Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1229
Peer-review started: February 21, 2021
First decision: April 19, 2021
Revised: April 28, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: October 15, 2021
Processing time: 233 Days and 20.6 Hours
Cholangiocarcinomas (CCAs) are diverse biliary epithelial tumours involving the intrahepatic, perihilar and distal parts of the biliary tree. The three entirely variable entities have distinct epidemiology, molecular characteristics, prognosis and strategy for clinical management. However, many cholangiocarcinoma tu
Core Tip: The significant role of autophagy in maintaining the energy balance of cancer cells in tumorigenesis remains controversial. A grown body of research data suggests that autophagy is a promising target for several cancer types, including cholangiocarcinomas (CCAs). A novel therapeutic approach which could involve autophagy ma
- Citation: Koustas E, Trifylli EM, Sarantis P, Papavassiliou AG, Karamouzis MV. Role of autophagy in cholangiocarcinoma: An autophagy-based treatment strategy. World J Gastrointest Oncol 2021; 13(10): 1229-1243
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1229.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1229
Cholangiocarcinoma (CCA) constitutes a highly malignant group of epithelial tu
Cholangiocarcinogenesis is a multistep event, resulted from deregulated signaling pathways and genomic aberrations[2,21]. Chronic biliary inflammation leads to the proinflammatory cytokine overexpression, like interleukin-6 (IL6), which has the role of growth factor in CCA[22,23]. FGFR gene fusion with MGEA5, TACC3, BICC1, PPHLN1 and ROS is reported, consisting of therapeutic targets[10,24-26]. A variety of mutations have been reported, like KRAS, TP53, RNF43, ROBO2, CDKN2A MLL3, SMAD4, ARID1A, and a recently reported in IDH, which also composes a druggable target[10,27]. KRAS and Tp53 mutations are associated with an aggressive behaviour of tumours and poor prognosis [E], while the latter is frequently coexisting with viral hepatitis B inflection[28,29]. Extrahepatic CCA, are frequently associated with ERBBE, ELF3 mutations and PRKACA-PRKACB fusions, while iCCA with IDH1/2, BRAF, ARID1A and FGFR gene fusions[30]. Epigenetic and microRNAs deregulation, are also reported. The former is frequently resulted by the mutation of MLLE, ARIDA1A and IDH[4,12,31], involved in chromatin remodeling and DNA methylation[3,32,33]. microRNAs up or down-regulation is closely involved in cell cycle function, including autophagy, as well as in invasion, metastasis and chemoresistance[34,35], while they constitute biomarkers for survival and prognosis prediction, especially miR-10b, miR-22 and miR-551b[10,36-38].
Autophagy is a multiphasic, homeostatic, self-degenerative cellular mechanism by which non-functional, clustered or mutant proteins and impaired organelles such as Endoplasmic reticulum, peroxisomes or mitochondria, are insulated into vesicles, which are further fused with lysosomes for the degeneration process[39]. Autophagy appears to have a dual role in cancer, either promotes or suppress carcinogenesis. This peculiar capacity has created new therapeutic strategies for cancer via interfering in autophagy steps[40]. Despite the fact that autophagy’s regulatory mechanism on tumors is still examined, many studies demonstrate propitious results of its thera
Based on several preclinical studies, disturbances in autophagy regulation are closely related to carcinogenesis in cholangiocytes, as well as with metastasis and dismal outcomes, while it can act as a potent anti-cancer drug target[42].
This review gathers information from the current clinical and preclinical research data, about autophagy modulation in CCA and the therapeutic strategies for this highly invasive malignancy.
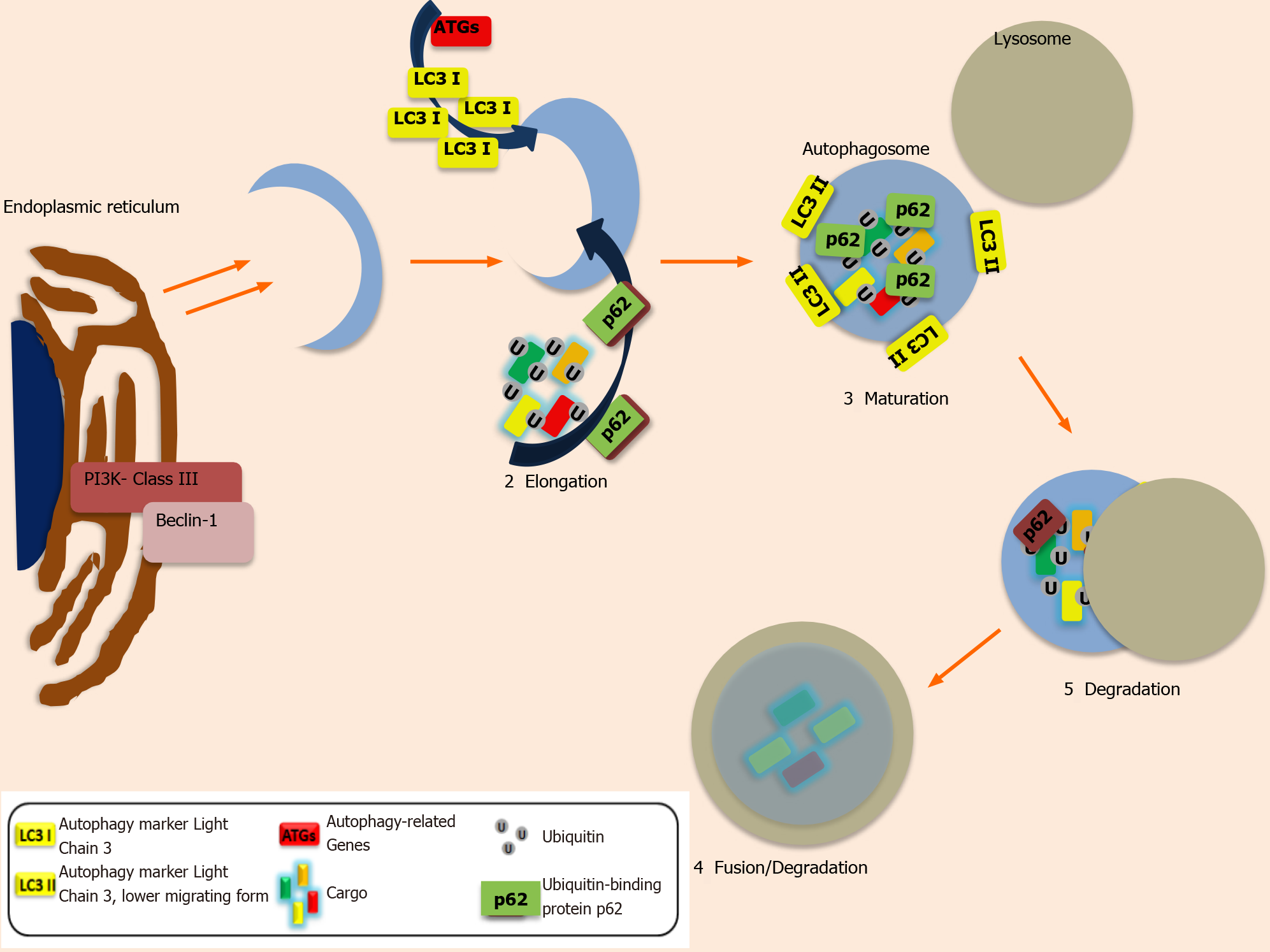
Autophagy (previously described as Macroautophagy) ensures cellular survival under stressful conditions[40]. Other less described entities of autophagy are: Microauto
Despite the fact that it is a physiological mechanism, it has a dual role (as it was mentioned before), either as a tumour suppressor or promoter of tumorigenesis and metastasis[43,44]. This complex procedure includes a series of steps in order to allow the engulfment of the cellular organelles by vesicles, the formation and the expansion of phagophore, the maturation into autophagosome and the fusion of the latter with the lysosome, with the formation of autolysosome, which is responsible for the degradation and recycling of the organelles[39]. The first step of the mechanism (induction) is initiated, by the inactivation of mammalian target of rapamycin (mTOR), allowing the activation of Unc-51-like kinase1 complex (ULK1) and the cargo selection and engulfment by vacuoles. The second step (nucleation), includes the activation and phosphorylation of activated class III PI3K complex by ULK1, with the formation of PI3K -Beclin-1 complex[39]. In the third step, phagophore starts to expand via mem
CCA is a highly diversified group of malignancies that exhibit various risk factors and an aberrant epigenetic and genetic landscape[2]. The well-established therapeutic strategies include surgical tumor resection, chemotherapy regimens, as well as locoregional therapies. Only a limited portion of patients (1/3) are eligible for tumor re
Genetic and epigenetic information, as well as the knowledge of the molecular pathways in CCA, which contribute to tumor resistance, relapse, as well as metastatic behavior, open up more therapeutic approaches via the usage of molecular agents, although with moderate overall survival enhancement[1,54,55]. Genomic profiling of iCCA sub-classifies it into: (i) inflammatory and (ii) proliferative classes. In the former, the activation of inflammatory pathways mainly occurs, while on the latter, the activation of oncogenes demonstrates a more worrisome prognosis[12].
The heterogeneity of CCAs subtypes is also demonstrated by Next-generation sequencing analysis, which indicates different genetic mutations based on CCA’s anatomical location. (iCCA vs extrahepatic: pCCA and dCCA). RAS mutation is more frequently exhibited in CCA, particularly in dCCA[56], however, there is a subclass of CCA, without exhibiting it. There is also emerging evidence of gene FGFR2 fusions involvement in cholangiocarcinogenesis, based on exome sequencing analysis[57]. Aberrations are also identified in the epigenetic level of gene regulation, such as histone modification, DNA hypermethylation and microRNAs (miRNAs) dysregulation, all implied in CCA Tumorigenesis[34].
As alluded to previously, a better understanding of the molecular, genomic and epigenetic affected pathways driving to CCA development and progression could give rise to new and improved generations of therapeutic approaches based on patient-stratification. Major factors implicated in CCA establishment are chronic biliary inflammation, ductal obstruction with cholestasis and bile duct injury[58]. As a consequence of chronic inflammation, proinflammatory cytokines’ overexpression occurs (TNF, IL-6, endotoxins). Persistent secretion of IL-6 by inflamed cholangiocytes and immune cells contributes to cancer establishment and progress. IL-6 oversecretion, induces nitric oxide production (via nitric oxide synthase), which is implied in DNA oxidation and damage[59], as well as stimulates the secretion of cyclic oxygenase (COX)-2-mediated prostaglandin, which promotes angiogenesis and disrupts the programmed cell death[60].
Autophagy has a crucial role in inflammation; however, their correlation is still being researched[44]. A large number of signaling pathways are involved in chronic inflammation, during cancer establishment, which influences the process of auto
Based on studies, many genetic mutations have been reported, implied in CCA. Harboring mutant KRAS has been identified in 40% of CCAs, particularly in dCCA with dismal outcomes[56]. Moreover, it is also related to lymphatic dissemination, lower long-term OS and higher grade, as was demonstrated in a study with a limited number of patients with iCCA and mutant KRAS gene (7.4%)[62]. In addition, based on an animal model study, concomitant mutations of KRAS and P53 are related to worse overall survival and malignant transformation in murine[63], while they con
The development of KRAS axis inhibitors, such as selumetinib, opens up new therapeutic strategies, which potentially could be enhanced via the addition of auto
Furthermore, c-MET inhibition is related to an increased level of autophagy, as it was demonstrated in lung cancer[68]. Similarly, mutant EGFR and ERBB genes are associated as well with poor outcome and invasiveness[69,70]. In many cancers, treated with inhibitors of tyrosine kinase, autophagy acts as a tumor suppressor[71]. The combination of autophagy and tyrosine kinase inhibitors could potentially improve the treatment results. Moreover, the fusion of FGFR2 genes is demonstrated in CCA[72], and they are correlated with decreased autophagy levels, leading to tumorigenesis. Inhibition of the above gene induces autophagy as a tumor suppressor mechanism in breast and lung malignancies, and its effect can be enhanced with the combination of autophagy inhibitors[73,74]. All the above data support that the com
In the initial phase of cholangiocarcinogenesis, Adenomatous Polyposis Coli (APC) mutation has been also reported[77], with the altered mechanism of autophagy[78] and during the establishment of cancer models[79]. Aberrations in the epigenetic level, such as histone modification, DNA hypermethylation, and miRNAs deregulation, are crucial for CCA establishment and development[80] while modulating the autophagy process[81], as well. The expression and the characteristics of the cilium are influenced by the increased expression of histone deacetylase 6 (HDAC6), which reduces its length and increases its proliferation. Inhibition of HDAC6 is correlated with reduced tumor progression and restoration of cilia[82,83]. Suppression of autophagy contri
Aggressiveness and dismal outcome of iCCA, are also reported in cases of modified HDAC1expression[85]. Significant autophagy regulators are the methylations of histone, which decelerate it[86]. Inactivation of tumor suppressors, caused by DNA methylation, is reported in cholangiocarcinogenesis. DNA hypermethylation of IDH1/2, is identified in some iCCA cases (10%), which leads to deregulation of cellular functions, such as their differentiation[87,88]. Mutation of IDH, identified in gliomas, demonstrates the interconnection of autophagy suppression and methylations of histone[81,86], which open up therapeutic opportunities via autophagy inhibitors[89]. Deregulation of many non-coding RNA sequences, such as miR-21, miR-29, miR-141 and others, present either up or down-regulation and they constitute biomarkers for tumor progression, invasion, cancer cell-death and chemoresistance in CCA[90,91]. Autophagy and its components, such as autophagy-associated proteins (ATG4, ATG9), beclin1, LC3 and ULK2, are also modulated via miRNAs[92,93]. Induction of autophagy, via the action of miR-124, resulted in an altered STAT3 sig
Autophagy modulators in combination with immunotherapy, targeted therapies and chemotherapy are positioning as a promising strategy to increase therapeutic benefits for cancer patients. Current treatment options for patients with CCA are limited to chemotherapy, thus, combinatorial scheme including autophagy modulators could offer an opportunity to increase survival of patients with CCA. Autophagy inhibition such as Hydroxy-chloroquine (HCQ) alters the mechanism of resistance and could potentially decrease CCA metastatic potential; therefore, clinical results of this study would be of great help for further design of novel therapeutic approaches involving autophagy inhibitors in CCA. Recent studies revealed the potential of the well-known autophagy marker, Beclin-1, as a prognostic factor in different cancers including CCA. It has emphasized the necessity to combine Beclin-1 expression with other autophagy-related proteins such as Bcl-2 family proteins Bcl-xL and BNIP3, HIF-1α, PI3KC3 or ATGs to increase its clinical value for patients with CCA.
Many studies demonstrate the correlation of autophagy mechanism with the microenvironment of tumors and the antitumor immune response, in many cancers, including CRC. Major histocompatibility complex (MCH) I/II Ag presentation is closely re
More particularly, it is demonstrated that therapy with Rapamycin intensifies radiotherapy effects on A549 malignant lung cells via autophagy activation and by expressing a dilatory effect on genome damage repairing[96]. Rapalogs, like evero
Furthermore, it is reported that another anti-proliferative agent, that inducts autophagy mechanism is the well-known metformin, which directs inhibition of autophagy, or via blocking beclin-1. Moreover, it is reported that metformin induces autophagy mechanism in the case of adenocarcinoma in the lung, as well as cell apoptosis via increasing tumor necrosis factor (TNF), the so-called TNF-Related-Apoptosis-Inducing Ligand (TRAIL), apoptosis[99]. In breast cancer therapy, without BRCA1 mutation, metformin is combined with another autophagy inhibitor, spautin-1, which sensitizes these tumors, for the mitochondrial-targeted disruptors. In this case, the combination of an autophagy activator and inhibitor, like metformin and spautin-1, responsively can modify the function of mitochondria differently, resulting in redu
Induction of autophagy can be achieved via another agent, like Obatoclax, com
Alkaloids are identified as another group of autophagy inducers in malignancies[103]. Some of them are liensinine, isoliensinine and cepharanthine[48], which target AMPK phosphorylation and mTOR blockage. These agents have been utilized in cases of MEFs, in which we are presenting resistance in the cell-apoptosis mechanism[102].
In addition to the well-established antioxidant function of omega-3polyunsaturated fatty acids (ω-3 PUFAs)[104], it has been shown that these safenatural compounds can induce 15-hydroxyprostaglandin dehydrogenase(15-PGDH) leading to inactivation of prostaglandin E2 (PGE2) that is knownto drive human cholangiocarcinoma[105]. The latter, combined with the fact that ω-3 PUFAs induce autophagy-mediated cell death in cancer cells support the use of ω-3 PUFAs as non-toxic adjuvant therapeutic agents for the treatment of human cholangiocarcinoma[106].
A wide range of studies about autophagy and its influence on the efficacy of other cancer treatments, such as chemotherapy, radiotherapy, or immunotherapy, has been reported in the last years[107]. These studies focused on this mechanism, used by cancer cells for their energy, metabolic regulation and survival[40,108]. The dual role of autophagy, either as tumor promoter, or tumor suppressor, opened up new oppor
The most widely known inhibitors are Chloroquine (CQ) and hydroxychloroquine (HCQ), which impede the fusion of autophagosomes with the lysosomes. Their effi
Moreover, the combination of immunotherapy and autophagy inhibitors, such as CQ with IL-2, has been proven beneficial with reduced toxicity, such as in animal-model studies of murine with hepatic metastasis. Furthermore, it was demonstrated that this dual therapeutic strategy, has a better survival rate in the long term as well as a better response by immune cells[107]. However, the response to CQ derivatives, including HCQ is variable, due to the lack of specificity, which leads to the interaction with other medical substances and the modification of tumor properties, like pH[109,112]. Additionally, the efficacy of autophagy inhibition by the above agents, cannot be evaluated due to the absence of biomarkers, which is a significant limitation in the clinical practice. This is the reason that new inhibitors with higher specificity have been developed[41,107].
There are some new, efficacious inhibitors, such as Lys05, also described as dimeric chloroquine, which is well–tolerated and exhibits a strong antitumor action via the modification of lysosome enzymes[112]. Another one is SAR405, an inhibitor of kinase, which is more specific and targets Vps18 and Vps34 vacuole proteins, which have a crucial role in the initiation of autophagy-mechanism. More particularly, the initiation step is regulated by Beclin-1 and Vps34, whereas Vps34 suppression, results in the impairment of lysosomal and vesicular transport[113]. Initiation-step can also be targeted, via the use of Beclin-1 inhibitors, which suppress the tumor progression, intensify the antitumor activity of Natural Killer (NK) cells and induce CCL5 cytokine overexpression by cancer cells, a condition that influences the transporting of NK cells towards the malignant tumors[107].
Based on studies in various malignancies, the inhibition of ULK1 (Unc-51 Like kinase-1) by SBI-0206965, has great antitumor potential due to its higher selectivity, resulting from the suppression of ULK1-phosphorylations[114]. Some other agents, are DCMI including desmethylclomipramine, verteporfin and clomipramine, impeding the fusion of autophagosome with lysosomes or acidification lysosomes[115], whereas the addition of DCMI to doxorubicin, in vitro, demonstrated higher effectiveness of the latter[116]. Moreover, spautin-1, is another effective inhibitor, which impedes the initiation step of autophagy, by suppressing the crucial for the process ubiquitin-specific peptidases USP13, USP10, as well as Beclin-1, which is deubiquitinated in Vps34 complex[99].
The microenvironment of tumors, is closely related to the autophagy mechanism, as well as with the antitumor immune response. According to this fact, the inhibition of autophagy could have a negative impact on the adaptive immune response against malignant tumors. However, Starobinetset al[117] in 2016 confuted this hypothesis by proving that inhibition of autophagy does not have an adverse impact on the adaptive anti-cancer immunity in melanoma and breast cancers. For this reason, inhibitors of autophagy can be combined with another chemotherapeutic agent without negatively influencing the antitumor response of T cells towards malignant tumors[117].
Herein, we provide two summarized tables about small agents that inhibit or activate autophagy. Autophagy manipulation is already used in research to develop putative chemotherapeutic strategies with a plethora of agents for different types of cancer (Tables 1 and 2).
| Agents | Mechanism of action |
| GDC-0941 | Inhibitor of class I PI3K |
| GDC-0980 | Dual inhibitor of PI3K and mTORC1 |
| Everolimus | mTORC1 inhibitor |
| Temsirolimus | mTORC1 inhibitor |
| Rapamycin | mTORC1 inhibitor |
| Tat–beclin 1 peptide | Releases beclin-1 into cytoplasm-regulate autophagosome formation |
| Metformin | AMPK activator |
| Fluspirilene | Antagonists of L-type Ca2+ channels |
| Loperamide | Antagonists of L-type Ca2+ channels |
| Amiodarone | Antagonists of L-type Ca2+ channels |
| Isoliensinine | Natural alkaloid |
| Cepharanthine | Natural alkaloid |
| Agents | Mechanism of action |
| 3-Methyladenine (3-MA) | Inhibitor of class III PI3K |
| LY294002 | PI3K inhibitor |
| Wortmannin | PI3K inhibitor |
| SB202190 | Cross-inhibition of the PI3K/mTOR and MAPKs pathway |
| MHY1485 | Activator of mTOR |
| Azithromycin | Inhibitor of v-ATPase, inhibition of lysosomal acidification |
| Bafilomycin A1 | Inhibitor of v-ATPase, inhibition of lysosomal acidification |
| Concanamycin A | Inhibitor of v-ATPase, inhibition of lysosomal acidification |
| Chloroquine (CQ) | Autophagosome-lysosome fusion |
| Hydroxy-chloroquine (HCQ) | Autophagosome-lysosome fusion |
| Clomipramine | Alter acidification of lysosomes |
| Verteporfin | Alter acidification of lysosomes |
| Paclitaxel | Microtubule stabilizer- inhibit phosphorylation of VPS34 at T159 |
| Spain-1 | Inhibits the activity of ubiquitin-specific peptidases, USP10 and USP13 |
| Monensin | Inhibit autophagosome-lysosome fusion |
It is a well-established knowledge that autophagy’s prominent role is strongly correlated with the degradation of dysfunctional cellular proteins and organelles. A plethora of studies in the field of cancer research and autophagy highlights the controversial role of this mechanism either as tumor suppressor or promoter mechanism in different types of cancer, including CCA. Several in vitro and in vivo studies in CCAs have associated autophagy with cholangiocarcinogenesis development and progre
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdel Moneim AE, Li Y S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 969] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 2. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1141] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 3. | Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma - Part I: Classification, diagnosis and staging. Dig Liver Dis. 2020;52:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Lendvai G, Szekerczés T, Illyés I, Dóra R, Kontsek E, Gógl A, Kiss A, Werling K, Kovalszky I, Schaff Z, Borka K. Cholangiocarcinoma: Classification, Histopathology and Molecular Carcinogenesis. Pathol Oncol Res. 2020;26:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 6. | Munoz-Garrido P, Rodrigues PM. The jigsaw of dual hepatocellular-intrahepatic cholangiocarcinoma tumours. Nat Rev Gastroenterol Hepatol. 2019;16:653-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Xue R, Chen L, Zhang C, Fujita M, Li R, Yan SM, Ong CK, Liao X, Gao Q, Sasagawa S, Li Y, Wang J, Guo H, Huang QT, Zhong Q, Tan J, Qi L, Gong W, Hong Z, Li M, Zhao J, Peng T, Lu Y, Lim KHT, Boot A, Ono A, Chayama K, Zhang Z, Rozen SG, Teh BT, Wang XW, Nakagawa H, Zeng MS, Bai F, Zhang N. Genomic and Transcriptomic Profiling of Combined Hepatocellular and Intrahepatic Cholangiocarcinoma Reveals Distinct Molecular Subtypes. Cancer Cell. 2019;35:932-947.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 8. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 9. | Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1543] [Article Influence: 308.6] [Reference Citation Analysis (0)] |
| 11. | Koo SH, Ihm CH, Kwon KC, Park JW, Kim JM, Kong G. Genetic alterations in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Genet Cytogenet. 2001;130:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, Peix J, Sole M, Tovar V, Alsinet C, Cornella H, Klotzle B, Fan JB, Cotsoglou C, Thung SN, Fuster J, Waxman S, Garcia-Valdecasas JC, Bruix J, Schwartz ME, Beroukhim R, Mazzaferro V, Llovet JM. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 13. | Suk WA, Bhudhisawasdi V, Ruchirawat M. The Curious Case of Cholangiocarcinoma: Opportunities for Environmental Health Scientists to Learn about a Complex Disease. J Environ Public Health. 2018;2018:2606973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Wilcox BA, Echaubard P. Balancing biomedical and ecological perspectives in research framing of liver fluke and cholangiocarcinoma in NE Thailand. Parasitol Int. 2017;66:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Fiorino S, Bacchi-Reggiani L, de Biase D, Fornelli A, Masetti M, Tura A, Grizzi F, Zanello M, Mastrangelo L, Lombardi R, Acquaviva G, di Tommaso L, Bondi A, Visani M, Sabbatani S, Pontoriero L, Fabbri C, Cuppini A, Pession A, Jovine E. Possible association between hepatitis C virus and malignancies different from hepatocellular carcinoma: A systematic review. World J Gastroenterol. 2015;21:12896-12953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One. 2017;12:e0186643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 17. | Fung BM, Lindor KD, Tabibian JH. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J Gastroenterol. 2019;25:659-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 18. | Matsumoto K, Onoyama T, Kawata S, Takeda Y, Harada K, Ikebuchi Y, Ueki M, Miura N, Yashima K, Koda M, Sakamoto T, Endo M, Horie Y, Murawaki Y. Hepatitis B and C virus infection is a risk factor for the development of cholangiocarcinoma. Intern Med. 2014;53:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Kongpetch S, Jusakul A, Ong CK, Lim WK, Rozen SG, Tan P, Teh BT. Pathogenesis of cholangiocarcinoma: From genetics to signalling pathways. Best Pract Res Clin Gastroenterol. 2015;29:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Jing W, Jin G, Zhou X, Zhou Y, Zhang Y, Shao C, Liu R, Hu X. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev. 2012;21:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 21. | Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W, McPherson JR, Allen GE, Ng CC, Wong BH, Myint SS, Rajasegaran V, Heng HL, Gan A, Zang ZJ, Wu Y, Wu J, Lee MH, Huang D, Ong P, Chan-on W, Cao Y, Qian CN, Lim KH, Ooi A, Dykema K, Furge K, Kukongviriyapan V, Sripa B, Wongkham C, Yongvanit P, Futreal PA, Bhudhisawasdi V, Rozen S, Tan P, Teh BT. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44:690-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 22. | Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Schmoldt A, Benthe HF, Haberland G. Digitoxin metabolism by rat liver microsomes. Biochem Pharmacol. 1975;24:1639-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 84] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Charest A, Lane K, McMahon K, Park J, Preisinger E, Conroy H, Housman D. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21). Genes Chromosomes Cancer. 2003;37:58-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Borad MJ, Champion MD, Egan JB, Liang WS, Fonseca R, Bryce AH, McCullough AE, Barrett MT, Hunt K, Patel MD, Young SW, Collins JM, Silva AC, Condjella RM, Block M, McWilliams RR, Lazaridis KN, Klee EW, Bible KC, Harris P, Oliver GR, Bhavsar JD, Nair AA, Middha S, Asmann Y, Kocher JP, Schahl K, Kipp BR, Barr Fritcher EG, Baker A, Aldrich J, Kurdoglu A, Izatt T, Christoforides A, Cherni I, Nasser S, Reiman R, Phillips L, McDonald J, Adkins J, Mastrian SD, Placek P, Watanabe AT, Lobello J, Han H, Von Hoff D, Craig DW, Stewart AK, Carpten JD. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10:e1004135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 26. | De Luca A, Esposito Abate R, Rachiglio AM, Maiello MR, Esposito C, Schettino C, Izzo F, Nasti G, Normanno N. FGFR Fusions in Cancer: From Diagnostic Approaches to Therapeutic Intervention. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Abou-Alfa GK, Mercade TM, Javle M, Kelley RK, Lubner S, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater JA, Harris WP, Murphy AG, Oh DY, Whisenant J, Wu B, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. LBA10_PR - ClarIDHy: A global, phase III, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Ann Oncol. 2019;30:872-873. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Nepal C, O'Rourke CJ, Oliveira DVNP, Taranta A, Shema S, Gautam P, Calderaro J, Barbour A, Raggi C, Wennerberg K, Wang XW, Lautem A, Roberts LR, Andersen JB. Genomic perturbations reveal distinct regulatory networks in intrahepatic cholangiocarcinoma. Hepatology. 2018;68:949-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Zou S, Li J, Zhou H, Frech C, Jiang X, Chu JS, Zhao X, Li Y, Li Q, Wang H, Hu J, Kong G, Wu M, Ding C, Chen N, Hu H. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 30. | Gingras MC, Covington KR, Chang DK, Donehower LA, Gill AJ, Ittmann MM, Creighton CJ, Johns AL, Shinbrot E, Dewal N, Fisher WE; Australian Pancreatic Cancer Genome Initiative, Pilarsky C, Grützmann R, Overman MJ, Jamieson NB, Van Buren G 2nd, Drummond J, Walker K, Hampton OA, Xi L, Muzny DM, Doddapaneni H, Lee SL, Bellair M, Hu J, Han Y, Dinh HH, Dahdouli M, Samra JS, Bailey P, Waddell N, Pearson JV, Harliwong I, Wang H, Aust D, Oien KA, Hruban RH, Hodges SE, McElhany A, Saengboonmee C, Duthie FR, Grimmond SM, Biankin AV, Wheeler DA, Gibbs RA. Ampullary Cancers Harbor ELF3 Tumor Suppressor Gene Mutations and Exhibit Frequent WNT Dysregulation. Cell Rep. 2016;14:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1073] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 32. | Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, Offerhaus GJA, Roa JC, Roberts LR, Gores GJ, Popescu I, Alexandrescu ST, Dima S, Fassan M, Simbolo M, Mafficini A, Capelli P, Lawlor RT, Ruzzenente A, Guglielmi A, Tortora G, de Braud F, Scarpa A, Jarnagin W, Klimstra D, Karchin R, Velculescu VE, Hruban RH, Vogelstein B, Kinzler KW, Papadopoulos N, Wood LD. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 33. | Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 34. | Liu HT, Gao P. The roles of microRNAs related with progression and metastasis in human cancers. Tumour Biol. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1765] [Cited by in RCA: 1701] [Article Influence: 189.0] [Reference Citation Analysis (0)] |
| 36. | Cao J, Sun L, Li J, Zhou C, Cheng L, Chen K, Yan B, Qian W, Ma Q, Duan W. A novel threemiRNA signature predicts survival in cholangiocarcinoma based on RNA-Seq data. Oncol Rep. 2018;40:1422-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7745] [Article Influence: 484.1] [Reference Citation Analysis (0)] |
| 38. | Zhong XY, Yu JH, Zhang WG, Wang ZD, Dong Q, Tai S, Cui YF, Li H. MicroRNA-421 functions as an oncogenic miRNA in biliary tract cancer through down-regulating farnesoid X receptor expression. Gene. 2012;493:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Koustas E, Karamouzis MV, Mihailidou C, Schizas D, Papavassiliou AG. Co-targeting of EGFR and autophagy signaling is an emerging treatment strategy in metastatic colorectal cancer. Cancer Lett. 2017;396:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Yun CW, Lee SH. The Roles of Autophagy in Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 684] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 41. | Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1946] [Article Influence: 243.3] [Reference Citation Analysis (0)] |
| 42. | Aredia F, Giansanti V, Mazzini G, Savio M, Ortiz LM, Jaadane I, Zaffaroni N, Forlino A, Torriglia A, Scovassi AI. Multiple effects of the Na(+)/H (+) antiporter inhibitor HMA on cancer cells. Apoptosis. 2013;18:1586-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32:955-963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 44. | Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res. 2014;74:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 352] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 45. | Koustas E, Sarantis P, Kyriakopoulou G, Papavassiliou AG, Karamouzis MV. The Interplay of Autophagy and Tumor Microenvironment in Colorectal Cancer-Ways of Enhancing Immunotherapy Action. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Perez-Montoyo H. Therapeutic Potential of Autophagy Modulation in Cholangiocarcinoma. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1313] [Article Influence: 164.1] [Reference Citation Analysis (0)] |
| 48. | Ávalos Y, Canales J, Bravo-Sagua R, Criollo A, Lavandero S, Quest AF. Tumor suppression and promotion by autophagy. Biomed Res Int. 2014;2014:603980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 49. | Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1616] [Article Influence: 161.6] [Reference Citation Analysis (0)] |
| 50. | Koustas E, Sarantis P, Papavassiliou AG, Karamouzis MV. Upgraded role of autophagy in colorectal carcinomas. World J Gastrointest Oncol. 2018;10:367-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308-5316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 907] [Cited by in RCA: 907] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 52. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine vs gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3166] [Article Influence: 211.1] [Reference Citation Analysis (1)] |
| 53. | Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, Bridgewater J, Okusaka T. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 54. | Benavides M, Antón A, Gallego J, Gómez MA, Jiménez-Gordo A, La Casta A, Laquente B, Macarulla T, Rodríguez-Mowbray JR, Maurel J. Biliary tract cancers: SEOM clinical guidelines. Clin Transl Oncol. 2015;17:982-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Simile MM, Bagella P, Vidili G, Spanu A, Manetti R, Seddaiu MA, Babudieri S, Madeddu G, Serra PA, Altana M, Paliogiannis P. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Simbolo M, Fassan M, Ruzzenente A, Mafficini A, Wood LD, Corbo V, Melisi D, Malleo G, Vicentini C, Malpeli G, Antonello D, Sperandio N, Capelli P, Tomezzoli A, Iacono C, Lawlor RT, Bassi C, Hruban RH, Guglielmi A, Tortora G, de Braud F, Scarpa A. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget. 2014;5:2839-2852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 57. | Arai Y, Totoki Y, Hosoda F, Shirota T, Hama N, Nakamura H, Ojima H, Furuta K, Shimada K, Okusaka T, Kosuge T, Shibata T. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 433] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 58. | Cheng Z, Lei Z, Shen F. Coming of a precision era of the staging systems for intrahepatic cholangiocarcinoma? Cancer Lett. 2019;460:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1033] [Cited by in RCA: 1233] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 60. | Nzeako UC, Guicciardi ME, Yoon JH, Bronk SF, Gores GJ. COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology. 2002;35:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, Zhang Z, Wang C, Chen G. Autophagy inhibition by sustained overproduction of IL6 contributes to arsenic carcinogenesis. Cancer Res. 2014;74:3740-3752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 62. | Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JM. Molecular Pathogenesis and Targeted Therapies for Intrahepatic Cholangiocarcinoma. Clin Cancer Res. 2016;22:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 63. | O'Dell MR, Huang JL, Whitney-Miller CL, Deshpande V, Rothberg P, Grose V, Rossi RM, Zhu AX, Land H, Bardeesy N, Hezel AF. Kras(G12D) and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res. 2012;72:1557-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 64. | Labib PL, Goodchild G, Pereira SP. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer. 2019;19:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 65. | Mertens JC, Rizvi S, Gores GJ. Targeting cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | Socoteanu MP, Mott F, Alpini G, Frankel AE. c-Met targeted therapy of cholangiocarcinoma. World J Gastroenterol. 2008;14:2990-2994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Pu XH, Yue S, Wu HY, Yang J, Fan XS, Fu Y, Ye Q, Chen J. C-MET in intrahepatic cholangiocarcinoma: High-Frequency amplification predicts protein expression and a unique molecular subtype. Pathol Res Pract. 2020;216:152857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Liu Y, Liu JH, Chai K, Tashiro S, Onodera S, Ikejima T. Inhibition of c-Met promoted apoptosis, autophagy and loss of the mitochondrial transmembrane potential in oridonin-induced A549 lung cancer cells. J Pharm Pharmacol. 2013;65:1622-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Xu L, Hausmann M, Dietmaier W, Kellermeier S, Pesch T, Stieber-Gunckel M, Lippert E, Klebl F, Rogler G. Expression of growth factor receptors and targeting of EGFR in cholangiocarcinoma cell lines. BMC Cancer. 2010;10:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Lai GH, Zhang Z, Shen XN, Ward DJ, Dewitt JL, Holt SE, Rozich RA, Hixson DC, Sirica AE. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129:2047-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Tanaka H, Hino H, Moriya S, Kazama H, Miyazaki M, Takano N, Hiramoto M, Tsukahara K, Miyazawa K. Comparison of autophagy inducibility in various tyrosine kinase inhibitors and their enhanced cytotoxicity via inhibition of autophagy in cancer cells in combined treatment with azithromycin. Biochem Biophys Rep. 2020;22:100750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Wu YM, Su F, Kalyana-Sundaram S, Khazanov N, Ateeq B, Cao X, Lonigro RJ, Vats P, Wang R, Lin SF, Cheng AJ, Kunju LP, Siddiqui J, Tomlins SA, Wyngaard P, Sadis S, Roychowdhury S, Hussain MH, Feng FY, Zalupski MM, Talpaz M, Pienta KJ, Rhodes DR, Robinson DR, Chinnaiyan AM. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 579] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 73. | van der Wekken AJ, Saber A, Hiltermann TJ, Kok K, van den Berg A, Groen HJ. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit Rev Oncol Hematol. 2016;100:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 74. | Chen Y, Xie X, Li X, Wang P, Jing Q, Yue J, Liu Y, Cheng Z, Li J, Song H, Li G, Liu R, Wang J. FGFR antagonist induces protective autophagy in FGFR1-amplified breast cancer cell. Biochem Biophys Res Commun. 2016;474:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Kang YK, Kim WH, Jang JJ. Expression of G1-S modulators (p53, p16, p27, cyclin D1, Rb) and Smad4/Dpc4 in intrahepatic cholangiocarcinoma. Hum Pathol. 2002;33:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Wang F, Xia X, Yang C, Shen J, Mai J, Kim HC, Kirui D, Kang Y, Fleming JB, Koay EJ, Mitra S, Ferrari M, Shen H. SMAD4 Gene Mutation Renders Pancreatic Cancer Resistance to Radiotherapy through Promotion of Autophagy. Clin Cancer Res. 2018;24:3176-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 77. | Cong WM, Bakker A, Swalsky PA, Raja S, Woods J, Thomas S, Demetris AJ, Finkelstein SD. Multiple genetic alterations involved in the tumorigenesis of human cholangiocarcinoma: a molecular genetic and clinicopathological study. J Cancer Res Clin Oncol. 2001;127:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Sasaki M, Nitta T, Sato Y, Nakanuma Y. Autophagy may occur at an early stage of cholangiocarcinogenesis via biliary intraepithelial neoplasia. Hum Pathol. 2015;46:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Qu X, Sheng J, Shen L, Su J, Xu Y, Xie Q, Wu Y, Zhang X, Sun L. Autophagy inhibitor chloroquine increases sensitivity to cisplatin in QBC939 cholangiocarcinoma cells by mitochondrial ROS. PLoS One. 2017;12:e0173712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Isomoto H. Epigenetic alterations associated with cholangiocarcinoma (review). Oncol Rep. 2009;22:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Peixoto P, Grandvallet C, Feugeas JP, Guittaut M, Hervouet E. Epigenetic Control of Autophagy in Cancer Cells: A Key Process for Cancer-Related Phenotypes. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Gradilone SA, Radtke BN, Bogert PS, Huang BQ, Gajdos GB, LaRusso NF. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 83. | Gradilone SA, Habringer S, Masyuk TV, Howard BN, Masyuk AI, Larusso NF. HDAC6 is overexpressed in cystic cholangiocytes and its inhibition reduces cystogenesis. Am J Pathol. 2014;184:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 84. | Kaliszczak M, van Hechanova E, Li Y, Alsadah H, Parzych K, Auner HW, Aboagye EO. The HDAC6 inhibitor C1A modulates autophagy substrates in diverse cancer cells and induces cell death. Br J Cancer. 2018;119:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Pant K, Peixoto E, Richard S, Gradilone SA. Role of Histone Deacetylases in Carcinogenesis: Potential Role in Cholangiocarcinoma. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 86. | Hu LF. Epigenetic Regulation of Autophagy. Adv Exp Med Biol. 2019;1206:221-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, Kwak EL, Clark JW, Ryan DP, Deshpande V, Dias-Santagata D, Ellisen LW, Zhu AX, Iafrate AJ. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 598] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 88. | Wang P, Dong Q, Zhang C, Kuan PF, Liu Y, Jeck WR, Andersen JB, Jiang W, Savich GL, Tan TX, Auman JT, Hoskins JM, Misher AD, Moser CD, Yourstone SM, Kim JW, Cibulskis K, Getz G, Hunt HV, Thorgeirsson SS, Roberts LR, Ye D, Guan KL, Xiong Y, Qin LX, Chiang DY. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091-3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 89. | Trejo-Solís C, Serrano-Garcia N, Escamilla-Ramírez Á, Castillo-Rodríguez RA, Jimenez-Farfan D, Palencia G, Calvillo M, Alvarez-Lemus MA, Flores-Nájera A, Cruz-Salgado A, Sotelo J. Autophagic and Apoptotic Pathways as Targets for Chemotherapy in Glioblastoma. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 90. | Li Z, Shen J, Chan MT, Wu WK. The role of microRNAs in intrahepatic cholangiocarcinoma. J Cell Mol Med. 2017;21:177-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Puik JR, Meijer LL, Le Large TY, Prado MM, Frampton AE, Kazemier G, Giovannetti E. miRNA profiling for diagnosis, prognosis and stratification of cancer treatment in cholangiocarcinoma. Pharmacogenomics. 2017;18:1343-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Gozuacik D, Akkoc Y, Ozturk DG, Kocak M. Autophagy-Regulating microRNAs and Cancer. Front Oncol. 2017;7:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 93. | Frankel LB, Lund AH. MicroRNA regulation of autophagy. Carcinogenesis. 2012;33:2018-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 94. | Ma J, Weng L, Wang Z, Jia Y, Liu B, Wu S, Cao Y, Sun X, Yin X, Shang M, Mao A. MiR-124 induces autophagy-related cell death in cholangiocarcinoma cells through direct targeting of the EZH2-STAT3 signaling axis. Exp Cell Res. 2018;366:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 95. | Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting autophagy in cancer. Cancer. 2018;124:3307-3318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 544] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 96. | Wang H, Li D, Li X, Ou X, Liu S, Zhang Y, Ding J, Xie B. Mammalian target of rapamycin inhibitor RAD001 sensitizes endometrial cancer cells to paclitaxel-induced apoptosis via the induction of autophagy. Oncol Lett. 2016;12:5029-5035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Byun S, Lee E, Lee KW. Therapeutic Implications of Autophagy Inducers in Immunological Disorders, Infection, and Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 98. | Koustas E, Papavassiliou AG, Karamouzis MV. The role of autophagy in the treatment of BRAF mutant colorectal carcinomas differs based on microsatellite instability status. PLoS One. 2018;13:e0207227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 99. | Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, Jin M, Zhu Z, Wang H, Yu J, Hao Y, Choi A, Ke H, Ma D, Yuan J. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 663] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 100. | Yeo SK, Paul R, Haas M, Wang C, Guan JL. Improved efficacy of mitochondrial disrupting agents upon inhibition of autophagy in a mouse model of BRCA1-deficient breast cancer. Autophagy. 2018;14:1214-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, Strasser A. BH3-Mimetic Drugs: Blazing the Trail for New Cancer Medicines. Cancer Cell. 2018;34:879-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 102. | Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 981] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 103. | Law BY, Chan WK, Xu SW, Wang JR, Bai LP, Liu L, Wong VK. Natural small-molecule enhancers of autophagy induce autophagic cell death in apoptosis-defective cells. Sci Rep. 2014;4:5510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 104. | Siasos G, Tousoulis D, Oikonomou E, Zaromitidou M, Verveniotis A, Plastiras A, Kioufis S, Maniatis K, Miliou A, Siasou Z, Stefanadis C, Papavassiliou AG. Effects of Ω-3 fatty acids on endothelial function, arterial wall properties, inflammatory and fibrinolytic status in smokers: a cross over study. Int J Cardiol. 2013;166:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 105. | Yao L, Han C, Song K, Zhang J, Lim K, Wu T. Omega-3 Polyunsaturated Fatty Acids Upregulate 15-PGDH Expression in Cholangiocarcinoma Cells by Inhibiting miR-26a/b Expression. Cancer Res. 2015;75:1388-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 106. | Kim S, Jing K, Shin S, Jeong S, Han SH, Oh H, Yoo YS, Han J, Jeon YJ, Heo JY, Kweon GR, Park SK, Park JI, Wu T, Lim K. ω3-polyunsaturated fatty acids induce cell death through apoptosis and autophagy in glioblastoma cells: In vitro and in vivo. Oncol Rep. 2018;39:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 107. | Janji B, Berchem G, Chouaib S. Targeting Autophagy in the Tumor Microenvironment: New Challenges and Opportunities for Regulating Tumor Immunity. Front Immunol. 2018;9:887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 108. | Qian HR, Shi ZQ, Zhu HP, Gu LH, Wang XF, Yang Y. Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget. 2017;8:62759-62768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 109. | Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, Mikkelson T, Wang D, Chang YC, Hu J, McAfee Q, Fisher J, Troxel AB, Piao S, Heitjan DF, Tan KS, Pontiggia L, O'Dwyer PJ, Davis LE, Amaravadi RK. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10:1359-1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 110. | Goulielmaki M, Koustas E, Moysidou E, Vlassi M, Sasazuki T, Shirasawa S, Zografos G, Oikonomou E, Pintzas A. BRAF associated autophagy exploitation: BRAF and autophagy inhibitors synergise to efficiently overcome resistance of BRAF mutant colorectal cancer cells. Oncotarget. 2016;7:9188-9221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 111. | Boone BA, Bahary N, Zureikat AH, Moser AJ, Normolle DP, Wu WC, Singhi AD, Bao P, Bartlett DL, Liotta LA, Espina V, Loughran P, Lotze MT, Zeh HJ 3rd. Safety and Biologic Response of Pre-operative Autophagy Inhibition in Combination with Gemcitabine in Patients with Pancreatic Adenocarcinoma. Ann Surg Oncol. 2015;22:4402-4410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 112. | Amaravadi RK, Winkler JD. Lys05: a new lysosomal autophagy inhibitor. Autophagy. 2012;8:1383-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 113. | Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, Bachelot MF, Lamberton A, Mathieu M, Bertrand T, Marquette JP, El-Ahmad Y, Filoche-Romme B, Schio L, Garcia-Echeverria C, Goulaouic H, Pasquier B. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol. 2014;10:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 114. | Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford ND, Shaw RJ. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol Cell. 2015;59:285-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 562] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 115. | Vakifahmetoglu-Norberg H, Xia HG, Yuan J. Pharmacologic agents targeting autophagy. J Clin Invest. 2015;125:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 116. | Rossi M, Munarriz ER, Bartesaghi S, Milanese M, Dinsdale D, Guerra-Martin MA, Bampton ET, Glynn P, Bonanno G, Knight RA, Nicotera P, Melino G. Desmethylclomipramine induces the accumulation of autophagy markers by blocking autophagic flux. J Cell Sci. 2009;122:3330-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 117. | Starobinets H, Ye J, Broz M, Barry K, Goldsmith J, Marsh T, Rostker F, Krummel M, Debnath J. Antitumor adaptive immunity remains intact following inhibition of autophagy and antimalarial treatment. J Clin Invest. 2016;126:4417-4429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |