Published online Jul 15, 2020. doi: 10.4251/wjgo.v12.i7.756
Peer-review started: March 3, 2020
First decision: April 18, 2020
Revised: May 1, 2020
Accepted: June 2, 2020
Article in press: June 2, 2020
Published online: July 15, 2020
Processing time: 133 Days and 16.8 Hours
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis can be performed in two ways: Open or closed abdominal technique.
To evaluate the impact of HIPEC method on post-operative and long-term survival outcomes.
Patients undergoing CRS with HIPEC from 2000-2017 were identified in the United States HIPEC collaborative database. Post-operative, recurrence, and overall survival outcomes were compared between those who received open vs closed HIPEC.
Of the 1812 patients undergoing curative-intent CRS and HIPEC, 372 (21%) patients underwent open HIPEC and 1440 (79%) underwent closed HIPEC. There was no difference in re-operation or severe complications between the two groups. Closed HIPEC had higher rates of 90-d readmission while open HIPEC had a higher rate of 90-d mortalities. On multi-variable analysis, closed HIPEC technique was not a significant predictor for overall survival (hazards ratio: 0.75, 95% confidence interval: 0.51-1.10, P = 0.14) or recurrence-free survival (hazards ratio: 1.39, 95% confidence interval: 1.00-1.93, P = 0.05) in the entire cohort. These findings remained consistent in the appendiceal and the colorectal subgroups.
In this multi-institutional analysis, the HIPEC method was not independently associated with relevant post-operative or long-term outcomes. HIPEC technique may be left to the discretion of the operating surgeon.
Core tip: Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) is the standard of care for carefully selected patients with peritoneal carcinomatosis. HIPEC can be performed in one of two ways: Open or closed abdominal technique. Our goal was to use a multi-institutional database to determine the impact of HIPEC method on post-operative and long-term survival outcomes. Among 1812 patients undergoing cytoreductive surgery-HIPEC in this multi-institutional analysis, the method of HIPEC delivery was not independently associated with relevant post-operative or long-term survival outcomes. The method of HIPEC may be left to the discretion of the primary surgeon.
- Citation: Leiting JL, Cloyd JM, Ahmed A, Fournier K, Lee AJ, Dessureault S, Felder S, Veerapong J, Baumgartner JM, Clarke C, Mogal H, Staley CA, Zaidi MY, Patel SH, Ahmad SA, Hendrix RJ, Lambert L, Abbott DE, Pokrzywa C, Raoof M, LaRocca CJ, Johnston FM, Greer J, Grotz TE. Comparison of open and closed hyperthermic intraperitoneal chemotherapy: Results from the United States hyperthermic intraperitoneal chemotherapy collaborative. World J Gastrointest Oncol 2020; 12(7): 756-767
- URL: https://www.wjgnet.com/1948-5204/full/v12/i7/756.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i7.756
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has become a recognized treatment option for well-selected patients with peritoneal carcinomatosis[1-3]. HIPEC permits the pharmacokinetic advantages of dose intensification, direct administration, enhanced tissue penetration, and synergistic cytotoxic effects of regional hyperthermia. The administration of HIPEC is generally done with either an open abdominal technique or a closed abdominal technique[4].
The open abdominal technique, or “coliseum technique”, is well described by Sugarbaker[5]. Briefly, closed suction drains are placed for inflow and outflow of hyperthermic chemotherapy. The abdominal wall skin edges are elevated with a retractor and the abdominal contents are directly agitated manually. In contrast, the closed technique involves placement of inflow and outflow catheters with temporary closure of the skin edges. The abdominal contents are agitated externally through the abdominal wall[6].
Proponents of the open method would argue that this technique allows for visualization of the abdominal cavity throughout the course of the treatment, allowing for more uniform distribution of heat and chemotherapy[7]. The disadvantages of this technique are that because of heat dissipation, it is more difficult to initially achieve a hyperthermic state, as well as the potential for contact, splash, and aerosolization exposure of cytotoxic agents to the operating team. The closed technique greatly limits the risk of exposure to the chemotherapy agent by the operating room staff, though it sacrifices visibility of the abdominal cavity in the process, potentially allowing for pooling of heat and chemotherapy. Another major advantage of the closed technique is the ability to rapidly achieve and maintain hyperthermia as there is minimal heat loss and the elevated intraabdominal pressure associated with the closed technique may improve tissue penetration[8].
The optimal method of HIPEC delivery has long been debated. Previous investigations have been limited to small single center retrospective reviews[9,10] or preclinical animal models[11-13]. Therefore, we sought to evaluate the impact of open vs closed HIPEC technique on short- and long-term outcomes, using a large multi-institutional database.
Patients who underwent curative-intent CRS and HIPEC from 2000 to 2017 were identified in the United States HIPEC collaborative database, a retrospectively-collected database from 12 high-volume institutions. Demographic, clinical, pathological, post-operative, and survival data was collected. Patients were divided into those who underwent open HIPEC and those who underwent closed HIPEC. Patients without available histology or overall survival data were excluded from the analysis.
Continuous variables were presented as mean and SD if normally distributed and median with interquartile range (IQR) if they were not normally distributed. Student’s t-test was used to compare continuous variables. Categorical variables were presented as total count and percentage and χ2 or Fisher’s Exact Test were used for comparison. Length of stay and intensive care unit (ICU) length of stay were dichotomized at > 75th percentile and ≤ 75th percentile for logistic regression models. PIC dose was dichotomized at > 90th percentile and ≤ 90th percentile for multivariable analysis.
Multi-variable logistic regression models were used for post-operative outcomes using clinically relevant variables and possible confounders. Estimates for overall survival (OS) and recurrence-free survival (RFS) were calculated using the Kaplan-Meier survival method. Univariate tests of association were made using a log-rank test. OS was assessed from the time of surgery to death and RFS was assessed from the time of surgery to the time of documented recurrence or last follow-up. Cox proportional hazard regression was used for multi-variable analysis of OS and RFS using variables with univariate significance (P < 0.05) and clinically relevant factors. P values of < 0.05 were considered statistically significant and all tests were 2-sided. Statistical analysis was performed on JMP software (JMP® Pro, Version 13.0.0, SAS Institute Inc., Cary, NC, United States). This study was approved by the Institutional Review Board at all participating institutions. No propensity score adjustment was made in the analyses as there were no variables available that were useful in the prediction of the use of open vs closed HIPEC. Statistical methods were reviewed by biomedical statisticians.
From 2000-2017, 1812 patients underwent curative-intent CRS with HIPEC. Of these, 372 (20.5%) underwent open HIPEC and 1440 (79.5%) underwent closed HIPEC. The frequency of technique changed over time. From 2000-2009, 80% of HIPEC procedures were done with an open abdomen compared to only 17% from 2010-2017 (P < 0.01). Univariate analysis revealed significant differences between the two groups (Table 1). Open HIPEC patients were more likely to be undergoing their first CRS (88.1% vs 75.6%, P < 0.01) and had better tumor biology with a greater prevalence of appendiceal (83.6% vs 60.4%, P < 0.01) and well differentiated (60.6% vs 51.9%, P < 0.01) tumors. Patients undergoing closed HIPEC were older (54.6 years vs 52.7 years, P = 0.02) with more racial diversity (79.2% white vs 94.1%, P < 0.01). Mean peritoneal carcinomatosis index (PCI) was also higher in the closed HIPEC group (14.7 vs 10.8, P < 0.01) though there were fewer completeness of cytoreduction (CC) scores of 2 or 3 (5% vs 14%, P < 0.01). Mitomycin-C was the most commonly used agent in both groups, though the average dose of Mitomycin-C was higher in the open HIPEC group (54 mg vs 40 mg, P < 0.01).
| Open | Closed | P value | ||||
| n = 372 | n = 1440 | |||||
| n (%) | n (%) | |||||
| Female gender | 165 (44.4) | 616 (42.8) | 0.58 | |||
| Mean age (SD) | 52.7 (12.4) | 54.6 (12.4) | 0.02 | |||
| Race | < 0.01 | |||||
| White | 350 (94.1) | 1140 (79.2) | ||||
| Black | 16 (4.3) | 83 (5.8) | ||||
| Asian | 2 (0.5) | 79 (5.5) | ||||
| Latino | 0 (0) | 71 (4.9) | ||||
| Other | 4 (1.1) | 50 (3.5) | ||||
| Missing | 0 (0) | 17 (1.2) | ||||
| Mean BMI (SD) | 28.4 (6.6) | 27.9 (6.6) | 0.22 | |||
| Previous CRS | < 0.01 | |||||
| 0 | 328 (88.1) | 1088 (75.6) | ||||
| 1 | 38 (10.2) | 298 (20.7) | ||||
| 2+ | 5 (1.3) | 51 (3.5) | ||||
| Missing | 1 (0.3) | 3 (0.2) | ||||
| Mean preoperative albumin (SD) | 4.0 (0.5) | 4.1 (0.5) | 0.17 | |||
| Histology | < 0.01 | |||||
| Appendiceal | 311 (83.6) | 870 (60.4) | ||||
| Colorectal | 42 (11.3) | 376 (26.1) | ||||
| Mesothelioma | 9 (2.4) | 142 (9.9) | ||||
| Other | 10 (2.7) | 52 (3.6) | ||||
| Tumor differentiation (appendiceal and colorectal) | < 0.01 | |||||
| Well | 214 (60.6) | 647 (51.9) | ||||
| Moderate or poor | 105 (29.8) | 511 (41.0) | ||||
| Missing | 34 (9.6) | 88 (7.1) | ||||
| ASA grade | < 0.01 | |||||
| 1 / 2 | 26 (7.0) | 249 (17.3) | ||||
| 3 / 4 | 346 (93.0) | 1024 (71.1) | ||||
| Missing | 0 (0) | 167 (11.6) | ||||
| Mean PCI (SD) | 10.8 (14.7) | 14.7 (8.7) | < 0.01 | |||
| Neoadjuvant chemotherapy | 0.47 | |||||
| Yes | 129 (34.7) | 508 (35.3) | ||||
| No | 239 (64.3) | 925 (64.2) | ||||
| Missing | 4 (1.1) | 7 (0.5) | ||||
| Mean operative time (h) (SD) | 6.7 (2.1) | 8.5 (2.7) | < 0.01 | |||
| Mean estimated blood loss (cc) (SD) | 491 (931) | 444 (560) | 0.37 | |||
| IP chemotherapy | 0.05 | |||||
| Mitomycin-C | 352 (94.6) | 1299 (90.2) | ||||
| Cisplatin | 14 (3.8) | 90 (6.3) | ||||
| Other | 6 (1.6) | 51 (3.5) | ||||
| Median MMC dose (mg) (IQR) | 54 (40-60) | 40 (33-40) | < 0.01 | |||
| Median cisplatin dose (mg) (IQR) | 75 (58-110) | 138 (86-250) | < 0.01 | |||
| Target temperature 40-43 ˚C | 362 (97.3) | 1415 (98.3) | 0.25 | |||
| Mean HIPEC duration (min) (SD) | 87 (10) | 89 (8) | 0.14 | |||
| CCR | < 0.01 | |||||
| CC 0/1 | 316 (84.9) | 1263 (87.7) | ||||
| CC 2/3 | 53 (14.2) | 74 (5.1) | ||||
| Missing | 3 (0.8) | 103 (7.2) | ||||
| Median LOS (IQR) | 9 (8-13) | 10 (8-13) | 0.50 | |||
| Complications | 0.27 | |||||
| Grade 0-II | 306 (82.3) | 1146 (79.6) | ||||
| Grade III-V | 66 (17.7) | 294 (20.4) | ||||
| 90-d readmission | < 0.01 | |||||
| Yes | 47 (12.6) | 341 (23.7) | ||||
| No | 321 (86.3) | 1089 (75.6) | ||||
| Missing | 4 (1.1) | 10 (0.7) | ||||
| Re-operation | 32 (8.6) | 131 (9.1) | 0.59 | |||
| Median days in ICU (IQR) | 2 (2-3) | 1 (0-3) | < 0.01 | |||
| 90-d mortality | 14 (3.8) | 25 (1.7) | 0.03 | |||
| Adjuvant chemotherapy | < 0.01 | |||||
| Yes | 47 (12.6) | 341 (23.7) | ||||
| No | 323 (86.8) | 1095 (76.0) | ||||
| Missing | 2 (0.5) | 4 (0.3) | ||||
Differences in short-term outcomes between patients undergoing open vs closed technique are reported in Table 1. Grade III or higher complications were similar between the two groups (17.7% vs 20.4%, P = 0.27). Open HIPEC patients had shorter operative times (6.7 h vs 8.5 h, P < 0.01) and fewer readmissions (12.6% vs 23.7%, P < 0.01) though their 90-d mortality rate was higher (3.8% vs 1.7%, P = 0.03). There was no significant difference in estimated blood loss and reoperation rates between the two groups.
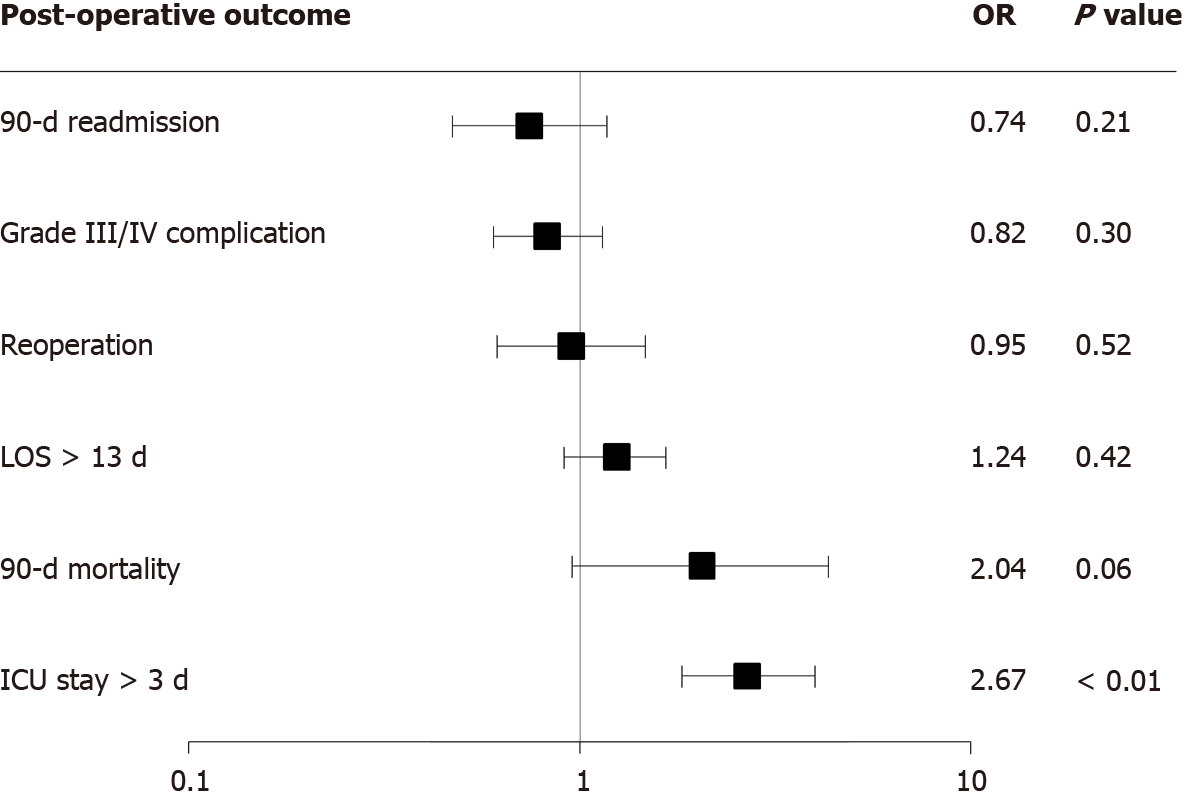
The impact of open vs closed HIPEC was assessed by logistic regression for the following post-operative factors: 90-d readmission, grade III or IV post-operative complication, reoperation, length of stay longer than the 75th percentile (13 d), 90-d mortality, and ICU stay longer than the 75th percentile (3 d). The odds ratio for open HIPEC when compared to closed HIPEC for individual multivariable models are seen in Figure 1. On logistic regression, the only post-operative outcome that was significant for HIPEC method was length of ICU stay where open HIPEC remained an independent predictor for longer stays in the ICU (OR: 2.67, 95%CI: 1.81-3.93, P < 0.01).
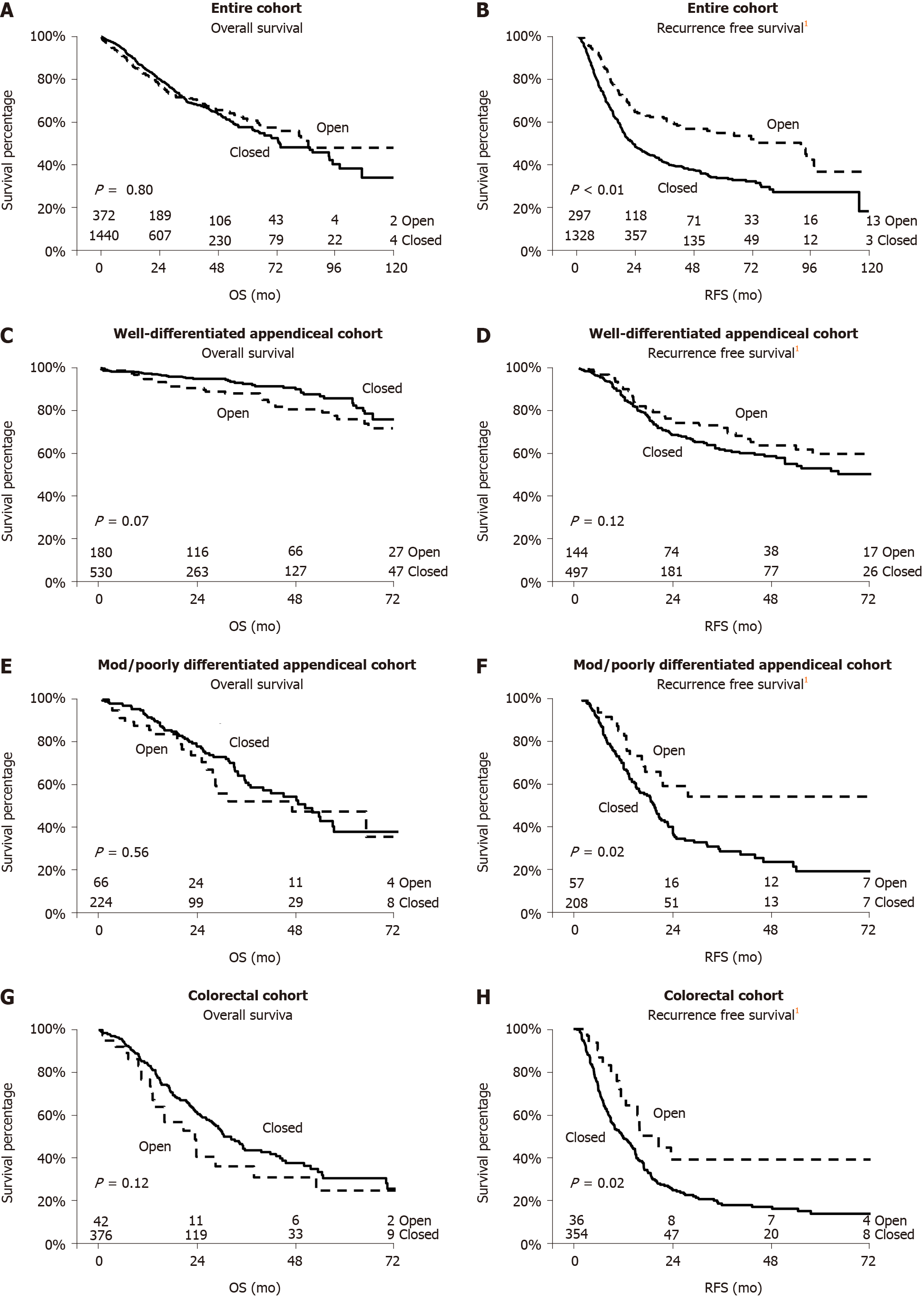
Median follow-up for the entire cohort was 20 mo (IQR: 8-39). On unadjusted Kaplan-Meier analysis of OS, there was no difference between open and closed HIPEC (Figure 2A). Median OS for the open HIPEC group was 85 mo and 73 mo for the closed HIPEC group (log-rank P = 0.80). For RFS, patients undergoing open HIPEC did better with a median RFS of 92 mo compared to 22 mo in the closed HIPEC group (log-rank P < 0.01) (Figure 2B). In an analysis of the well-differentiated appendiceal cancer subgroup (n = 710) and the moderate to poorly differentiated subgroup (n = 290), unadjusted OS was not significantly different (Figure 2C and E), however, the RFS was significantly better in the open HIPEC group for the moderate to poorly differentiated tumors (Figure 2F). Similarly, for the colorectal cancer subgroup (n = 418), OS was again not significantly different between the two groups with better RFS in the open group (Figure 2G and H).
To better understand the impact of HIPEC method on survival, multi-variable Cox proportional regression analysis was done for OS of the entire cohort, appendiceal cohort, and colorectal cohort (Table 2). In all three cohorts, HIPEC method was not an independent predictor for OS. Predictors of worse OS in all three cohorts were PCI ≥ 20 and a CC score of 2 or 3.
| Factor | OS entire cohort | OS appendiceal cohort | OS colorectal cohort | |||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | ||
| Age | < 65 | Reference | Reference | Reference | ||||||
| ≥ 65 | 1.07 | 0.79-1.45 | 0.65 | 1.21 | 0.80-1.81 | 0.37 | 0.87 | 0.54-1.39 | 0.57 | |
| Previous CRS | No | Reference | Reference | Reference | ||||||
| Yes | 1.22 | 0.91-1.64 | 0.18 | 1.51 | 1.01-2.24 | 0.04 | 1.10 | 0.69-1.73 | 0.68 | |
| Histology | Appendiceal | Reference | ||||||||
| Colorectal | 2.75 | 1.99-3.78 | < 0.01 | |||||||
| Differentiation | Well | Reference | Reference | Reference | ||||||
| Mod / poor | 3.22 | 2.28-4.55 | < 0.01 | 3.92 | 2.63-5.82 | < 0.01 | 1.55 | 0.79-3.06 | 0.20 | |
| ASA | 1-2 | Reference | Reference | Reference | ||||||
| 3-4 | 1.98 | 1.17-3.32 | 0.01 | 1.83 | 0.94-3.56 | 0.07 | 2.01 | 0.84-4.79 | 0.11 | |
| PCI | < 20 | Reference | Reference | Reference | ||||||
| ≥ 20 | 1.86 | 1.34-2.57 | < 0.01 | 1.86 | 1.21-2.88 | < 0.01 | 1.94 | 1.17-3.21 | 0.01 | |
| PIC dose | Low dose | Reference | Reference | Reference | ||||||
| High dose | 1.35 | 0.91-2.02 | 0.13 | 1.16 | 0.67-2.01 | 0.58 | 1.49 | 0.65-3.42 | 0.33 | |
| CCR | 0-1 | Reference | Reference | Reference | ||||||
| 2-3 | 2.33 | 1.49-3.63 | < 0.01 | 2.15 | 1.28-3.61 | < 0.01 | 5.74 | 1.99-16.56 | < 0.01 | |
| Complications | 0 – II | Reference | Reference | Reference | ||||||
| III – V | 1.61 | 1.20-2.14 | < 0.01 | 1.23 | 0.81-1.86 | 0.31 | 1.96 | 1.26-3.05 | < 0.01 | |
| Adjuvant chemo | No | Reference | Reference | Reference | ||||||
| Yes | 1.16 | 0.86-1.56 | 0.31 | 1.33 | 0.87-2.05 | 0.18 | 0.98 | 0.65-1.48 | 0.94 | |
| PIC method | Open | Reference | Reference | Reference | ||||||
| Closed | 0.75 | 0.51-1.10 | 0.14 | 0.78 | 0.48-1.25 | 0.31 | 0.67 | 0.29-1.53 | 0.34 | |
Similarly, a multi-variable regression analysis was done for RFS on the three groups: Entire cohort, appendiceal only, and colorectal only (Table 3). Again, HIPEC method was found to be an insignificant factor in RFS (entire cohort: HR: 1.36, P = 0.05; appendiceal: HR: 1.13, P = 0.52, colorectal: HR: 1.75, P = 0.08). Predictors of worse RFS in the entire cohort were previous CRS, colorectal histology, moderate to poor differentiation, ASA ≥ 3, PCI ≥ 20, and CC score of 2 or 3.
| Factor | RFS entire cohort | RFS appendiceal cohort | RFS colorectal cohort | |||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | ||
| Age | < 65 | Reference | Reference | Reference | ||||||
| ≥ 65 | 1.00 | 0.78-1.26 | 0.99 | 0.95 | 0.69-1.29 | 0.75 | 1.03 | 0.70-1.50 | 0.87 | |
| Previous CRS | No | Reference | Reference | Reference | ||||||
| Yes | 1.27 | 1.01-1.59 | 0.04 | 1.35 | 1.01-1.81 | 0.04 | 1.17 | 0.80-1.70 | 0.39 | |
| Histology | Appendiceal | Reference | ||||||||
| Colorectal | 2.20 | 1.72-2.82 | < 0.01 | |||||||
| Differentiation | Well | Reference | Reference | Reference | ||||||
| Mod / Poor | 1.77 | 1.37-2.29 | < 0.01 | 1.89 | 1.41-2.55 | < 0.01 | 0.91 | 0.54-1.52 | 0.73 | |
| ASA | 1-2 | Reference | Reference | Reference | ||||||
| 3-4 | 1.56 | 1.12-2.18 | 0.01 | 1.82 | 1.20-2.77 | < 0.01 | 1.23 | 0.69-2.21 | 0.46 | |
| PCI | < 20 | Reference | Reference | Reference | ||||||
| ≥ 20 | 1.59 | 1.24-2.02 | < 0.01 | 1.78 | 1.33-2.39 | < 0.01 | 1.27 | 0.80-2.03 | 0.31 | |
| PIC dose | Low dose | Reference | Reference | Reference | ||||||
| High dose | 1.06 | 0.71-1.59 | 0.74 | 0.87 | 0.53-1.41 | 0.58 | 1.32 | 0.66-2.63 | 0.42 | |
| CCR | 0-1 | Reference | Reference | Reference | ||||||
| 2-3 | 1.90 | 1.02-3.55 | 0.04 | 2.35 | 1.17-4.72 | 0.02 | 1.04 | 0.25-4.27 | 0.95 | |
| Complications | 0 – II | Reference | Reference | Reference | ||||||
| III – V | 1.23 | 0.96-1.56 | 0.09 | 1.20 | 0.87-1.65 | 0.24 | 1.10 | 0.74-1.64 | 0.62 | |
| Adjuvant chemo | No | Reference | Reference | Reference | ||||||
| Yes | 1.46 | 1.16-1.84 | < 0.01 | 2.23 | 0.63-3.05 | < 0.01 | 0.92 | 0.66-1.28 | 0.62 | |
| PIC method | Open | Reference | Reference | Reference | ||||||
| Closed | 1.39 | 1.00-1.93 | 0.05 | 1.13 | 0.77-1.66 | 0.52 | 1.75 | 0.93-3.29 | 0.08 | |
The optimal method for HIPEC administration has long been debated without a clear answer. This study is the first to directly compare both post-operative and long-term survival outcomes between these two methods in a large cohort of patients across multiple institutions. We have demonstrated that in this mixed histological cohort, HIPEC method has little, if any, impact on post-operative outcomes. Additionally, we have shown that after accounting for significant confounding variables, HIPEC method did not impact overall or recurrence free survival for the entire group, as well as having no impact on survival in the appendiceal and the colorectal subgroups. Therefore, the method of HIPEC delivery may be left to the discretion of the operating surgeon as it does not appear to significantly influence short- or long-term outcomes, though there is a growing need to standardize HIPEC technique as we continue to move forward with clinical trials.
One of the major arguments for open HIPEC is that it results in better tissue penetration when compared to closed HIPEC. A preclinical study in pigs demonstrated better tissue penetration and higher systemic concentrations with an open technique compared to a closed technique[13]. A second preclinical study from the same group again showed a deeper penetration of oxaliplatin when the open technique was used[11]. Another argument for open HIPEC is the ability for better temperature homogeneity[7]. On the other hand, a downside of open HIPEC is achieving and maintaining hyperthermic temperatures given the dissipation of heat from the open abdomen[10]. Our study would suggest that these different parameters are unlikely to confer a survival advantage for either HIPEC method. The method of HIPEC used was not a significant factor when other confounding variables were accounted for. Other factors that have previously been shown to significantly impact survival, particularly PCI, completeness of cytoreduction, and post-operative complications, were found to be significant in this study[14-16].
A concern with open HIPEC remains the lack of barrier between the cytotoxic agents and the operating staff. In a small preclinical study, analysis of operating room gloves after administration of open HIPEC was unable to detect chemotherapeutics on the inner surface of the glove[11]. Other studies have been unable to detect cytotoxic therapeutics in the blood or urine of individuals involved in the administration of open HIPEC[17,18]. This would suggest that there is limited risk of contamination with cytotoxic agents if appropriate precautions are taken when dealing with these toxic compounds, although no official guidelines exist as to what these precautions should be[19].
One previous retrospective study of around 100 patients compared intraoperative patient parameters between open HIPEC to closed HIPEC[9]. They did not find any differences in morbidity or mortality, and though patients who underwent closed HIPEC tended toward more stable hemodynamics than those undergoing open HIPEC, this did not reach statistical significance[9]. Our study did not look at specific hemodynamics but we found no difference in EBL between patients in the open vs the closed HIPEC groups. Time was longer in the closed HIPEC group (8.5 h vs 6.7 h) though this may have been more of a result of the greater PCI and need for operative procedures than the technical aspects of HIPEC administration. Additionally, patients undergoing open HIPEC in our study were more likely to have extended stays in the ICU and higher 90-d mortality. This may be a reflection in practice differences with routine admission to the ICU and higher mortality early in the HIPEC experience. There were no other differences in post-operative outcomes between the two techniques.
With regards to intraoperative parameters, the vast majority of patients in both groups received Mitomycin-C with a small percentage of patients receiving Cisplatin. Patients in the open HIPEC group had a higher average dose of Mitomycin-C (54 mg vs 40 mg) when compared to patients in the closed HIPEC group, which may be accounted for by the fact that the majority of the open HIPEC cases were performed early in the experience. There were no differences in temperature or duration.
The method of HIPEC was not associated with any significant differences in OS on univariate or multi-variable analysis. There was a difference in RFS on univariate analysis with better RFS for patients undergoing open HIPEC (92 mo vs 22 mo, P < 0.01). This significance did not, however, remain on multi-variable analysis after controlling for other significant variables, including differentiation, histology, and PCI. This is likely due to the fact that patients in the open HIPEC group were more likely to have well differentiated appendiceal tumors with lower average PCIs.
There are several limitations in this multi-institutional study. Practice and treatment algorithms, including indications for surgery, likely differed across time and between institutions in this study, though this allows the results to be more generalizable to the entire country, rather than representing a single institution. Selection bias is always a risk with retrospective studies. We did investigate the use of propensity scoring to account for these factors but found that it was not particularly helpful in this analysis given the lack of reliable factors that influenced the HIPEC method received. This is likely due to the fact that surgeons perform one method or the other based on training and not on patient or tumor factors. We utilized multi-variable analyses in an attempt to mitigate this bias. The study population was a heterogeneous group of diverse histologies, though subgroup analysis was performed on those histologies with adequate numbers for analysis (e.g. appendiceal and colorectal). Lastly, we could not address the impact of HIPEC technique on incisional or wound recurrences as the database did not have that granularity of data.
In conclusion, the method of HIPEC, open vs closed technique, was not independently associated with relevant post-operative or long-term outcomes in this multi-institutional analysis of patients undergoing CRS and HIPEC for peritoneal carcinomatosis. While the technique of HIPEC may be left to the discretion of the surgeon, a continued emphasis on patient selection, obtaining a complete cytoreduction, and prevention of clinically relevant post-operative complications will optimize patient outcomes.
Appropriately selected patients with peritoneal carcinomatosis are treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). HIPEC is administered in either an open or a closed fashion.
The two techniques to administer HIPEC both have advantages and disadvantages. The open technique allows for full visualization of the abdomen during the HIPEC administration, though it is more difficult to maintain hyperthermia as well as increased potential for contamination with cytotoxic agents. The closed technique, on the other hand, allows for greater ability for temperature control and limits exposure though at the cost of visibility.
The objective of this study was to determine if one of these techniques was superior to the other in terms of both short- and long-term outcomes. Previous studies have been limited either preclinical animal models or single-center studies.
A multi-institutional database from 12 academic institutions across the country was utilized for this study. Patients who underwent curative-intent CRS and HIPEC were identified and demographic, clinical, post-operative, and survival data was obtained. Kaplan-Meier survival method was used to determine estimates for overall and recurrence-free survival. Cox proportional hazard regression was used for multi-variable analysis was also used for overall and recurrence-free survival.
There was no difference in severe complications or rates of re-operation between the open and the closed HIPEC groups. Open HIPEC had higher mortality within 90 d while closed HIPEC had higher rates of readmission. The HIPEC technique used was also not an independent factor for overall or recurrence-free survival on multi-variable analysis.
We found that HIPEC technique was not an independent factor for overall or recurrence-free survival, as well as not contributing significantly to relevant post-operative outcomes. Our goal was to determine if there was an optimal HIPEC regimen in order to provide patients with the best possible outcomes.
The HIPEC technique used can be left to the discretion of the operating surgeon, though continued effort to standardize HIPEC administration would benefit our ability to study patient outcomes. The optimal HIPEC regimen remains unknown and may vary depending on the clinical situation.
This work was presented at the 2019 International Symposium on Regional Cancer Therapies in Phoenix, AZ.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Coco D, Sommariva A S-Editor: Zhang L L-Editor: A E-Editor: Li X
| 1. | Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24:4011-4019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 2. | Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Helm JH, Miura JT, Glenn JA, Marcus RK, Larrieux G, Jayakrishnan TT, Donahue AE, Gamblin TC, Turaga KK, Johnston FM. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22:1686-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Glehen O, Cotte E, Kusamura S, Deraco M, Baratti D, Passot G, Beaujard AC, Noel GF. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Sugarbaker PH. Technical Handbook for the Integration of Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy into the Surgical Management of Gastrointestinal and Gynecologic Malignancy. 4th ed. Michigan: Laudann Company; 2005. |
| 6. | Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, Trillet-Lenoir V, Sayag-Beaujard AC, François Y, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 274] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Elias D, Antoun S, Goharin A, Otmany AE, Puizillout JM, Lasser P. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int J Surg Investig. 2000;1:431-439. [PubMed] |
| 8. | Goldenshluger M, Zippel D, Ben-Yaacov A, Dux J, Yalon T, Zendel A, Rayman S, Mor E, Berkenstadt H, Fogel-Grinvald H, Ventorrero M, Nissan A. Core Body Temperature but Not Intraabdominal Pressure Predicts Postoperative Complications Following Closed-System Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Administration. Ann Surg Oncol. 2018;25:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Halkia E, Tsochrinis A, Vassiliadou DT, Pavlakou A, Vaxevanidou A, Datsis A, Efstathiou E, Spiliotis J. Peritoneal carcinomatosis: intraoperative parameters in open (coliseum) versus closed abdomen HIPEC. Int J Surg Oncol. 2015;2015:610597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Rodríguez Silva C, Moreno Ruiz FJ, Bellido Estévez I, Carrasco Campos J, Titos García A, Ruiz López M, González Poveda I, Toval Mata JA, Mera Velasco S, Santoyo Santoyo J. Are there intra-operative hemodynamic differences between the Coliseum and closed HIPEC techniques in the treatment of peritoneal metastasis? A retrospective cohort study. World J Surg Oncol. 2017;15:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Facy O, Combier C, Poussier M, Magnin G, Ladoire S, Ghiringhelli F, Chauffert B, Rat P, Ortega-Deballon P. High pressure does not counterbalance the advantages of open techniques over closed techniques during heated intraperitoneal chemotherapy with oxaliplatin. Surgery. 2015;157:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Badrudin D, Sideris L, Perrault-Mercier C, Hubert J, Leblond FA, Dubé P. Comparison of open and closed abdomen techniques for the delivery of intraperitoneal pemetrexed using a murine model. J Surg Oncol. 2018;117:1318-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Ortega-Deballon P, Facy O, Jambet S, Magnin G, Cotte E, Beltramo JL, Chauffert B, Rat P. Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin? An experimental comparison of open and closed techniques. Ann Surg Oncol. 2010;17:1957-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Baratti D, Kusamura S, Iusco D, Bonomi S, Grassi A, Virzì S, Leo E, Deraco M. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum. 2014;57:858-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Coccolini F, Catena F, Glehen O, Yonemura Y, Sugarbaker PH, Piso P, Montori G, Ansaloni L. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur J Surg Oncol. 2015;41:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Näslund Andréasson S, Anundi H, Thorén SB, Ehrsson H, Mahteme H. Is Platinum Present in Blood and Urine from Treatment Givers during Hyperthermic Intraperitoneal Chemotherapy? J Oncol. 2010;2010:649719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Stuart OA, Stephens AD, Welch L, Sugarbaker PH. Safety monitoring of the coliseum technique for heated intraoperative intraperitoneal chemotherapy with mitomycin C. Ann Surg Oncol. 2002;9:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Ferron G, Simon L, Guyon F, Glehen O, Goere D, Elias D, Pocard M, Gladieff L, Bereder JM, Brigand C, Classe JM, Guilloit JM, Quenet F, Abboud K, Arvieux C, Bibeau F, De Chaisemartin C, Delroeux D, Durand-Fontanier S, Goasguen N, Gouthi L, Heyd B, Kianmanesh R, Leblanc E, Loi V, Lorimier G, Marchal F, Mariani P, Mariette C, Meeus P, Msika S, Ortega-Deballon P, Paineau J, Pezet D, Piessen G, Pirro N, Pomel C, Porcheron J, Pourcher G, Rat P, Regimbeau JM, Sabbagh C, Thibaudeau E, Torrent JJ, Tougeron D, Tuech JJ, Zinzindohoue F, Lundberg P, Herin F, Villeneuve L; BIG-RENAPE Working Group. Professional risks when carrying out cytoreductive surgery for peritoneal malignancy with hyperthermic intraperitoneal chemotherapy (HIPEC): A French multicentric survey. Eur J Surg Oncol. 2015;41:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |