Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1518
Peer-review started: April 27, 2021
First decision: June 13, 2021
Revised: June 26, 2021
Accepted: August 27, 2021
Article in press: August 27, 2021
Published online: October 15, 2021
Processing time: 168 Days and 23.8 Hours
Probiotics are used to manage a number of gastrointestinal disorders due to their beneficial properties. Clinical reports showed that probiotics also improve the life quality of patients with colorectal cancer (CRC) subjected to oncologic treatment. In a CRC animal model, probiotics supplementation has the potential to decrease the formation of aberrant crypts and ameliorate tumor malignancy, enhancing the antitumor effect of 5-fluorouracil (5-FU) chemotherapy. Based on these data, we hypothesize that the administration of probiotics impact positively in the overall survival and life quality of rats with CRC under the treatment of capecitabine, which is the pro drug of 5-FU.
To evaluate the probiotics effects in a rat CRC model treated with capecitabine and followed until the end of life.
1,2-Dimethylhidrazine dihydrochloride (1,2-DMH) was employed as carcinogen inductor of CRC. Fifty male Wistar-Lewis rats were randomly assigned to one of five following groups: Control (n = 5), Control + probiotics (Control-P group, n = 5), 1,2-DMH alone (DMH group, n = 10), 1,2-DMH + capecitabine (DMH-C group, n = 10), 1,2-DMH + probiotics (DMH-P group, n = 10) and 1,2-DMH + capeci
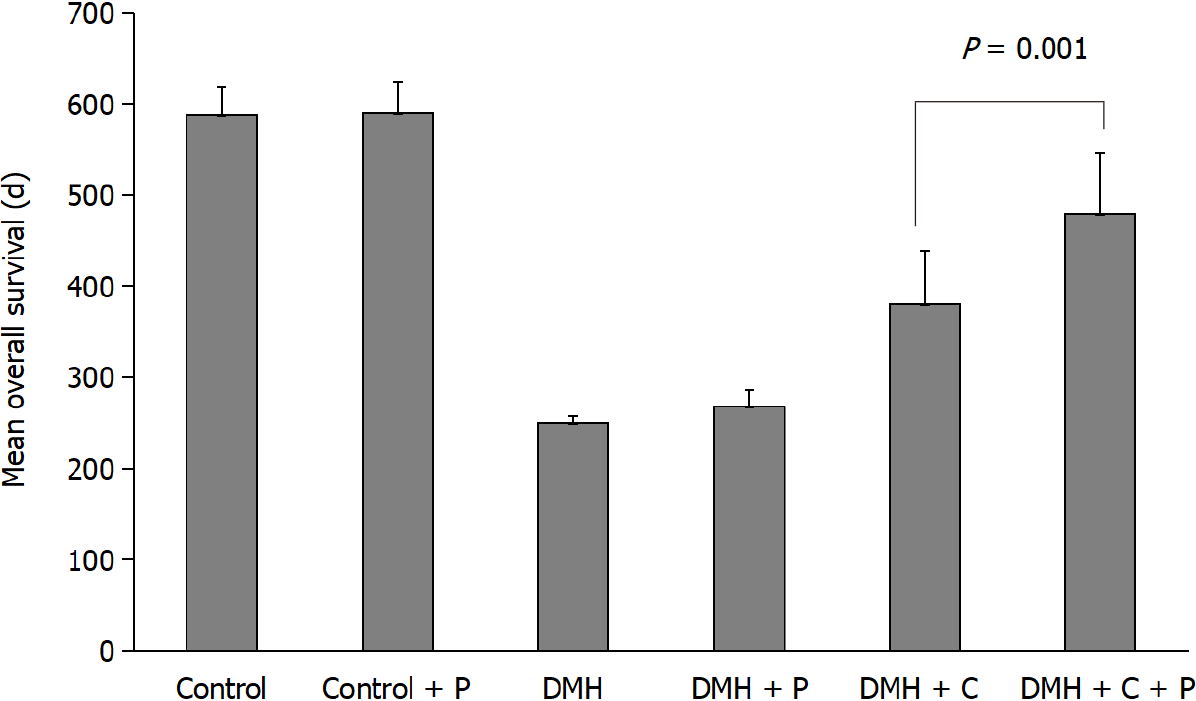
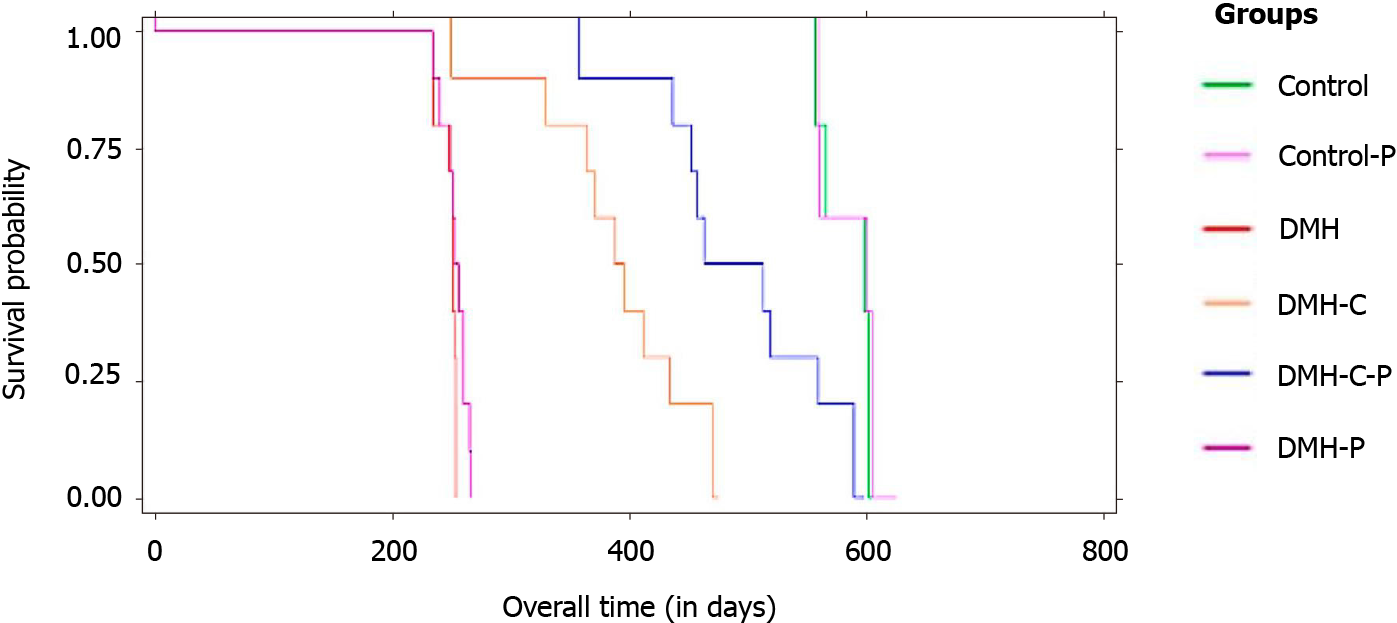
The data of mean overall survival for DMH, DMH-P, DMH-C, DMH-C-P, Control and Control-P groups were 250 d [95% confidence interval (CI): 242.5-253.1], 268 d (95%CI: 246.3-271.4), 380 d (95%CI: 337.8-421.9), 480 d (95%CI: 436.9-530.7), 588 d (95%CI: 565.8-609.3) and 590 d (95%CI: 564.3-612.9), respectively, with a significant difference between DMH-C and DMH-C-P groups (P = 0.001). Comparing all groups by Kaplan-Meier estimator, we found a significantly different in the overall survival of DMH and DMH-P groups respect to DMH-C (P = 0.001) and DMH-C-P (P = 0.001) groups; interestingly, there were no meaningful differences between Control, Control-P and DMH-C-P groups (P = 0.012). The tendency of change in body weight gain of the rats at 90 d of finishing DMH administration was similar in Control group compared with DMH-C and DMH-C-P groups; however, and of relevance, DMH-C-P group has experienced a higher body weight gain at the end of animal’s life than DMH-C group (P = 0.001). In DMH-C-P group we found a positive effect of probiotics in clinical manifestations since diarrhea, constipation and blood stool were absenting. Also, the tumor burden was lower in DMH-C-P than DMH-C, DMH-P or DMH groups (1.25 vs 1.81 vs 3.9 vs 4.8 cm2, respectively). DMH-C and DMH-C-P groups showed only mucinous carcinoma type while in other DMH groups the tumor types were variable. However, mucinous carcinoma from DMH-C-P group showed invasion until muscularis propria layer. Interestingly, metastatic lymph node was observed in DMH, DMH-C and DMH-P groups but not in DMH-C-P. All animals in Control group died from natural causes without objective injuries. All animals of DMH and DMH-P groups died from tumor complications (i.e., obstruction or intestinal perforation); however, this cause was seen only in 44.5% of DMH-C and DMH-C-P groups
Probiotics administration improves life quality of rats with CRC under cape
Core Tip: Today it is still unclear which are the most effective forms of probiotics administration at long-term to reduce the incidence of human colorectal cancer (CRC) or which microorganisms are the most suitable; the amount, time and frequency that should be consumed in the diet. Also, which is their influence on side effect from chemotherapy in CRC? Herein we show, for the first time, that probiotics have a positive impact on the overall survival of rats with CRC under capecitabine treatment. These animals have also a benefit in weight gain, clinical manifestations, developing cancer, number, localization and tumor burden.
- Citation: Gigola G, Carriere P, Novoa Díaz MB, Perdigon G, Zwenger AO, Gentili C. Survival effect of probiotics in a rat model of colorectal cancer treated with capecitabine. World J Gastrointest Oncol 2021; 13(10): 1518-1531
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1518.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1518
Colorectal carcinoma (CRC) is the fourth most commonly diagnosed cancer in the Western world and is the result of a multistep process whose progression is associated with the gradual accumulation of genetic and epigenetic mutations[1,2]. This disease is sporadic in more than 90% of cases and develops gradually, proceeding from normal epithelium to adenomatous polyps and invasive carcinoma. Several genetic predispositions and environmental factors can increase the risk of CRC onset[3]. Between environmental factors, the human microbiota is emerging as a new potential factor; indeed, compared to healthy controls, CRC patients have an increased number of pro-inflammatory opportunistic pathogens and microorganisms that commonly are associated with metabolic disorders[4]. On the other hand, some microbiota may deplete their strategic microbial partners unbalancing the intestinal homeostasis[5-7].
Probiotics are becoming increasingly important in basic and clinical research, and are also subject of considerable economic interest due to their expanding popularity. The Food and Agriculture Organization of the United Nations together with the World Health Organization define probiotics as ‘live micro-organisms which, when ad
CRC represents a major public health problem and curative surgery is feasible in three-quarters of patients, but despite this, about one half of the patients subsequently develop incurable recurrent cancer. Treatment methods for CRC include also chemotherapy and radiotherapy. 5-fluorouracil (5-FU) is one of the most important chemotherapeutic agents used to treat this disease but the clinical benefit of 5-FU is limited because of resistance of colon tumor cells and adverse side effects[16]. Capecitabine is a pro-drug of 5-FU and regimens containing it have been widely used in adjuvant setting as well as in incurable disease. However, excessive adverse effects caused by their prolonged use can lead to serious health problems with the consequent suspension of treatment[17]. Therefore, the combination therapies are necessary to improve the management of cancer and decrease systemic toxicity.
Probiotics have been identified as potential factors leading to reduce the risks of CRC[18]; so, and based on their possible anticancer properties, they could be used in combination with conventional CRC therapies. Clinical reports showed a significant benefit of administering probiotics in surgically seating[19,20] and in post-resection CRC patients treated with adjuvant 5-FU therapy[21]. Also, probiotic supplementation could have an antitumor effect of decreasing the formation of aberrant crypts and ameliorate tumor malignancy enhancing the apoptosis-induction capacity of 5-FU[22,23].
Despite these previous studies, the impact of probiotics on the overall survival and life quality of rats with CRC under capecitabine treatment is still unknown. So, the aim of this work was to evaluate the probiotics effects in this animal model until the end of life.
Eight weeks-old male Wistar-Lewis rats, body weight between 180-220 g were employed and obtained from the inbred population of Animal Care House, National University of South, Argentina. Sex was selected according to the literature showing that male rats are more sensitive to the pro-carcinogen 1,2-Dimethylhidrazine dihydrochloride (1,2-DMH) than female rats[24]. All experiments with animals were su
1,2-DMH purchased from Sigma Chemical Co., St. Louis, MO, was employed as carcinogen inductor of colorectal cancer. 1,2-DMH was reconstituted in EDTA saline solution (1 mmol/L), and adjusting the pH at 7.0 with H2CO3Na solution (1 mol/L). Then, and as it was previously established by our laboratory[25], animals received 1,2-DMH at dose of 20 mg/kg body weight intramuscularly weekly for 8 wk to achieve colorectal cancer in 100% of animals at 90 d after the last 1,2-DMH administration.
To reproduce the human colorectal cancer treatment, the Capecitabine (Xeloda®, a 5-FU pro-drug) was given through an orogastric sonde (gavage) (Fine Science Tools, Reusable feeding needle 18061-50, 1.25 mm de diameter, 50 mm long) at a median toxic dose (TD50 for rats, 359 mg/kg body weight) for 14 d with 7 d of resting until the end of animal’s life or its humanitarian sacrifice.
Before starting the experimental work that is showed in the present manuscript, we carried out a preliminary test and in view of the results from that initial assay, we performed a statistical analysis using InfoStat software and it was determined with a significant value (α = 0.01) that the appropriate number of rats per group to use was 10[26]. It should be noted that the number of animals per group in Control and Control plus probiotics groups was 5 instead of 10 because our previous results revealed that all animals from these groups showed the same behavior without any abnormality or complications and all of them died from natural causes without objective injuries[26]. So, and in accordance with the National Institutes of Health guide for the care and use of Laboratory animals, we decided to reduce the number of animals in 5.
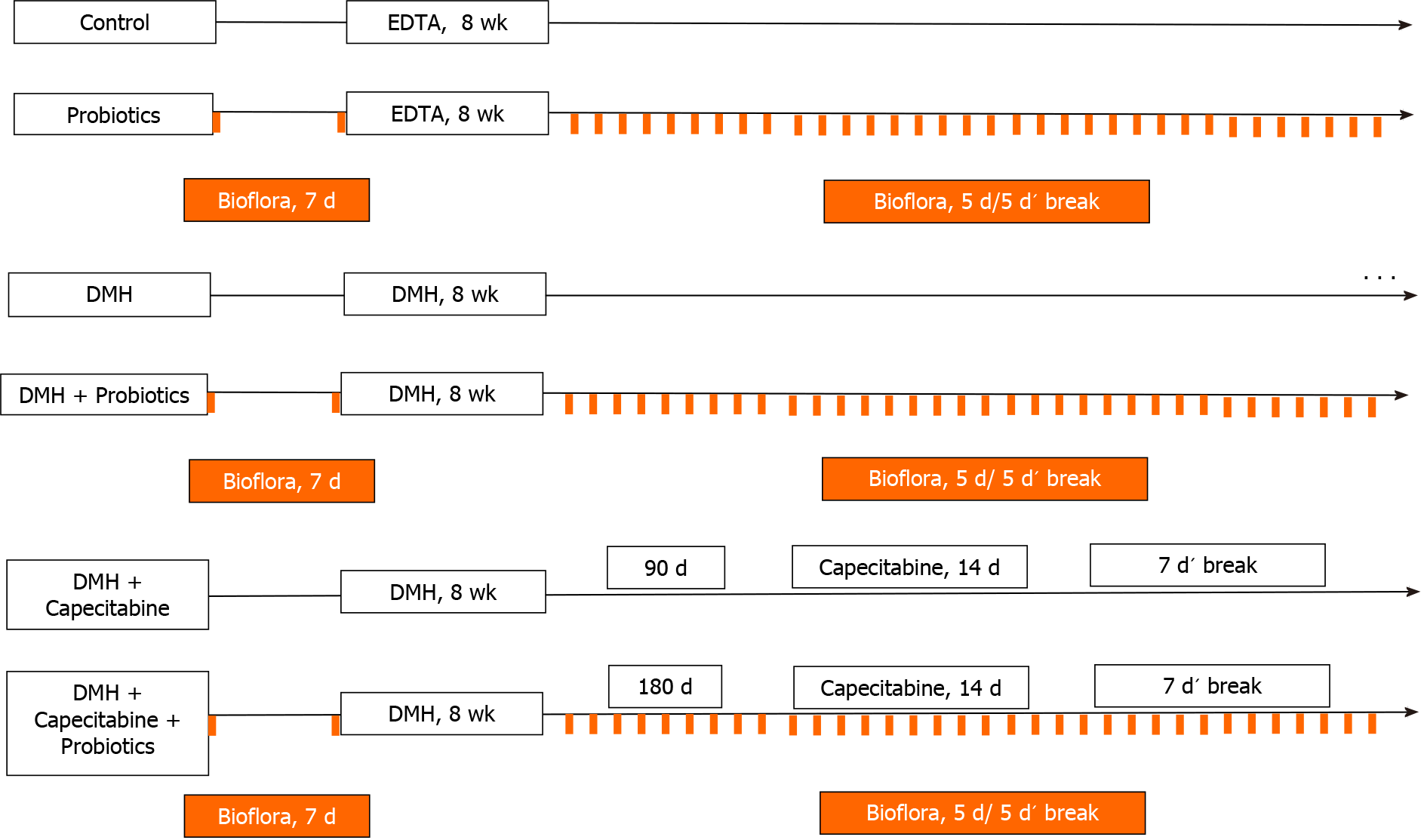
Taking account the previous results described above, 50 rats were randomly assigned to one of five following groups: (1) Animal Care House Control group (control, n = 5): animals received a single dose of EDTA saline solution (1 mmol/L) (pH 7.0) via subcutaneous, weekly for 8 wk (see Figure 1); (2) Animal Care House Control group + probiotics (Control-P, n = 5): animals received EDTA saline solution as described above plus probiotics. Lactobacillus plantarum (L. plantarum), Lactobacillus casei, Streptococcus faecalis and Bifidobacteriumbrevis (Biofloraâ, supplied by BioSidus Lab) were used in the experiment. As it was previously established by our laboratory[26], 1 mL of probiotics was given orally through a syringe for 7 d before EDTA saline solution administration and after cyclically (5 consecutive days followed for 5 d’ break) until the end of animal’s life (see Figure 1). This cyclically administration was performed based on our previous studies[27]; (3) DMH group (DMH, n = 10): Animals received 1,2- DMH as described previously in Materials and Methods Section (see Figure 1); (4) DMH + Probiotics (DMH-P, n = 10) Animals received 1,2-DMH plus probiotics. As describe above, 1 mL of probiotics was given orally for 7 d before starting carcinogenesis induction and after cyclically (5 consecutive days followed for 5 d’ break) until the end of animal’s life or its humanitarian sacrifice (see Figure 1); (5) DMH + Capecitabine group (DMH-C, n = 10): Animals received 1,2-DMH plus capecitabine as described previously in Materials and Methods Section. Taking account, the time to achieve the colorectal cancer illness in our animal model[25], the capecitabine treatment was started 90 d after the last day of 1,2-DMH administration (see Figure 1); and (6) DMH + Capecitabine + Probiotics group (DMH-C-P, n = 10): Animals received carcinogenesis inductor plus capecitabine plus probiotics at the same manner and doses that were described above. Capecitabine regimen was started 180 d after the administration of the last 1,2-DMH dose due the influence of probiotics on the delay of colorectal carcinogenesis, as described previously by us and by others researchers[18,26] (see Figure 1).
During the experiment, all animals were exposed to 12 h of light and darkness respectively (200 lux/1 m from the floor), at an equal atmospheric pressure, 20°C to 22°C and 40% to 70% of humidity in a proper ventilated animal room. Also, they had access to the same food and water ad libitum. As we established previously[28], animal’s evolution was followed by daily reports and body weights were recorded once per week until the end of the experiment. Humanitarian euthanasia was carried out through anesthetic overdose with acepromazine (Acedan® Holliday-Scott) ad
After sacrifice, a macroscopic analysis was carried out by trained personnel. Large intestine, lymph node, liver and lung were removed and explored. Any abnormality detected was included in the histopathological analysis. Each tissue was processed and fixed in buffered neutral formalin (100 g/L) for 6-12 h, then were dehydrated in an ascending series of ethanol concentration, cleared in xylol, and embedded in paraffin wax. Then, sections of 5 μm were severed and mounted on glass slides. Sections were routinely stained with haematoxylin and eosin for light microscope examination.
All animals were strictly followed until the end of their life or their humanitarian euthanasia due to several complications of malignancy. Overall survival was taken account from the end of tumor induction until death or humanitarian sacrifice.
The statistical methods of this study were performed by the following author of this work: Ariel Zwenger, PhD, Oncology Specialist, Health Assistant Researcher at the National Scientific and Technical Research Council - CONICET – Argentina. Dr. Zwenger has experience in statistical analysis. All parametric data were expressed as the mean ± SD. The statistical significance of differences was analyzed using one-way ANOVA. Data were analyzed with InfoStat software. The results were considered statistically significant at P < 0.05. Overall survival was evaluated with the Kaplan-Meier estimator with the log-rank test.
As shown in Figure 2, the mean overall survival for Control and Control-P groups were 588 d (95%CI: 565.8-609.3) and 590 d (95%CI: 564.3-612.9), respectively, while for the other experimental groups were the following: 250 d (95%CI: 242.5-253.1) for DMH group, 268 d (95%CI: 246.3-271.4) for DMH-P group, 380 d (95%CI: 337.8-421.9) for DMH-C group and 480 d (95%CI: 436.9-530.7) for DMH-C-P group. There was a significant difference between the values from DMH-C and DMH-C-P groups (P = 0.001). The values in both Control groups were similar to that usually observed in animals from the Animal Care House.
Comparing all groups (Figure 3) by Kaplan-Meier estimator, we found a significant difference in the overall survival between DMH group and the other groups (sig
A rat from DMH-C group and a rat from DMH-C-P group perished during experiment because of acute chemical pneumonia: a serious adverse effect expected in the first experimental administration of capecitabine through gavage.
As shown in Table 1, and as expected, 80% of the DMH group presented diverse gastrointestinal complications because of their malignancy while capecitabine group showed only diarrhea. However, clinical manifestations were absent in DMH-C-P group.
| Control (n = 5) | Control-P (n = 5) | DMH (n = 10) | DMH-C (n = 9) | DMH-P (n = 10) | DMH-C-P (n = 9) | |
| Clinical manifestations | ||||||
| Diarrhea | - | - | 3 (30) | 2 (22) | 1 (10) | - |
| Constipation | - | - | 3 (30) | - | 3 (30) | - |
| Blood stool | - | - | 2 (20) | - | 4 (40) | - |
| No manifestations | - | - | 2 (20) | 7 (78) | 2 (20) | 9 (100) |
| Tumor location | ||||||
| Left colon | - | - | 7 (70) | 3 (33) | 5 (63) | 2 (22) |
| Right colon | - | - | 1 (10) | 1 (11) | 2 (25) | 2 (22) |
| Rectum | - | - | 2 (20) | - | 1 (12) | - |
| Histological type | ||||||
| Adenocarcinoma | - | - | 5 (50) | - | 6 (75) | - |
| Mucinous | - | - | 2 (20) | 4 (44) | 2 (25) | 4 (44) |
| Mixed | - | - | 2 (20) | - | - | - |
| SRCC | - | - | 1 (10) | - | - | - |
| Average tumor size/animal | - | - | 2.5 | 1.0 | 2.1 | 1.0 |
| Tumoral burden (cm2) | - | - | 4.8 | 1.81 | 3.9 | 1.25 |
| Number of nodes involved in the total number of animals | - | - | 4 | 2 | 4 | - |
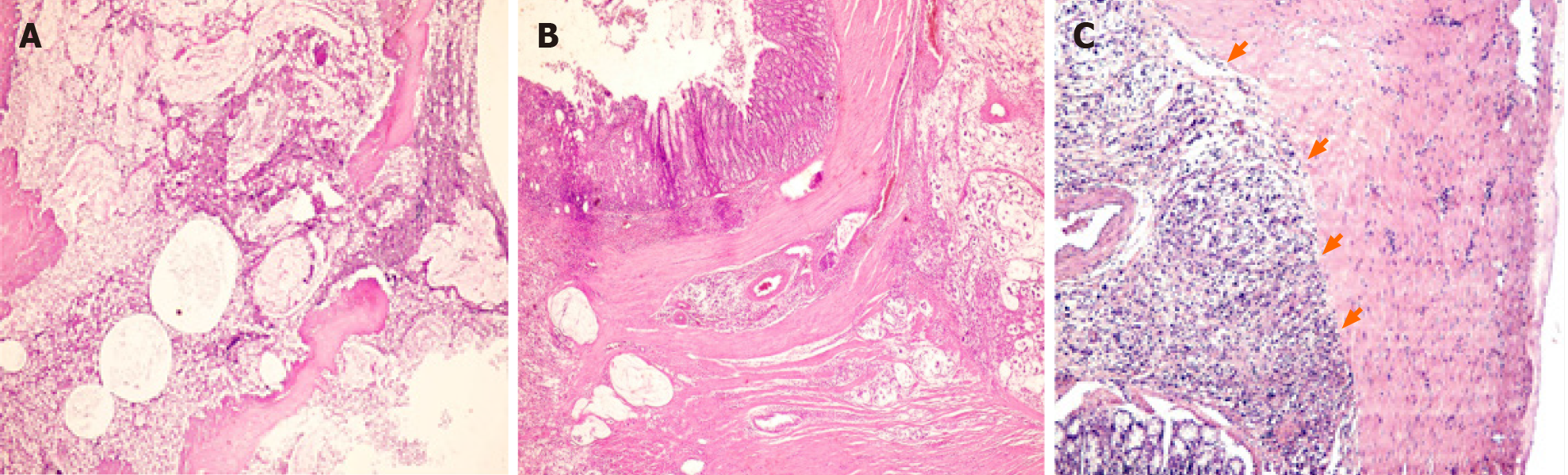
The prevalence of left colon cancer declined significantly from DMH group to DMH-C-P group (70% vs 22%); however, the right colon cancer showed a slight opposite tendency. DMH and DMH-P groups developed rectum cancer. Interestingly, DMH-C and DMH-C-P groups showed only mucinous carcinoma type while in DMH groups the tumor types were variable. However, only mucinous carcinoma from probiotics animals group showed invasion until muscularis propria layer (see Table 1 and Figure 4).
Average tumor size per animal and tumor burden diminished in DMH-C-P group respect to DMH group. Four rats from DMH-C group had mucinous adenocarcinoma and in two of them (50%) we found metastatic lymph nodes which were mucinous type. Interestingly, DMH-C-P group did not develop any lymph node metastasis.
Table 2 shows the average body weight in each group. Based on these data, average body weight of all animals was the following: at the beginning of the experiment: 319.1 ± 24.6 g, at 90 d of finishing DMH administration: 430.3 ± 29.3 g and at the end of life: 550 ± 76.7 g.
| Groups | At beginning (g) | At 90 d (g) | At the end of life (g) |
| Control | 329.8 ± 14.41 | 459.6 ± 15.6 | 563.0 ± 40.5 |
| Control-P | 280.2 ± 11.3 | 459.6 ± 14.7 | 619.3 ± 42.0 |
| DMH | 361.0 ± 17.6 | 451.2 ± 22.5 | 592.8 ± 52.2 |
| DMH-P | 361.0 ± 17.6 | 473.7 ± 23.7 | 592.8 ± 52.2 |
| DMH-C | 299.0 ± 15.7 | 412.4 ± 17.1 | 493.0 ± 67.0 |
| DMH-P-C | 292.1 ± 34.6 | 412.7 ± 26.1 | 559.8 ± 91.9 |
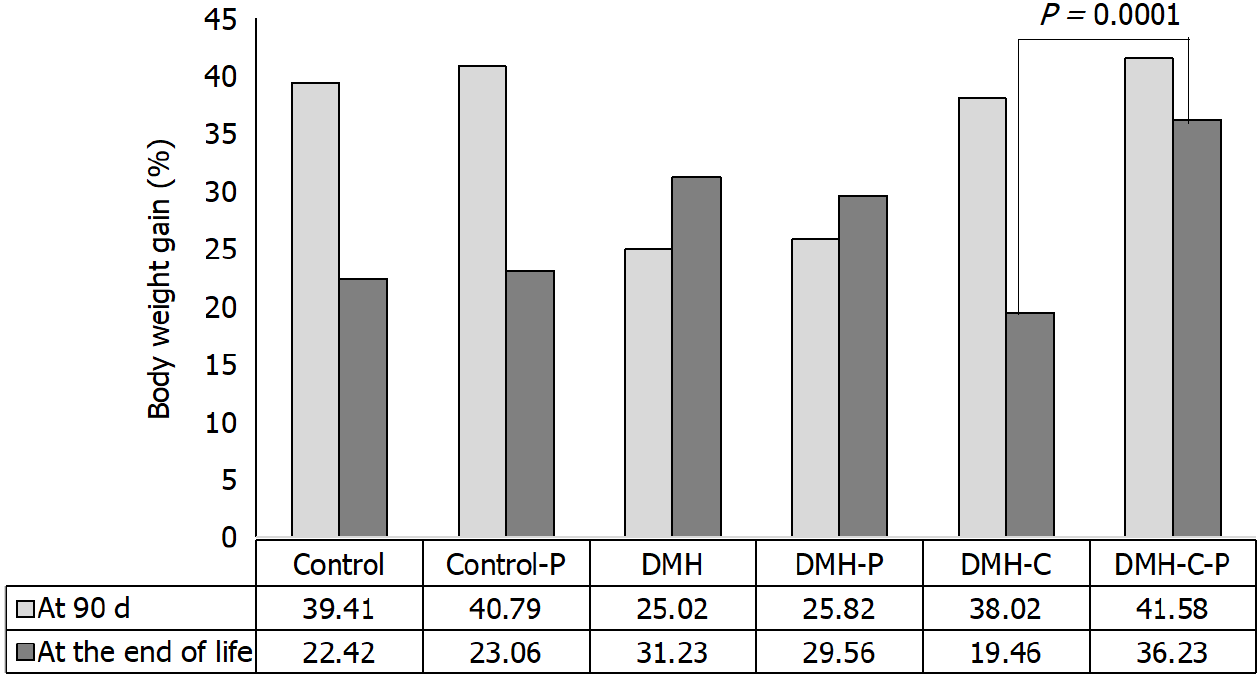
We then compared the body weight gains of rats from DMH-C and DMH-C-P groups because these animals lived a similar life span. As shown in Figure 5, the tendency of change in body weight gain of the rats at 90 d of finishing DMH administration was similar in control group compared with DMH-C and DMH-C-P groups; however, and of relevance, DMH-C-P group has experienced a higher body weight gain at the end of animal’s life than DMH-C group (P = 0.0001).
All animals of DMH and DMH-P groups died from tumor complications (i.e., ob
The present study was developed to clarify the impact of probiotics in the outcome of rats with CRC which were treated with capecitabine and followed until the end of their life.
To our knowledge this experimental model shows, for the first time, that the administration of probiotics has a positive effect on the overall survival of a CRC model under capecitabine treatment. In DMH-C-P group this benefit on survival could be because of the antitumor activity of probiotics by delaying the tumor promotion and its progression which is reached when probiotics are given before and after tumor induction and also, when they are consumed through long-term and cyclical form[26,27]. Nonetheless, Kibe and collaborators showed that the increased intestinal level of polyamines, produced by colonic microbiota stimulated by probiotics, could increase longevity by improving intestinal health and inhibiting systemic chronic low-grade inflammation[30]. Certain probiotic strains such as Lactobacillus (i.e., casei, plantarum, delbruekii, lactis, acidophilus), Bifidobacterium (i.e., longum, animalis), and Streptococcus thermophilus, among others, are able to reduce the probability of colon cancer pro
The chemotherapy alters the composition of the intestinal microbiota, which is critical for metabolism of various intestinal enzymes and regulation of immune functions[34]. Regimens based on 5-FU or capecitabine are frequently associated with diarrhea which can lead to a metabolic or nutritional imbalance and neutropenia associated with an increasing risk to host’s life. There are very few clinical trials focused in the study of probiotics supplementation effects in patients under 5-FU treatment. Österlund and colleagues showed, in early stage of CRC patients who underwent surgery and treated with 5-FU adjuvant, a low grade 3 or 4 of diarrhea and abdominal discomfort with L. rhamnosus GG supplementation[21]. Respect to animal studies, Yeung and colleagues showed that mice with intraperitoneally-injected 5-FU developed diarrhea, but their symptoms were improved when they received a probiotic suspension[35]. Despite these studies, there are no human data or animal experiments evaluating whether probiotics can help improve tolerance to capecitabine therapy. In our work we didn’t find diarrhea or any other symptoms in animals with probiotics supplementation, attributed either to capecitabine regimens or to the primary tumor. We must remark that these animals have been followed until the end of their lives without being subjected to a curative surgery like they did in Österlund study or the most basic research.
Several lines of evidence in healthy human and animal studies, point towards a biological role of probiotics in weight gain, however in CRC setting this relationship seems to be less clear[36]. Our study showed an increase of body weight gain in animals with probiotics supplementation (DMH-C-P) respect to animals from DMH plus capecitabine at the end of life. A meta-analysis carried out by Millon and colleagues showed that only some Lactobacillus species such as acidophilus, ingluviei or fermentum are linked to weight gain in healthy humans. These probiotics could improve absorption and digestion of food particles in the intestine, among others causes[37].
In our work we found that 70% of DMH group developed cancer in the left colon (distal colon) and 10% in the right colon (proximal colon), in addition to 20% in the rectum. Similar results were showed by Ma et al[38] regarding the tumor location in rats with 1-2 DMH administration. They observed that when total colon was exposed to this pro-carcinogen, 73 % of tumors occurred distally and only 12 % occurred proximally[38]; furthermore, they suggested that the observed differences between proliferation patterns in distal and proximal colon may be associated with the higher incidence of tumors in the distal colon[39].
The available literature describes that tumors in the proximal colon and distal colon show different molecular and histological characteristics; also the therapy responses are totally different between these tumor entities[40]. CRC patients with tumors in left colon are more benefited with adjuvant chemotherapies such as 5-FU-based regimes and have a better prognosis while CRC patients with tumor in right colon do not respond well to conventional chemotherapies. According to these data, although we observed in DMH group that the percentage of left colon tumors was 70%, this percentage is much lower in both DMH groups subjected to capecitabine treatment, DMH-C and DMH-C-P groups (70% DMH group vs 33% DMH-C group and 22% DMH-C-P). With respect to DMH-P group, the reduction of the percentage was less remarkable (63%) since the action of the probiotics delays the appearance of tumors, but does not prevent it. In line with this and with the data from Baran et al[40], the tumor location in the right colon does not vary substantially in all groups.
We observed that DMH and DMH-P groups developed rectum cancer. Consistent with the well establish benefit of the capecitabine as chemotherapeutic agent in the treatment of rectal cancer patients[41], we didn’t find tumors in the groups that receiving this drug (DMH-C and DMH-C-P).
Molecular studies support the hypothesis that mucinous CRC represents a biologically distinct entity. These tumors are characterized by frequent and stronger expression of the MUC2 gene and a higher frequency of K-ras mutations than non-mucinous carcinomas. Furthermore, MUC2 expression has been implicated in the resistance to anticancer drugs such as 5-FU[42]. This could explain somehow why within the animals treated with capecitabine we found only mucinous carcinomas. As expected in this type of tumors, metastatic lymph nodes found in the DMH-C groups were mucinous type. Compared to the most common non-mucinous variety, mu
Administration of Lactobacillus and Bifidobacterium strains to rats has been evaluated due to have immunomodulatory effects through positive regulation of IL-10 (an anti-inflammatory cytokine) and negative regulation of TNF-α and IL-6 (which are pro-inflammatory cytokines)[44]. Furthermore, it has been shown that Lactobacillus acidophilus could decrease the expression of stromal CXCR4-derived factor 1 receptor mRNA, and that the bacteria-free solution originating from L. plantarum suppresses NF-κB pathways, both events associated with mesenchymal epithelial transition and invasive potential in various types of cancer, suggesting a role in preventing meta
Probiotics may have a protective effect on colorectal carcinogenesis. However, it is not clear which are the most effective forms of administration at long-term to reduce the incidence of CRC in humans. Also, it is not fully studied which microorganisms are the most suitable; the amount, time and frequency that should be consumed in the diet. We have shown in this study a positive effect of probiotics in weight gain, clinical manifestations, delay of developing cancer, number, localization and tumor burden, and survival in CRC rat model treated with capecitabine. Furthermore, there were no effects in the group supplemented with probiotics leading to interfere the effect of the chemotherapeutic agent neither was observed biological mechanisms that affect the cell cycle progression beyond the carcinogen effect induced by the drug. Further experimental studies are required to understand the specific mechanisms involved in the influence of probiotics on CRC.
Colorectal cancer (CRC) is one of the leading causes of mortality due to malignant diseases worldwide. Capecitabine, the prodrug of 5-fluorouracil (5-FU), is one of the most important chemotherapeutic agents used in CRC treatment. Prolonged use of regimens containing capecitabine can lead to systemic toxicity with the consequent discontinuation of the treatment.
To improve the management of CRC patients, it is necessary the incorporation of therapies that mitigate the side effects of the conventional CRC treatment and reduce its resistance. Probiotics have beneficial properties when they are used in the management of many gastrointestinal diseases. Also, it is known that probiotics are able to reduce undesirable effects of 5-FU in CRC patients and to benefit CRC patients treated surgically. In a rat CRC model, probiotic supplementation potentiated the antitumor effect of 5-FU chemotherapy on colon. The positive impact of probiotics in a preclinical model of CRC under capecitabine treatment was unknown when we started our experimental work.
The aim of this study was to evaluate the impact of a mixture of probiotics strains in the outcome of a rat CRC model treated with capecitabine and monitored until the end of life.
Male Wistar-Lewis rats with CRC induced by 1,2-dimethylhydrazine dihydrochloride (1,2-DMH) were grouped as follow: 1.2-DMH alone (DMH group, n = 10), 1,2-DMH + capecitabine (DMH-C group, n = 10), 1,2-DMH + probiotics (DMH-P group, n = 10), 1,2-DMH + capecitabine + probiotics (DMH-C-P group, n = 10). Two groups of male Wistar-Lewis rats were used as controls: untreated group (Control n = 5) and Control + probiotics group (Control-P, n = 5). During the experiment, the following were analyzed in all groups: survival time, clinicopathological characteristics, quality of life and cause of death.
The administration of probiotics showed a benefit in survival time, weight gain, clinical manifestations and cancer development.
The fact that the animals were followed until the end of life allow to conclude that this study is the first that shows the positive impact of probiotics in the overall survival of rats with CRC under capecitabine treatment.
The use of probiotics could improve the overall survival and quality of life of patients with CRC treated with capecitabine.
This work was made thanks to the support received from Universidad Nacional del Sur and BioSidus laboratory. The authors want to express their appreciation to the biochemists Gabriel Melatini and Gonzalo Martin Arrieta for their collaboration in the experimental process. The authors also appreciate the collaboration of Dr. Pérez JE and Dr. Maturi HV as supervisors of this work.
Manuscript source: Invited manuscript
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Batyrbekov K, Gao W S-Editor: Wu YXJ L-Editor: A P-Editor: Yuan YY
| 1. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4900] [Article Influence: 700.0] [Reference Citation Analysis (1)] |
| 2. | Hong SN. Genetic and epigenetic alterations of colorectal cancer. Intest Res. 2018;16:327-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Thanikachalam K, Khan G. Colorectal Cancer and Nutrition. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 478] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 4. | Saus E, Iraola-Guzmán S, Willis JR, Brunet-Vega A, Gabaldón T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol Aspects Med. 2019;69:93-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 5. | Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 667] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 6. | Farhana L, Banerjee HN, Verma M, Majumdar APN. Role of Microbiome in Carcinogenesis Process and Epigenetic Regulation of Colorectal Cancer. Methods Mol Biol. 2018;1856:35-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Yu X, Yu E, Wang N, Cai Q, Shuai Q, Yan F, Jiang L, Wang H, Liu J, Chen Y, Li Z, Jiang Q. Changes in gut microbiota and plasma inflammatory factors across the stages of colorectal tumorigenesis: a case-control study. BMC Microbiol. 2018;18:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Araya M, Morelli L, Reid G; Sanders ME, Stanton C. Probiotics in food. Health and nutritional properties and guidelines for evaluation. Pineiro M, Ben EmbarekP, editors. FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada, 2002. Rome: FAO/WHO, 2006. |
| 9. | Brasiel PGA, Dutra Luquetti SCP, Peluzio MDCG, Novaes RD, Gonçalves RV. Preclinical Evidence of Probiotics in Colorectal Carcinogenesis: A Systematic Review. Dig Dis Sci. 2020;65:3197-3210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Hajela N, Ramakrishna BS, Nair GB, Abraham P, Gopalan S, Ganguly NK. Gut microbiome, gut function, and probiotics: Implications for health. Indian J Gastroenterol. 2015;34:93-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 12. | Dimidi E, Christodoulides S, Scott SM, Whelan K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv Nutr. 2017;8:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 308] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 13. | do Carmo MS, Santos CID, Araújo MC, Girón JA, Fernandes ES, Monteiro-Neto V. Probiotics, mechanisms of action, and clinical perspectives for diarrhea management in children. Food Funct. 2018;9:5074-5095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Sun JR, Kong CF, Qu XK, Deng C, Lou YN, Jia LQ. Efficacy and safety of probiotics in irritable bowel syndrome: A systematic review and meta-analysis. Saudi J Gastroenterol. 2020;26:66-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Huidrom S. Therapeutic Approach of Probiotics in Children with Atopic Dermatitis. Antiinflamm Antiallergy Agents Med Chem. 2021;20:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Yuan S, Li L, Yang D, Xu C, Wang S, Zhang D. Novel proapoptotic agent SM-1 enhances the inhibitory effect of 5-fluorouracil on colorectal cancer cells in vitro and in vivo. Oncol Lett. 2017;13:4762-4768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Aguado C, García-Paredes B, Sotelo MJ, Sastre J, Díaz-Rubio E. Should capecitabine replace 5-fluorouracil in the first-line treatment of metastatic colorectal cancer? World J Gastroenterol. 2014;20:6092-6101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Drago L. Probiotics and Colon Cancer. Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Bajramagic S, Hodzic E, Mulabdic A, Holjan S, Smajlovic SV, Rovcanin A. Usage of Probiotics and its Clinical Significance at Surgically Treated Patients Sufferig from Colorectal Carcinoma. Med Arch. 2019;73:316-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, Jiang Y, Zhang H, Wang Y, Zheng Q. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery - a double-blind study. Aliment Pharmacol Ther. 2011;33:50-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Osterlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P, Kouri M, Elomaa I, Joensuu H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 22. | Baldwin C, Millette M, Oth D, Ruiz MT, Luquet FM, Lacroix M. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer. 2010;62:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Genaro SC, Lima de Souza Reis LS, Reis SK, Rabelo Socca EA, Fávaro WJ. Probiotic supplementation attenuates the aggressiveness of chemically induced colorectal tumor in rats. Life Sci. 2019;237:116895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Turusov VS, Lanko NS, Parfenov YD, Gordon WP, Nelson SD, Hillery PS, Keefer LK. Carcinogenicity of deuterium-labeled 1,2-dimethylhydrazine in mice. Cancer Res. 1988;48:2162-2167. [PubMed] |
| 25. | Gigola G, Melatini G, Ullua N, Cardozo C, Martín AG. Comparación de dos protocolos de inducción de cáncer colorrectal en ratas Wistar Lewis con 1,2-Dimetilhidrazina. Oncología Clínica. 2011;16:1-4. |
| 26. | Gigola G. Dietary supplementation with probiotics in rats with colon cancer induced by dimethylhydrazine and under treatment with chemotherapy. PhD Thesis, National University of the South. 2014. [cited 26 April 2021] Available from: http://repositoriodigital.uns.edu.ar/bitstream/123456789/3629/1/Tesis%20Graciela%20Gigola.pdf. |
| 27. | de Moreno de Leblanc A, Perdigón G. Yogurt feeding inhibits promotion and progression of experimental colorectal cancer. Med Sci Monit. 2004;10:BR96-B104. [PubMed] |
| 28. | Pérez JE, Oresti GM, Melatini G, Berton P, UllúaN, Gandini NA. Historias clínicas y partes diarios para investigación: herramientas para estudios de carcinogénesis en modelos animales. Oncología Clínica. 2006;11:1278-1282. |
| 29. | Guide for the Care and Use of Laboratory Animals. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US); 2011. [PubMed] [DOI] [Full Text] |
| 30. | Kibe R, Kurihara S, Sakai Y, Suzuki H, Ooga T, Sawaki E, Muramatsu K, Nakamura A, Yamashita A, Kitada Y, Kakeyama M, Benno Y, Matsumoto M. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep. 2014;4:4548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Raman M, Ambalam P, Kondepudi KK, Pithva S, Kothari C, Patel AT, Purama RK, Dave JM, Vyas BR. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes. 2013;4:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Marotta F, Naito Y, Minelli E, Tajiri H, Bertuccelli J, Wu CC, Min CH, Hotten P, Fesce E. Chemopreventive effect of a probiotic preparation on the development of preneoplastic and neoplastic colonic lesions: an experimental study. Hepatogastroenterology. 2003;50:1914-1918. [PubMed] |
| 33. | Azad MAK, Sarker M, Wan D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res Int. 2018;2018:8063647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 34. | Ervin SM, Ramanan SV, Bhatt AP. Relationship Between the Gut Microbiome and Systemic Chemotherapy. Dig Dis Sci. 2020;65:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Yeung CY, Chan WT, Jiang CB, Cheng ML, Liu CY, Chang SW, Chiang Chiau JS, Lee HC. Correction: Amelioration of Chemotherapy-Induced Intestinal Mucositis by Orally Administered Probiotics in a Mouse Model. PLoS One. 2015;10:e0141402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Bertkova I, Hijova E, Chmelarova A, Mojzisova G, Petrasova D, Strojny L, Bomba A, Zitnan R. The effect of probiotic microorganisms and bioactive compounds on chemically induced carcinogenesis in rats. Neoplasma. 2010;57:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 38. | Ma Q, Williamson KE, O'rourke D, Rowlands BJ. The effects of l-arginine on crypt cell hyperproliferation in colorectal cancer. J Surg Res. 1999;81:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Ma QY, Williamson KE, Rowlands BJ. Variability of cell proliferation in the proximal and distal colon of normal rats and rats with dimethylhydrazine induced carcinogenesis. World J Gastroenterol. 2002;8:847-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Baran B, Mert Ozupek N, Yerli Tetik N, Acar E, Bekcioglu O, Baskin Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterology Res. 2018;11:264-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 41. | Yang XH, Li KG, Wei JB, Wu CH, Liang SX, Mo XW, Chen JS, Tang WZ, Qu S. Retrospective study of preoperative chemoradiotherapy with capecitabine vs capecitabine plus oxaliplatin for locally advanced rectal cancer. Sci Rep. 2020;10:12539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Hugen N, Simons M, Halilović A, van der Post RS, Bogers AJ, Marijnissen-van Zanten MA, de Wilt JH, Nagtegaal ID. The molecular background of mucinous carcinoma beyond MUC2. J Pathol Clin Res. 2015;1:3-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Nozoe T, Anai H, Nasu S, Sugimachi K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol. 2000;75:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 44. | Karamese M, Aydin H, Sengul E, Gelen V, Sevim C, Ustek D, Karakus E. The Immunostimulatory Effect of Lactic Acid Bacteria in a Rat Model. Iran J Immunol. 2016;13:220-228. [PubMed] |
| 45. | Motevaseli E, Dianatpour A, Ghafouri-Fard S. The Role of Probiotics in Cancer Treatment: Emphasis on their In Vivo and In Vitro Anti-metastatic Effects. Int J Mol Cell Med. 2017;6:66-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |