Published online Jul 15, 2020. doi: 10.4251/wjgo.v12.i7.768
Peer-review started: February 3, 2020
First decision: March 24, 2020
Revised: April 13, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: July 15, 2020
Processing time: 163 Days and 6 Hours
Preoperative neoadjuvant chemoradiation therapy (NACRT) is applied for resectable pancreatic cancer (RPC). To maximize the efficacy of NACRT, it is essential to ensure the accurate placement of fiducial markers for image-guided radiation. However, no standard method for delivering fiducial markers has been established to date, and the nature of RPC during NACRT remains unclear.
To determine the feasibility, safety and benefits of endoscopic ultrasound-guided (EUS) fiducial marker placement in patients with RPC.
This was a prospective case series of 29 patients (mean age, 67.5 years; 62.1% male) with RPC referred to our facility for NACRT. Under EUS guidance, a single gold marker was placed into the tumor using either a 19- or 22-gauge fine-needle aspiration needle. The differences in daily marker positioning were measured by comparing simulation computed tomography and treatment computed tomography.
In all 29 patients (100%) who underwent EUS fiducial marker placement, fiducials were placed successfully with only minor, self-limiting bleeding during puncture observed in 2 patients (6.9%). NACRT was subsequently administered to all patients and completed in 28/29 (96.6%) cases, with one patient experiencing repeat cholangitis. Spontaneous migration of gold markers was observed in 1 patient. Twenty-four patients (82.8%) had surgery with 91.7% (22/24) R0 resection, and two patients experienced complete remission. No inflammatory changes around the marker were observed in the surgical specimen. The daily position of gold markers showed large positional changes, particularly in the superior-inferior direction. Moreover, tumor location was affected by food and fluid intake as well as bowel gas, which changes daily.
EUS fiducial marker placement following NACRT for RPC is feasible and safe. The RPC is mobile and is affected by not only aspiration, but also food and fluid intake and bowel condition.
Core tip: Currently, chemoradiation therapy for pancreatic cancer is mainly performed for patients with unresectable or borderline resectable pancreatic cancer (RPC). Although image-guided radiation therapies rely on fiducial marker placement, no standard delivery method has been established, and the nature of RPC during chemoradiation therapy remains unclear. In the present study, we report the feasibility and safety of endoscopic ultrasound-guided fiducial marker placement for RPC as well as the specificity of RPC, including daily tumor positional changes, which are affected by not only respiration but also food intake, fluid intake and bowel condition.
- Citation: Ashida R, Fukutake N, Takada R, Ioka T, Ohkawa K, Katayama K, Akita H, Takahashi H, Ohira S, Teshima T. Endoscopic ultrasound-guided fiducial marker placement for neoadjuvant chemoradiation therapy for resectable pancreatic cancer. World J Gastrointest Oncol 2020; 12(7): 768-781
- URL: https://www.wjgnet.com/1948-5204/full/v12/i7/768.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i7.768
To suppress recurrence after surgery and improve the prognosis of patients with resectable pancreatic cancer (RPC), preoperative neoadjuvant chemoradiation therapy (NACRT) is actively carried out[1,2]. Thanks to recent advances in radiation therapy, radiation treatments are administered using image-guided radiation therapies, such as intensity-modulated radiation therapy, respiration synchronization and irradiation tracking with the CyberKnife system. Furthermore, treatments with high radiation doses, such as stereotactic body radiation therapy and heavy particle therapy, are becoming increasingly more common[3,4]. While these radiation therapies are expected to be effective, they retain the risk of complications such as bleeding, ulceration and perforation, necessitating more precise irradiation. For this reason, the accurate placement of fiducial markers is essential, and the establishment of safe procedures for fiducial marker placement is required.
Endoscopic ultrasound-guided fiducial marker placement (EUS-FP) was first reported by Pishvaian et al[5] in a 2006 study in which fiducials were successfully placed in six of seven pancreatic cancer (PC) patients. Since then, EUS-FP has been reported for many cancer types, such as mediastinal tumors, prostate cancer and gastrointestinal (GI) malignancies (including PCs)[6,7]. Several reports have been published on EUS-FP for locally advanced and recurrent PC with technical success rates ranging from 85% to 100%[5,7-10].
At our hospital, we have aggressively administered NACRT for RPC and have reported promising results[11,12]. However, the risk of complications such as ulceration and GI tract bleeding remains. Therefore, the present study examined the feasibility, safety and potential benefits of EUS-FP for patients with RPC who are undergoing preoperative NACRT.
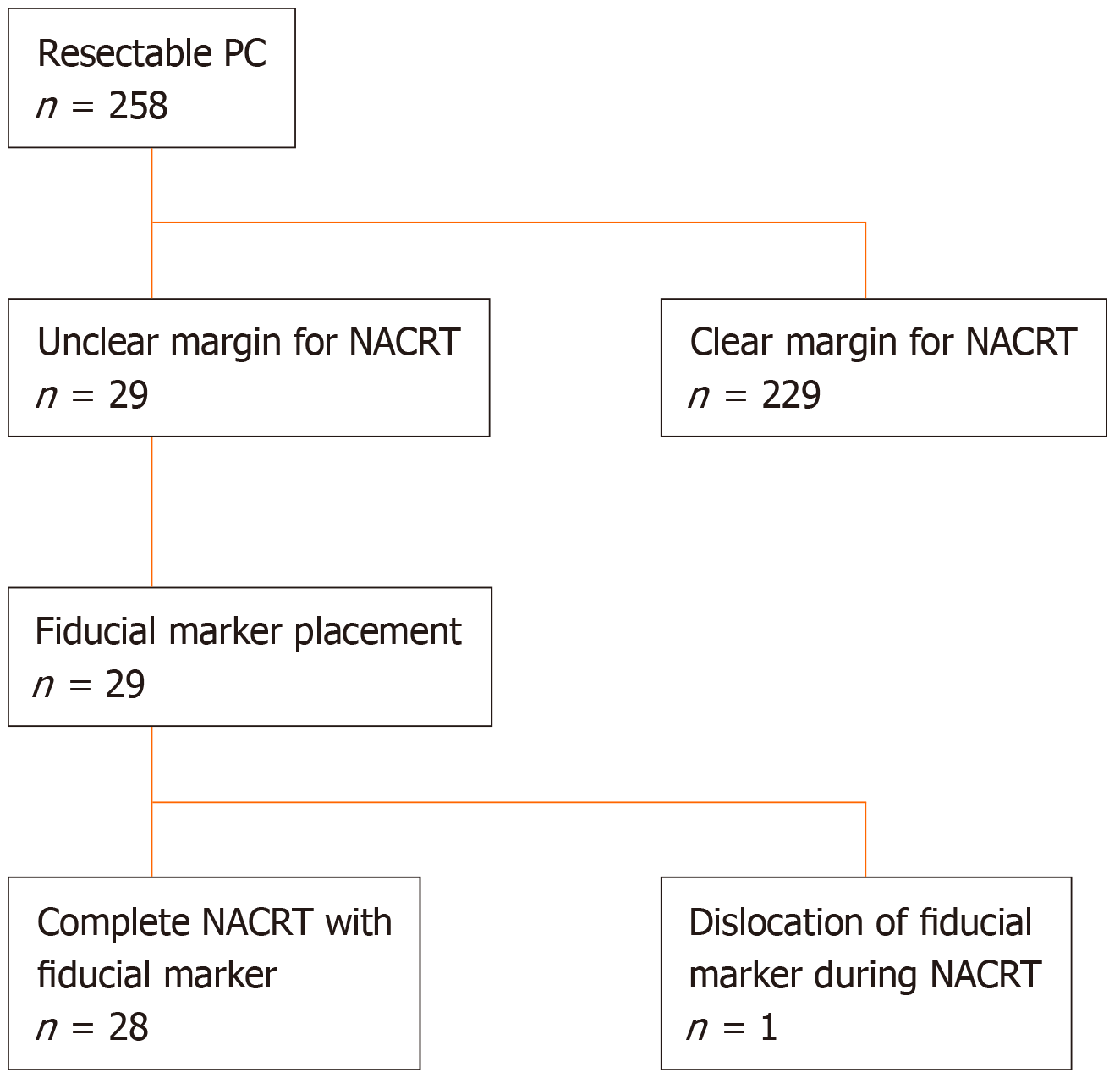
Between April 2013 and June 2017, 258 patients diagnosed with RPC through pathological assessment were scheduled to undergo NACRT at the Osaka International Cancer Institute. RPC was defined as follows: (1) A tumor without extension into the superior mesenteric artery, celiac artery or common hepatic artery; (2) A tumor with an abutment/encasement or a short segment of occlusion into the superior mesenteric vein and portal vein; or (3) A tumor which achieved down staging after neoadjuvant chemotherapy from T4 to above criteria.
Among these, 29 patients with an unclear cancer margin that was thought to be difficult to identify by cone-beam computed tomography (CBCT) during radiation therapy were referred for EUS-FP (Figure 1). A multidisciplinary team consisting of surgeons, radiation oncologists and experienced endosonographers determined the eligibility for NACRT and feasibility of EUS-FP based on reviews of abdominal cross-sectional EUS imaging and pancreatic protocol computed tomography (CT). The protocol of this study was approved by the Osaka International Cancer Institute Institutional Review Board (IRB No. 1302271178), and written informed consent was obtained from the patients before beginning the procedure (UMIN000010418). Exclusion criteria were: (1) An inability to safely receive conscious sedation; (2) An inability to reach the lesion site due to anatomic reasons (postsurgical changes, stricture not amenable to dilation); (3) An inability to understand and sign informed consent; (4) Pregnancy; and (5) Coagulopathy (international normalized ratio of 1.5 or platelet count < 5 × 104/μL).
For all patients, EUS was performed using a linear-array echoendoscope (GF-UCT 240-AL5 or GF-UCT 260-AL5; Olympus Corp., Tokyo, Japan) under conscious sedation. Fiducial markers were placed using an EUS-fine-needle aspiration (FNA) needle (EXPECT: 22G or Flex needle 19G; Boston Scientific, United States). For this study, Visicoil markers (RadioMed Corporation, Bartlett, TN, United States) measuring 0.75 mm or 0.5 mm in diameter × 5 mm in length or Gold Anchor™ markers (Naslund Medical AB, Huddinge, Sweden) measuring 0.28 mm × 10 mm were implanted. Visicoil markers were placed as described previously[13]. Gold Anchor markers also came preloaded on a percutaneously designed delivery device, but this device was small enough to be back-loaded directly into an FNA needle.
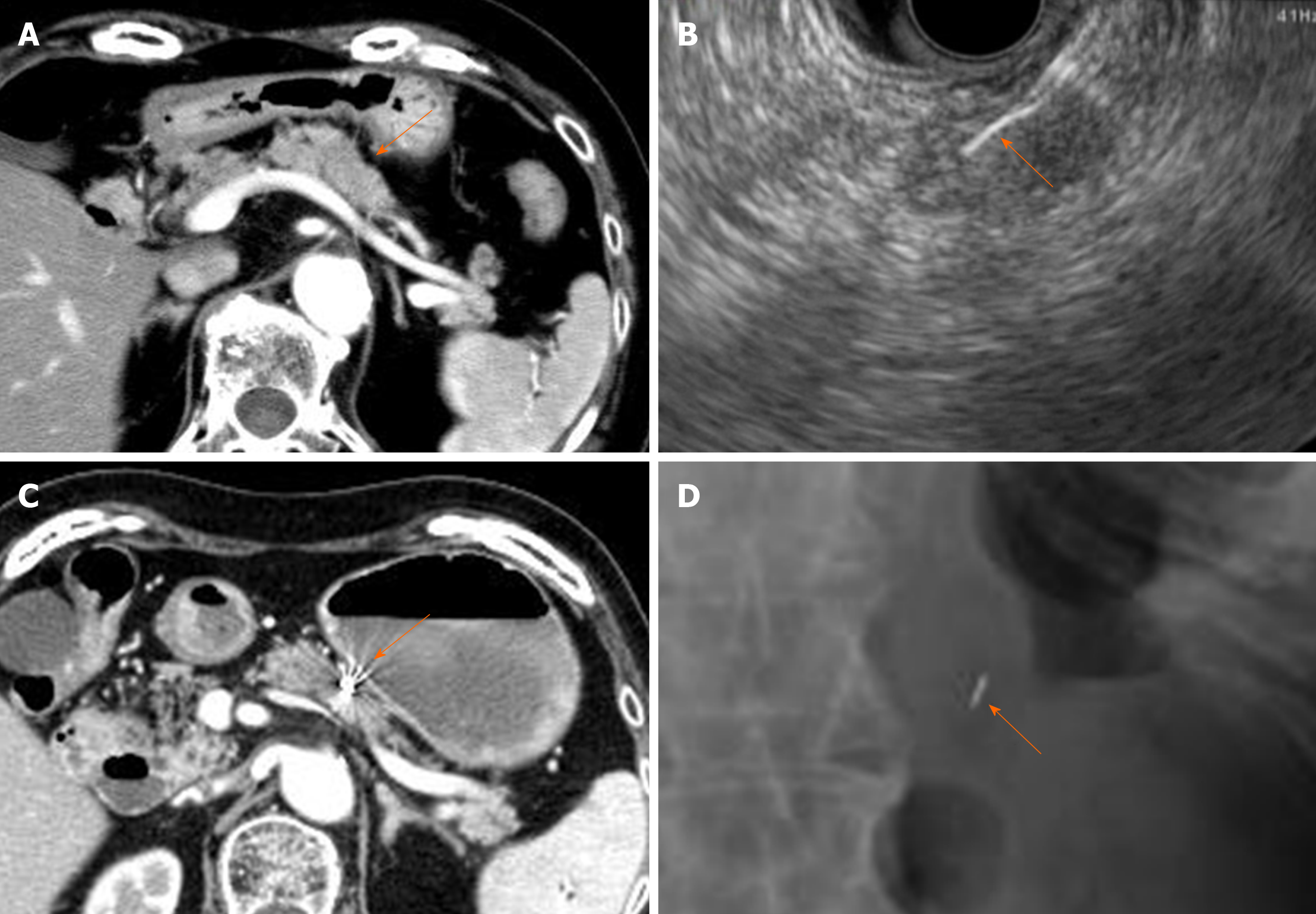
The FNA needle loaded with a marker was advanced through the operating channel of the echoendoscope. Upon needle insertion into the target lesion, the fiducial was deployed by advancing the stylet under EUS guidance. This procedure was performed by experienced endosonographers (Ashida R, Fukutake N) who have performed EUS-FNA in more than 500 cases. To confirm the success of placement and the location of the fiducial markers, abdominal radiography was performed after the procedure (Figure 2). Intravenous antibiotics (Sulperazone, 1 g × 2 d) were administered prophylactically to all patients. All patients were admitted for this procedure and monitored overnight for symptoms such as fever, abdominal pain and nausea.
After fiducial marker placement, irradiation fields were constructed with the aid of 3D radiation planning. The clinical target volume included the primary pancreatic cancer lesion, retropancreatic soft tissues, the para-aortic region and the celiac and superior mesenteric arteries, which could not be removed surgically. The planning target volume was generated by adding 5-10 mm margins in three directions (anterior-posterior, superior-inferior, left-right). A total radiation dose of 60 Gy was delivered in daily fractions of 2.4 Gy five times per week (25 fractions total). During the treatment schedule, CBCT or four-dimensional CT images were acquired, and the marker displacement against the simulation CT acquired for the treatment planning was measured (details of the method were reported differently[14]). Gemcitabine (1000 mg/m2) was administered to 21 patients on days 1, 8 and 15 of each 28-d cycle. S-1 monotherapy was administered to 2 patients for 4 wk at 2-wk intervals. One patient switched from gemcitabine to S-1 due to side effects such as a severe skin reaction. One patient was administered a combination of gemcitabine (1000 mg/m2) and S-1 (120 mg/d) for three cycles. These preoperative treatments were administered at our outpatient clinic. Five patients received a combination of gemcitabine (600 mg/m2) and nab-paclitaxel (75 mg/m2), which were administered on days 1, 8 and 15 of each 28-d cycle.
Histologic grading of the extent of residual tumor was evaluated using the Evans classification[15], which is a 4-tiered grading system that quantifies the extent of residual tumor based on the grading by assessing the percentage of viable tumor cells (destruction of tumor cells) in the posttreatment specimens as follows: Grade I, little (< 10%) or no tumor cell destruction; grade IIa, destruction of 10% to 50% of tumor cells; grade IIb, destruction of 51% to 90% of tumor cells; grade III, few (< 10%) viable tumor cells; and grade IV, no viable tumor cells.
Primary outcome measurements included technical success rate, complications and technical limitations of EUS-FP. Secondary outcome was the percentage of cases that successfully completed NACRT and subsequent surgery after marker placement. Technical success was defined as the ability to place fiducials in the desired location. A chart review for procedure-related complications was performed until NACRT following surgery had been accomplished.
A total of 29 patients (18 males, 11 females) with pathologically confirmed resectable adenocarcinoma with unclear margin for NACRT were referred for EUS-guided fiducial marker placement, as shown in Figure 1. The mean age of the patients was 66.9 ± 9.2 (range, 38-78) years. Fifteen (51.7%) tumors were located in the head of the pancreas, thirteen (44.8%) were in the body of the pancreas, and one (3.4%) was in the tail. The mean size of the tumors was 20.2 ± 7.7 cm (range 8.5-40 cm). The number of patients for each location and the size of marker are described in Table 1.
| Ca-se | Age in yr/sex | Tumor size in mm | cStage, UICC | Tumor location | Puncture route | Marker type | Marker size in mm | Needle gauge in G |
| 1 | 78/F | 25 | IIA | B | G | Visicoil | 0.75 × 5 | 19 |
| 2 | 63/F | 24 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 3 | 77/F | 18 | IIB | H | G | Visicoil | 0.5 × 5 | 22 |
| 4 | 55/M | 30 | IIA | B | G | Visicoil | 0.75 × 5 | 19 |
| 5 | 72/M | 20 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 6 | 68/F | 32 | III | B | G | Visicoil | 0.75 × 5 | 19 |
| 7 | 68/F | 14 | IIA | B | G | Visicoil | 0.5 × 5 | 22 |
| 8 | 70/M | 18 | IIA | H | D1 | Visicoil | 0.5 × 5 | 22 |
| 9 | 64/F | 15 | IIA | B | G | Visicoil | 0.5 × 5 | 22 |
| 10 | 73/F | 15 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 11 | 78/F | 11 | IIA | B | D1 | Visicoil | 0.5 × 5 | 22 |
| 12 | 68/M | 25 | IIB | B | G | Visicoil | 0.5 × 5 | 22 |
| 13 | 60/M | 20 | IIA | B | G | Visicoil | 0.5 × 5 | 22 |
| 14 | 78/F | 22 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 15 | 52/M | 15 | IIA | H | D1 | Visicoil | 0.5 × 5 | 22 |
| 16 | 73/M | 35 | IIA | B | G | Visicoil | 0.5 × 5 | 22 |
| 17 | 61/M | 15 | IIA | B | G | Visicoil | 0.5 × 5 | 22 |
| 18 | 66/M | 29 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 19 | 38/M | 19 | IIA | H | G | Visicoil | 0.5 × 5 | 22 |
| 20 | 58/M | 18 | IIA | H | D1 | Visicoil | 0.5 × 5 | 22 |
| 21 | 71/F | 12 | IIB | B | G | Visicoil | 0.5 × 5 | 22 |
| 22 | 57/M | 19 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 23 | 64/M | 21 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 24 | 78/M | 11 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 25 | 66/M | 21 | IIA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 26 | 68/M | 14 | IA | H | D2 | Visicoil | 0.5 × 5 | 22 |
| 27 | 77/M | 12 | IIA | T | G | Gold Anchor | 0.28 × 10 | 22 |
| 28 | 74/M | 40 | IIB | B | G | Gold Anchor | 0.28 × 10 | 22 |
| 29 | 66/F | 8.5 | IIA | B | G | Gold Anchor | 0.28 × 10 | 22 |
All fiducials were successfully deployed. Marker placement with a 19G FNA needle and 0.75 mm × 5 mm Visicoil was performed in 3 (10.3%) patients with tumors in the body of the pancreas. A 22G FNA needle with 0.5 mm × 5 mm Visicoil was used in 15 (51.7%) patients with tumors in the head and 8 (27.6%) with tumors in the body of the pancreas. A 22G FNA needle with 0.28 mm × 10 mm Gold Anchor was applied to 2 (6.9%) patients with tumors in the body of the pancreas and 1 patient with a tumor in the tail of the pancreas. All implanted markers were identifiable on abdominal radiographs after the placement procedure and simulation CT for radiation therapy (Figure 2).
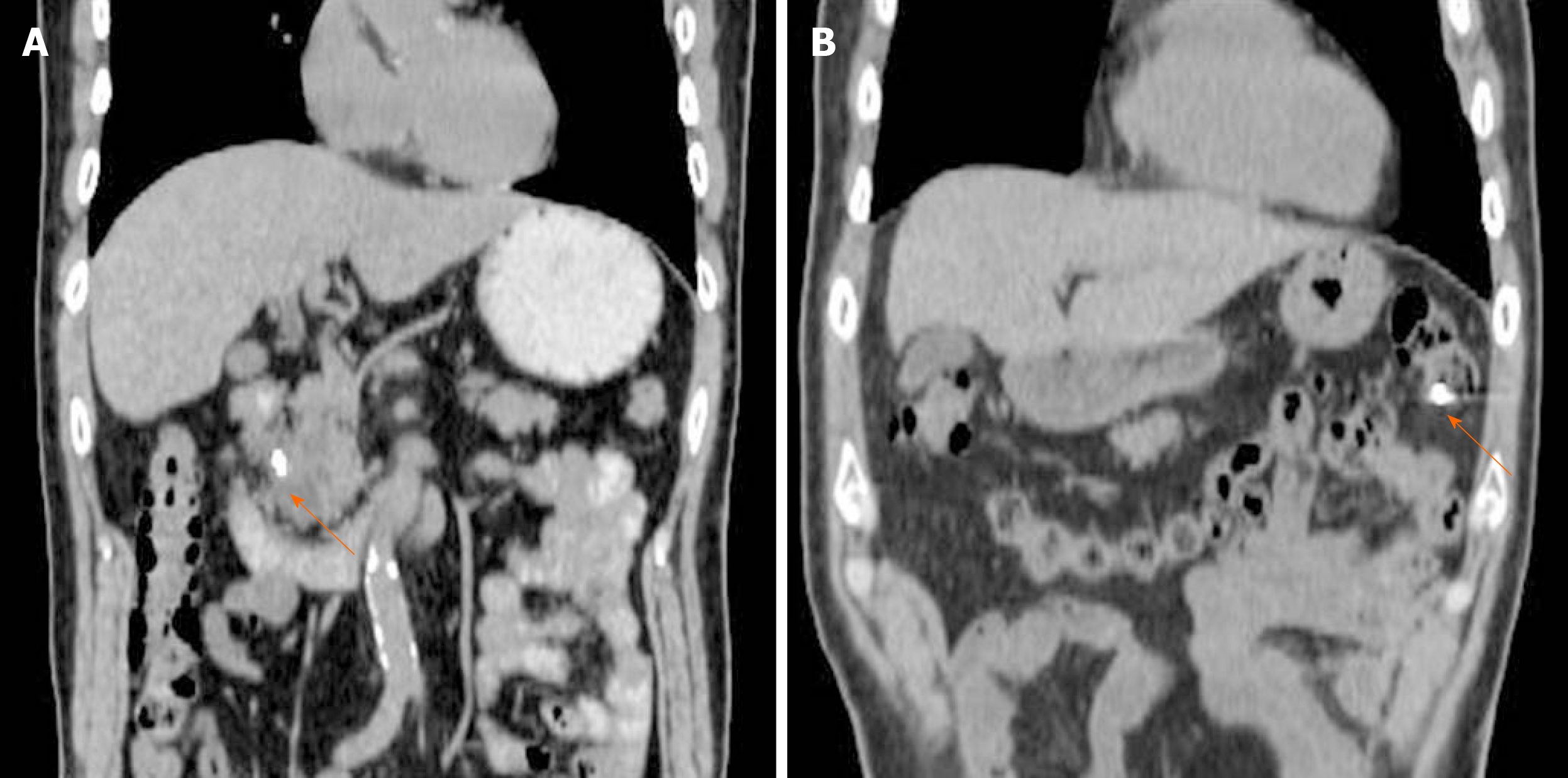
In terms of procedure-related complications, 2 cases (6.9%) of self-limiting minor luminal bleeding were seen with no significant decrease in hemoglobin. Otherwise, no complications occurred during the 24 h of monitoring immediately following marker placement. Marker migration occurred in one case (3.4%) 11 d after marker placement during NACRT, although no migration-related complications occurred (Figure 3). One patient developed a fever due to cholangitis 2 d after the procedure and was admitted to the hospital. This patient was treated with antibiotics and endoscopic biliary drainage, and the infection was resolved. However, this patient developed recurrent cholangitis, and physicians thought that continuing NACRT would be too difficult. Therefore, this patient underwent surgery as soon as radiation therapy was completed.
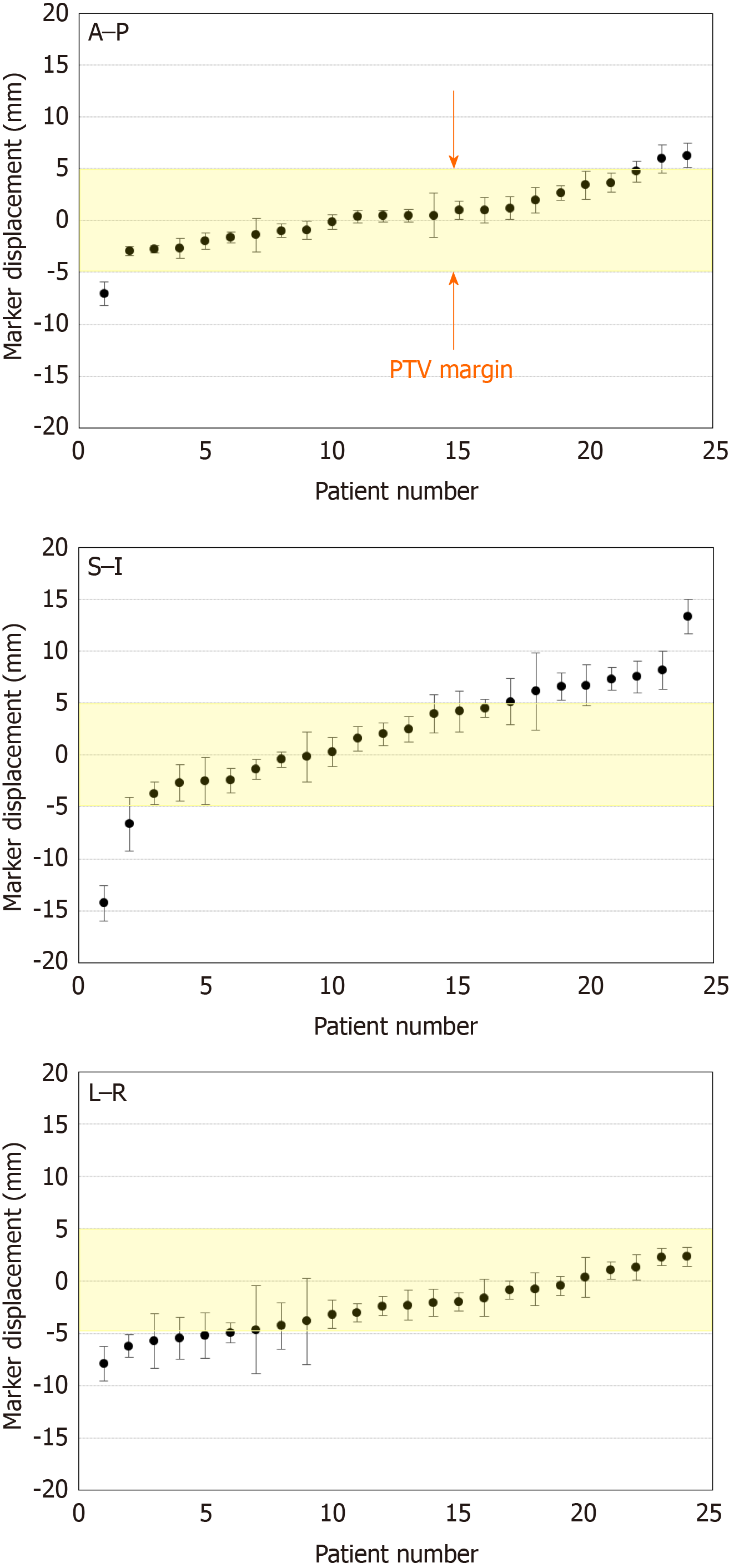
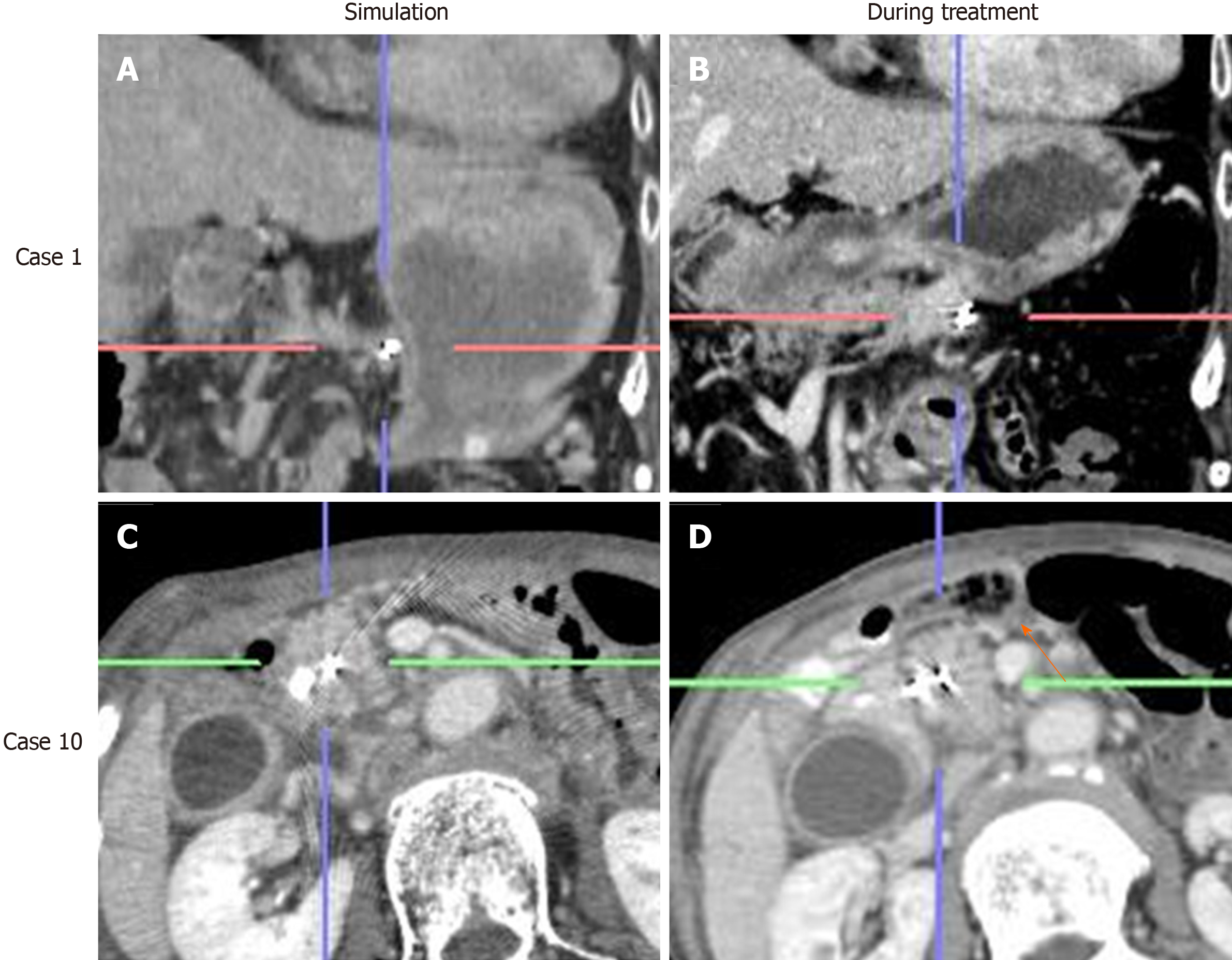
Among 24 out of 29 cases, the marker displacement between the simulation CT and CBCT or 4DCT images during the treatment schedule is shown in Figure 4. For the anterior-posterior and left-right directions, 2 patients showed 95% confidence intervals > 5 mm, which was the minimal planning target volume margin for radiation therapy. The positional differences of RPC between simulation and treatment were remarkable, particularly in the superior-inferior direction, and 6 patients showed a confidence interval > 5 mm. Moreover, it became clear that cancer location was affected by the change in stomach shape due to either food or fluid intake. Even lesions in the pancreatic head were affected by bowel gas as shown in Figure 5.
After fiducial placement, all patients completed NACRT, except for one patient who developed recurrent cholangitis (Table 2). During NACRT, 4 patients developed GI complications, such as gastric ulcers or erosion, and one patient had both a pressure fracture at L1 and L3 and interstitial lung disease. Two patients declined surgery, two patients had liver metastasis, and one patient developed dissemination during NACRT. In total, 24 patients underwent surgery (24/29; 82.8%), among whom 16 underwent pancreaticoduodenectomy, 7 underwent distal pancreatectomy, and 1 underwent total pancreatectomy with 91.7% of these patients experiencing R0 resection.
| Case | CRT | Complication during EUS-FP | Complication during NACRT | Surgery | Operation type | pStage, UICC | Evans classification |
| 1 | G-RT | 0 | 0 | Surgery | DP | IA | III |
| 2 | G-RT | 0 | Repeat cholangitis | Surgery | PD | IIB | I |
| 3 | G-RT | Minor bleeding | 0 | Surgery | PD | IIA | IIa |
| 4 | G-RT | 0 | 0 | Surgery | DP, left adrenal | IIA | III |
| 5 | G-RT | 0 | GE | Surgery | PD | IIA | IIa |
| 6 | G-RT | 0 | GU | Dissemination | - | - | - |
| 7 | GS-RT | 0 | GE | Surgery | DP, left adrenal | IIA | IIa |
| 8 | G-RT | 0 | 0 | Surgery | PD | IIB | IIa |
| 9 | G-RT | 0 | 0 | Surgery | DP | No residual carcinoma | IV |
| 10 | G-RT | 0 | 0 | Surgery | PD | IIA | IIb |
| 11 | G-RT | 0 | GU | Surgery | PD | IIA | IIa |
| 12 | G-RT | Minor bleeding | 0 | Surgery | TP | 0(cis) | III |
| 13 | G-RT | 0 | 0 | Liver met | - | - | - |
| 14 | G-RT | 0 | Pressure fractureL1.3, ILD | Surgery | PD | IIA | IIb |
| 15 | G-RT | 0 | 0 | Surgery | PD | IIB | IIb |
| 16 | GNab-RT | 0 | 0 | Declined | - | - | - |
| 17 | G-RT | 0 | 0 | Liver met | - | - | - |
| 18 | G-RT | 0 | 0 | Surgery | PD | IIB | IIb |
| 19 | GNab-RT | 0 | 0 | Surgery | PD | IA | IIb |
| 20 | G-RT | 0 | 0 | Surgery | PD | No residual carcinoma | IV |
| 21 | GNab-RT | 0 | 0 | Surgery | DP | IIA | IIb |
| 22 | GNab-RT | 0 | 0 | Surgery | PD | IIB | IIa |
| 23 | GNab-RT | 0 | 0 | Surgery | PD | IIA | IIa |
| 24 | G-RT→S-RT | 0 | 0 | Surgery | PD | IIA | I |
| 25 | G-RT | 0 | Migration | Surgery | PD | IA | I |
| 26 | G-RT | 0 | NA | Declined | - | - | - |
| 27 | G-RT | 0 | 0 | Surgery | PD | IA | IIb |
| 28 | G-RT | 0 | 0 | Surgery | DP | IIB | IIb |
| 29 | S-RT | 0 | 0 | Surgery | DP | IA | III |
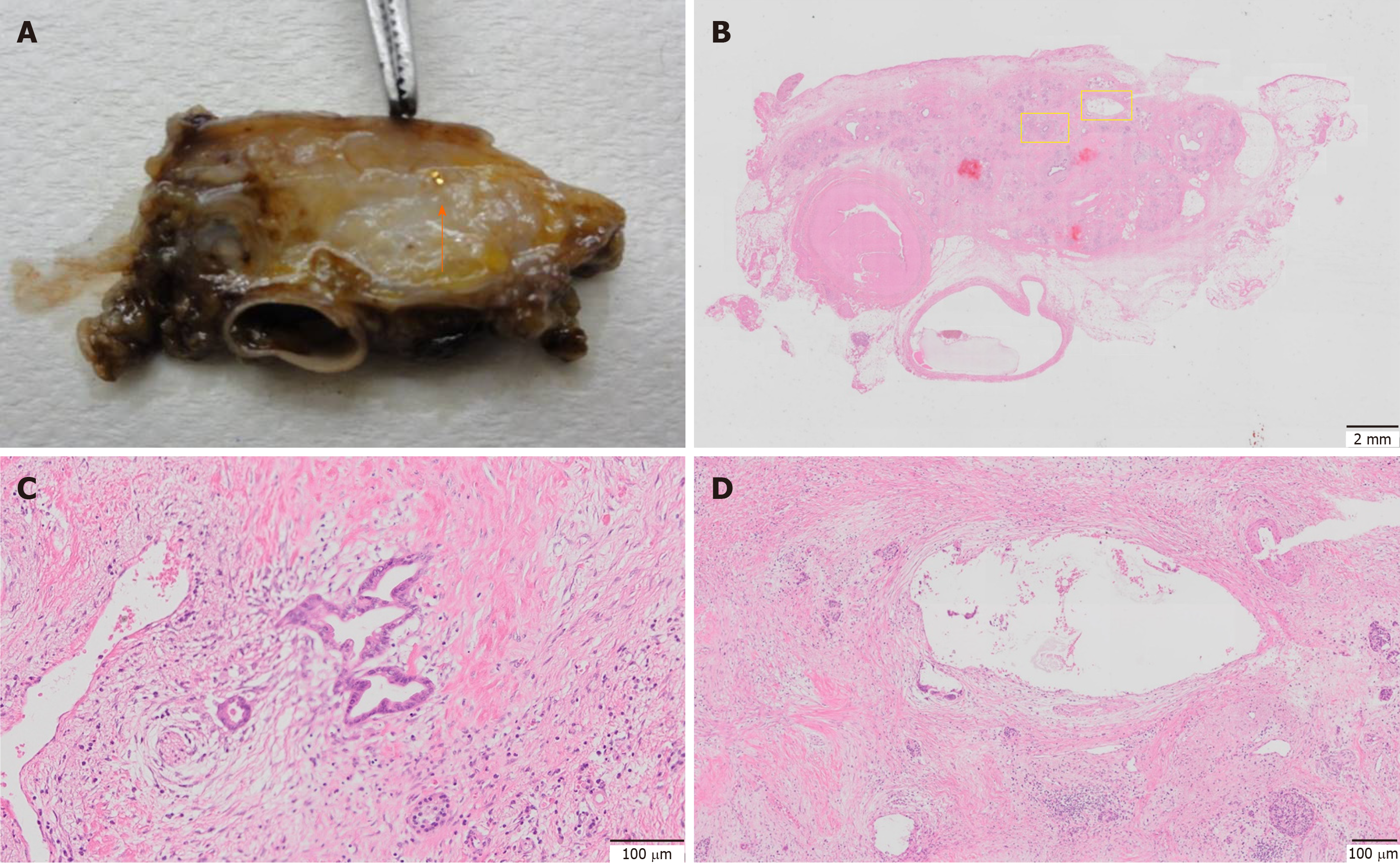
To investigate the effects of marker placement on tumor tissue, all surgical specimens underwent pathological examination. Except for one case of migration, placement of the gold markers was confirmed in the resected specimens. No significant changes, such as the accumulation of inflammatory cells or fibrosis, were observed around the markers in any pathological specimen (Figure 6). Regarding the effectiveness of NACRT, there were 2 cases with complete remission. Regarding Evans classification, 2 patients showed grade IV, 4 patients showed grade III, 7 patients showed grade IIa, 8 patients showed grade IIb, and 3 patients showed grade I[15].
PC is currently the fourth leading cause of cancer-related death worldwide and has a very poor prognosis. Patients with RPC who received preoperative treatment had an estimated median survival of 23.3 mo, whereas those who did not undergo preoperative treatment had an estimated median survival of 16.9–20.2 mo[16]. Therefore, preoperative neoadjuvant therapy is increasingly recognized as important.
Among the available preoperative regimens, NACRT is thought to confer a lower incidence of local recurrence after subsequent resection because it may provide macroscopic and microscopic levels of down staging[12,17]. However, radiation therapy requires high-level techniques, and complications such as GI bleeding or perforation of the GI tract are serious problems that could preclude surgery. Therefore, it is thought to be important to place fiducial markers in order to confirm the radiation field.
Fiducial markers have been inserted either during surgery or percutaneously under CT or US guidance[18,19]. However, surgery is invasive, and percutaneous procedures involve risks such as bleeding or needle tract tumor seeding. In recent years, the placement of fiducial markers under EUS guidance has been recommended due to its safety and accuracy. Although several reports related to EUS-FP have been published for unresectable locally advanced PC or borderline RPC, to date no study has focused only on RPC. Therefore, the present study focused on the feasibility and safety of EUS-FP for RPC undergoing NACRT.
In terms of feasibility, EUS-FP was successfully performed in all 29 patients (100%) with a single marker placement in the middle of the tumor to confirm the tumor location daily for radiation therapy. During the early period of this study, a 19-gauge FNA needle loaded with a Visicoil 0.75 mm in diameter × 0.5 cm in length was used for lesions of the pancreatic body or tail through the stomach, and a 22-gauge needle loaded with a Visicoil 0.5 mm in diameter × 0.5 cm in length was used for the lesions in the pancreatic head through the duodenum. However, after several cases were successfully treated using the smaller Visicoil with adequate delineation by CBCT, all cases were shifted to the use of small markers due to their ease of manipulation although a thicker fiducial marker would be more clearly visible during radiation therapy.
Previous reports have stated that during preparation the Visicoil markers must be removed once from the percutaneous puncture needle and reloaded into the FNA needle. However, we experienced some cases in which markers were bent during back-loading. Following these instances of Visicoil malfunction, we switched the marker from Visicoil to Gold Anchor so that the markers could be reloaded directly into the FNA needle. To avoid the recurrence of such malfunctions, it will be necessary to develop a dedicated device for EUS-FP. In addition, when multiple markers must be placed, it is currently necessary to place them one by one despite the increasing risk of complications, such as needle tract seeding due to the repeated puncturing of a tumor using the same needle. Although several techniques such as the antegrade, front-loading approach[8] or the hydrostatic technique using a BNX needle aspiration system (Covidien, Dublin, Ireland) have been reported[20], dedicated needles with multiple preloaded markers need to become more widely available for commercial use as quickly as possible[21].
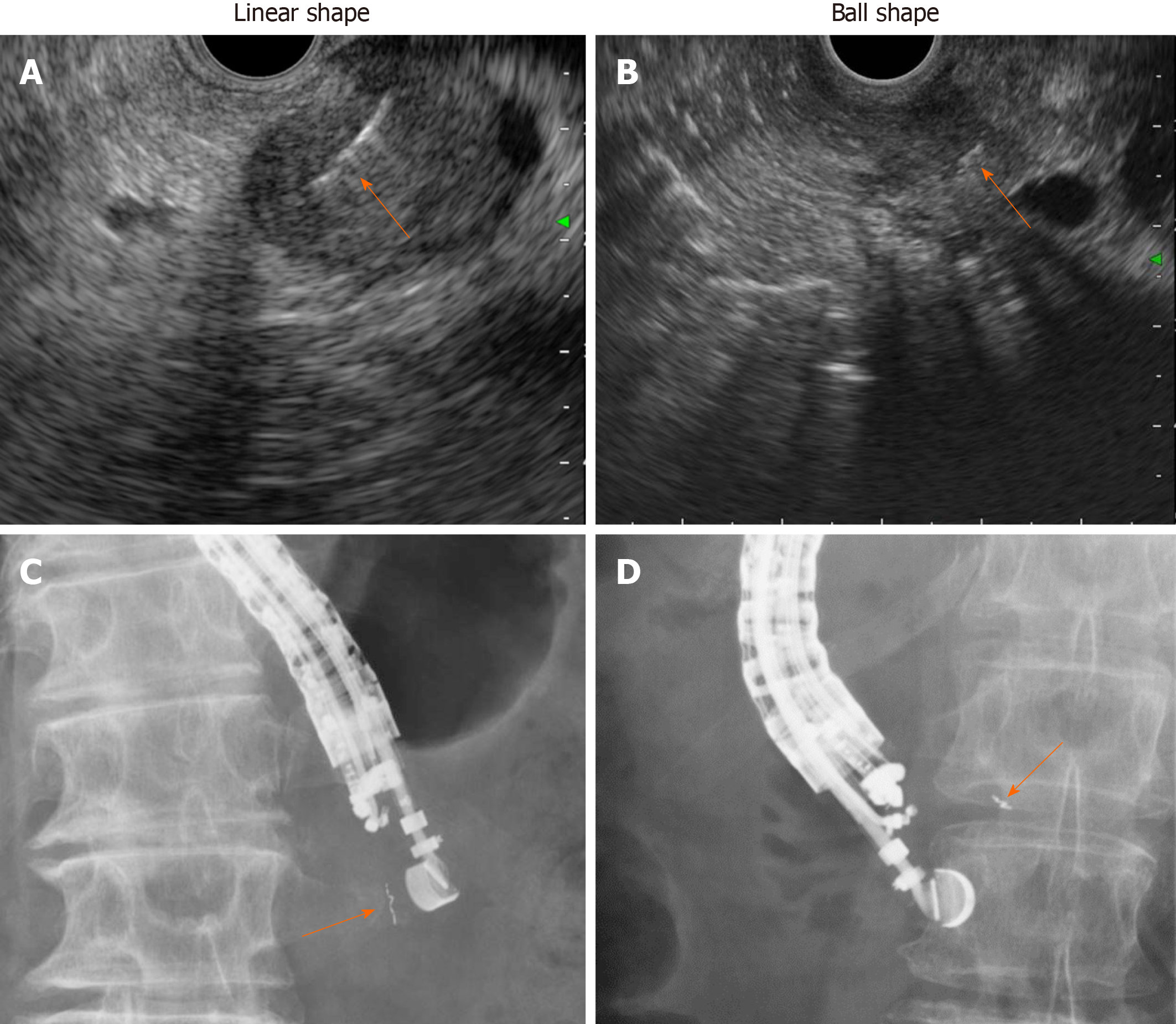
Similar to previously reported results[6-10], the overall complication rate was 6.9% in the present study and included self-limiting minor luminal bleeding. One patient (3.4%) showed marker migration 11 d after placement during NACRT (Figure 3). Migration depends on the type of fiducial marker used, and previous studies have shown that coiled fiducials migrate less than traditional fiducials[22]. However, the notched design of the Gold Anchor might be advantageous because the fiducial shape can be modified to either a ball or linear shape depending on the insertion technique, and the fiducial consequently remains more stable in the tumor (Figure 7)[23]. Our radiation oncologists prefer the ball shape to the linear shape due to better visibility on CBCT.
All surgical specimens were examined to investigate the impact of marker placement on the tumor tissue. Except in one case of migration, all the gold markers were confirmed to be present at the initial placement site in the resected specimen. No significant changes around the markers, such as the accumulation of inflammatory cells or fibrosis, were observed in any pathological specimen (Figure 6). Therefore, the influence of the marker placement itself on NACRT or resection was small.
In terms of the benefits of placing fiducial markers in RPC, the results of the present study demonstrate that RPC is more mobile than initially expected, particularly along the superior-inferior axis, which is not dependent on respiratory effects, but on food intake or bowel gas. As shown in Figure 4, these differences show large individual variation.
It was previously considered that the positional change in the pancreas was small because the pancreas is a retroperitoneal organ. Moreover, unresectable PC is usually less mobile because of its infiltration into the retroperitoneum. However, as shown in Figure 5, RPC is very mobile, not only due to respiratory effects but also due to food or even water intake. Therefore, we adjusted the radiation protocol to no longer allow patients to eat before undergoing radiation. This may increase the efficacy of radiation therapy as well as reduce the side effects.
The results of the present study suggest several areas for further investigation. First, the optimal location and number of fiducial markers that should be inserted into the tumor for adequate image guided radiotherapy are still unclear. This question may depend on the type of radiation treatment. For example, at our institution, a single gold marker was placed to confirm the tumor location daily. However, multiple marker placements are necessary if synchronizing respiration or tracking irradiation is to be performed. Furthermore, if radiation with strong GI toxicity, such as heavy particle therapy or proton beam therapy, is planned, it may be necessary to place the marker in the vicinity of the GI tract so that appropriate precautions can be made during radiation planning to avoid irradiating the digestive tract, which can cause GI bleeding or perforation.
In addition, the actual advantage of placing fiducial markers on the NACRT remains unclear. As the present study was a single arm study and the radiation field was not changed from the conventional method, it was difficult to discuss the superiority of image guided radiotherapy on tumor size reduction or overall survival. However, given the large tumor movement during daily treatment, image guided radiotherapy with fiducial markers may become an indispensable treatment for improving the prognosis of PC in the future.
In summary, EUS-FP is feasible and safe in patients with RPC who are undergoing NACRT. This technique not only has a high technical success rate and a very low complication rate, it provides accurate tumor location information during daily radiation therapy.
Preoperative neoadjuvant chemoradiation therapy (NACRT) is applied for resectable pancreatic cancer (RPC). To maximize the efficacy of NACRT, it is essential to ensure the accurate placement of fiducial markers for image-guided radiation. However, no standard method for delivering fiducial markers has been established.
For the accurate placement of fiducial markers, endoscopic ultrasound-guided fiducial marker placement (EUS-FP) have been published for unresectable locally advanced PC or borderline RPC showing promising results. However, there is no study that has focused only on RPC. Therefore, the present study focused on the feasibility and safety of EUS-FP for RPC undergoing NACRT as well as the nature of RPC during NACRT.
A total of 29 patients (18 males, 11 females) with pathologically confirmed resectable adenocarcinoma with unclear margin for NACRT were referred for EUS-FP.
Under EUS guidance, a single gold marker was placed into the tumor using either a 19- or 22-gauge fine-needle aspiration needle. The differences in daily marker positioning were measured by comparing simulation computed tomography and treatment computed tomography.
In all 29 patients (100%) who underwent EUS-FP, fiducials were placed successfully with only minor, self-limiting bleeding during puncture observed in 2 patients (6.9%). NACRT was subsequently administered to all patients and completed in 28/29 (96.6%) cases, with one patient experiencing repeat cholangitis. Spontaneous migration of gold markers was observed in 1 patient. Twenty-four patients (82.8%) had surgery with 91.7% (22/24) R0 resection, and two patients experienced complete remission. No inflammatory changes around the marker were observed in the surgical specimen. The daily position of gold markers showed large positional changes, particularly in the superior-inferior direction. Moreover, tumor location was affected by food and fluid intake as well as bowel gas, which changes daily.
EUS-FP for RPC not only has a high technical success rate and a very low complication rate, it provides accurate tumor location information during daily radiation therapy.
A prospective randomized controlled study with a larger sample size of patients is needed to further evaluate the role EUS-FP following NACRT for RPC.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy (Fellow) and Japanese Society of Gastroenterology (Fellow).
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao ZF, Lin Q, Sun SY S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzese JL, Wolff RA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 547] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 2. | Satoi S, Toyokawa H, Yanagimoto H, Yamamoto T, Kamata M, Ohe C, Sakaida N, Uemura Y, Kitade H, Tanigawa N, Inoue K, Matsui Y, Kwon AH. Neo-adjuvant chemoradiation therapy using S-1 followed by surgical resection in patients with pancreatic cancer. J Gastrointest Surg. 2012;16:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Shinoto M, Yamada S, Terashima K, Yasuda S, Shioyama Y, Honda H, Kamada T, Tsujii H, Saisho H; Working Group for Pancreas Cancer. Carbon Ion Radiation Therapy With Concurrent Gemcitabine for Patients With Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2016;95:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | de Geus SWL, Eskander MF, Kasumova GG, Ng SC, Kent TS, Mancias JD, Callery MP, Mahadevan A, Tseng JF. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer. 2017;123:4158-4167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Fernandez DC, Hoffe SE, Barthel JS, Vignesh S, Klapman JB, Harris C, Almhanna K, Biagioli MC, Meredith KL, Feygelman V, Rao NG, Shridhar R. Stability of endoscopic ultrasound-guided fiducial marker placement for esophageal cancer target delineation and image-guided radiation therapy. Pract Radiat Oncol. 2013;3:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Choi JH, Seo DW, Park DH, Lee SK, Kim MH. Fiducial placement for stereotactic body radiation therapy under only endoscopic ultrasonography guidance in pancreatic and hepatic malignancy: practical feasibility and safety. Gut Liver. 2014;8:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Ammar T, Coté GA, Creach KM, Kohlmeier C, Parikh PJ, Azar RR. Fiducial placement for stereotactic radiation by using EUS: feasibility when using a marker compatible with a standard 22-gauge needle. Gastrointest Endosc. 2010;71:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Varadarajulu S, Trevino JM, Shen S, Jacob R. The use of endoscopic ultrasound-guided gold markers in image-guided radiation therapy of pancreatic cancers: a case series. Endoscopy. 2010;42:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Ohigashi H, Ishikawa O, Eguchi H, Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H, Tomita Y, Nishiyama K. Feasibility and efficacy of combination therapy with preoperative full-dose gemcitabine, concurrent three-dimensional conformal radiation, surgery, and postoperative liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg. 2009;250:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Takahashi H, Ohigashi H, Gotoh K, Marubashi S, Yamada T, Murata M, Ioka T, Uehara H, Yano M, Ishikawa O. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg. 2013;258:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Owens DJ, Savides TJ. EUS placement of metal fiducials by using a backloaded technique with bone wax seal. Gastrointest Endosc. 2009;69:972-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Ohira S, Isono M, Ueda Y, Hirata T, Ashida R, Takahashi H, Miyazaki M, Takashina M, Koizumi M, Teshima T. Assessment with cone-beam computed tomography of intrafractional motion and interfractional position changes of resectable and borderline resectable pancreatic tumours with implanted fiducial marker. Br J Radiol. 2017;90:20160815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ, Ames FC. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 542] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1170] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 17. | He J, Blair AB, Groot VP, Javed AA, Burkhart RA, Gemenetzis G, Hruban RH, Waters KM, Poling J, Zheng L, Laheru D, Herman JM, Makary MA, Weiss MJ, Cameron JL, Wolfgang CL. Is a Pathological Complete Response Following Neoadjuvant Chemoradiation Associated With Prolonged Survival in Patients With Pancreatic Cancer? Ann Surg. 2018;268:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Sotiropoulou E, Stathochristopoulou I, Stathopoulos K, Verigos K, Salvaras N, Thanos L. CT-guided fiducial placement for cyberknife stereotactic radiosurgery: an initial experience. Cardiovasc Intervent Radiol. 2010;33:586-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Kim JH, Hong SS, Kim JH, Park HJ, Chang YW, Chang AR, Kwon SB. Safety and efficacy of ultrasound-guided fiducial marker implantation for CyberKnife radiation therapy. Korean J Radiol. 2012;13:307-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Khara HS, Pineda-Bonilla JJ, Chaput KJ, Johal AS. Endoscopic ultrasound-guided placement of fiducial markers using a novel "wet-fill technique" without a bone wax seal. Endoscopy. 2013;45 Suppl 2 UCTN:E426-E427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Draganov PV, Chavalitdhamrong D, Wagh MS. Evaluation of a new endoscopic ultrasound-guided multi-fiducial delivery system: a prospective non-survival study in a live porcine model. Dig Endosc. 2013;25:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Khashab MA, Kim KJ, Tryggestad EJ, Wild AT, Roland T, Singh VK, Lennon AM, Shin EJ, Ziegler MA, Sharaiha RZ, Canto MI, Herman JM. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Dávila Fajardo R, Lekkerkerker SJ, van der Horst A, Lens E, Bergman JJ, Fockens P, Bel A, van Hooft JE. EUS-guided fiducial markers placement with a 22-gauge needle for image-guided radiation therapy in pancreatic cancer. Gastrointest Endosc. 2014;79:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |