Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1317
Peer-review started: March 6, 2021
First decision: June 4, 2021
Revised: June 19, 2021
Accepted: September 3, 2021
Article in press: September 3, 2021
Published online: October 15, 2021
Processing time: 221 Days and 11.5 Hours
Gallbladder cancer is a rare, aggressive malignancy that has a poor overall prognosis. Effective treatment consists of early detection and surgical treatment. With the wide spread treatment of gallbladder disease with minimally invasive techniques, the rate of incidental gallbladder cancer has seen an equitable rise along with stage migration towards earlier disease. Although the treatment remains mostly surgical, newer modalities such as regional therapy as well as directed therapy based on molecular medicine has led to improved outcomes in patients with advanced disease. We aim to summarize the management of gallbladder cancer along with the newer developments in this formidable disease process.
Core Tip: Gallbladder cancer is a rare, aggressive malignancy that has an overall poor prognosis. Effective treatment consists of early detection and surgical treatment. With the wide spread treatment of gallbladder disease with minimally invasive techniques, the rate of incidental gallbladder cancer has seen an equitable rise along with stage migration towards earlier disease. Although the treatment remains mostly surgical, newer modalities such as regional therapy as well as directed therapy based on molecular medicine has led to improved outcomes in patients with advanced disease.
- Citation: Okumura K, Gogna S, Gachabayov M, Felsenreich DM, McGuirk M, Rojas A, Quintero L, Seshadri R, Gu K, Dong XD. Gallbladder cancer: Historical treatment and new management options. World J Gastrointest Oncol 2021; 13(10): 1317-1335
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1317.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1317
Gallbladder cancer (GBC) is an uncommon malignancy (1.2%) accounting for about 220000 cases worldwide in 2018[1]. Although the incidence of the malignancy is low, there is significant geographic variability with cases concentrated in Latin America such as Bolivia and Peru, and Southeast Asia such as Thailand. It is also the only gastrointestinal malignancy that is more common in women than men[1]. Being the most common biliary tract malignancy, it accounts for 1.7% of all cancer mortalities reflecting the poor prognosis associated with this diagnosis. Adenocarcinoma of the gallbladder is also significant from management standpoint due to the technical challenges in surgical treatment as well as lack of significant breakthroughs in adjuvant therapies.
In this review, we will examine the causes of GBC along with new innovative treatments in the management of GBC. The various genetic predispositions are examined in detail in terms of their particular risks.
GBC is a rare disease with a worldwide incidence of 2/100000 individuals[2], and females are 2-3 times more likely to develop this disease than males[3]. While GBC is a very rare disease in western countries, the numbers are particularly high in some regions of the world (e.g., South America, Eastern Europe, India, Pakistan, etc.). Overall, GBC is the fifth most common cancer of the gastrointestinal tract[2].
GBC as well as cholangiocarcinoma (CCA) are both different types of biliary tract cancer, which includes all tumors originating from the biliary tract epithelium. GBC is the most common biliary tract cancer (equaling 80%-90% of all biliary tract cancers), followed by CCA[4]. CCA can be categorized by its anatomical origin into intra- and extrahepatic CCA. Special types of extrahepatic CCA are the Klatskin tumor and distal CCA[5].
The etiology of GBC is multifactorial. So far, various genetic variants as well as different environmental factors have been associated with a higher risk of developing GBC[6] (Table 1). To name a few: Chronic infections of the gallbladder, gallstones, polyps of the gallbladder, obesity, various dietary factors, exposure to special chemicals or metals and infections with salmonella are risk factors for GBC[7-9]. Furthermore, GBC is known to be more frequent in women than in men, which may be caused by hormonal influences that increase the levels of cholesterol in the bile. This, in turn, is postulated to lead to development of gallstones[10]. On a genetic level, using either a candidate gene study approach or a genome-wide association approach, multiple gene candidates have been identified as possible candidates for GBC[6]. Genetic alterations including KRAS, TP53, and c-ERB-B2 are associated with poor prognosis with GBC[6]. Several other factors, such as those involved in lipid movement through the liver, gallbladder and bile ducts are still under discussion and are focuses of a few ongoing studies.
| Patient predisposition | Environmental factors | Patient factors/conditions |
| Female sex | Chronic bacterial infections | Diabetes |
| Age | Aflatoxins | High body mass index |
| Race/ethnicity | Ochratoxin | Primary sclerosing cholangitis |
| Genetics (variants) | Arsenic | Porcelain gallbladder |
| Liver fluke | Gallbladder polyps | |
| Geography | Crohn’s disease | |
| Anomalous biliary ductal insertion | ||
| Gallstones | ||
| Sjogren’s syndrome |
Over 90% of GBC are adenocarcinomas. Histopathological types include papillary, mucinous, squamous or adeno-squamous carcinomas. The most common type of differentiation is the biliary type, followed by the intestinal and the gastric-foveolar type[2]. In terms of the location of GBC, it is isolated to the fundus in 60%, in the body in 30%, and in the neck of the gallbladder in 10% of times[4].
Due to the rare nature of the disease, GBC is often discovered incidentally. This occurs either during routine cholecystectomy or post-operatively on final pathology performed for other indications. Intra-operative diagnosis is found to be as low as 0.28% by Glauser et al[11], with the majority discovered on pathology post-operatively. Re-operative surgery needs to be contemplated to achieve an R0 resection, improve adequacy of margin resection, and/or to perform concurrent lymphadenectomy[12].
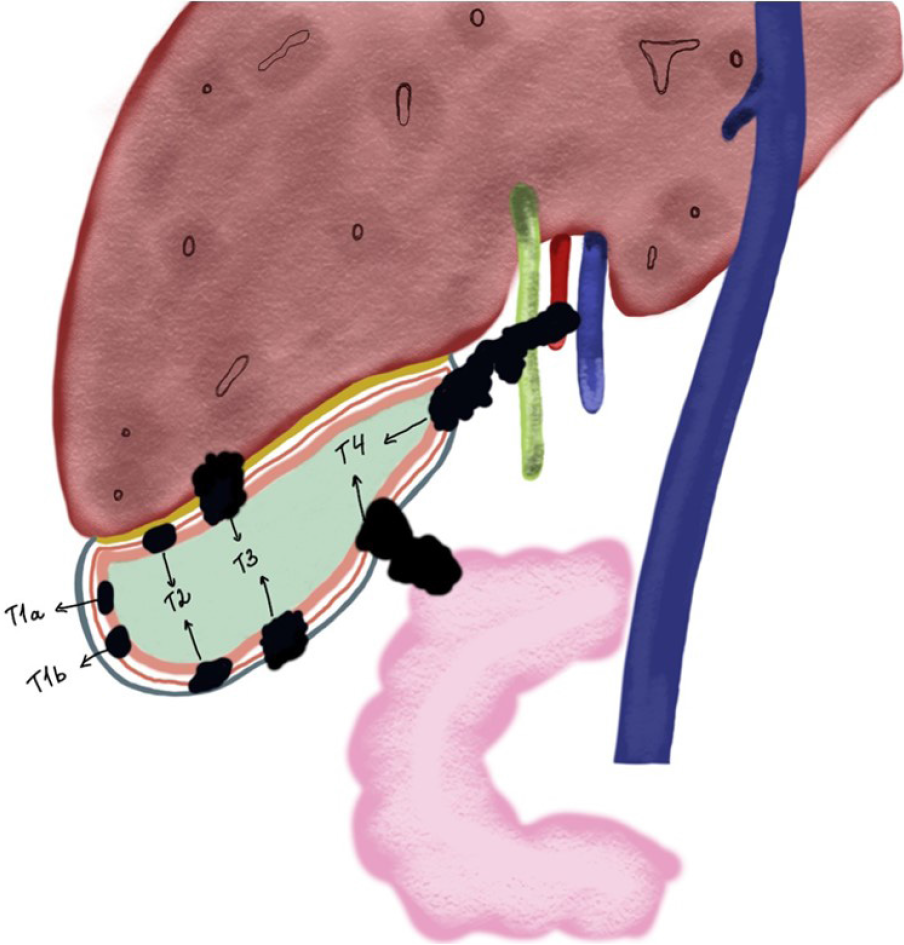
The tumor depth of invasion is the single biggest factor in determining the need for further surgical intervention. For GBC with Tis or T1a on pathology, no further surgery is required as the initial cholecystectomy is frequently curative. For GBC with T1b and above, re-operation is recommended[12] (Figure 1). Re-operation with extended re-resection of the gallbladder bed and lymph nodes increases survival for patients with T2 and T3 lesions. Interestingly enough, for patients with T1b lesions, extended re-resection does appear to increase survival over simple cholecystectomy alone[11]. Goetze and Paolucci[13] suggested that with each increasing tumor depth, more radical resection was needed. For T1b lesions a wedge-resection of 2-3 cm margins had superior survival compared to radical re-resection, while for patients with T2 and T3 tumors, survival was better with segment IVb and V resection with lymph node dissection around the hepatoduodenal ligament[13]. In fact, with radical re-resection, survival is comparable to the survival of patients who have primary resection rather than re-resection for incidental disease[14]. While re-resection increases survival, resection of the common bile duct has not been shown to increase survival or margin-free resection[15].
Although these re-resections have shown survival benefits, particularly in T1b or stage II-III, GBC with T4 or N1 Lesions still have very poor prognosis. For GBC overall, neoadjuvant therapy and clinical trials are more important for survival than radical re-resection[14]. For these patients, a re-operation with radical resection may provide some palliation if biliary compression is causing secondary sequelae[16]. With each increasing tumor grade, the chance of having curative re-operation decreases[17]. Re-operation appears to have the best survival rates if done within 4-8 wk following the initial cholecystectomy[18].
Historically, there were concerns that laparoscopic cholecystectomy increased the risk of intra-peritoneal metastases in cases of occult GBC. This is no longer felt to be the case. Survival rates have now been shown to be similar between laparoscopic and open cholecystectomy, with no differences in recurrence of cancer in the abdominal wall[19,20]. What is important, however, is the tumor stage and whether or not bile spillage occured during the index surgery[19].
One of the biggest questions during re-operative surgery following an incidental GBC is whether or not to perform a port site resection. In a large meta-analysis of 26 studies, Choi et al[21] found that 8.1% of patients had positive cancer cells at the port sites. Despite the fact that port site metastases are associated with decreased survival, Maker et al[22] determined that port site resection should not be a mandatory step in management, as it did not improve survival or disease-free recurrence. Ethun et al[23] reached the same conclusion, with no increase in survival with port site excision and the same rate of distant recurrence of GBC despite port-site resection. In a study of 218 patients with incidental GBC, port-site excision was not associated with improved survival[24].
Interestingly enough, patients with GBC found incidentally have been found to have better survival than patients with known cancer going into a cholecystectomy. D’Hondt et al[25] found that curative surgery and five-year survival rates were both higher in patients who had incidental GBC. This is potentially due to lower tumor stages in patients with incidentally discovered GBC.
GBC is relatively asymptomatic in its early stages. The presentation of GBC has been divided into three common scenarios: (1) Identification by final pathology following a routine cholecystectomy; (2) Discovery during the index surgery; and (3) Suspicion before surgery due to atypical symptomatology[26].
In a United States population-based study of incidental GBC, Pitt et al[27] showed that four factors are strongly associated with the finding of cancer at cholecystectomy: Age ≥ 65 years, female, Asian or African American race, and elevated alkaline phosphatase preoperatively.
The diagnosis of GBC should be considered in an elderly patient with constant right upper quadrant abdominal pain with weight loss or anorexia. Weight loss, anorexia and particularly jaundice are signs of advanced disease[28]. Hawkins et al[29] showed that the presence of jaundice with localized GBC are associated with worse outcome.
Tumor makers such as carcinoembryonic antigen (CEA) and carbonic anhydrase 19-9 (CA 19-9) may be associated with GBC, but are unreliable screening tools. An elevated CEA can be specific for presence of gastrointestinal malignancy (90%) but lacks sensitivity (50%) when CEA was used as a screening test for cancer in patients presenting with gallbladder diseases[30]. CA 19-9 is another tumor maker used in patients with pancreatico-biliary tumors with up to 75% sensitivities and specificities when the level exceeds 20 U/mL[31]. Due to their limitations, serum tumor markers are useful in the setting of follow up, for earlier detection of recurrent disease. The use of tumor markers in the form of CEA and CA 19-9 has been useful in the diagnostic algorithm for GBC although they pertain to a larger group of pancreatic biliary associated malignancies and not specific to the GBC alone[32].
When the clinical presentation is worrisome for a GBC, ultrasonography (US) is frequently the first imaging modality of choice. These findings on the US are worrisome: Calcification, a mass protruding into the lumen, loss of interface between gallbladder and liver, direct liver infiltration, gallbladder polyps ≥ 10 mm, or an abnormal thickened gallbladder wall[33]. The overall accuracy of US to diagnose and stage the local and distant extent of suspected GBC is limited[34]. Additional imaging such as computed tomography (CT) or magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) is necessary to complete staging work up for GBC (Figure 2). Subsequent biopsy, either percutaneous or endoscopic, may guide therapeutic interventions.
On cross sectional imaging, we need to evaluate the potential for resectability by the extent of invasion into liver parenchyma, vascular involvement (hepatic artery and portal vein) and suspected nodal involvement[35]. Once the diagnosis is established, based on the staging of GBC, the extent of surgical resection and regional lymphadenectomy is determined. Endoscopic ultrasound (EUS) is an emerging modality to assess GBC. EUS is more accurate than trans-abdominal US and EUS is useful both detection and staging of tumor extension[36,37].
The role of a positron emission tomography (PET) scan is controversial in patients with potential resectable disease[12]. PET scanning may help to distinguish between benign and malignant diseases. Furthermore, although PET has been reported to have low sensitivity of extrahepatic disease, particularly peritoneal carcinomatosis, it is still a useful adjunct to detect metastatic disease[38]. The recent study from Goel et al[39] showed that up to 23.4% patients had a change in the management following PET-CT and they suggested PET-CT should be included in preoperative work up.
Over the years, a variety of staging systems for GBC has been proposed. Realizing that the histological grade of the tumor impacted survival, Nevin et al[40] reported a series of 66 patients with GBC and described a method combining staging with histological grading of this cancer. This staging method was modified and later used[41]. Currently, the Tumor, Node, Metastasis (TNM) staging system of the combined American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) is the standard classification scheme[35] (Tables 2 and 3).
| T stage | Primary tumor |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor invades the lamina propria or muscular layer |
| T1a | Tumor invades lamina propria |
| T1b | Tumor invades muscle layer |
| T2 | Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum) or tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver |
| T2a | Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum) |
| T2b | Tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver |
| T3 | Tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or other adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts |
| T4 | Tumor invades main portal vein or hepatic artery or invades two or more extrahepatic organs or structures |
| N stage | Regional lymph nodes |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to one to three regional lymph nodes |
| N2 | Metastasis to four or more regional lymph nodes |
| M stage | Distant metastasis |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Stage | T | N | M | Description |
| 0 | Tis | N0 | M0 | Cancer in situ |
| I | T1 | N0 | M0 | Tumor is only in the gallbladder and has not spread |
| II | T2 | N0 | M0 | Tumor has extended to the perimuscular connective tissue but has not spread elsewhere |
| IIIA | T3 | N0 | M0 | Tumor has spread beyond the gallbladder but not to nearby arteries or veins. It has not spread to any lymph nodes or other parts of the body |
| IIIB | T1-3 | N1 | M0 | Tumor of any size has spread to nearby lymph nodes but not to nearby arteries and/or veins or to other parts of the body |
| IVA | T4 | N0 or N1 | M0 | Tumor has spread to nearby arteries, veins, and/or nearby lymph nodes, but it has not spread to other parts of the body |
| IVB | Any T | Any N | M1 | Any tumor that has spread to other parts of the body |
| Any T | N2 | M0 | Any tumor that has distant lymph node spread, even if it has not spread to distant organs |
In AJCC staging system, GBC is classified into four stages based on the depth of invasion into the gallbladder wall and the extend of spread to surrounding organs and lymph nodes (Figure 3). In the revised 8th edition of the AJCC staging system, T2 gallbladder carcinoma was divided into two groups: tumors on the peritoneal side (T2a) and tumors on the hepatic side (T2b)[35]. This revision was made based on two retrospective studies showing that invasion into the hepatic side carries with worse prognosis, compared to tumors located on the peritoneal side[42,43]. However, it is important to note that it can be difficult to determine the exact location of the tumor, contributing to difficulty in predicting patient outcome. In the most updated edition, staging of lymph nodes was also changed. Regional lymph node involvement is now staged according to number of positive nodes, instead of staging based on anatomic location of involved lymph nodes.
GBC requires a multimodal approach to its management. Surgery remains the gold standard for curative intent. Several factors need to be taken into account when choosing surgical management in patients with GBC: health of the patient, extent of surgical intervention needed, and post-operative outcomes anticipated. Extent of the disease and location of the tumor are important factors determining the extent of surgery in addition to the type of procedure need to ensure radical excision of the cancer with no residual disease. The location of the cancer influences surgical procedure because tumors located closer to the cystic plate have a propensity to invade the liver whereas those located in the inferior wall may spread to the peritoneum early (Figure 1).
Table 4 summarizes the types of surgical procedures performed for GBC, ranging from simple cholecystectomy to multi-visceral resection. These surgical procedures can be divided into curative and palliative procedures. In early stages of GBC (T1a or less), a simple cholecystectomy, regardless of whether the approach is open or minimally invasive (laparoscopic or robotic), is sufficient with almost 100% 5-year survival rate, as the current evidence supports[44,45]. In general, open approach rather than laparoscopy is recommended by most experts to minimize the risk of bile spillage. Nonetheless, laparoscopy has a key role in staging, especially in patients with preoperatively diagnosed GBCs with elevated CA 19-9 levels[46]. Agarwal et al[47] studied the role of diagnostic laparoscopy for GBCs and found that 23.2% of patients had disseminated disease and could avoid laparotomy. Currently, routine port-site excision is not recommended after laparoscopic cholecystectomy as we discussed above. In fact, the data from the US Extrahepatic Biliary Malignancy Consortium found no difference in distant recurrence rates and 3-year overall survival (OS) rates between patients who underwent port-site excision and those who did not[23].
| Procedure | Description | Indications |
| Curative procedures | ||
| Simple cholecystectomy | Dissection, ligation, and transection of cystic duct and artery at the level of Calot triangle and dissection of the cystic plate | Benign gallbladder conditions, gallbladder polyps, porcelain gallbladder, GBC (T0, Tis, and T1a) |
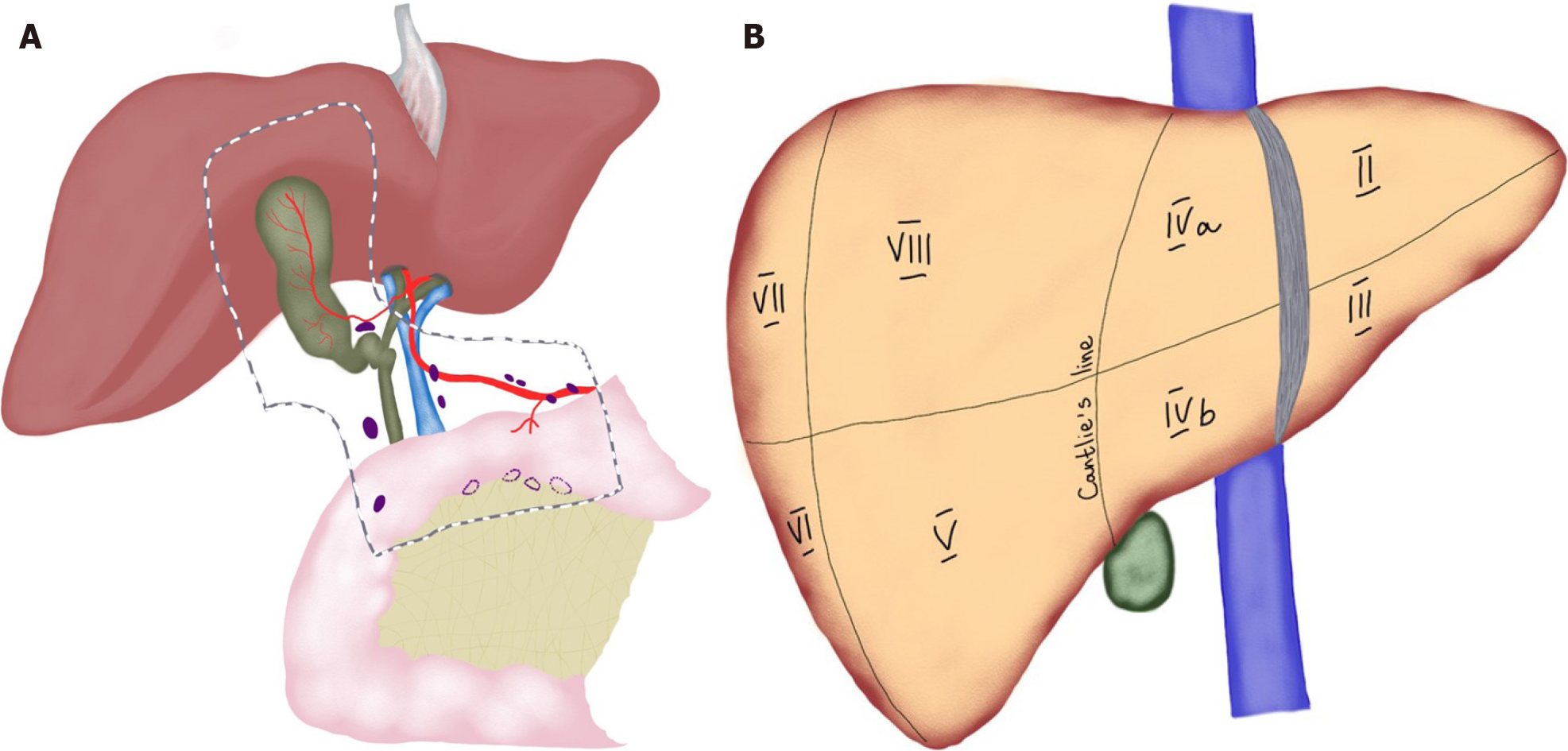
| Extended cholecystectomy | Simple cholecystectomy + hepatic wedge resection at the level of gallbladder fossa (2-3 cm in depth) | T1b and higher GBC |
| IVb/V hepatic bisegmentectomy | Resection of liver segments IVb and V en bloc with the gallbladder with intra-parenchymal transection of the middle hepatic vein | GBC invading liver parenchyma |
| Extended liver resections | Most commonly right hepatectomy, rarely left hepatectomy | GBC invading structures of porta hepatis |
| Bile duct resection | Resection of the extrahepatic bile duct + Roux-en-Y hepaticojejunostomy | GBC invading extrahepatic bile ducts or positive cystic duct margin at frozen section pathology |
| Lymphadenectomy | Removal of lymph nodes from N1 and N2 zones | T1b and higher GBC, N+ GBC |
| Multivisceral resection | May involve right colectomy, pancreaticoduodenectomy, resection of abdominal wall, etc. | Locally advanced GBC |
| Palliative procedures | ||
| Biliodigestive anastomoses | Roux-en-Y hepaticojejunostomy | Locally advanced unresectable GBC presenting with jaundice |
| Digestive anastomoses | Gastro-enteric anastomosis, ileo-transverse colon anastomosis | Locally advanced unresectable GBC presenting with intestinal obstruction |
In GBC with the T stage of T1b or higher, more radical procedures are required. The extent of radical cholecystectomy may vary from extended cholecystectomy, when liver margin at the gallbladder fossa is resected, to right hepatectomy or multi-visceral resection[12,48] (Figure 3). Contraindications for curative surgery include the fo
In patients with preoperatively detected GBC with no distant metastases, laparotomy is the traditional treatment of choice as it allows for extended resections. This was historically due to suboptimal accuracy in radiologic staging. More recently, current evidence suggests laparoscopy to be the feasible with advances in minimally invasive surgery[44,45]. Simple cholecystectomy is usually sufficient in T1a cancers when muscular layer is not involved and is associated with 73%-100% OS[49,50]. Intraoperative frozen section pathology of the cystic duct is a decisive factor for bile duct resection, if positive. In a recent study, bile duct resection was not found to improve lymph node harvest or OS[51]. Moreover, bile duct resection was associated with increased rates of postoperative morbidity in the AFC-GBC-2009 cohort[15]. Therefore, it should be reserved for cases with positive cystic duct margin. In T1b GBCs when the muscular layer is involved, extended cholecystectomy is indicated due to higher rates of lymph node metastases (15% vs 2.5%), higher loco-regional recurrence rates (60% vs 50%), and higher rates of liver invasion (13% vs 0%)[48]. Nonetheless, a recent SEER database study did not find the survival benefit of extended cholecystectomy to be as significant as the number of harvested lymph nodes more than six[52]. In T2 GBCs when peri-muscular connective tissue is involved, extended cholecystectomy with lymphadenectomy in N2 zone (hepatoduodenal ligament, along proper hepatic artery, and posterior superior pancreatic lymph nodes) is indicated as T2 cancers are associated with a higher rate of liver invasion (10%), higher rate of lymph node involvement (up to 60%), and higher risk of residual disease (up to 76%)[53] (Figure 1). It is known that survival in patients with T2 GBC correlates with the number of lymph nodes harvested[54]. In fact, data from previous reports suggested that a minimum of six lymph nodes should be removed in order to consider the nodal status negative[55]. With regard to resectable T3 and T4 cancers, surgeons were reluctant in the past to operate on these patients due to high postoperative morbidity and poor survival outcomes[56]. However, data from Japan indicating improved survival in these patients (15%-63% in T3 and 7%-25% in T4 cancers) advocated for surgical management[57]. Moreover, some authors have advocated for even more extensive multivisceral resections involving hepatectomy, colectomy, pancreaticodudenectomy, or nephrectomy in potentially resectable disease and have reported a median survival of 17 mo in such patients[58]. Nonetheless, postoperative morbidity (in half of the patients) and mortality (in every fifth patient) were significant. Dissimilarly, D’Angelica et al[59] in the United States did not find any survival benefit in patients undergoing multivisceral resection. In general, lymph nodes outside the hepatoduodenal ligament are usually not subject to resection since outcomes following radical lymphadenectomy are even worse than with N2 disease[12].
Surgery is the only curative treatment option in GBC, but recurrence is very common despite curative resection[60]. Therefore, considerable interest exists in applying neoadjuvant[61] or adjuvant therapy in the multi-modal management of GBC. Although there is a paucity of high-quality prospective studies, several chemotherapy regimens are recommended by NCCN in the neoadjuvant setting. These include gemcitabine/cisplatin, gemcitabine/oxaliplatin, capecitabine/oxaliplatin, and combinations of gemcitabine, capecitabine, and 5-FU[12].
American Hepato-Pancreato-Biliary Association (AHPBA) sponsored consensus meeting advocated for neoadjuvant therapy in patients with clinical T3/T4/N1 disease[62]. Patients receiving neoadjuvant treatment can potentially downsize their tumor or avoid unnecessary surgery in those with occult metastases. However, the latest meta-analysis on 8 studies totaling 474 patients found that there was insufficient data to support the routine use of neoadjuvant therapy for advanced GBC[12]. Only one-third of all the patients were down-staged completely to be able to achieve an R0 resection. There is a need for multicenter prospective studies on this topic.
After complete resection, locoregional and/or distant recurrences are very common. The burden of disease recurrence rate at locoregional sites is 33.9%-79.2%, distant sites, 52.6%-79.8%, and at both sites is 18.9%-50%, respectively[63]. Adjuvant chemoradiotherapy seems to offer a potential solution to improve OS, but high-quality data seems to be lacking. The literature supporting gemcitabine and fluropyrimidine based adjuvant chemotherapy comes from four randomized control trials (RCT)[64-67]. More recently, the 3 phase III RCTs trial BILCAP trial deserves special mention. This trial is considered pivotal in updating international treatment guidelines, in defining the role of capecitabine based ACT as a reference regimen in resectable GBC. These results set the benchmark for subsequent phase III trials testing other combination chemotherapy regimens in the adjuvant setting[68]. Intra-arterial chemotherapy (IAC) has a well-established role in decreasing liver metastases from colorectal cancer and is known to improve long-term survival[69]. More recently, IAC (based on oxaliplatin and gemcitabine regimen) has been shown to effectively and safely improve 3-year disease-free survival, OS, and hepatic metastases-free survival of stage 2-3 GBC[69].
Unfortunately, the majority of patients present with unresectable or metastatic disease when palliative treatment is the only option[70]. However, the emergence of targeted therapies is rapidly changing the treatment paradigm for biliary tract cancer[71].
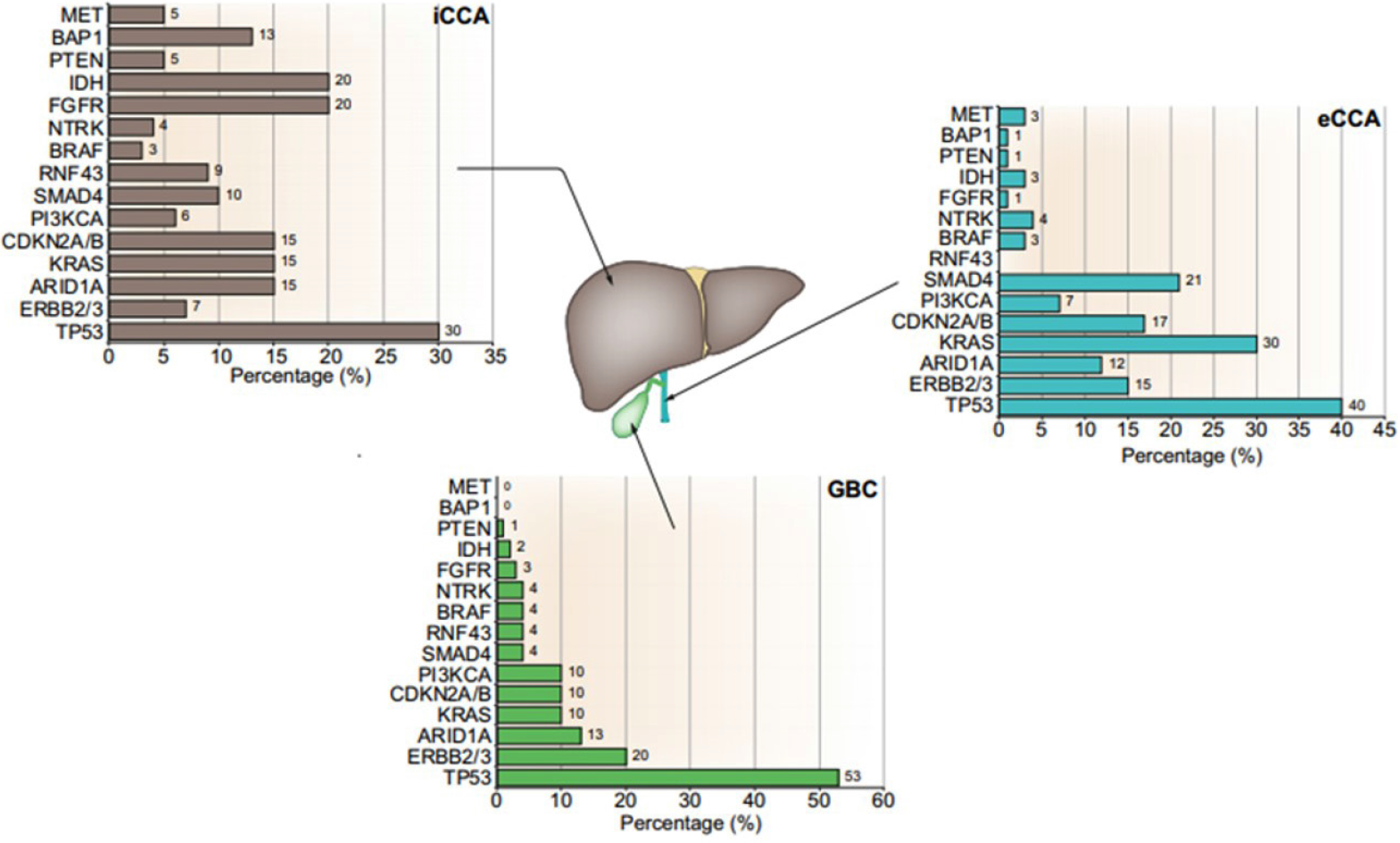
In recent years, ‘precision medicine’ for advanced or refractory advanced GBC is an exciting area of research and clinical trials. Essentially, it involves targeting specific molecular alterations with immunotherapy[72]. Figure 4 shows that alterations in genes such as IDH1/2, FGFR2, FGFR4, EGFR, ERBB2, and PIK3CA are frequent in biliary tract cancer and can be targets for treatment[71]. It is important to note that immunotherapy for GBC is still in infancy as compared to options for intrahepatic CCA.
Management of metastatic disease and unresectable begins with preoperative evaluation and biopsy to confirm the diagnosis[12]. With biopsy specimen, microsatellite instability and or mismatch repair testing should be routinely performed. The GBCs with MMR deficiency benefit from PD-1 receptor blockade such as pembrolizumab[73]. Pembrolizumab is a monoclonal antibody against PD-1, which has been shown to have a long-lasting antitumor activity and low toxicity in several-advanced cancers[74]. A recent study has shown that high PD-L1 expression was associated with a better response to pembrolizumab based therapy[75]. The combination of pertuzumab and trastuzumab was also investigated in 11 patients with previously treated biliary tract cancer, with HER2 amplification (8 patients) or HER2 mutations (3 patients). Risk reduction of 7.5% and 33.3% were achieved in these patients, respectively[76]. For patients with BRAF (V600E) mutations, dual inhibition with dabrafenib and trametinib have been investigated[77]. Within the biliary tract cancer subgroup (33 patients) in that study, partial response rates of 42% and 36% with a dual inhibitor were achieved, respectively. However, none of the studies so far has shown the superiority of immunotherapy over first or second-line systemic chemo
Given the severity of GBC, new surgical approaches and techniques are constantly being explored. Innovations in how these patients are treated are sought to decrease morbidity, and hopefully, eventually decrease mortality as well.
Laparoscopic and robotic surgical management of GBC greatly could reduce morbidity with decreased estimated blood loss and length of hospital stay[78,79]. Initially, laparoscopic approaches raised further questions due to increase in port site cancer occurrences in addition to the lack of dexterity of laparoscopic instruments[49]. Current consensus for minimally invasive surgery approach is still controversial among hepatobiliary/pancreas surgeons, especially in advanced GBCs[80]. Yoon et al[81] showed that laparoscopic extended cholecystectomy including lymphadenectomy for GBC achieved compatible outcomes with that of open surgery over long-term follow-up. Development of robotic surgery overcame some of the limitations of laparoscopic surgery and demonstrated equivalent outcomes to open approaches while retaining the benefits of minimally invasive approach[78,79]. Laparoscopic surgery for liver resection or bile duct resection showed comparable oncological outcomes for the experts and the instrumentation in the robotic platforms would be expected to show similar outcomes. The robotic installment allows increased dexterity required for proper lymphadenectomy and 3D high-definition visualization which could possibly aid in better visualization of anatomical structures.
Recent studies on robotic surgery for GBC have shown favorable results in several limited case series[79,82,83]. As such, there is a need for larger studies. While a randomized trial for a rare pathology is not feasible, larger cohort studies could still be conducted[82,83]. At present, the technical approach for the robotic surgery of GBC has been described in the literature, such as that of Belli et al[84]. Similar to the laparoscopic approach, the patient is positioned in reverse-Trendelenburg, with five ports being used which include the 3 ports for robotic instrumentation, 1 for the camera and 1 Laparoscopic port for an assistant surgeon[84].
Indocyanine green (ICG) is a fluorescent molecule that is water soluble and injected intravenously. ICG is taken up by the liver and excreted in biliary system allowing visualization of liver perfusion and imaging of the extrahepatic biliary tree. The use of ICG is not limited to the robotic surgery, however, it has been used widespread in the laparoscopic surgery prior to its adoption in robotic surgery. In the literature, its use has been described for GBC to identify the biliary system and lymph nodes[85]. ICG is useful to identify anatomical border of liver sections and ICG allows to perform anatomical resections in minimally invasive surgery setting. Yu et al[86] showed no difference between anatomical resection and non-anatomical resection in GBC. Si et al[87] reported anatomical resection for intrahepatic CCA had similar complication rates, but showed better survival outcomes in tumors with stage IB or II without vascular invasions. Hepatocellular carcinoma might have benefit from anatomical resection[88,89]. For oncology prospective, it is better to perform anatomical resection and ICG makes anatomical resection easier. Current evidence showed that no difference in outcomes, however, we need to study using ICG and compare the outcomes.
Several ablative therapies have been utilized for management of GBC when it is isolated to the liver parenchyma. Modalities such as microwave ablation, radiofrequency ablation (RFA), alcohol ablation all have had limited success. Ablative therapies work by destruction of liver parenchyma through targeted tissue necrosis. Because of the need to avoid injury to the central biliary tree, these modalities have been mostly limited to local regional recurrence or metastatic sites for GBC[90,91]. Microwave ablation, RFA and alcohol ablation may also be useful to treat palliative purposes for advanced stage GBC, however, the literature is limited on this topic and further research is needed to better understand the benefits of such an approach.
With improvements in preoperative imaging, augmented reality allows precise preoperative planning for surgical resections with superimposed intraoperative imaging. This technology takes multiple preoperative images, integration into a 3D reconstruction, followed by preoperative planning and incorporation intraoperatively to improve identification of structures and precision of resections. Several software systems have been developed to generate such simulations which can simulate surgical resections[92]. Intraoperatively, the images are superimposed directly over the patient’s anatomy and the technology has been used in a case series by Peterhans et al[93]. Augmented reality has also been used as an aid in visualization by helping to see through structures, aiding in port placement, and identification of intrahepatic vasculature. Additionally, artificial intelligence, while still in its infancy, may also assist in locating and identifying structures in complex surgeries[94]. Such technology could lead to improved margins of resection and preservation of unaffected and critical structures in patients.
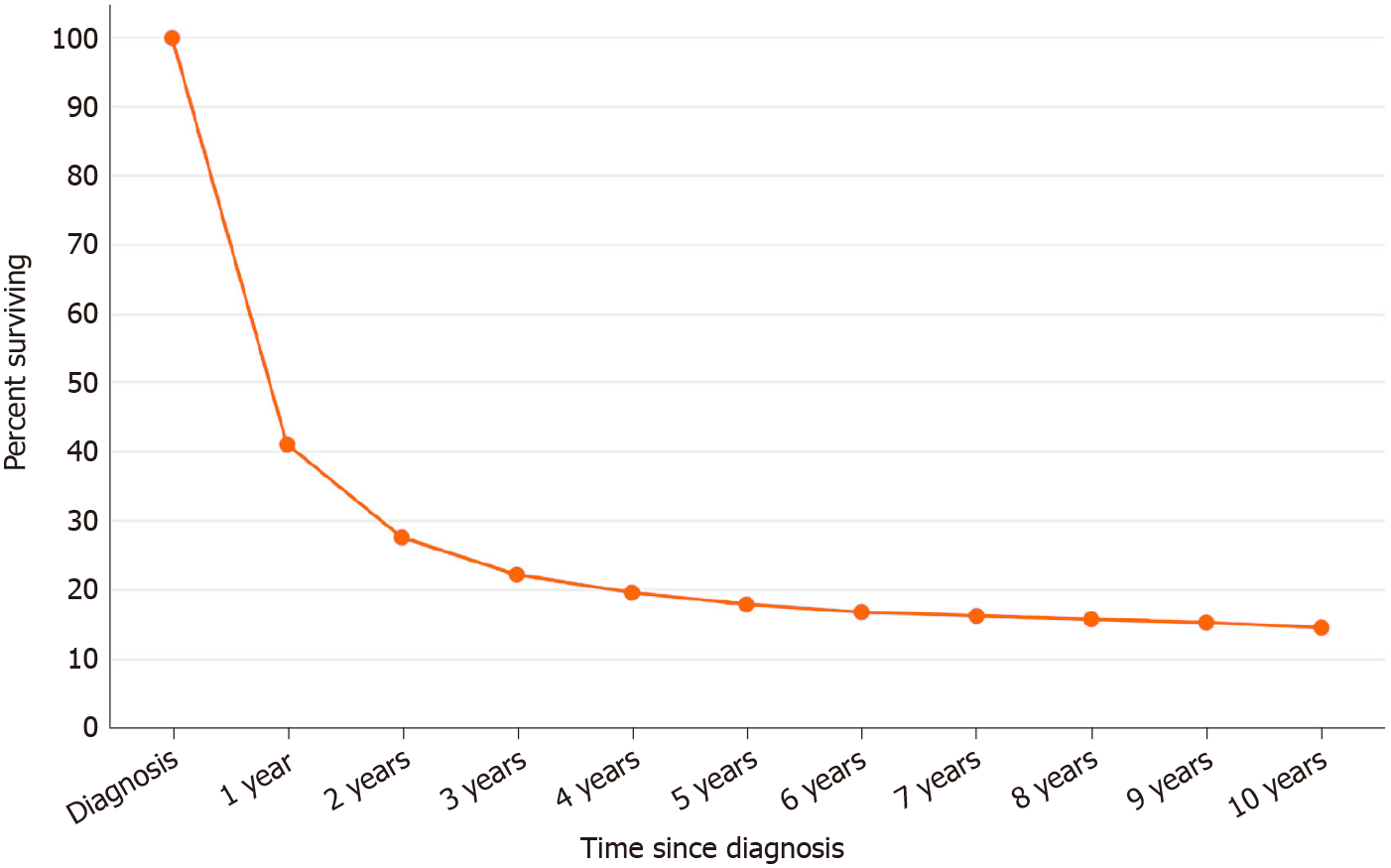
The prognosis of GBC remains guarded. Most GBC are diagnosed as advanced conditions. Surgical resection is an important factor for management of GBC. According to SEER database between 2000 and 2016, five-year survival is 17.9%[95] (Figure 5). The age-adjusted incidence of gallbladder has remain unchanged around 1.2 per 100000, however the mortality has been decreasing significantly over the last 2 decades (P < 0.01)[95].
We have reviewed the outcomes of previous published studies for GBC (Table 5). The overall five-year survival for all patients with GBC is around 20 percent (SEER 19.2%, Figure 5)[95]. Patients with localized disease who are amenable to surgical treatment have over 65% survival in five years[95]. However, survival of patients with distant metastasis remains dismal at 2%-13%. Therefore, potentially resectable patients have a significant advantage over patients without surgical options.
| Ref. | n | Year | Status | Survival time (mo) |
| Gall et al[96], 1991 | 113 | 1970-1989 | Curative resection | 42 |
| Non-curative resection (metastasis +) | 12.5 | |||
| Cubertafond et al[97], 1994 | 724 | 1980-1989 | Curative resection 25% | Overall 3 |
| Non-curative resection 75% | ||||
| Jarnagin et al[98], 2003 | 97 | 1990-2001 | Curative resection | 31.3 |
| Hawkins et al[29], 2004 | 240 | 1995-2002 | Curative resection | 34 |
| Non-curative resection | 8 | |||
| Nishio et al[99], 2007 | 166 | 1977-2004 | Curative resection | 12 |
| Non-curative resection | 6 | |||
| Duffy et al[28], 2008 | 206 | 1995-2005 | Overall | 10.3 |
| Incidental GBC | 15.7 | |||
| Curative surgery | 30.3 | |||
| Curative surgery + adjuvant therapy | 23.4 | |||
| D’Hondt et al[25], 2013 | 102 | 1998-2008 | Overall | 7.2 |
| Incidental GBC | 25.8 | |||
| Non-Incidental GBC | 4.4 | |||
| Butte et al[100], 2011 | 261 | 1999-2007 | Overall | DSS 16.97 |
| Ito et al[55], 2011 | 122 | 1992-2007 | R0 resection | DSS 41 |
| Butte et al[101], 2014 | 135 | 1998-2009 | Overall | DFS 25.9 |
| Residual disease | 11.2 | |||
| Non-residual disease | 93.4 | |||
| Ethun et al[18], 2017 | 449 | 2000-2014 | Incidental GBC | 27.6 |
| Zhang et al[102], 2018 | 1422 | 2010-2014 | Resection | 13 |
| Yu et al[86], 2019 | 81 | 2006-2015 | T3 Anatomical resection | 54 |
| T3 Wedge resection | 49 | |||
| Ref. | n | Year | Status | Survival rate (%) |
| Ogura et al[103], 1991 | 1686 | 1979-1988 | Radical resection | 66.2 (3 yr) 50.7 (5 yr) |
| Non-radical resection | 14.1 (3 yr) 6.2 (5 yr) | |||
| Cubertafond et al[97], 1994 | 724 | 1980-1989 | Curative resection 25% | Overall 14 (1 yr) 5 (5 yr) |
| Non-curative resection 75% | ||||
| Carriaga and Henson[104], 1995 | 4412 | 1973-1987 | Overall | 12.3 (5 yr) |
| Bartlett et al[105], 1996 | 149 | 1985-1993 | Resection | 51 (5 yr) |
| Fong et al[17], 2000 | 410 | 1986-2000 | Resection | 38 (5 yr) |
| Non-resection | 4 (5 yr) | |||
| Nakeeb et al[106], 2002 | 140 | 1990-2001 | Resection | 31 (5 yr) |
| Pawlik et al[107], 2007 | 115 | 1984-2006 | Resection | 12 (5 yr) |
| Kayahara and Nagakawa[57], 2007 | 4770 | 1988-1997 | Overall | 39 (5 yr) |
| Shih et al[108], 2007 | 107 | 1995-2004 | Overall | 15 (5 yr) |
| Incidental GBC | 33 (5 yr) | |||
| D’Angelica et al[59], 2009 | 109 | 1988-2002 | Overall | 42 (5 yr) |
| Fuks et al[15], 2011 | 218 | 1998-2008 | Incidental GBC | 41 (5 yr) |
| Hari et al[109], 2013 | 1115 | 1988-2008 | Cholecystectomy | 50 (5 yr) |
| Cholecystectomy + LN dissection | 70 (5 yr) | |||
| Radical cholecystectomy | 79 (5 yr) | |||
| Barreto et al[110], 2014 | 163 | 2003-2010 | Overall | DFS 79.6 (2 yr) |
| Shindoh et al[42], 2015 | 252 | 1981-2011 | T2 | 42.6 (5 yr) |
GBC remains a formidable disease. Surgical management has traditionally been challenging due to the technical difficulties associated with liver resection and biliary reconstruction. In addition, effective cytotoxic agents were limited. However, newer developments in management are leading to significant changes in the prognosis of this disease. Minimally invasive techniques in management such as robotic surgery coupled with ablative techniques offer quicker recovery for patients undergoing surgery for loco-regional disease. Perioperative management for incidentally discovered GBC is being better defined based on more accurate pathologic staging. Targeted therapies are offering renewed enthusiasm among oncologists with its rapidly evolving landscape. Nonetheless, much remains undefined in management of GBC and further studies are urgently needed in this malignancy.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Da Costa AC, Ghannam WM, Gupta V, Saengboonmee C S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55833] [Article Influence: 7976.1] [Reference Citation Analysis (132)] |
| 2. | Shaffer EA. Gallbladder cancer: the basics. Gastroenterol Hepatol (NY). 2008;4:737-741. [PubMed] |
| 3. | Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 575] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 4. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 490] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 5. | Oneda E, Abu Hilal M, Zaniboni A. Biliary Tract Cancer: Current Medical Treatment Strategies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol. 2017;23:3978-3998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 212] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (4)] |
| 7. | Jain K, Sreenivas V, Velpandian T, Kapil U, Garg PK. Risk factors for gallbladder cancer: a case-control study. Int J Cancer. 2013;132:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Shrikhande SV, Barreto SG, Singh S, Udwadia TE, Agarwal AK. Cholelithiasis in gallbladder cancer: coincidence, cofactor, or cause! Eur J Surg Oncol. 2010;36:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 9. | Park M, Song DY, Je Y, Lee JE. Body mass index and biliary tract disease: a systematic review and meta-analysis of prospective studies. Prev Med. 2014;65:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Barreto SG, Haga H, Shukla PJ. Hormones and gallbladder cancer in women. Indian J Gastroenterol. 2009;28:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Glauser PM, Strub D, Käser SA, Mattiello D, Rieben F, Maurer CA. Incidence, management, and outcome of incidental gallbladder carcinoma: analysis of the database of the Swiss association of laparoscopic and thoracoscopic surgery. Surg Endosc. 2010;24:2281-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, Anders R, Are C, Brown D, Chang DT. Hepatobiliary cancers, version 2.2019 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2019;17:302-310. |
| 13. | Goetze TO, Paolucci V. Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc. 2010;24:2156-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 14. | Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, Kuvshinoff B. Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Fuks D, Regimbeau JM, Le Treut YP, Bachellier P, Raventos A, Pruvot FR, Chiche L, Farges O. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World J Surg. 2011;35:1887-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Varshney S, Butturini G, Gupta R. Incidental carcinoma of the gallbladder. Eur J Surg Oncol. 2002;28:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 17. | Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Ethun CG, Postlewait LM, Le N, Pawlik TM, Buettner S, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Jin LX, Weber SM, Salem A, Martin RC, Scoggins C, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Kooby DA, Maithel SK. Association of Optimal Time Interval to Re-resection for Incidental Gallbladder Cancer With Overall Survival: A Multi-Institution Analysis From the US Extrahepatic Biliary Malignancy Consortium. JAMA Surg. 2017;152:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Sarli L, Contini S, Sansebastiano G, Gobbi S, Costi R, Roncoroni L. Does laparoscopic cholecystectomy worsen the prognosis of unsuspected gallbladder cancer? Arch Surg. 2000;135:1340-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | de Aretxabala XA, Roa IS, Mora JP, Orellana JJ, Riedeman JP, Burgos LA, Silva VP, Cuadra AJ, Wanebo HJ. Laparoscopic cholecystectomy: its effect on the prognosis of patients with gallbladder cancer. World J Surg. 2004;28:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Choi KS, Choi SB, Park P, Kim WB, Choi SY. Clinical characteristics of incidental or unsuspected gallbladder cancers diagnosed during or after cholecystectomy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (2)] |
| 22. | Maker AV, Butte JM, Oxenberg J, Kuk D, Gonen M, Fong Y, Dematteo RP, D'Angelica MI, Allen PJ, Jarnagin WR. Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol. 2012;19:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Ethun CG, Postlewait LM, Le N, Pawlik TM, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick BA, Weber SM, Salem A, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Cardona K, Maithel SK. Routine port-site excision in incidentally discovered gallbladder cancer is not associated with improved survival: A multi-institution analysis from the US Extrahepatic Biliary Malignancy Consortium. J Surg Oncol. 2017;115:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Fuks D, Regimbeau JM, Pessaux P, Bachellier P, Raventos A, Mantion G, Gigot JF, Chiche L, Pascal G, Azoulay D, Laurent A, Letoublon C, Boleslawski E, Rivoire M, Mabrut JY, Adham M, Le Treut YP, Delpero JR, Navarro F, Ayav A, Boudjema K, Nuzzo G, Scotte M, Farges O. Is port-site resection necessary in the surgical management of gallbladder cancer? J Visc Surg. 2013;150:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | D'Hondt M, Lapointe R, Benamira Z, Pottel H, Plasse M, Letourneau R, Roy A, Dagenais M, Vandenbroucke-Menu F. Carcinoma of the gallbladder: patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience. Eur J Surg Oncol. 2013;39:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Pitt SC, Jin LX, Hall BL, Strasberg SM, Pitt HA. Incidental gallbladder cancer at cholecystectomy: when should the surgeon be suspicious? Ann Surg. 2014;260:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH, O'Reilly EM. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 29. | Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Strom BL, Maislin G, West SL, Atkinson B, Herlyn M, Saul S, Rodriguez-Martinez HA, Rios-Dalenz J, Iliopoulos D, Soloway RD. Serum CEA and CA 19-9: potential future diagnostic or screening tests for gallbladder cancer? Int J Cancer. 1990;45:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Ritts RE Jr, Nagorney DM, Jacobsen DJ, Talbot RW, Zurawski VR Jr. Comparison of preoperative serum CA19-9 levels with results of diagnostic imaging modalities in patients undergoing laparotomy for suspected pancreatic or gallbladder disease. Pancreas. 1994;9:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Gamboa AC, Maithel SK. The Landmark Series: Gallbladder Cancer. Ann Surg Oncol. 2020;27:2846-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Toda K, Souda S, Yoshikawa Y, Momiyama T, Ohshima M. Significance of laparoscopic excisional biopsy for polypoid lesions of the gallbladder. Surg Laparosc Endosc. 1995;5:267-271. [PubMed] |
| 34. | Pandey M, Sood BP, Shukla RC, Aryya NC, Singh S, Shukla VK. Carcinoma of the gallbladder: role of sonography in diagnosis and staging. J Clin Ultrasound. 2000;28:227-232. [PubMed] [DOI] [Full Text] |
| 35. | Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC cancer staging manual. 8th ed. New York: Springer, 2017: 1032. |
| 36. | Sadamoto Y, Kubo H, Harada N, Tanaka M, Eguchi T, Nawata H. Preoperative diagnosis and staging of gallbladder carcinoma by EUS. Gastrointest Endosc. 2003;58:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee. Anderson MA, Appalaneni V, Ben-Menachem T, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Fisher LR, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan K, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Shergill AK, Dominitz JA, Cash BD. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Goel S, Aggarwal A, Iqbal A, Gupta M, Rao A, Singh S. 18-FDG PET-CT should be included in preoperative staging of gall bladder cancer. Eur J Surg Oncol. 2020;46:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Nevin JE, Moran TJ, Kay S, King R. Carcinoma of the gallbladder: staging, treatment, and prognosis. Cancer. 1976;37:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Donohue JH, Nagorney DM, Grant CS, Tsushima K, Ilstrup DM, Adson MA. Carcinoma of the gallbladder. Does radical resection improve outcome? Arch Surg. 1990;125:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 144] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Shindoh J, de Aretxabala X, Aloia TA, Roa JC, Roa I, Zimmitti G, Javle M, Conrad C, Maru DM, Aoki T, Vigano L, Ribero D, Kokudo N, Capussotti L, Vauthey JN. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg. 2015;261:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 43. | Lee H, Choi DW, Park JY, Youn S, Kwon W, Heo JS, Choi SH, Jang KT. Surgical Strategy for T2 Gallbladder Cancer According to Tumor Location. Ann Surg Oncol. 2015;22:2779-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Lee SE, Jang JY, Lim CS, Kang MJ, Kim SW. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol. 2011;17:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Pilgrim C, Usatoff V, Evans PM. A review of the surgical strategies for the management of gallbladder carcinoma based on T stage and growth type of the tumour. Eur J Surg Oncol. 2009;35:903-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Davidson JT 4th, Jin LX, Krasnick B, Ethun CG, Pawlik TM, Poultsides GA, Idrees K, Weber SM, Martin RCG, Shen P, Hatzaras I, Maithel SK, Fields RC; and the U. S. Extrahepatic Biliary Malignancy Consortium. Staging laparoscopy among three subtypes of extra-hepatic biliary malignancy: a 15-year experience from 10 institutions. J Surg Oncol. 2019;119:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Agarwal AK, Kalayarasan R, Javed A, Gupta N, Nag HH. The role of staging laparoscopy in primary gall bladder cancer--an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg. 2013;258:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Abramson MA, Pandharipande P, Ruan D, Gold JS, Whang EE. Radical resection for T1b gallbladder cancer: a decision analysis. HPB (Oxford). 2009;11:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 49. | Suzuki K, Kimura T, Ogawa H. Long-term prognosis of gallbladder cancer diagnosed after laparoscopic cholecystectomy. Surg Endosc. 2000;14:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Yildirim E, Celen O, Gulben K, Berberoglu U. The surgical management of incidental gallbladder carcinoma. Eur J Surg Oncol. 2005;31:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Gani F, Buettner S, Margonis GA, Ethun CG, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick B, Weber SM, Salem A, Martin RC, Scoggins C, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Maithel SK, Pawlik TM. Assessing the impact of common bile duct resection in the surgical management of gallbladder cancer. J Surg Oncol. 2016;114:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Xu L, Tan H, Liu X, Huang J, Liu L, Si S, Sun Y, Zhou W, Yang Z. Survival benefits of simple vs extended cholecystectomy and lymphadenectomy for patients with T1b gallbladder cancer: An analysis of the surveillance, epidemiology, and end results database (2004 to 2013). Cancer Med. 2020;9:3668-3679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Kapoor VK. Incidental gallbladder cancer. Am J Gastroenterol. 2001;96:627-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Downing SR, Cadogan KA, Ortega G, Oyetunji TA, Siram SM, Chang DC, Ahuja N, Leffall LD Jr, Frederick WA. Early-stage gallbladder cancer in the Surveillance, Epidemiology, and End Results database: effect of extended surgical resection. Arch Surg. 2011;146:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Ito H, Ito K, D'Angelica M, Gonen M, Klimstra D, Allen P, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Cubertafond P, Mathonnet M, Gainant A, Launois B. Radical surgery for gallbladder cancer. Results of the French Surgical Association Survey. Hepatogastroenterology. 1999;46:1567-1571. [PubMed] |
| 57. | Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer. 2007;110:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Dixon E, Vollmer CM Jr, Sahajpal A, Cattral M, Grant D, Doig C, Hemming A, Taylor B, Langer B, Greig P, Gallinger S. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg. 2005;241:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 59. | D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 60. | Wang J, Narang AK, Sugar EA, Luber B, Rosati LM, Hsu CC, Fuller CD, Pawlik TM, Miller RC, Czito BG, Tuli R, Crane CH, Ben-Josef E, Thomas CR Jr, Herman JM. Evaluation of Adjuvant Radiation Therapy for Resected Gallbladder Carcinoma: A Multi-institutional Experience. Ann Surg Oncol. 2015;22 Suppl 3:S1100-S1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Chaudhari VA, Ostwal V, Patkar S, Sahu A, Toshniwal A, Ramaswamy A, Shetty NS, Shrikhande SV, Goel M. Outcome of neoadjuvant chemotherapy in "locally advanced/borderline resectable" gallbladder cancer: the need to define indications. HPB (Oxford). 2018;20:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 62. | Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, Coimbra FJ, Jarnagin WR. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015;17:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 63. | Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - A systematic review. Eur J Surg Oncol. 2019;45:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 64. | Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, Depisch D, Lang F, Scheithauer W. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004;15:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Ducreux M, Van Cutsem E, Van Laethem JL, Gress TM, Jeziorski K, Rougier P, Wagener T, Anak O, Baron B, Nordlinger B; EORTC Gastro Intestinal Tract Cancer Group. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3167] [Article Influence: 211.1] [Reference Citation Analysis (1)] |
| 67. | Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J, Koshiji M, Nambu Y, Furuse J, Miyazaki M, Nimura Y. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 568] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 68. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 822] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 69. | Margonis GA, Gani F, Buettner S, Amini N, Sasaki K, Andreatos N, Ethun CG, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick B, Weber SM, Salem A, Martin RC, Scoggins C, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Maithel SK, Pawlik TM. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford). 2016;18:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 70. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 484] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 71. | Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: Ready for "prime time" in biliary tract cancer. J Hepatol. 2020;73:170-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 72. | Verlingue L, Malka D, Allorant A, Massard C, Ferté C, Lacroix L, Rouleau E, Auger N, Ngo M, Nicotra C, De Baere T, Tselikas L, Ba B, Michiels S, Scoazec JY, Boige V, Ducreux M, Soria JC, Hollebecque A. Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer. 2017;87:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 73. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4951] [Article Influence: 618.9] [Reference Citation Analysis (0)] |
| 74. | Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4861] [Article Influence: 486.1] [Reference Citation Analysis (0)] |
| 75. | Ahn S, Lee JC, Shin DW, Kim J, Hwang JH. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep. 2020;10:12348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 76. | Javle MM, Hainsworth JD, Swanton C, Burris HA, Kurzrock R, Sweeney C, Meric-Bernstam F, Spigel DR, Bose R, Guo S. Pertuzumab+ trastuzumab for HER2-positive metastatic biliary cancer: Preliminary data from MyPathway. J Clin Oncol. 2017;35:402. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Berking C, Livingstone E, Weichenthal M, Leiter U, Wittmann K, Eigentler T, Mohr P, Kiecker F, Loquai C, Debus D. Efficacy and safety of dabrafenib and trametinib in patients with metastatic BRAFV600 mutation-positive melanoma in the real-world setting: Interim results of the non-interventional COMBI-r study. Ann Oncol. 2019;30:v544-v545. |
| 78. | Goel M, Khobragade K, Patkar S, Kanetkar A, Kurunkar S. Robotic surgery for gallbladder cancer: Operative technique and early outcomes. J Surg Oncol. 2019;119:958-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Vega EA, De Aretxabala X, Qiao W, Newhook TE, Okuno M, Castillo F, Sanhueza M, Diaz C, Cavada G, Jarufe N, Munoz C, Rencoret G, Vivanco M, Joechle K, Tzeng CD, Vauthey JN, Vinuela E, Conrad C. Comparison of oncological outcomes after open and laparoscopic re-resection of incidental gallbladder cancer. Br J Surg. 2020;107:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 80. | Han HS, Yoon YS, Agarwal AK, Belli G, Itano O, Gumbs AA, Yoon DS, Kang CM, Lee SE, Wakai T, Troisi RI. Laparoscopic Surgery for Gallbladder Cancer: An Expert Consensus Statement. Dig Surg. 2019;36:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 81. | Yoon YS, Han HS, Cho JY, Choi Y, Lee W, Jang JY, Choi H. Is Laparoscopy Contraindicated for Gallbladder Cancer? J Am Coll Surg. 2015;221:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 82. | Shen BY, Zhan Q, Deng XX, Bo H, Liu Q, Peng CH, Li HW. Radical resection of gallbladder cancer: could it be robotic? Surg Endosc. 2012;26:3245-3250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Georgakis GV, Novak S, Bartlett DL, Zureikat AH, ZEH III HJ, Hogg ME. The Emerging Role of Minimally-Invasive Surgery For Gallbladder Cancer: A Comparison to Open Surgery. Conn Med. 2018;82. |
| 84. | Belli A, Patrone R, Albino V, Leongito M, Piccirillo M, Granata V, Pasta G, Palaia R, Izzo F. Robotic surgery of gallbladder cancer. Mini Surg. 2020;4. |
| 85. | Ahmad A. Use of indocyanine green (ICG) augmented near-infrared fluorescence imaging in robotic radical resection of gallbladder adenocarcinomas. Surg Endosc. 2020;34:2490-2494. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Yu LH, Yuan B, Fu XH, Yu WL, Liu J, Zhang YJ. Does Anatomic Resection Get More Benefits than Wedge Hepatectomy on the Prognosis for pT3 Unsuspected Gallbladder Cancer? J Laparoendosc Adv Surg Tech A. 2019;29:1414-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Si A, Li J, Yang Z, Xia Y, Yang T, Lei Z, Cheng Z, Pawlik TM, Lau WY, Shen F. Impact of Anatomical Versus Non-anatomical Liver Resection on Short- and Long-Term Outcomes for Patients with Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2019;26:1841-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 88. | Cucchetti A, Cescon M, Ercolani G, Bigonzi E, Torzilli G, Pinna AD. A comprehensive meta-regression analysis on outcome of anatomic resection vs nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol. 2012;19:3697-3705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 89. | Moris D, Tsilimigras DI, Kostakis ID, Ntanasis-Stathopoulos I, Shah KN, Felekouras E, Pawlik TM. Anatomic vs non-anatomic resection for hepatocellular carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol. 2018;44:927-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 90. | Mizandari M, Pai M, Xi F, Valek V, Tomas A, Quaretti P, Golfieri R, Mosconi C, Guokun A, Kyriakides C, Dickinson R, Nicholls J, Habib N. Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol. 2013;36:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 91. | Lee YW, Kim HJ, Lee SY, Heo J, Jung MK. Palliative Measures with Ethanol Gallbladder Ablation and Endobiliary Radiofrequency Ablation Followed by Endoscopic Biliary Stent Placement in an Advanced Case of Common Bile Duct Cancer: A Case Report. Korean J Gastroenterol. 2020;75:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Tang R, Ma LF, Rong ZX, Li MD, Zeng JP, Wang XD, Liao HE, Dong JH. Augmented reality technology for preoperative planning and intraoperative navigation during hepatobiliary surgery: A review of current methods. Hepatobiliary Pancreat Dis Int. 2018;17:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 93. | Peterhans M, vom Berg A, Dagon B, Inderbitzin D, Baur C, Candinas D, Weber S. A navigation system for open liver surgery: design, workflow and first clinical applications. Int J Med Robot. 2011;7:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 94. | Bari H, Wadhwani S, Dasari BVM. Role of artificial intelligence in hepatobiliary and pancreatic surgery. World J Gastrointest Surg. 2021;13:7-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Surveillance, Epidemiology, and End Results Program. SEER*Explorer: An interactive website for SEER cancer statistics. [cited 14 September 2020]. In: National Cancer Institute [Internet]. Available from: https://seer.cancer.gov/explorer/. |
| 96. | Gall FP, Köckerling F, Scheele J, Schneider C, Hohenberger W. Radical operations for carcinoma of the gallbladder: present status in Germany. World J Surg. 1991;15:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 209] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 98. | Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, Wagman R, Blumgart LH, Fong Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 99. | Nishio H, Nagino M, Ebata T, Yokoyama Y, Igami T, Nimura Y. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg. 2007;14:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 100. | Butte JM, Matsuo K, Gönen M, D'Angelica MI, Waugh E, Allen PJ, Fong Y, DeMatteo RP, Blumgart L, Endo I, De La Fuente H, Jarnagin WR. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J Am Coll Surg. 2011;212:50-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 101. | Butte JM, Kingham TP, Gönen M, D'Angelica MI, Allen PJ, Fong Y, DeMatteo RP, Jarnagin WR. Residual disease predicts outcomes after definitive resection for incidental gallbladder cancer. J Am Coll Surg. 2014;219:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 102. | Zhang W, Hong HJ, Chen YL. Establishment of a Gallbladder Cancer-Specific Survival Model to Predict Prognosis in Non-metastatic Gallbladder Cancer Patients After Surgical Resection. Dig Dis Sci. 2018;63:2251-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 103. | Ogura Y, Mizumoto R, Isaji S, Kusuda T, Matsuda S, Tabata M. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg. 1991;15:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 182] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 104. | Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 105. | Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 270] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 106. | Nakeeb A, Tran KQ, Black MJ, Erickson BA, Ritch PS, Quebbeman EJ, Wilson SD, Demeure MJ, Rilling WS, Dua KS, Pitt HA. Improved survival in resected biliary malignancies. Surgery. 2002;132:555-563; discission 563-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 107. | Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, Adams RB, Staley CA, Trindade EN, Schulick RD, Choti MA, Capussotti L. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478-1486; discussion 1486-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 108. | Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, Campbell KA, Yeo CJ, Talamini MA. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 109. | Hari DM, Howard JH, Leung AM, Chui CG, Sim MS, Bilchik AJ. A 21-year analysis of stage I gallbladder carcinoma: is cholecystectomy alone adequate? HPB (Oxford). 2013;15:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 110. | Barreto SG, Pawar S, Shah S, Talole S, Goel M, Shrikhande SV. Patterns of failure and determinants of outcomes following radical re-resection for incidental gallbladder cancer. World J Surg. 2014;38:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |