Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1532
Peer-review started: April 15, 2021
First decision: June 4, 2021
Revised: June 11, 2021
Accepted: August 16, 2021
Article in press: August 16, 2021
Published online: October 15, 2021
Processing time: 181 Days and 11.6 Hours
Adjuvant chemoradiotherapy (ACRT) with oral capecitabine and intensity-modulated radiotherapy (IMRT) were well tolerated in a phase I study in patients who had undergone partial or total gastrectomy for locally advanced gastric cancer (GC). This phase II study aimed to further determine the efficacy and toxicity of this combination after radical resection and D1/D2 lymph node dissection (LND) for patients with locally advanced GC.
To further determine the efficacy and toxicity of this combination after radical resection and D1/D2 LND for patients with locally advanced GC.
Forty patients (median age, 53 years; range, 24-71 years) with pathologically confirmed adenocarcinoma who underwent D1/D2 LND were included in this study. The patients received ACRT comprising IMRT (total irradiation dose: 45 Gy delivered in daily 1.8-Gy fractions on 5 d a week over 5 wk) and capecitabine chemotherapy (dose: 800 mg/m² twice daily throughout the duration of ra
The 3-year DFS and OS were 66.2% and 75%, respectively. The median time to recurrence was 19.5 mo (range, 6.1-68 mo). Peritoneal implantation (n = 10) was the most common recurrence pattern, and the lung was the most common site of extra-abdominal metastases (n = 5). Nine patients developed grade 3 or 4 toxicities during ACRT. Two patients discontinued ACRT, while eleven underwent ACRT without receiving the entire course of capecitabine. There were no treatment-related deaths.
The ACRT protocol described herein showed acceptable safety and efficacy for patients with locally advanced GC who received radical gastrectomy and D1/2 LND.
Core Tip: In our previous phase I study, we found that an adjuvant chemoradiotherapy (ACRT) regimen of 45 Gy radiotherapy concurrent with oral capecitabine was well tolerated in patients with locally advanced gastric cancer who had received partial or total gastrectomy. The maximum tolerated and recommended dose of capecitabine was 800 mg/m2 twice daily with oral administration. We performed this phase II study to further assess the efficacy and toxicity of this ACRT regimen as an adjuvant therapy after radical resection and D1/D2 lymph node dissection for patients with locally advanced gastric cancer.
- Citation: Wang X, Wang WH, Wang SL, Song YW, Liu YP, Tang Y, Li N, Liu WY, Fang H, Li YX, Zhao DB, Chi Y, Yang L, Jin J. Efficacy and toxicity of capecitabine combined with intensity-modulated radiotherapy after D1/D2 lymph node dissection in patients with gastric cancer. World J Gastrointest Oncol 2021; 13(10): 1532-1543
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1532.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1532
The Intergroup trial (INT0116) demonstrated a major survival benefit of using a combination of conventional radiotherapy (RT) and fluorouracil (5-FU) chemotherapy on the 3-year disease-free survival (DFS) in patients with locally advanced gastric cancer (GC) after radical surgery (R0) and D0/D1 lymph node dissection (LND)[1]. However, more than half of the patients developed grade 3/4 hematologic toxicity and one-third developed gastrointestinal (GI) toxicity, which may affect the prognosis. Thus, it is important to combine advanced radiation techniques with a low-toxicity chemotherapy regimen to improve compliance to adjuvant chemoradiotherapy (ACRT) among postoperative GC patients who show poor tolerability for adjuvant treatment because of partial or total loss of the stomach.
Capecitabine, which belongs to fluoropyrimidines, has been widely used for chemotherapy and concurrent with radiotherapy in GC[2,3]. It is comparable to 5-FU and has a safer side effect profile and convenient oral administration[3,4]. High tumor response rates (26%-34%) have been reported with capecitabine monotherapy in phase II studies[5-7], and the drug has been found to be more efficacious when used in combination with platinum-based drugs in some phase III trials in patients with advanced GC[4-8].
Modern intensity-modulated radiotherapy (IMRT) planning systems have made it possible to deliver radiation doses more accurately to the planning target volume (PTV) and spare critical normal tissues to a substantial degree. IMRT has also been confirmed to be superior to two- or three-dimensional RT.
In our previous phase I study, we found that the ACRT regimen of 45 Gy ra
Participants were recruited if they met the following inclusion criteria: received partial (proximal or distal subtotal gastrectomy) or total gastrectomy with D1/D2 LND; had not received neoadjuvant anti-cancer treatment; postoperative pathologic diagnosis of adenocarcinoma; pathologic classification of T3-4N0 or any TN+M0 according to the 7th edition of the American Joint Committee on Cancer TNM classification; age ≤ 75 years and good performance (Eastern Cooperative Oncology Group performance status ≤ 1); no prior or concurrent history of malignant disease (except non-melanoma skin cancers or in situ carcinoma of the cervix); no prior abdominal radiation; and leukocyte count ≥ 3.5 × 109/L, neutrophil count ≥ 1.5 × 109/L, platelet count ≥ 100 × 109/L, hemoglobin level ≥ 10.0 g/L, and normal alanine aminotransferase/aspartate aminotransferase and creatinine level.
All patients entering the trial received physical examinations, computed tomo
All patients were recommended D2 LND, which requires resection of all perigastric LNs, left gastric artery, common hepatic artery, celiac artery, proximal splenic artery, and proper hepatic artery, depending on the primary tumor location.
The prescription dose and fraction were 45 Gy in daily 1.8 Gy (5 d a week over 5 wk) by IMRT techniques. To enable visualization of the small intestine, patients needed to fast for 4 h before CT simulation, and take an oral positive contrast (300 mL) 30 min before the simulation. A normalized meal (300 mL of ready-to-eat canned porridge) was given to the patients 15 min before CT simulation and each treatment daily to decrease heterogeneity in gastric filling. The patients were placed in the supine position with thermoplastic immobilization masks; intravenous contrast was re
Clinical target volume (CTV) for each patient was contoured in accordance with recommendations from the Japanese Gastric Cancer Association depending on the extension and location of the primary tumor and the LN region involved status[10]. The CTV generally covered anastomoses, duodenal stump, tumor bed (only for stage T4b, if present), and regional LNs (Table 1). The remnant stomach was not routinely included in the target volume. The PTV typically includes the CTV plus a 5-7 mm margin in the radial direction and a 10 mm margin in the superior-inferior direction. Dose limitations for an organ at risk (OAR) were as follows: volume percentage receiving over 30 Gy (V30) < 40% for the liver, V20 < 30% or a mean dose of < 20 Gy for both kidneys, and V30 < 30% for the heart; the maximal dose for the spinal cord planning OAR volume was < 40 Gy. The maximal dose was less than the prescribed dose for the small intestine and colon. An experienced physicist did the IMRT plans design using a five-to-seven–field, coplanar, sliding window technique using the Pinnacle system, version 8.0.
Oral capecitabine was delivered twice daily (after breakfast and after dinner) at a dose of 800 mg/m² from the beginning to end of the radiation period based on the results of a previous phase I study[9]. Adjuvant chemotherapy (ACT) was required for a maximum of 6 mo and was conducted before or after ACRT depending on the performance status, clinical comorbidities, and toxicity profile of the patient; however, the regimens were open.
The primary endpoint of our phase II study was DFS, which was defined as the length of time after surgery ends that the patient’s disease progresses or dies from any cause. The secondary endpoints were overall survival (OS), toxic effects, and treatment compliance. We hypothesized that the 3-year DFS rate would improve from 50% to 70% based on the results of INT0116. The use of Fleming’s design (P1 = 0.50 and P2 = 0.70, setting α = 0.05 [two-sided], 80% power) revealed that 37 study participants were needed. At least 40 patients were required for this study with assumption of a 10% dropout rate.
The first site of recurrence was recorded to analyze treatment failure patterns. Locoregional recurrence was defined as reappearance of cancer at the anastomosis site, remnant stomach, duodenal stump, tumor bed, or regional LNs within the radiation field. Outside radiation field LNs region relapse, peritoneal implantation, liver metastasis or any other extra-abdominal site metastasis were regarded as distant metastases. Survival analysis were assessed with Kaplan–Meier curves using SPSS for Windows, version 20.0 (IBM SPSS Inc., Armonk, NY, United States).
From October 2011 to June 2013, 40 patients were recruited for the study. The patients’ general characteristics are shown in Table 2. The median age was 53 years (range, 24-71 years). Thirty-seven (92.5%) patients had positive LNs. The median number of positive LNs was 7 (range, 1-26 nodes), and the median number of LNs resected was 24 (range, 15-56 nodes). D2 LND was performed in 22 (55%) patients. The median interval between surgery and ACRT was 5.5 mo (1.4-8 mo).
| Characteristic | n | % |
| Age in yr, median (range) | 53 (24-71) | |
| Men | 28 | 70.0 |
| Tumor size in cm, median (range) | 5.0 (2-20) | |
| Location of primary tumor | ||
| Upper 1/3rd of stomach | 8 | 20 |
| Middle 1/3rd of stomach | 8 | 20 |
| Lower 1/3rd of stomach | 14 | 35 |
| ≥ 2 sites involved | 10 | 25 |
| Surgery type | ||
| Partial gastrectomy | 36 | 90 |
| Total gastrectomy | 4 | 10 |
| Positive LNs, median (range) | 7 (1-26) | |
| LNs resected, median (range) | 24 (15-56) | |
| LN ratio, median (range) | 0.27 (0-0.86) | |
| Extent of dissection | ||
| D1 | 18 | 45 |
| D2 | 22 | 55 |
| Lauren type | ||
| Intestinal type | 12 | 30 |
| Diffuse type | 16 | 40 |
| Mixed type | 12 | 30 |
| Tumor differentiation | ||
| Good | 1 | 2.5 |
| Moderate | 8 | 20 |
| Poor | 31 | 77.5 |
| Lymphatic/vascular invasion | ||
| Absent | 16 | 40 |
| Present | 24 | 60 |
| Perineural invasion | ||
| Absent | 30 | 75 |
| Present | 10 | 25 |
| Signet ring cells | ||
| Absent | 29 | 72.5 |
| Present | 11 | 27.5 |
| Tumor deposit | ||
| Absent | 34 | 85 |
| Present | 6 | 15 |
| Stage (AJCC 7th) | ||
| IIa | 2 | 5 |
| IIb | 6 | 15 |
| IIIa | 11 | 27.5 |
| IIIb | 11 | 27.5 |
| IIIc | 10 | 25 |
| Stage (AJCC 6th) | ||
| Ib | 2 | 5 |
| II | 14 | 35 |
| IIIa | 10 | 25 |
| IIIb | 2 | 5 |
| IV | 12 | 30 |
The patients received the following ACT regimens based on docetaxel and/or oxaliplatin and 5-FU analogues, with a median of six cycles (range, 3–10 cycles) before or after ACRT: oxaliplatin/cisplatin and S-1 (n = 18, 45%); docetaxel, oxaliplatin, and capecitabine/S-1/5-FU (n = 13, 32.5%); oxaliplatin and capecitabine (n = 6, 15%); and oxaliplatin, 5-FU, and leucovorin (n = 3, 7.5%).
During ACRT, nine patients (22.5%) developed grade 3-4 toxicities and there were no treatment-related deaths. The most common grade 3-4 toxicities were leukopenia (5 patients, 12.5%), vomiting (4 patients, 10%), nausea (3, 7.5%), esophagitis (3, 7.5%), and thrombocytopenia (3, 7.5%).
Two patients discontinued ACRT due to disease progression (total dose, 25.2 Gy) and serious fatigue (total dose, 5.4 Gy). The remaining 38 patients (95%) received 45 Gy as planned, including 3 patients who developed grade 3 thrombocytopenia (2 cases) and grade 3 vomiting (1 case) but finally completed RT (not with capecitabine) after a break. Besides the treatment discontinuation mentioned above, an additional 8 patients did not finish the whole course of capecitabine because of the reasons below: leukopenia (maximum grade, 3), 2 patients; thrombocytopenia (maximum grade, 3), 2 patients; anemia (maximum grade, 2), 1 patient; gastritis (maximum grade, 3), 1 patient; vomiting (maximum grade, 3), 1 patient; and anorexia (maximum grade, 3), 1 patient. The overall toxicities are showed in Table 3.
| Toxicity | Grade 1-2, n (%) | Grade 3-4, n (%) |
| Nausea | 22 (45) | 3 (7.5) |
| Vomiting | 15 (37.5) | 4 (10) |
| Anorexia | 27 (67.5) | 2 (5) |
| Esophagitis | 6 (15) | 3 (7.5) |
| Diarrhea | 5 (12.5) | 0 |
| Abdominal pain | 1 (2.5) | 1 (2.5) |
| Gastritis | 9 (22.5) | 2 (5) |
| Fatigue | 21 (52.5) | 1 (2.5) |
| Weight loss | 8 (20) | 0 |
| HFS | 14 (35) | 0 |
| Leukopenia | 27 (67.5) | 5 (12.5) |
| Neutropenia | 7 (17.5) | 1 (2.5) |
| Anemia | 3 (7.5) | 0 |
| Thrombocytopenia | 17 (42.5) | 3 (7.5) |
| ALT/AST | 2 (5) | 0 |
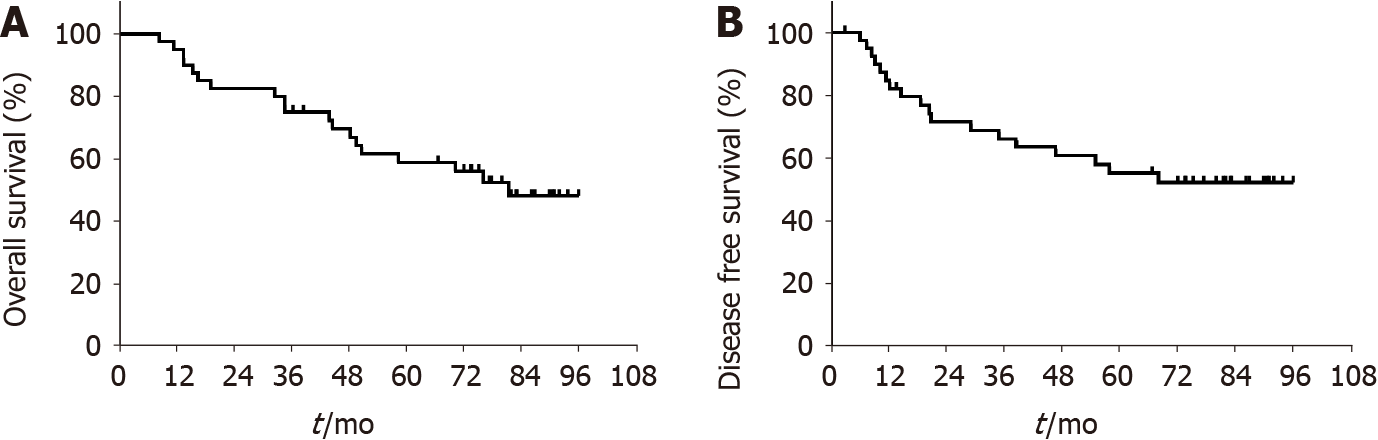
In total, 19 patients died during the follow-up period (median 80 mo; range, 8.4-96 mo): 18 died of disease and 1 of gastrorrhagia. The 3-year DFS, the primary endpoint of this study, was 66.2 (95% confidence interval [CI]: 58.6-73.8). The survival outcomes of OS, locoregional recurrence-free survival (RFS), and distant metastasis-free survival are listed in Figure 1 and Table 4. During the follow-up period, the following recurrence patterns were observed in the 18 patients (45%, 18/40): peritoneal im
Our results suggest that ACRT with 45 Gy IMRT and concurrent oral capecitabine at a dose of 800 mg/m2 twice daily had an acceptable efficacy and toxicity profile in patients with locally advanced GC after radical gastrectomy and D1/2 LND. The 3-year DFS was 66.2%, which did not reach the primary hypothesis endpoint of our phase II study.
The role of ACRT in patients with locally advanced GC remains debatable. The benefits or drawbacks of this scenario mainly depend on whether a D1 or D2 lymphadenectomy has been performed. The INT0116 study was the first trial to prove the benefit of ACRT in patients after radical gastrectomy and D0/1 LND; it showed that the 3-year OS and DFS increased from 41% to 50% and 31% to 48%, respectively[1]. Even after 10 years of follow-up, ACRT was associated with superior DFS and OS[11]. Dikken et al[12] suggested that the addition of ACRT after D1 LND has a major impact on local recurrence in GC. Zhang et al[13] suggested that patients with D1 or D1 plus LND benefit from adjuvant RT, and adjuvant RT may be beneficial for some patients with D2 LND. Yu et al[14] re-analyzed the ARTIST study and concluded that adjuvant RT after D2 resection in GC reduces locoregional recurrence risk, especially in group 3 LNs, and improves locoregional RFS Patients with positive LN benefited more from the adjuvant RT than the other subgroup[14]. The National Comprehensive Cancer Network (NCCN) guideline recommends ACRT as an adjuvant treatment in patients with less than D2 LND.
In China, D2 LND is considered a routine surgical procedure for locally advanced GC, because it is the most widely accepted surgical procedure in Asian and European countries[15]. However, given the many differences between centers or institutions in terms of hospital volume, patient populations, surgical practices and training, pos
In the past decade, capecitabine has been widely used in GI cancer, as it has a much safer side effect profile and does not require invasive delivery[21,22]. Oral capecitabine was not inferior to infusional 5-FU in randomized control trials for patients with advanced GC[4]. Therefore, capecitabine has been considered as a standard che
The investigation of issues related to the sequence of ACT or ACRT is important since poor compliance to adjuvant treatment after gastrectomy is the main problem that may affect patient prognosis. Theoretically, for patients with high-risk patho
The most commonly observed grade 3/4 hematologic and GI toxicities in this study were leukopenia (12.5%) and vomiting (10%), which were much less frequent than those in INT 0116 (54% and 32% of the patients developed grade 3/4 hematologic and GI toxicity) and CALGB 80801 study (about 50% and 16% of the patients developed grade 3/4 hematologic and GI toxicity)[1,30]. The exclusion of the remnant stomach from the target volume and the use of IMRT technology and capecitabine mo
The 3-year DFS of the ACRT arm in INT 0116 was used in the power calculation for the present phase II study, as this is the only randomized trial evaluating the effect of ACRT in GC patients with an LND level less than D2. However, the final 3-year DFS in our study was 66.2%, which did not meet the primary endpoint (3-year DFS = 70%). This could be attributable to the maximum number of positive LNs found (as high as 7) and the fact that only 55% of our patients had D2 LND. Despite previous findings, our results are still better than those obtained with ACRT treatment by Janson et al[27,28]. The 2-year OS of their phase II trials evaluating capecitabine/cisplatin che
This study had several limitations that warrant emphasis. Due to the poor patient recruitment for this study, we did not limit the regimens or cycles of adjuvant chemotherapy administered before or after ACRT. Accordingly, this may have in
In conclusion, we considered that ACRT with 800 mg/m2/d oral capecitabine twice daily combined with 45 Gy IMRT was safe and efficacious. The use of advanced techniques such as IMRT or tomotherapy, an appropriate irradiation field, and low-toxicity single-agent chemotherapy regimens such as capecitabine chemotherapy is highly recommended. A randomized phase III study in our hospital comparing ACT with ACRT for node-positive locally advanced GC after D2 LND is ongoing (NCT 02648841), and its results are highly awaited.
Capecitabine has been widely used for chemotherapy and concurrent with ra
We performed this phase II study to further assess the efficacy and toxicity of this ACRT regimen as an adjuvant therapy after radical resection and D1/D2 lymph node dissection (LND) for locally advanced GC patients.
The aim of this study was to evaluate the efficacy and toxicity of IMRT combined with capecitabine after radical resection and D1/D2 LND for patients with locally advanced GC.
Forty patients with locally advanced GC, who underwent radical resection and D1/D2 LNDwere included in this study. The patients received ACRT comprising IMRT (total irradiation dose: 45 Gy delivered in daily 1.8-Gy fractions on 5 d a week over 5 wk) and capecitabine chemotherapy (dose: 800 mg/m² twice daily throughout the duration of RT). The primary study endpoint was disease-free survival (DFS) and the secondary endpoints were overall survival (OS), toxic effects, and treatment compliance.
The 3-year DFS and OS were 66.2% and 75%, respectively. Nine patients developed grade 3 or 4 toxicities during ACRT. Two patients discontinued ACRT, while 11 underwent ACRT without receiving the entire course of capecitabine.
ACRT with oral capecitabine and IMRTwas safe and efficacious.
The use of IMRT and low-toxicity single-agent chemotherapy regimens such as capecitabine is highly recommended in patients who had undergone partial or total gastrectomy for locally advanced GC. Moreover, to further determine the efficacy of this combination therapy, a randomized phase III study in our hospital is ongoing.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dimofte GM, Kim HS, Lee KG, Mohamed SY S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2436] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 2. | Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin vs capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 Lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 3. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Ji J, Yeh TS, Button P, Sirzén F, Noh SH; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1290] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 4. | Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud PI. Capecitabine/cisplatin vs 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 5. | Hong YS, Song SY, Lee SI, et al. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15:1344-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Sakamoto J, Chin K, Kondo K, et al. Phase II study of a 4-week capecitabine regimen in advanced or recurrent gastric cancer. Anticancer Drugs. 2006;17:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Lee JL, Kang YK, Kang HJ, Lee KH, Zang DY, Ryoo BY, Kim JG, Park SR, Kang WK, Shin DB, Ryu MH, Chang HM, Kim TW, Baek JH, Min YJ. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;99:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1691] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 9. | Wang X, Jin J, Li YX, Ren H, Fang H, Wang SL, Liu YP, Wang WH, Yu ZH, Song YW, Liu XF. Phase I study of postoperative radiotherapy combined with capecitabine for gastric cancer. World J Gastroenterol. 2014;20:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 960] [Reference Citation Analysis (0)] |
| 11. | Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH, Bae JM, Kim S, Kim ST, Park JO, Park YS, Lim HY, Kang WK. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol. 2015;33:3130-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 12. | Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, Boot H, Putter H, Peeters KC, van de Velde CJ, Verheij M. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Zhang N, Fei Q, Gu J, Yin L, He X. Progress of preoperative and postoperative radiotherapy in gastric cancer. World J Surg Oncol. 2018;16:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Yu JI, Lim DH, Ahn YC, Lee J, Kang WK, Park SH, Park JO, Park YS, Lim HY, Kim ST, Kim S, Sohn TS, Choi MG, Bae JM, Nam H. Effects of adjuvant radiotherapy on completely resected gastric cancer: A radiation oncologist's view of the ARTIST randomized phase III trial. Radiother Oncol. 2015;117:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1069] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 16. | Wang X, Jin J, Li Y, et al. Analysis of recurrence for locally advanced gastric or gastroesophageal cancer patients after receiving curative gastrectomy (>D1) and its indication for adjuvant chemoradiotherapy. Chin J Radiat Oncol. 2011;20:133-137. |
| 17. | Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114-7124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 475] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Huang ZN, Chen QY, Zheng CH, et al. Are the indications for postoperative radiotherapy in the NCCN guidelines for patients with gastric adenocarcinoma too broad? A study based on the SEER database. BMC Cancer. 2018;18:1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Datta J, McMillan MT, Ecker BL, Karakousis GC, Mamtani R, Plastaras JP, Giantonio BJ, Drebin JA, Dempsey DT, Fraker DL, Roses RE. Implications of Lymph Node Staging on Selection of Adjuvant Therapy for Gastric Cancer in the United States: A Propensity Score-matched Analysis. Ann Surg. 2016;263:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 20. | Zhu WG, Xua DF, Pu J, Zong CD, Li T, Tao GZ, Ji FZ, Zhou XL, Han JH, Wang CS, Yu CH, Yi JG, Su XL, Ding JX. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol. 2012;104:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, Husseini F, Jodrell D, Koralewski P, Kröning H, Maroun J, Marschner N, McKendrick J, Pawlicki M, Rosso R, Schüller J, Seitz JF, Stabuc B, Tujakowski J, Van Hazel G, Zaluski J, Scheithauer W. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 860] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 22. | Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E, Fritz E, Hieber U, Lindemann HW, Grunewald M, Kremers S, Constantin C, Hipp M, Hartung G, Gencer D, Kienle P, Burkholder I, Hochhaus A. Chemoradiotherapy with capecitabine vs fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 23. | Lee HS, Choi Y, Hur WJ, Kim HJ, Kwon HC, Kim SH, Kim JS, Lee JH, Jung GJ, Kim MC. Pilot study of postoperative adjuvant chemoradiation for advanced gastric cancer: adjuvant 5-FU/cisplatin and chemoradiation with capecitabine. World J Gastroenterol. 2006;12:603-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Jansen EP, Boot H, Saunders MP, Crosby TD, Dubbelman R, Bartelink H, Verheij M, Cats A. A phase I-II study of postoperative capecitabine-based chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;69:1424-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Soyfer V, Geva R, Michelson M, Inbar M, Shacham-Shmueli E, Corn BW. The impact of overall radiotherapy treatment time and delay in initiation of radiotherapy on local control and distant metastases in gastric cancer. Radiat Oncol. 2014;9:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | McMillan MT, Ojerholm E, Roses RE, Plastaras JP, Metz JM, Mamtani R, Karakousis GC, Fraker DL, Drebin JA, Stripp D, Ben-Josef E, Datta J. Adjuvant Radiation Therapy Treatment Time Impacts Overall Survival in Gastric Cancer. Int J Radiat Oncol Biol Phys. 2015;93:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Jansen EP, Boot H, Dubbelman R, Bartelink H, Cats A, Verheij M. Postoperative chemoradiotherapy in gastric cancer -- a Phase I/II dose-finding study of radiotherapy with dose escalation of cisplatin and capecitabine chemotherapy. Br J Cancer. 2007;97:712-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Jansen EPM, Boot H, Dubbelman R, Verheij M, Cats A. Postoperative chemoradiotherapy in gastric cancer--a phase I-II study of radiotherapy with dose escalation of weekly cisplatin and daily capecitabine chemotherapy. Ann Oncol. 2010;21:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Kim TH, Park SR, Ryu KW, Kim YW, Bae JM, Lee JH, Choi IJ, Kim YJ, Kim DY. Phase 3 trial of postoperative chemotherapy alone vs chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys. 2012;84:e585-e592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Fuchs CS, Niedzwiecki D, Mamon HJ, Tepper JE, Ye X, Swanson RS, Enzinger PC, Haller DG, Dragovich T, Alberts SR, Bjarnason GA, Willett CG, Gunderson LL, Goldberg RM, Venook AP, Ilson D, O'Reilly E, Ciombor K, Berg DJ, Meyerhardt J, Mayer RJ. Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy With Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results From CALGB 80101 (Alliance). J Clin Oncol. 2017;35:3671-3677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 31. | Nam H, Lim DH, Kim S, Kang WK, Sohn TS, Noh JH, Kim YI, Park CH, Park CK, Ahn YC, Huh SJ. A new suggestion for the radiation target volume after a subtotal gastrectomy in patients with stomach cancer. Int J Radiat Oncol Biol Phys. 2008;71:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Trip AK, Nijkamp J, van Tinteren H, Cats A, Boot H, Jansen EP, Verheij M. IMRT limits nephrotoxicity after chemoradiotherapy for gastric cancer. Radiother Oncol. 2014;112:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Minn AY, Hsu A, La T, Kunz P, Fisher GA, Ford JM, Norton JA, Visser B, Goodman KA, Koong AC, Chang DT. Comparison of intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy as adjuvant therapy for gastric cancer. Cancer. 2010;116:3943-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Sharfo AWM, Stieler F, Kupfer O, Heijmen BJM, Dirkx MLP, Breedveld S, Wenz F, Lohr F, Boda-Heggemann J, Buergy D. Automated VMAT planning for postoperative adjuvant treatment of advanced gastric cancer. Radiat Oncol. 2018;13:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Wang X, Tian Y, Tang Y, Hu ZH, Zhang JJ, Fu GS, Ma P, Ren H, Zhang T, Li N, Liu WY, Fang H, Li YX, Jin J. Tomotherapy as an adjuvant treatment for gastroesophageal junction and stomach cancer may reduce bowel and bone marrow toxicity compared to intensity-modulated radiotherapy and volumetric-modulated arc therapy. Oncotarget. 2017;8:39727-39735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Onal C, Dölek Y, AkkuşYıldırım B. Dosimetric comparison of 3-dimensional conformal radiotherapy, volumetric modulated arc therapy, and helical tomotherapy for postoperative gastric cancer patients. Jpn J Radiol. 2018;36:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Stumpf PK, Amini A, Jones BL, Koshy M, Sher DJ, Lieu CH, Schefter TE, Goodman KA, Rusthoven CG. Adjuvant radiotherapy improves overall survival in patients with resected gastric adenocarcinoma: A National Cancer Data Base analysis. Cancer. 2017;123:3402-3409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |