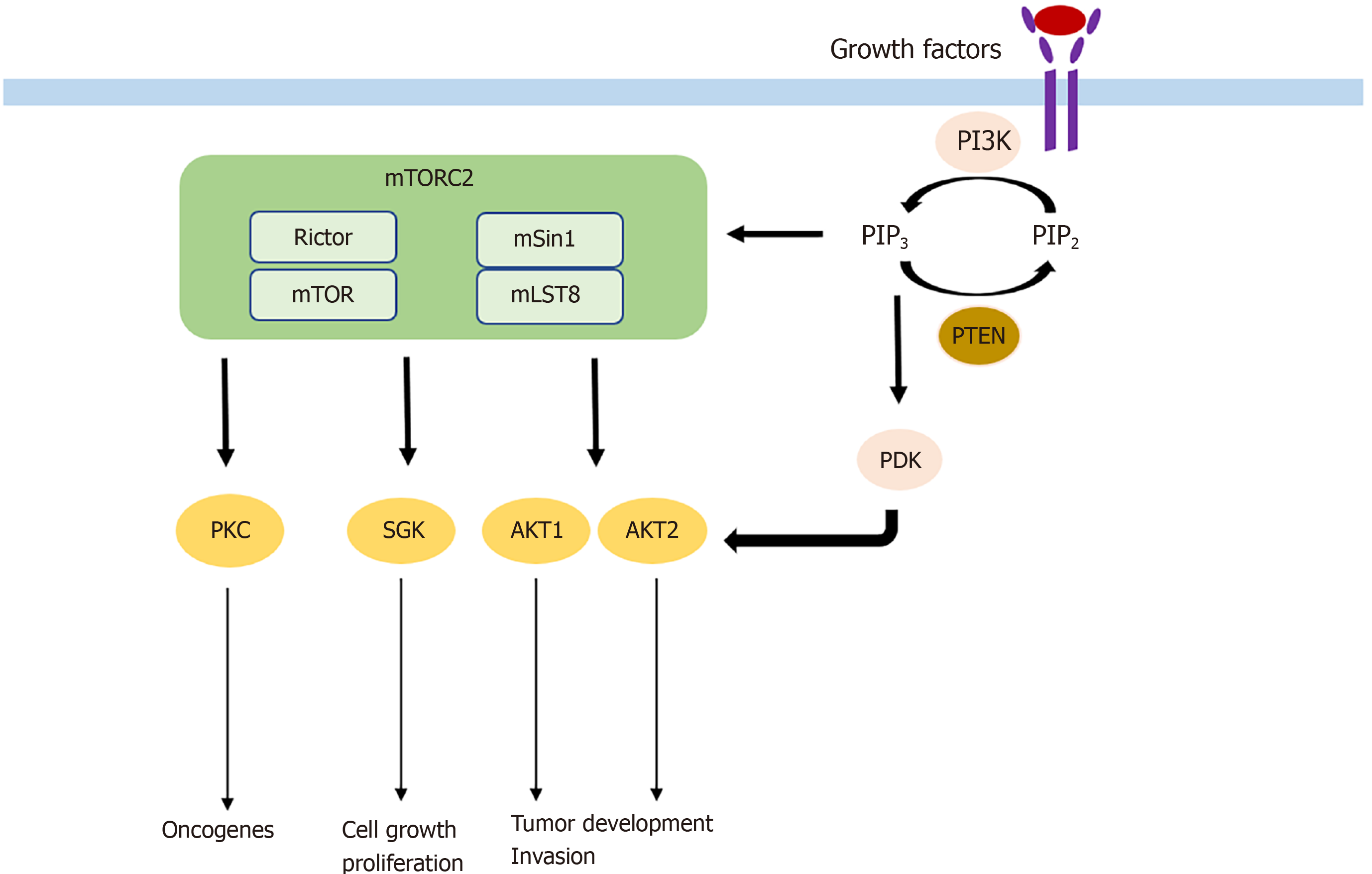
mTORC2 consists of its specific subunits Rictor and mSin1. Similar to Raptor, Rictor controls mTORC2 stability, subcellular localization, and substrate identification. It is essential for mTORC2 function, as silencing Rictor leads to significant inhibition of AKT, the key substrate of mTORC2. mSin1 negatively regulates mTORC2 until PI3K-mediated growth factor signaling locates mSin1/mTORC2 to the plasma membrane and relieves its inhibition. In turn, PI3K-generated PIP3 activates mTORC2. Activated mTORC2/Rictor leads to phosphorylation of AKT at the Ser473 residue. AKT can also be phosphorylated at the Thr308 residue by PDK1, which is in turn attracted by PIP3. Importantly, AKT is one of the most frequently activated proteins in cancer, and its full activity is only achieved if both sites are phosphorylated[13,14]. On a functional level, AKT is involved in many processes. Association with cell migration, invasion, increased tumor growth, and cell survival while inhibiting apoptosis and promoting proliferative processes including glucose uptake and glycolysis have been described[15,16]. Additional substrates of mTORC2 are the AGC kinases like the PKC (e.g., PKCα, PKCδ, PKCε) family, which control tumorigenesis, cell migration, and cytoskeletal remodeling, and SGK isoforms that are implicated in cell survival and resistance to chemotherapy.
Along with the essential role of Rictor for mTORC2 functioning, it also acts independently of mTORC2. Thr1135, one of the 37 phosphorylation sites of Rictor, was shown to be stimulated directly by growth factor signaling and to be sensitive to rapamycin, as it is targeted by S6K1, one of the downstream effectors of mTORC1[17,18]. This mechanism is assumed to represent a regulatory link between mTORC1 and mTORC2 signaling and to be part of a reciprocally influenced feedback loop mecha
Besides being implicated in many physiological processes such as glucose and lipid homeostasis, adipogenesis, maintaining muscle mass and function, brain and immune function, deregulation of mTORC1 signaling is not only associated with diseases such as diabetes, neurodegeneration, and cancer. As described above, mTORC1 is activated by the oncogenic pathways PI3K/Akt and Ras-MAPK cascade. However, the on
The implications of mTORC1 in cancer are widely studied because of the availability of rapamycin as a selective mTORC1 inhibitor. However, rapamycin analogs (rapalogs) have only shown limited efficacy in cancer therapy. While inhibition of mTORC1 by rapamycin blocks phosphorylation of S6K1, phosphorylation of 4EBP1 is not fully blocked[29]. Therefore, 4EBP1-regulated translation of proteins involved in tumorigenesis is not inhibited. Another reason for the limited efficacy of rapamycin and rapalogs is explained by compensatory upregulation of AKT through phosphorylation of its Thr308 and Ser473 residue[30,31] as inactivated S6K1 no longer prevents suppression of insulin-PI3K signaling[32,33]. Moreover, treatment with rapalogs may increase micropinocytosis and autophagy leading to enhanced cell proliferation and survival[34,35].
Compared with mTORC1, much less is known about the role of mTORC2 in cancer, although existing data suggest mTORC2 to be of importance. Particularly, it is one of the key effectors of the PI3K/AKT/mTOR pathway and stimulates cell growth, cell survival, metabolism, and cytoskeletal organization.
As described above, AKT is the key downstream target of mTORC2 signaling and one of the most commonly activated proteins in cancer[36,37] with its isoforms AKT1 and AKT2 being the main effectors in tumorigenesis[38]. With more than 200 AKT substrates, the different isoforms seem to have unique roles in tumorigenesis. While AKT1 increases tumor development and reduces tumor invasion, the expression of AKT2 has the opposite effect[39]. Frequently, hyperactivated AKT is found in different kinds of cancers. For example, hyperactivation of AKT caused by a somatic mutation was found to induce B-cell lymphoma, in contrast to wild-type AKT[40]. The somatic mutation was also found in breast, colorectal, and ovarian cancer. In addition to somatic mutations, hyperactivation of AKT may also occur because of activating upstream mutations in PI3K or deletions in PTEN, a tumor suppressor[41]. AKT has several substrates in common with SGK family members, another downstream target of mTORC2. Hence, SGK is similarly implicated in cell growth and proliferation[42]. Its isoform SGK3 is dependent on PDK1 signaling to induce tumor growth and adopted the role of AKT in tumorigenesis in PI3K mutated cancer cells[43]. AKT and SGK, which are different isoforms of PKC, are also involved in tumorigenesis. PKC-ε, PKC-λ/ι, and PKC-β are known oncogenes with PKC-λ/ι and PKC-β for example being involved in colon carcinogenesis[44,45]. Mechanisms by which mTORC2 is involved in tumorigenesis are shown in Figure 1.
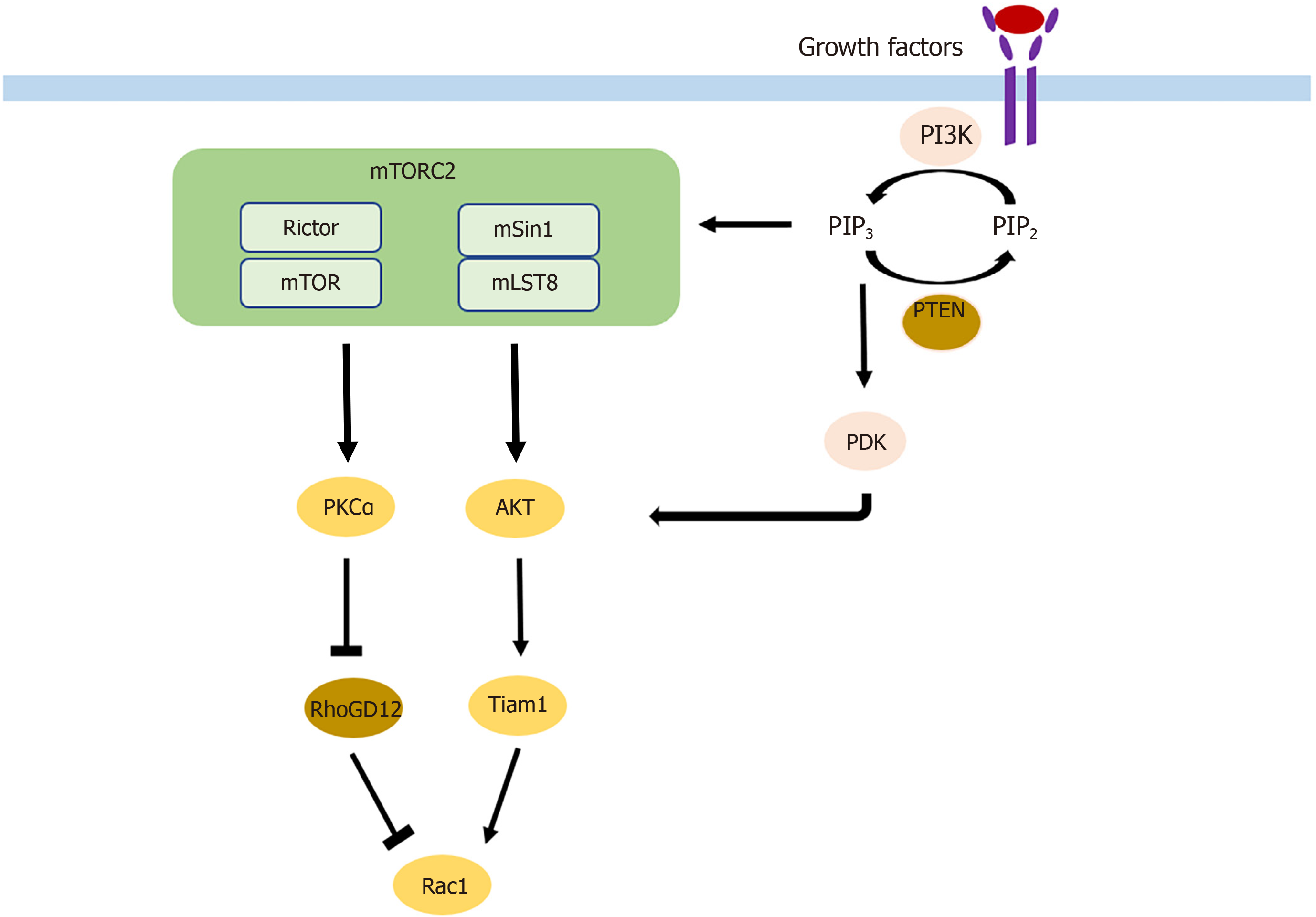
Cell migration and invasion are the two key components of metastasis that are affected by mTORC2 through different pathways. On the one hand, phosphorylation of AKT leads to activation of Rac1 through activation of Tiam1[46]. On the other hand, Rac1 is also upregulated by the suppression of its inhibitor RhoGD12, not only through AKT but also independent of AKT through PKCα activation[46,47] (Figure 2). Rac1 and RhoA are small GTPases known to have crucial roles in actin cytoskeletal rearrangement and cell migration, mainly by stimulating lamellipodia formation[48]. Upon mTORC2 knockdown, expression of Rac1 and RhoA is decreased, leading to a reduction of colorectal metastasis[49]. AKT1 is thus the AKT isoform implicated in metastasis. Silencing only AKT1 and not AKT2 reduces migration and invasion[50]. The gain of invasive behavior is explained by the EMT, and was reversible upon mTORC2 and mTORC1 inhibition, which was followed by an increase in cell-cell contacts and E-cadherin; vimentin, SMA, fibronectin, and MMP9 decreased[49].
Metabolic reprogramming is a hallmark of cancer, and it allows tumor cells to receive and maintain their energy supply for rapid tumor growth[51]. mTORC2 was shown to control c-Myc, a regulator of the Warburg effect, by phosphorylation of class IIa HDAC and acetylation of FoxO in both an AKT dependent and independent manner, thereby increasing glycolysis[52]. Increases of glucose and acetate cause acetylation of Rictor, which in turn maintains mTORC2 signaling[53]. Besides glycolysis, mTORC2 also controls cystine uptake and glutathione metabolism. Phosphorylation of SLC7A11 thereby allows tumor cells to focus mainly on survival rather than proliferation if the extracellular environment changes[54]. In addition to energy supply, metabolic reprogramming can also be involved in drug resistance, as mTORC2 was shown to act as a central link between glucose metabolism and resistance to EGFR tyrosine kinase inhibitors[55].
As mentioned above, metabolic reprogramming is one of the mechanisms of mTORC2-mediated drug resistance in cancer cells. Glucose metabolism has been linked not only to resistance to EGFR tyrosine kinase inhibitors[55] but also has caused Rictor acetylation that can be achieved by either glucose or acetate. Rictor acetylation induces auto-activation of mTORC2 signaling despite the absence of upstream growth factor signaling leading to resistance to EGFR-, PI3K- and AKT-targeted therapies[56]. Furth
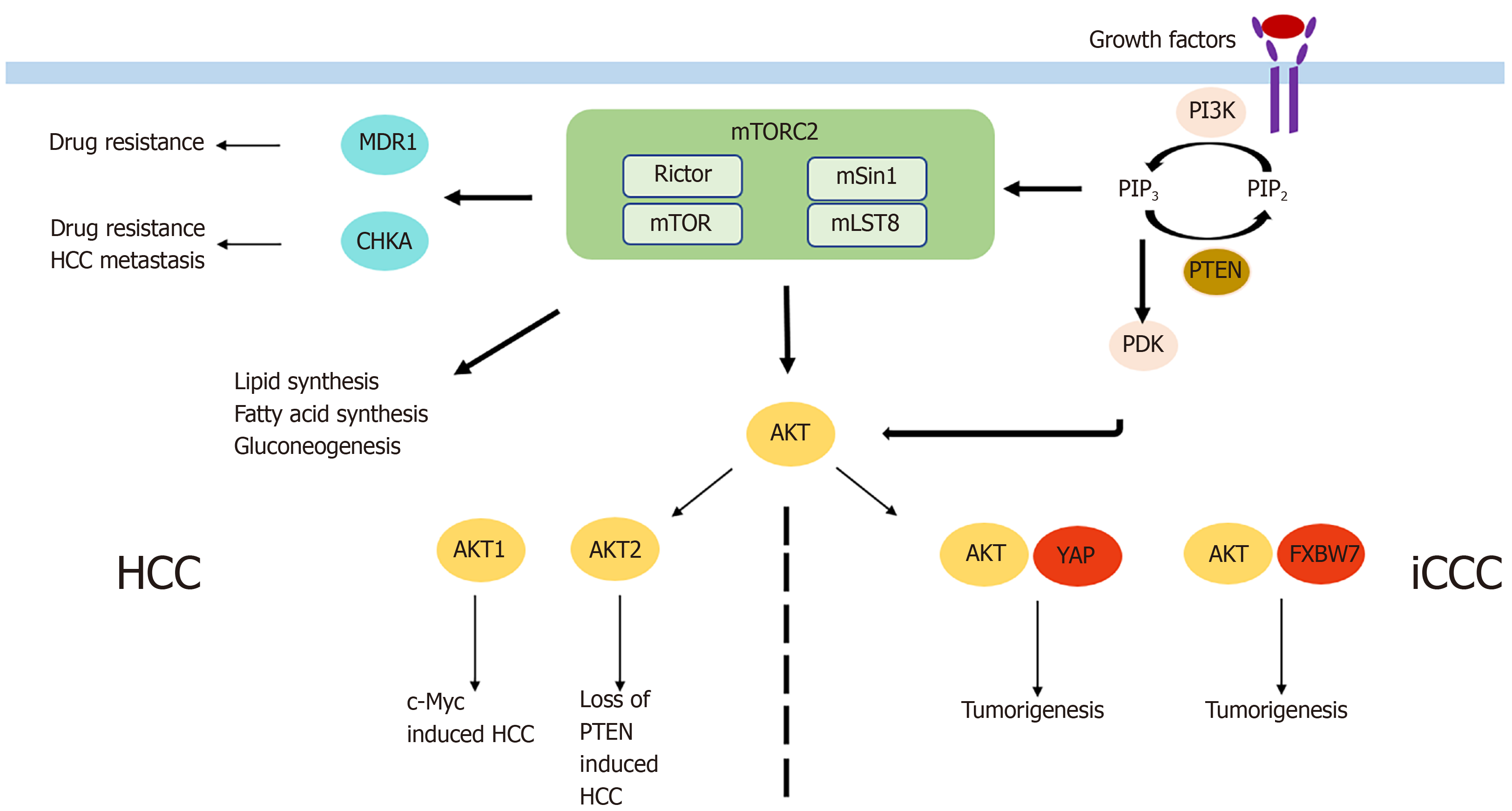
HCC is the most common primary liver cancer and one of the main cancer-related deaths worldwide, with increasing incidence in recent years[58]. Systemic treatment options, with the multikinase inhibitors sorafenib and lenvatinib as the only approved drugs, are very limited in case of unresectability or unavailability to local treatment options. Activation of mTORC2 as determined by immunohistochemistry of phospho-AKT was detectable in 60% of HCCs[59]. Chromosomal gain of Rictor was described in 25% of HCCs, and its high expression was associated with a poor prognosis in HCC patients[60]. Similarly, Kaibori et al[61] found high expression of Rictor mRNA and protein and association with Rictor/Raptor ≥ 0,3 was a prognostic factor indicating poor recurrence-free survival. Rictor knockdown was shown to inhibit HCC cell growth in vitro[62], and AKT overexpression in vivo led to increased HCC devel
While these data show an important role of mTORC2/Rictor in the tumorigenesis and tumor progression of HCC, it is also involved in pre-tumor conditions. For example, Reyes-Gordillo et al[69] showed that the AKT isoforms were activated in an in vivo two-hit model of alcoholic liver disease, leading to an increase of mTORC2 and inflammatory, proliferative, and fibrogenic genes. In line with the results, blocking of AKT1 and AKT2 led to a decrease in progression of liver fibrosis. In addition, mTORC2 was involved in the progression of nonalcoholic fatty liver disease (NAFLD) by dysregulation of white adipose tissue. Thereby, de novo lipogenesis, lipolysis, glycolysis, and increased glucose uptake by GLUT4 are the mechanisms by which mTORC2 regulates adiposity and NAFLD[70]. Besides alcoholic and nonalcoholic liver disease, viral hepatitis is one of the main risk factors for the development of HCC. In that context, increased AKT activity was demonstrated for hepatitis B and C. In hepatitis B, activation of AKT by the hepatitis B virus protein HBx leads to a persistent, noncytopathic virus replication[71]. In hepatitis C, its NS3/4A protease increases AKT activity by enhancing EGF-induced signal transduction[72].
iCCC is a highly aggressive tumor entity with increasing incidence in recent years[73]. As systemic treatment only has partial benefits in advanced stages of iCCC[74], surgical resection remains the only curative option. Only a few studies examining the role of mTORC2 in iCCC exist. mTORC2 was found to be activated in almost 70% of iCCCs as determined by immunohistochemistry of phospho-AKT[75]. When examining the AKT isoforms, protein expression of phospho-AKT1 was shown in 34% of patients with iCCC and was associated with a favorable prognosis[76]. This unexpected result might be dependent on the mechanism of AKT activation triggering different downstream targets or a potential distinct role of AKT1 in iCCC. However, after Rictor knockdown, growth of iCCC cells in vitro was impaired and activated AKT was shown to cooperate with YAP to induce iCCC in mice[75]. Moreover, in liver-specific Rictor knockout mice, cholangiocarcinogenesis induced by AKT/YapS127A was completely abolished, while wild-type mice had a lethal tumor burden at the same time point[75]. Therefore, Zhang et al[77] used the pan-mTOR inhibitor MLN0128 and noticed significantly increased apoptosis but only slight effects on proliferation in iCCC in vitro and in vivo. Significantly enhanced apoptosis and consequently impaired cell proliferation in iCCC was also found after siRNA-mediated Rictor knockdown and simultaneous treatment with sorafenib via increase of FoxO1. Wang et al[78] reported another mechanism of tumorigenesis, which supported the oncogenic potential of mTORC2 signaling in iCCC. Briefly, activated AKT in combination with downregulation of the tumor suppressor FXBW7, increased cholangiocarcinogenesis. Interestingly, silencing cMyc in AKT/Fbxw7ΔF mice completely impaired iCCC growth[78]. Furthermore, the results of a study by Yang et al[79] examining the impact of FXBW7 on EMT and metastasis of iCCC and perihilar CCC (pCCC) is also interesting even though it did not directly connect FXBW7 to mTORC2. In that study, silencing of FXBW7 lead to promotion of EMT, stem-like property, and metastasis of iCCC and pCCC.
While mTORC2 seems to be also involved in the pre-tumoral conditions of HCC including (non) alcoholic liver disease and viral hepatitis, no data exist on the role of mTORC2 chronic cholangitis, primary or secondary biliary cirrhosis as risk factors for the development of iCCC. In summary, mTORC2/Rictor seems to play a role in the development and progression of HCC and iCCC via different mechanisms (Figure 3). However, more research is necessary to determine its exact role and to define potential targets for antineoplastic therapy.
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths[80], with liver metastases being one of the most important predictors of poor long-term outcome. While it was shown that Rictor mRNA and protein are overexpressed in CRC[49] and expression is correlated with tumor progression, Dukes stage, lymph node metastasis, and impaired overall survival[10,81], there was no difference in Rictor expression between primary tumors and metastatic liver lesions[49]. However, Rictor expression in primary tumors with metastatic liver lesions was significantly higher than it was in primary tumors without metastatic disease[49]. Further, not only Rictor but also Raptor seems to be involved in colorectal liver metastases (CRLM), as knockdown of Rictor, as well as knockdown of Raptor, led to decreased migration and invasion of colorectal cancer cells in vitro[49]. In addition, in vivo knockdown of Raptor and Rictor in CRC cell lines impaired the formation of even micrometastases[49]. A study by Gulhati et al[49] did not focus on the development of liver metastases, but they showed that mTORC2 via Rictor regulated actin cytoskeleton reorganization and cell migration through Rac1 and RhoA signaling. However, that is not the only mTORC2-associated mechanism involved in the formation of CRLM. TELO2, known to be essential for mTOR complex integrity, was found to be associated with colorectal tumorigenesis, migration, and invasion, as Rictor knockdown led to reduced TELO2-induced migratory and invasive behavior of colorectal cancer cells[82]. Moreover, colorectal metastasis is not controlled only via Rictor but also by mSin1. Wang et al[83] showed that the tumor suppressor Pdcd4 inhibited Sin1 translation leading to reduced mTORC2 activation and inhibited invasion of CRC cells. While the studies support the important oncogenic and metastatic potential of mTORC2 in CRC, it is also involved in resistance to systemic chemotherapeutic agents[81]. In particular, resistance of CRC cells to irinotecan, one of the three drugs of FOLFIRI, was resolved by treatment with mTORC1/2 inhibitors. Reita et al[84] demonstrated that the combination of irinotecan and a mTORC1/2 blocker reduced migration and invasion in vitro as well as the development of liver metastases in vivo more effectively than irinotecan alone. Consistently, the knockdown of Rictor increased the sensitivity to irinotecan in SMAD4-negative colon cancer cells[85].
Breast cancer accounts for almost one in four cancer cases among women, thereby representing the leading cause of cancer in over 100 countries worldwide[80]. Ma et al[86] recently reviewed the evidence that after the bony skeleton and the lung, breast cancer metastasizes most often to the liver, leading to very limited survival if untreated. Although many systemic therapies exist for metastatic breast cancer, overactive PI3K/AKT/mTOR signaling was shown to be associated with resistance to therapy and with tumor progression[87-89]. The findings revealed 92% Rictor positivity in breast cancer lymph node metastases[90] as well as decreased tumor growth and migration but increased apoptosis upon Rictor knockdown[91]. Functionally, different pathways of mTORC2/Rictor involvement in breast cancer metastasis have been described. mTORC2 activates Rac1 through AKT phosph
Melanoma liver metastasis occurs in up to 20% of patients with cutaneous melanoma and is one of the main prognostic factors of poor survival[96,97]. mTORC2/Rictor is not only involved in PI3K dependent melanoma development[98] and metabolic reprogramming[99] but also in melanoma liver metastases. Rictor mRNA and protein were shown to be overexpressed in invasive melanoma[100] and to be significantly enhanced in metastatic compared with nonmetastatic melanoma[101]. Consistent with those findings, siRNA-mediated Rictor knockdown as well as pharmacological inhibition of mTORC2 not only led to reduced tumor cell motility, migration, and invasion in vitro[100,101] but also reduced melanoma liver metastasis in vivo[101,102]. Rictor depletion was shown to reduce AKT phosphorylation at the Ser473 and Thr308 residues and to inhibit the expression of MMP-2 and MMP-9[100,102]. Moreover, upon Rictor inhibition, interaction with stromal components such as hepatic stellate cells and HGF-induced melanoma cell activation/motility was impaired[101].
Liver metastases occur in about one-fifth of patients with metastatic renal cancer[103], and surgical therapy remains the only strategy to improve survival (see Pinotti et al[104] for review). However, mTORC2 signaling might be a potential therapeutic target, as it is involved in the formation of renal cancer liver metastasis. Sun et al[105] showed that the proinflammatory cytokines TNFα and IL-6 increased upregulation of Rictor through the NF-κB pathway, thereby enhancing chemotaxis, invasion, and migration of renal cancer cells. Upon Rictor knockdown, the formation of renal cancer liver and lung metastases was significantly reduced[105]. Increased migration and invasion were also associated with activation of mTORC2/Akt/GSK3β/β-catenin signaling through TCTP overexpression[106]. Furthermore, pharmacological mTORC2 inhibition led to reduced migration by regulation of HIF2α and increase of cell-cell-junctions via E-cadherin[107].
Gastric cancer is the third leading cause of cancer-related deaths worldwide[80] with the liver being the most common side of gastric cancer metastasis[108]. Similarly, pancreatic cancer is one of the most fatal diseases with a 5-year survival rate of only 7%[109]. Rictor expression in gastric tumor samples was shown to correlate with TNM stage, lymph node metastasis, and poor long-term outcome. Positive staining of Akt at the Ser473 residue was associated with distant metastasis[7,110]. Also, Wang et al[111] reported the role of mTORC2 in gastric cancer metastasis, as DDR2 was found to enhance invasion and EMT through mTORC2 activation and AKT phosphorylation. Upon Rictor knockdown, proliferation, migration, and invasion of gastric cancer cells were significantly reduced while apoptosis was enhanced[110]. Regarding pancreatic cancer, Rictor protein expression was associated with overall survival after surgical resection. Patients with high or medium Rictor expression had significantly shorter survival compared with those with low expression[9]. Upon siRNA-mediated Rictor knockdown, pancreatic tumor cell proliferation and vascularization were significantly impaired and a trend toward fewer liver metastases was observed[9].
Compared with mTORC1, little is known about the role of mTORC2 and its distinct subunit Rictor, in cancer. However, the present review underlines the importance and high relevance of mTORC2 not only in tumorigenesis of primary liver cancer but also in the formation of metastatic liver lesions with different primaries. Thereby, mTORC2/Rictor and AKT, its main downstream effector, are associated with various steps of the metastatic cascade, including EMT, migration and invasion, and angiogenesis, and tumor cell proliferation through different signaling pathways. However, a more refined understanding of the implications of mTORC2 in primary and secondary liver cancer is essential to convert this knowledge into the development of specific mTORC2 targeting therapies.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues PM S-Editor: Wang LL L-Editor: Filipodia P-Editor: Yu HG
| 1. | Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1486] [Article Influence: 185.8] [Reference Citation Analysis (0)] |
| 2. | Lu X, Paliogiannis P, Calvisi DF, and Chen X. Role of the Mammalian Target of Rapamycin Pathway in Liver Cancer: From Molecular Genetics to Targeted Therapies. Hepatology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Kim LC, Cook RS, and Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 324] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 4. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4097] [Article Influence: 585.3] [Reference Citation Analysis (6)] |
| 5. | Valle JW, Kelley RK, Nervi B, Oh DY, and Zhu AX. Biliary tract cancer. Lancet. 2021;397:428-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 619] [Article Influence: 154.8] [Reference Citation Analysis (2)] |
| 6. | Mejia JC, Pasko J. Primary Liver Cancers: Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma. Surg Clin North Am. 2020;100:535-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Bian Y, Wang Z, Xu J, Zhao W, Cao H, and Zhang Z. Elevated Rictor expression is associated with tumor progression and poor prognosis in patients with gastric cancer. Biochem Biophys Res Commun. 2015;464:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Lin XM, Hu L, Gu J, Wang RY, Li L, Tang J, Zhang BH, Yan XZ, Zhu YJ, Hu CL, Zhou WP, Li S, Liu JF, Gonzalez FJ, Wu MC, Wang HY, and Chen L. Choline Kinase α Mediates Interactions Between the Epidermal Growth Factor Receptor and Mechanistic Target of Rapamycin Complex 2 in Hepatocellular Carcinoma Cells to Promote Drug Resistance and Xenograft Tumor Progression. Gastroenterology. 2017;152: 1187-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Schmidt KM, Hellerbrand C, Ruemmele P, Michalski CW, Kong B, Kroemer A, Hackl C, Schlitt HJ, Geissler EK, Lang SA. Inhibition of mTORC2 component RICTOR impairs tumor growth in pancreatic cancer models. Oncotarget. 2017;8:24491-24505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Wang L, Qi J, Yu J, Chen H, Zou Z, Lin X, and Guo L. Overexpression of Rictor protein in colorectal cancer is correlated with tumor progression and prognosis. Oncol Lett. 2017;14:6198-6202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Zhao D, Jiang M, Zhang X, Hou H. The role of RICTOR amplification in targeted therapy and drug resistance. Mol Med. 2020;26:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009;8:3831-3837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Liu P, Gan W, Chin YR, Ogura K, Guo J, Zhang J, Wang B, Blenis J, Cantley LC, Toker A, Su B, and Wei W. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015;5:1194-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 14. | Magaway C, Kim E, Jacinto E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 15. | Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 657] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 16. | McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657-5670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 357] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Boulbes D, Chen CH, Shaikenov T, Agarwal NK, Peterson TR, Addona TA, Keshishian H, Carr SA, Magnuson MA, Sabatini DM, and Sarbassov dos D. Rictor phosphorylation on the Thr-1135 site does not require mammalian target of rapamycin complex 2. Mol Cancer Res. 2010;8:896-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 20. | Gkountakos A, Pilotto S, Mafficini A, Vicentini C, Simbolo M, Milella M, Tortora G, Scarpa A, Bria E, Corbo V. Unmasking the impact of Rictor in cancer: novel insights of mTORC2 complex. Carcinogenesis. 2018;39:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Serrano I, McDonald PC, Lock FE, Dedhar S. Role of the integrin-linked kinase (ILK)/Rictor complex in TGFβ-1-induced epithelial-mesenchymal transition (EMT). Oncogene. 2013;32:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Bera A, Das F, Ghosh-Choudhury N, Kasinath BS, Abboud HE, Choudhury GG. microRNA-21-induced dissociation of PDCD4 from rictor contributes to Akt-IKKβ-mTORC1 axis to regulate renal cancer cell invasion. Exp Cell Res. 2014;328:99-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1581] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 24. | Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 952] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 25. | Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 947] [Cited by in RCA: 1092] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 26. | Ricoult SJ, Yecies JL, Ben-Sahra I, Manning BD. Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene. 2016;35:1250-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 27. | Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, Prescianotto-Baschong C, Sauer U, Jenoe P, Hall MN. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339:1320-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 28. | Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 601] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 29. | Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 778] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 30. | Lang SA, Hackl C, Moser C, Fichtner-Feigl S, Koehl GE, Schlitt HJ, Geissler EK, Stoeltzing O. Implication of RICTOR in the mTOR inhibitor-mediated induction of insulin-like growth factor-I receptor (IGF-IR) and human epidermal growth factor receptor-2 (Her2) expression in gastrointestinal cancer cells. Biochim Biophys Acta. 2010;1803:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Lang SA, Moser C, Fichnter-Feigl S, Schachtschneider P, Hellerbrand C, Schmitz V, Schlitt HJ, Geissler EK, and Stoeltzing O. Targeting heat-shock protein 90 improves efficacy of rapamycin in a model of hepatocellular carcinoma in mice. Hepatology. 2009;49:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, and Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1327] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 33. | Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 861] [Cited by in RCA: 888] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 34. | Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1605] [Cited by in RCA: 1586] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 35. | Palm W, Park Y, Wright K, Pavlova NN, Tuveson DA, Thompson CB. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell. 2015;162:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 36. | Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4880] [Cited by in RCA: 4817] [Article Influence: 267.6] [Reference Citation Analysis (0)] |
| 37. | Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2247] [Cited by in RCA: 2558] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 38. | Xu K, Liu P, and Wei W. mTOR signaling in tumorigenesis. Biochim Biophys Acta. 2014;1846:638-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057-5064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, and Thomas JE. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439-444. [PubMed] [DOI] [Full Text] |
| 41. | Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1532] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 42. | Bruhn MA, Pearson RB, Hannan RD, Sheppard KE. Second AKT: the rise of SGK in cancer signalling. Growth Factors. 2010;28:394-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, Dunn IF, Schinzel AC, Sandy P, Hoersch S, Sheng Q, Gupta PB, Boehm JS, Reiling JH, Silver S, Lu Y, Stemke-Hale K, Dutta B, Joy C, Sahin AA, Gonzalez-Angulo AM, Lluch A, Rameh LE, Jacks T, Root DE, Lander ES, Mills GB, Hahn WC, Sellers WR, Garraway LA. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 44. | Murray NR, Jamieson L, Yu W, Zhang J, Gökmen-Polar Y, Sier D, Anastasiadis P, Gatalica Z, Thompson EA, Fields AP. Protein kinase Ciota is required for Ras transformation and colon carcinogenesis in vivo. J Cell Biol. 2004;164:797-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Liu Y, Su W, Thompson EA, Leitges M, Murray NR, Fields AP. Protein kinase CbetaII regulates its own expression in rat intestinal epithelial cells and the colonic epithelium in vivo. J Biol Chem. 2004;279:45556-45563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Morrison Joly M, Williams MM, Hicks DJ, Jones B, Sanchez V, Young CD, Sarbassov DD, Muller WJ, Brantley-Sieders D, Cook RS. Two distinct mTORC2-dependent pathways converge on Rac1 to drive breast cancer metastasis. Breast Cancer Res. 2017;19:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 47. | Agarwal NK, Chen CH, Cho H, Boulbès DR, Spooner E, Sarbassov DD. Rictor regulates cell migration by suppressing RhoGDI2. Oncogene. 2013;32:2521-2526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4808] [Cited by in RCA: 4803] [Article Influence: 177.9] [Reference Citation Analysis (0)] |
| 49. | Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T, Evers BM. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246-3256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 464] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 50. | Kim EK, Yun SJ, Ha JM, Kim YW, Jin IH, Yun J, Shin HK, Song SH, Kim JH, Lee JS, Kim CD, Bae SS. Selective activation of Akt1 by mammalian target of rapamycin complex 2 regulates cancer cell migration, invasion, and metastasis. Oncogene. 2011;30:2954-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 52. | Masui K, Tanaka K, Akhavan D, Babic I, Gini B, Matsutani T, Iwanami A, Liu F, Villa GR, Gu Y, Campos C, Zhu S, Yang H, Yong WH, Cloughesy TF, Mellinghoff IK, Cavenee WK, Shaw RJ, Mischel PS. mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell Metab. 2013;18:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 53. | Masui K, Cavenee WK, and Mischel PS. mTORC2 and Metabolic Reprogramming in GBM: at the Interface of Genetics and Environment. Brain Pathol. 2015;25:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Gu Y, Albuquerque CP, Braas D, Zhang W, Villa GR, Bi J, Ikegami S, Masui K, Gini B, Yang H, Gahman TC, Shiau AK, Cloughesy TF, Christofk HR, Zhou H, Guan KL, Mischel PS. mTORC2 Regulates Amino Acid Metabolism in Cancer by Phosphorylation of the Cystine-Glutamate Antiporter xCT. Mol Cell. 2017;67:128-138.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 55. | Chiang CT, Demetriou AN, Ung N, Choudhury N, Ghaffarian K, Ruderman DL, Mumenthaler SM. mTORC2 contributes to the metabolic reprogramming in EGFR tyrosine-kinase inhibitor resistant cells in non-small cell lung cancer. Cancer Lett. 2018;434:152-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Masui K, Tanaka K, Ikegami S, Villa GR, Yang H, Yong WH, Cloughesy TF, Yamagata K, Arai N, Cavenee WK, Mischel PS. Glucose-dependent acetylation of Rictor promotes targeted cancer therapy resistance. Proc Natl Acad Sci U S A. 2015;112:9406-9411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 57. | Tanaka K, Babic I, Nathanson D, Akhavan D, Guo D, Gini B, Dang J, Zhu S, Yang H, De Jesus J, Amzajerdi AN, Zhang Y, Dibble CC, Dan H, Rinkenbaugh A, Yong WH, Vinters HV, Gera JF, Cavenee WK, Cloughesy TF, Manning BD, Baldwin AS, Mischel PS. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1:524-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 58. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1871] [Article Influence: 207.9] [Reference Citation Analysis (4)] |
| 59. | Hu J, Che L, Li L, Pilo MG, Cigliano A, Ribback S, Li X, Latte G, Mela M, Evert M, Dombrowski F, Zheng G, Chen X, Calvisi DF. Co-activation of AKT and c-Met triggers rapid hepatocellular carcinoma development via the mTORC1/FASN pathway in mice. Sci Rep. 2016;6:20484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 60. | Xu Z, Xu M, Liu P, Zhang S, Shang R, Qiao Y, Che L, Ribback S, Cigliano A, Evert K, Pascale RM, Dombrowski F, Evert M, Chen X, Calvisi DF, and Chen X. The mTORC2-Akt1 Cascade Is Crucial for c-Myc to Promote Hepatocarcinogenesis in Mice and Humans. Hepatology. 2019;70:1600-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 61. | Kaibori M, Shikata N, Sakaguchi T, Ishizaki M, Matsui K, Iida H, Tanaka Y, Miki H, Nakatake R, Okumura T, Tokuhara K, Inoue K, Wada J, Oda M, Nishizawa M, Kon M. Influence of Rictor and Raptor Expression of mTOR Signaling on Long-Term Outcomes of Patients with Hepatocellular Carcinoma. Dig Dis Sci. 2015;60:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, Battiston C, Van Laarhoven S, Fiel MI, Di Feo A, Hoshida Y, Yea S, Toffanin S, Ramos A, Martignetti JA, Mazzaferro V, Bruix J, Waxman S, Schwartz M, Meyerson M, Friedman SL, Llovet JM. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972-1983, 1983.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 591] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 63. | Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J, Brozzetti S, Staniscia T, Chen X, Dombrowski F, Evert M. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 472] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 64. | Galicia VA, He L, Dang H, Kanel G, Vendryes C, French BA, Zeng N, Bayan JA, Ding W, Wang KS, French S, Birnbaum MJ, Rountree CB, Stiles BL. Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology. 2010;139:2170-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Xu Z, Hu J, Cao H, Pilo MG, Cigliano A, Shao Z, Xu M, Ribback S, Dombrowski F, Calvisi DF, and Chen X. Loss of Pten synergizes with c-Met to promote hepatocellular carcinoma development via mTORC2 pathway. Exp Mol Med. 2018;50:e417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Guri Y, Colombi M, Dazert E, Hindupur SK, Roszik J, Moes S, Jenoe P, Heim MH, Riezman I, Riezman H, Hall MN. mTORC2 Promotes Tumorigenesis via Lipid Synthesis. Cancer Cell. 2017;32:807-823.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 297] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 67. | Khan MW, Biswas D, Ghosh M, Mandloi S, Chakrabarti S, Chakrabarti P. mTORC2 controls cancer cell survival by modulating gluconeogenesis. Cell Death Discov. 2015;1:15016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Chen BW, Chen W, Liang H, Liu H, Liang C, Zhi X, Hu LQ, Yu XZ, Wei T, Ma T, Xue F, Zheng L, Zhao B, Feng XH, Bai XL, Liang TB. Inhibition of mTORC2 Induces Cell-Cycle Arrest and Enhances the Cytotoxicity of Doxorubicin by Suppressing MDR1 Expression in HCC Cells. Mol Cancer Ther. 2015;14:1805-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Reyes-Gordillo K, Shah R, Arellanes-Robledo J, Cheng Y, Ibrahim J, and Tuma PL. Akt1 and Akt2 Isoforms Play Distinct Roles in Regulating the Development of Inflammation and Fibrosis Associated with Alcoholic Liver Disease. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | Festuccia WT. Regulation of Adipocyte and Macrophage Functions by mTORC1 and 2 in Metabolic Diseases. Mol Nutr Food Res. 2021;65:e1900768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Rawat S, Bouchard MJ. The hepatitis B virus (HBV) HBx protein activates AKT to simultaneously regulate HBV replication and hepatocyte survival. J Virol. 2015;89:999-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 72. | Brenndörfer ED, Karthe J, Frelin L, Cebula P, Erhardt A, Schulte am Esch J, Hengel H, Bartenschlager R, Sällberg M, Häussinger D, Bode JG. Nonstructural 3/4A protease of hepatitis C virus activates epithelial growth factor-induced signal transduction by cleavage of the T-cell protein tyrosine phosphatase. Hepatology. 2009;49:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 74. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3165] [Article Influence: 211.0] [Reference Citation Analysis (1)] |
| 75. | Zhang S, Song X, Cao D, Xu Z, Fan B, Che L, Hu J, Chen B, Dong M, Pilo MG, Cigliano A, Evert K, Ribback S, Dombrowski F, Pascale RM, Cossu A, Vidili G, Porcu A, Simile MM, Pes GM, Giannelli G, Gordan J, Wei L, Evert M, Cong W, Calvisi DF, and Chen X. Pan-mTOR inhibitor MLN0128 is effective against intrahepatic cholangiocarcinoma in mice. J Hepatol. 2017;67:1194-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 76. | Lee D, Do IG, Choi K, Sung CO, Jang KT, Choi D, Heo JS, Choi SH, Kim J, Park JY, Cha HJ, Joh JW, Choi KY, and Kim DS. The expression of phospho-AKT1 and phospho-MTOR is associated with a favorable prognosis independent of PTEN expression in intrahepatic cholangiocarcinomas. Mod Pathol. 2012;25:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Yokoi K, Kobayashi A, Motoyama H, Kitazawa M, Shimizu A, Notake T, Yokoyama T, Matsumura T, Takeoka M, Miyagawa SI. Survival pathway of cholangiocarcinoma via AKT/mTOR signaling to escape RAF/MEK/ERK pathway inhibition by sorafenib. Oncol Rep. 2018;39:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Wang J, Wang H, Peters M, Ding N, Ribback S, Utpatel K, Cigliano A, Dombrowski F, Xu M, Chen X, Song X, Che L, Evert M, Cossu A, Gordan J, Zeng Y, Calvisi DF. Loss of Fbxw7 synergizes with activated Akt signaling to promote c-Myc dependent cholangiocarcinogenesis. J Hepatol. 2019;71:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 79. | Yang H, Lu X, Liu Z, Chen L, Xu Y, Wang Y, Wei G, and Chen Y. FBXW7 suppresses epithelial-mesenchymal transition, stemness and metastatic potential of cholangiocarcinoma cells. Oncotarget. 2015;6:6310-6325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 80. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 81. | Shuhua W, Chenbo S, Yangyang L, Xiangqian G, Shuang H, Tangyue L, and Dong T. Autophagy-related genes Raptor, Rictor, and Beclin1 expression and relationship with multidrug resistance in colorectal carcinoma. Hum Pathol. 2015;46:1752-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 82. | Guo Z, Zhang X, Zhu H, Zhong N, Luo X, Zhang Y, Tu F, Zhong J, Wang X, He J, Huang L. TELO2 induced progression of colorectal cancer by binding with RICTOR through mTORC2. Oncol Rep. 2021;45:523-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 83. | Wang Q, Zhu J, Wang YW, Dai Y, Wang YL, Wang C, Liu J, Baker A, Colburn NH, and Yang HS. Tumor suppressor Pdcd4 attenuates Sin1 translation to inhibit invasion in colon carcinoma. Oncogene. 2017;36:6225-6234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 84. | Reita D, Bour C, Benbrika R, Groh A, Pencreach E, Guérin E, Guenot D. Synergistic Anti-Tumor Effect of mTOR Inhibitors with Irinotecan on Colon Cancer Cells. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Wong CK, Lambert AW, Ozturk S, Papageorgis P, Lopez D, Shen N, Sen Z, Abdolmaleky HM, Győrffy B, Feng H, Thiagalingam S. Targeting RICTOR Sensitizes SMAD4-Negative Colon Cancer to Irinotecan. Mol Cancer Res. 2020;18:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Ma R, Feng Y, Lin S, Chen J, Lin H, Liang X, Zheng H, Cai X. Mechanisms involved in breast cancer liver metastasis. J Transl Med. 2015;13:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 87. | Morrison Joly M, Hicks DJ, Jones B, Sanchez V, Estrada MV, Young C, Williams M, Rexer BN, Sarbassov dos D, Muller WJ, Brantley-Sieders D, Cook RS. Rictor/mTORC2 Drives Progression and Therapeutic Resistance of HER2-Amplified Breast Cancers. Cancer Res. 2016;76:4752-4764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 88. | Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452-4461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 89. | Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 349] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 90. | Zhang F, Zhang X, Li M, Chen P, Zhang B, Guo H, Cao W, Wei X, Cao X, Hao X, and Zhang N. mTOR complex component Rictor interacts with PKCzeta and regulates cancer cell metastasis. Cancer Res. 2010;70:9360-9370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 91. | Li H, Lin J, Wang X, Yao G, Wang L, Zheng H, Yang C, Jia C, Liu A, Bai X. Targeting of mTORC2 prevents cell migration and promotes apoptosis in breast cancer. Breast Cancer Res Treat. 2012;134:1057-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Zhang Z, Yang M, Chen R, Su W, Li P, Chen S, Chen Z, Chen A, Li S, and Hu C. IBP regulates epithelial-to-mesenchymal transition and the motility of breast cancer cells via Rac1, RhoA and Cdc42 signaling pathways. Oncogene. 2014;33:3374-3382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 93. | Chen S, Han Q, Wang X, Yang M, Zhang Z, Li P, Chen A, Hu C, and Li S. IBP-mediated suppression of autophagy promotes growth and metastasis of breast cancer cells via activating mTORC2/Akt/FOXO3a signaling pathway. Cell Death Dis. 2013;4:e842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Daulat AM, Bertucci F, Audebert S, Sergé A, Finetti P, Josselin E, Castellano R, Birnbaum D, Angers S, Borg JP. PRICKLE1 Contributes to Cancer Cell Dissemination through Its Interaction with mTORC2. Dev Cell. 2016;37:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Zhang S, Qian G, Zhang QQ, Yao Y, Wang D, Chen ZG, Wang LJ, Chen M, and Sun SY. mTORC2 Suppresses GSK3-Dependent Snail Degradation to Positively Regulate Cancer Cell Invasion and Metastasis. Cancer Res. 2019;79:3725-3736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Aubin JM, Rekman J, Vandenbroucke-Menu F, Lapointe R, Fairfull-Smith RJ, Mimeault R, Balaa FK, Martel G. Systematic review and meta-analysis of liver resection for metastatic melanoma. Br J Surg. 2013;100:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Tas F, Erturk K. Recurrence behavior in early-stage cutaneous melanoma: pattern, timing, survival, and influencing factors. Melanoma Res. 2017;27:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Laugier F, Finet-Benyair A, André J, Rachakonda PS, Kumar R, Bensussan A, Dumaz N. RICTOR involvement in the PI3K/AKT pathway regulation in melanocytes and melanoma. Oncotarget. 2015;6:28120-28131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Chen XY, Li DF, Han JC, Wang B, Dong ZP, Yu LN, Pan ZH, Qu CJ, Chen Y, Sun SG, and Zheng QS. Reprogramming induced by isoliquiritigenin diminishes melanoma cachexia through mTORC2-AKT-GSK3β signaling. Oncotarget. 2017;8:34565-34575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 100. | Liang X, Sun R, Zhao X, Zhang Y, Gu Q, Dong X, Zhang D, Sun J, and Sun B. Rictor regulates the vasculogenic mimicry of melanoma via the AKT-MMP-2/9 pathway. J Cell Mol Med. 2017;21:3579-3591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | Schmidt KM, Dietrich P, Hackl C, Guenzle J, Bronsert P, Wagner C, Fichtner-Feigl S, Schlitt HJ, Geissler EK, Hellerbrand C, Lang SA. Inhibition of mTORC2/RICTOR Impairs Melanoma Hepatic Metastasis. Neoplasia. 2018;20:1198-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Guenzle J, Akasaka H, Joechle K, Reichardt W, Venkatasamy A, Hoeppner J, Hellerbrand C, Fichtner-Feigl S, Lang SA. Pharmacological Inhibition of mTORC2 Reduces Migration and Metastasis in Melanoma. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 103. | Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P, Menon M, Montorsi F, Karakiewicz PI. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 480] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 104. | Pinotti E, Montuori M, Giani A, Uggeri F, Garancini M, Gianotti L, Romano F. Surgical treatment of liver metastases from kidney cancer: a systematic review. ANZ J Surg. 2019;89:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Sun B, Chen L, Fu H, Guo L, Guo H, and Zhang N. Upregulation of RICTOR gene transcription by the proinflammatory cytokines through NF-κB pathway contributes to the metastasis of renal cell carcinoma. Tumour Biol. 2016;37:4457-4466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Bae SY, Kim HJ, Lee KJ, and Lee K. Translationally controlled tumor protein induces epithelial to mesenchymal transition and promotes cell migration, invasion and metastasis. Sci Rep. 2015;5:8061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 107. | Chen L, Wang KJ, Li H. Re: Inhibition of mTORC2 but not mTORC1 up-regulates E-cadherin expression and inhibits cell motility by blocking HIF-2α expression in human renal cell carcinoma: S. Maru, Y. Ishigaki, N. Shinohara, T. Takata, N. Tomosugi and K. Nonomura J Urol 2013; 189: 1921-1929. J Urol. 2013;190:1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 108. | Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307-52316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 109. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12989] [Article Influence: 1443.2] [Reference Citation Analysis (2)] |
| 110. | Bian YH, Xu J, Zhao WY, Zhang ZZ, Tu L, Cao H, Zhang ZG. Targeting mTORC2 component rictor inhibits cell proliferation and promotes apoptosis in gastric cancer. Am J Transl Res. 2017;9:4317-4330. [PubMed] [DOI] [Full Text] |
| 111. | Wang YG, Xu L, Jia RR, Wu Q, Wang T, Wei J, Ma JL, Shi M, and Li ZS. DDR2 Induces Gastric Cancer Cell Activities via Activating mTORC2 Signaling and Is Associated with Clinicopathological Characteristics of Gastric Cancer. Dig Dis Sci. 2016;61:2272-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |