Published online Jul 15, 2020. doi: 10.4251/wjgo.v12.i7.741
Peer-review started: February 4, 2020
First decision: March 24, 2020
Revised: April 6, 2020
Accepted: May 27, 2020
Article in press: May 27, 2020
Published online: July 15, 2020
Processing time: 161 Days and 18.9 Hours
Although the role of p53 in the evolution and prognosis of gastric cancer (GC) has been extensively examined, the exact mechanism of action is incompletely understood. In the last years, p53-target genes were supposed to be involved in the p53 pathway. One of them is the tumor-suppressor gene Maspin, which codifies the protein with the same name. Maspin activity depends on its subcellular localization. To our knowledge, the possible role of TP53 gene in Maspin subcellular localization, in GC cells, has not yet been studied in a large number of human samples.
To evaluate the possible role of wild-type and mutated p53 in Maspin subcellular localization.
The present study included 266 consecutive patients with GC in which TP53 gene status, and mutations in exons 2 to 11, respectively, were analyzed and correlated with immunohistochemical expression of p53 and Maspin.
None of the 266 cases showed mutations in exon 9. The rate of TP53 mutations was 33.83%. The mutation rate was slightly higher in distally-located GCs, with a lower degree (≤ 5 buds/ high power fields) of dyscohesivity (P < 0.01). The wild-type cases had a longer survival, compared with mutant GCs, especially in patients without lymph node metastases, despite the high depth of tumor infiltration (P = 0.01). The Dukes-MAC-like staging system was proved to have the most significant independent prognostic value (P < 0.01). The statistical correlations proved that TP53 gene mutations in exon 7 might induce knockdown of Maspin, but wild-type p53 can partially restore nuclear Maspin expression and decrease the metastatic potential of gastric adenocarcinoma cells.
Downregulated Maspin might be induced by mutations in exon 7 of the TP53 gene but wild-type p53 can partially restore nuclear Maspin expression. These findings should be proved in experimental studies.
Core tip: In this paper we tried to emphasize the possible prognostic role of TP53 status in gastric cancer, and its relation with Maspin protease. For the first time, we have proved that TP53 gene mutations in exon 7 might induce knockdown of Maspin, but wild-type p53 can partialy restore nuclear Maspin expression and decrease the metastatic potential of gastric adenocarcinoma cells.
- Citation: Gurzu S, Jung I, Sugimura H, Stefan-van Staden RI, Yamada H, Natsume H, Iwashita Y, Szodorai R, Szederjesi J. Maspin subcellular expression in wild-type and mutant TP53 gastric cancers. World J Gastrointest Oncol 2020; 12(7): 741-755
- URL: https://www.wjgnet.com/1948-5204/full/v12/i7/741.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i7.741
In many cancers, including gastric carcinomas (GC), TP53 is known to be frequently mutated[1,2]. Despite this well-known fact, the prognostic role of TP53 is still controversial. Moreover, its interaction with other biomarkers is constantly assessed but the results are controversial.
It is known that TP53 plays roles in genomic stability[1] but the mechanism of interaction with other p53-target genes such Maspin is unclear[2,3]. Maspin (Serpine B5) is a serine protease that is known to be involved in tumor cell proliferation and has antiangiogenic and anti-apoptotic properties against tumor cells[2-4]. Its regulatory functions depend on the subcellular localization (nucleus vs cytoplasm)[3,5]. In this study, we examined the p53 and Maspin immunohistochemical (IHC) expression, correlated with the mutation rate of TP53 gene, in GC samples. The independent prognostic role of these markers was also determined.
The present study included 266 GC cases, which were retrospectively enrolled from the Clinical County Emergency Hospital of Targu-Mureș, Romania. The Ethical Committee of the Clinical County Emergency Hospital, Targu-Mureș, Romania, approved retrospective evaluation of the cases. In these patients, surgical intervention was performed between 2006 and 2016, and consisted of partial or total curative gastrectomy, with lymph node dissection. No cases with stump carcinoma, associated peptic ulcer or patients receiving preoperative chemo- or radiotherapy were included.
In these 266 patients, age and gender were correlated with tumor characteristics such localization, stage, macroscopic type, microscopic type, histologic grade of differentiation, and the presence/absence of associated intestinal metaplasia. For macroscopic classification, we used Borrmann’s system and criteria proposed by the Japan Gastroenterological Endoscopy Society as follows: Type I-polypoid; type II-ulcerated; type III-ulcero-infiltrative; and type IV-diffusely infiltrative.
All of the examined samples were surgical specimens. We did not include biopsy samples. The histopathological characteristics were determined in carcinoma samples classified based on the combined Lauren and World Health Organization tumor classifications[6]. The following two main histologic subtypes were included[6]: Intestinal type GCs [well-differentiated (G1)/moderately differentiated (G2)/poorly (G3) differentiated GCs/papillary carcinoma] and diffuse GC (non-mucinous poorly cohesive, signet ring cell carcinoma and microglandular GCs, defined as diffuse proliferation of small tubular structures).
Based on the rules used for colorectal carcinomas[5,7], which were adapted for GC[8], we also assessed the grade of dyscohesivity (tumor budding degree). In terms of the invasion front, we took into account at least 5 high power fields (HPF), and then classified the cases into five groups: 0–nodular growth intestinal type GCs (adenocarcinoma without buds); 1–adenocarcinoma with 1-4 dyscohesive cells in the invasion front (low grade); 2-adenocarcinoma with 5-9 dyscohesive cells in the invasion front (high grade); 3-adenocarcinoma with over 10 dyscohesive cells in the invasion front (high grade); and 4 – diffuse growth-GCs.
Although the cases were diagnosed from 2003-2016, they were re-staged, based on the two currently used systems. The first system was the 8th edition of the WHO/AJCC staging system[7]. The cases were classified based on invasion of the mucosa (pT1a), submucosa (pT1b), muscularis (pT2), serosa (pT3) and crossing serosa (pT4). At the same time, pN staging took into account the absence of lymph node metastases (pN0) and the number of involved lymph nodes (pN1-3)[7].
The second system used for tumor staging was proposed by our team in 2017 and is called the Dukes-MAC-like staging system[9]. This system consists of the classification of GCs, based on the depth of invasion (T) and lymph node status (N), in eight groups, as follows: 1-T1N0; 2-T1N1-3; 3-T2N0; 4-T2N1-3; 5-T3N0; 6-T3N1-3; 7-T4N0; 8-T4N1-3[9].
The IHC staining was performed on paraffin-embedded tissues. After deparaffinization, the tissues were processed with the Novolink Polymer Detection System (Novocastra, Newcastle Upon Tyne, United Kingdom) according to the manufacturer’s instructions. We used the antibodies Maspin (clone EAW24, Novocastra, Newcastle-upon-Tyne, United Kingdom) and p53 (clone DO-7, LabVision, Fremont, CA, United States). Development was carried with DAB (diaminobenzidine) solution (Novocastra). For the negative controls, incubation was performed with the omission of specific antibodies.
Two pathologists (Gurzu S, Jung I), using criteria described previously in the literature, performed the IHC assessments. For p53, three groups were considered, based on the intensity and extent of stained tumor cell nuclei: Negative (< 5%), low (5%-50%) and high p53 expression (> 50%). For Maspin, we used a system of quantification, which was previously published by our team and is based on the subcellular localization of this protease, and cases were classified as negative, with cytoplasm positivity, nuclear predominance and mixed expression (dual positivity, in cytoplasm and nuclei)[3,4,5,10].
The DNA extracted from paraffin blocks was used for genetic examination which was performed at the Department of Tumor Pathology of Hamamatsu University School of Medicine, Japan. DNA isolation was carried out using the Qiagen kit and the manufacturer’s protocol. TP53 gene sequencing was performed with direct sequencing using polymerase chain reaction (PCR) product amplified by the primer sets for each exon. Fragments covering exon 2 to 11 and boundary regions of the p53 gene were amplified by PCR with HotStarTaq DNA polymerase (Qiagen, Valencia, CA, United States). The PCR products were purified with Exo-SAP-IT (Thermo Fisher Scientific) and directly sequenced with a BigDye Terminator Cycle Sequencing Reaction Kit and the ABI 3130xL Genetic Analyzer (Thermo Fisher Scientific).
Statistical analysis was performed using GraphPad Prism 8 software. For descriptive statistics, the median value ± standard deviation was used. The χ2 test, ANOVA, Fisher’s exact test and Spearman’s test were used to check the correlations. The median follow-up period was 36 mo (range: 2–61 mo). Patients who died in the first month after surgery were not included in the analysis. Univariate survival rate was evaluated using Kaplan-Meier survival curves. P < 0.05 (with a 95% confidence interval) was considered statistically significant.
In the present study, which included 266 patients with GC, we noted a predominance of males vs females (M:F = 1.9:1). GC was diagnosed at the median age of 62.52 ± 14.34 years (range 22 to 98 years), in both males and females. Up to 55% of cases had ulcero-infiltrative adenocarcinomas of the proximal and middle third stomach, which did not develop on the background of intestinal metaplasia. Although in both young and elderly patients, a similar distribution between the proximal and distal stomach was observed, Spearman’s correlation showed that the predominance of involvement in the distal stomach was slightly increased in patients diagnosed aged over 60 years (P < 0.05). In elderly patients, 109/266 GC cases (40.98%) developed on the background of intestinal metaplasia, whereas only 47/266 (17.67%) of GCs in patients ≤ 60 years presented with associated metaplasia (P < 0.01). In line with these results, metaplasia-related intestinal type carcinomas were more frequent in elderly, compared with younger patients (P < 0.01).
Examination of the budding degree showed that over half of cases (62.03%) had high-grade dyscohesivity or infiltrative growth. Nodular growth was found in only 12.78% of cases (Table 1). Only 37 of 266 cases (13.91%) were identified in the early stages (pT1+2). Over 80% of cases had metastases in at least one lymph node, independent of the depth of tumor infiltration (Table 1).
| Clinicopathological parameter | Number (n = 266), n (%) | |
| Age (yr) | ≤ 60 | 96 (36.09) |
| > 60 | 170 (63.91) | |
| Gender | Male | 175 (65.78) |
| Female | 91 (34.22) | |
| Localization | Proximal stomach | 108 (40.60) |
| Middle third | 47 (17.67) | |
| Distal third and antrum | 95 (35.71) | |
| Diffuse localization | 16 (6.02) | |
| Macroscopic aspect (Bormann’s type) | Polypoid | 21 (7.89) |
| Ulcerated | 62 (23.31) | |
| Ulcero-infiltrative | 151 (56.77) | |
| Diffusely infiltrative | 32 (12.03) | |
| Microscopic aspect | Adenocarcinoma G1+2 | 75 (28.20) |
| Adenocarcinoma G3 | 76 (28.57) | |
| Papillary carcinoma | 10 (3.76) | |
| Non-mucinous poorly cohesive | 53 (19.92) | |
| Signet ring cell carcinoma | 25 (9.40) | |
| Diffuse micro-glandular | 27 (10.15) | |
| Budding degree (Dyscohesivity rate) | Nodular growth | 34 (12.78) |
| 1-4 buds/HPF | 17 (6.39) | |
| 5-9 buds/HPF | 50 (18.80) | |
| ≥ 10 buds/HPF | 60 (22.56) | |
| Diffuse growth | 105 (39.47) | |
| Associated intestinal metaplasia | Yes | 87 (32.71) |
| No | 179 (67.29) | |
| pT stage | pT1 | 22 (8.27) |
| pT2 | 15 (5.64) | |
| pT3 | 66 (24.81) | |
| pT4 | 163 (61.28) | |
| pN stage | pN0 | 53 (19.92) |
| pN1 | 45 (16.92) | |
| pN2 | 51 (19.17) | |
| pN3 | 117 (43.99) | |
| Dukes-MAC-like stage | T1N0 | 10 (3.76) |
| T1N1-3 | 12 (4.51) | |
| T2N0 | 7 (2.63) | |
| T2N1-3 | 8 (3) | |
| T3N0 | 13 (4.89) | |
| T3N1-3 | 53 (19.93) | |
| T4N0 | 23 (8.65) | |
| T4N1-3 | 140 (52.63) | |
Of the 266 examined cases, 40 proved to have no p53 expression. Of the remaining 226 p53-positive cases, 157 showed nuclear expression in over 50% of the tumor cells (Table 2), without correlations with the examined clinicopathological factors (Table 3). The TP53 gene showed mutations in 90/266 (33.83%) cases, the other 176 cases (66.17%) were wild-type (wt) cases (Table 2). Independent of the mutated exon and other parameters, statistical analysis showed a slight predominance of p53 wt cases with tumors of the distal stomach which also showed a high degree (> 5 buds/HPF) of dyscohesivity (Table 3).
| Parameter | Number (n = 266), n (%) | |
| Maspin expression in tumor core | Negative | 39 (14.66) |
| Cytoplasm | 125 (46.99) | |
| Mixed (nucleus+cytoplasm) | 85 (31.96) | |
| Nuclear | 17 (6.39) | |
| Maspin expression in the invasion front | Negative | 21 (7.90) |
| Cytoplasm | 0 (0) | |
| Mixed (nucleus+cytoplasm) | 201 (75.56) | |
| Nuclear | 44 (16.54) | |
| p53 – nuclear expression | Negative | 40 (15.04) |
| 5%-50% | 69 (25.94) | |
| > 50% | 157 (59.02) | |
| TP53 gene status | Wild-type | 176 (66.17) |
| Mutations | 90 (33.83) | |
| Clinicopathological parameter | Number (n = 266) | aP53 nuclear expression (n = 266) | aP value | bTP53 gene status (n = 266) | bP value | ||||
| negative (n = 40) | 10%-50%(n = 69) | > 50% (n = 157) | Wild type(n = 176) | Mutated (n = 90) | |||||
| Age (yr) | ≤ 60 | 96 | 15 | 28 | 53 | > 0.05 | 70 | 26 | > 0.05 |
| > 60 | 170 | 25 | 41 | 104 | 106 | 64 | |||
| Localization | Proximal/middle | 155 | 22 | 34 | 99 | > 0.05 | 95 | 60 | 0.05 |
| Distal and antrum | 95 | 15 | 33 | 47 | 72 | 23 | |||
| Diffuse localization | 16 | 3 | 3 | 10 | 9 | 7 | |||
| Microscopy | G1+2 | 75 | 9 | 20 | 46 | > 0.05 | 43 | 32 | > 0.05 |
| G3 | 76 | 14 | 23 | 39 | 51 | 25 | |||
| Papillary | 10 | 1 | 2 | 7 | 5 | 5 | |||
| Poorly cohesive | 53 | 5 | 14 | 34 | 35 | 18 | |||
| Signet ring cell | 25 | 8 | 2 | 15 | 19 | 6 | |||
| Micro-glandular | 27 | 3 | 8 | 16 | 23 | 4 | |||
| Budding degree (Dyscohesivity) | Nodular | 34 | 4 | 8 | 22 | > 0.05 | 17 | 17 | 0.05 |
| ≤ 5 buds/HPF | 17 | 2 | 7 | 8 | 9 | 8 | |||
| 5-9 buds/HPF | 50 | 5 | 19 | 26 | 30 | 20 | |||
| ≥ 10 buds/HPF | 60 | 11 | 13 | 36 | 42 | 18 | |||
| Diffuse | 105 | 18 | 22 | 65 | 78 | 27 | |||
| pT stage | pT1,2 | 37 | 6 | 7 | 24 | > 0.05 | 24 | 13 | > 0.05 |
| pT3,4 | 229 | 34 | 62 | 133 | 152 | 77 | |||
| pN stage | pN0 | 53 | 11 | 13 | 29 | > 0.05 | 32 | 21 | > 0.05 |
| pN1-3 | 213 | 29 | 56 | 128 | 144 | 69 | |||
| Dukes-MAC-like stage | 1 + 3 - T1,2N0 | 17 | 2 | 3 | 12 | > 0.05 | 12 | 5 | > 0.05 |
| 2 + 4 - T1,2N1-3 | 20 | 3 | 3 | 14 | 11 | 9 | |||
| 5 + 7 – T3,4N0 | 36 | 7 | 10 | 19 | 20 | 16 | |||
| 6 + 8 – T3,4N1-3 | 193 | 28 | 53 | 112 | 133 | 60 | |||
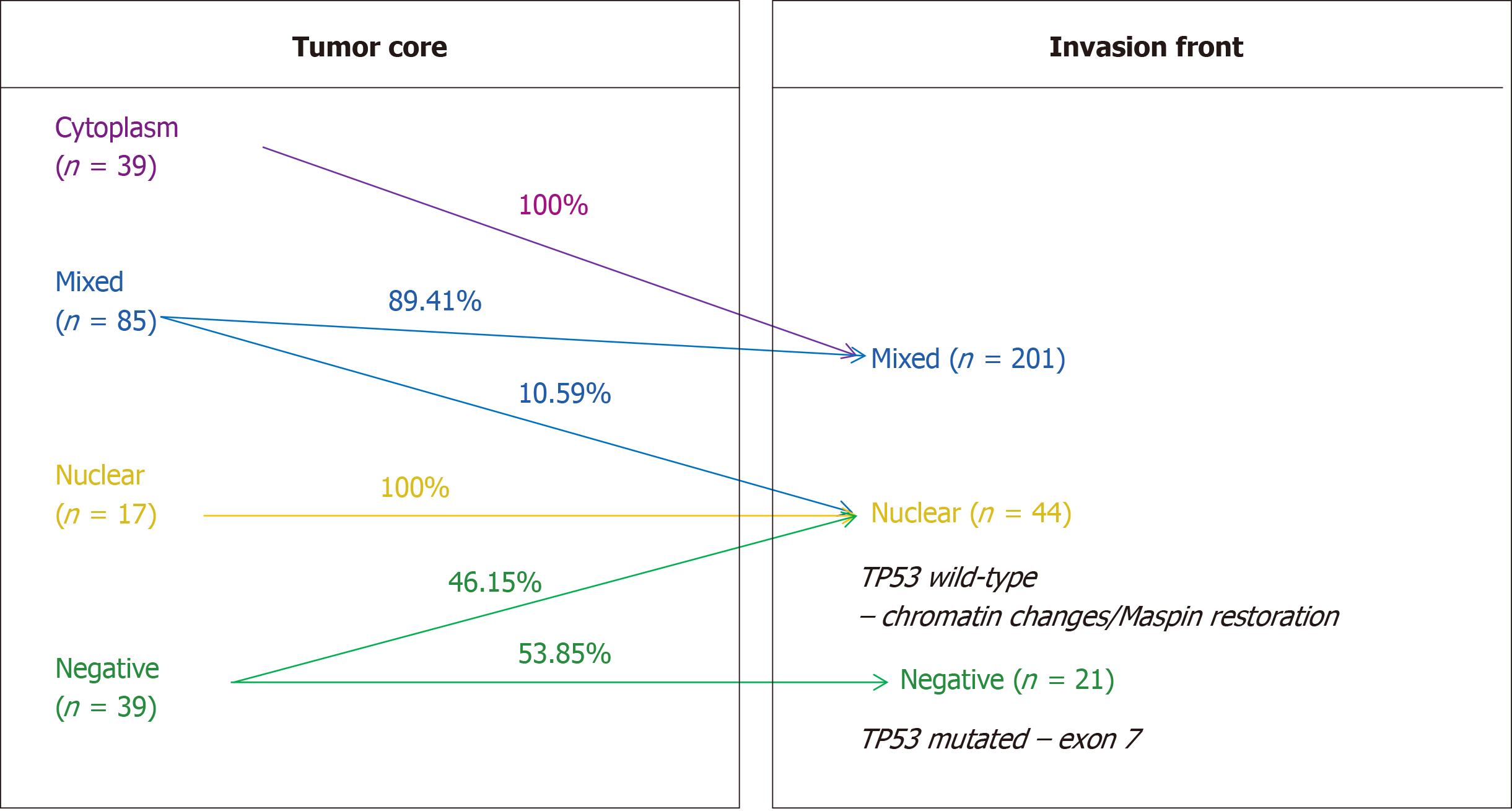
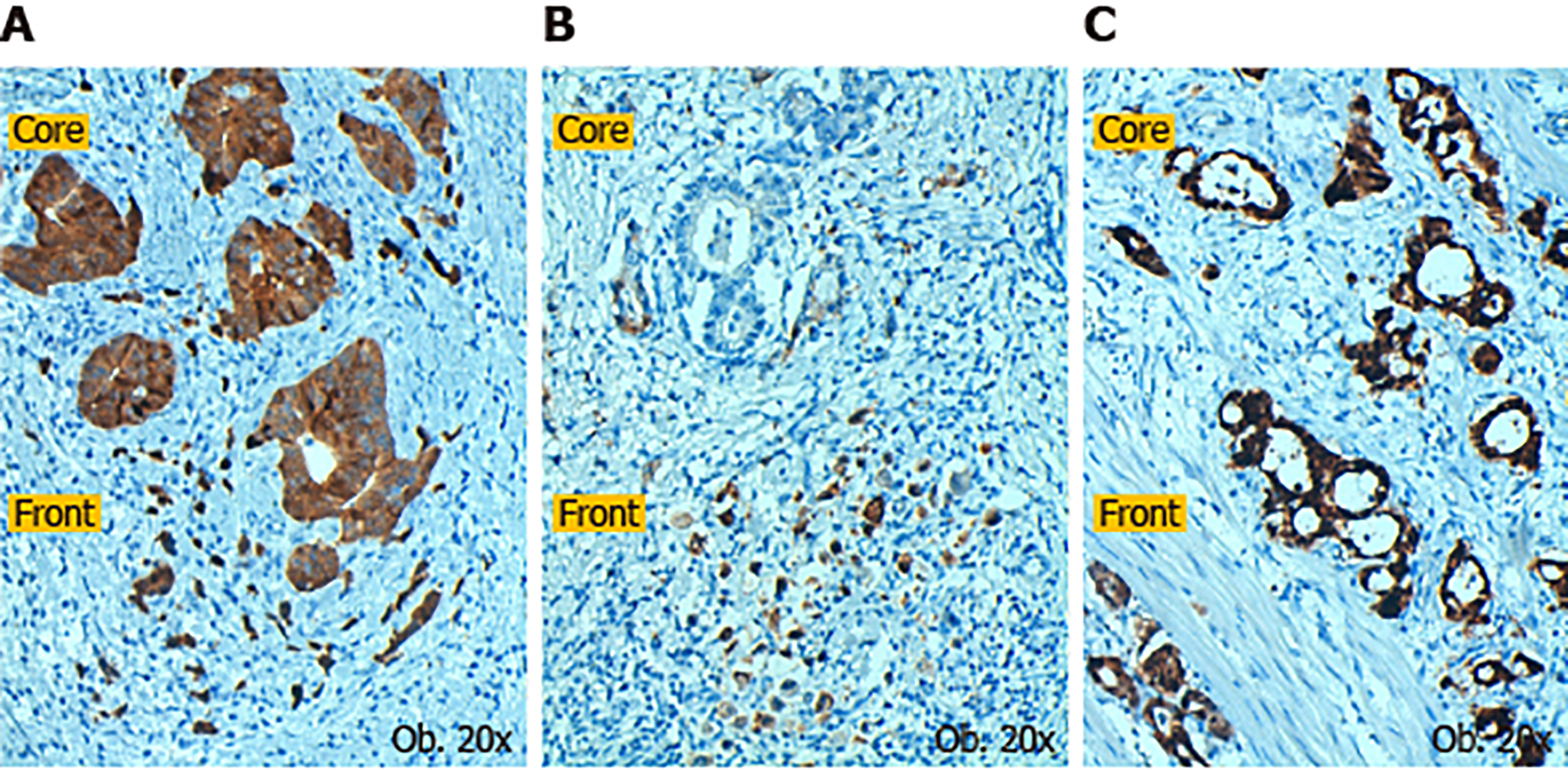
Subcellular expression of Maspin positivity was determined in both the tumor core and invasion front. In the tumor core, it was observed that most of the cases demonstrated mixed expression (nuclear + cytoplasm) of Maspin, followed by the cytoplasm and negative cases. With regard to the invasion front, Maspin subcellular expression was significantly modified, compared with the tumor core (P < 0.01) (Table 1). It was noted that nuclear expression was completely retained (in the cases with nuclear Maspin in the core) and all of the cases with cytoplasm staining in the tumor core, gained nuclear expression in the tumor front. Some of the mixed cases lost cytoplasm staining and other negative cases, in the core, showed nuclear positivity in the invasion zone.
The statistical correlations showed a predominance of cases with nuclear positivity in the tumor core in younger patients with poorly cohesive carcinomas, whereas Maspin cytoplasmic expression was most frequently seen in patients over 60 years with intestinal-type adenocarcinomas with nodular growth or low grade dyscohesivity (≤ 5 buds/HPF). All of the “linitis plastica” cases showed pure nuclear Maspin in the invasion front (Table 4).
| Clinicopathological parameter | Number(n = 266) | aTumor core (n = 266) | aP value | bInvasion front (n = 266) | bP value | ||||||
| Negative (n = 39) | Cytoplasm(n = 125) | Mixed (n = 85) | Nuclear (n = 17) | Negative (n = 21) | Mixed(n = 201) | Nuclear (n = 44) | |||||
| Age (yr) | ≤ 60 | 96 | 8 | 37 | 39 | 12 | < 0.01 | 5 | 85 | 6 | > 0.05 |
| > 60 | 170 | 31 | 88 | 46 | 5 | 16 | 116 | 38 | |||
| Localization | Proximal/middle | 155 | 21 | 80 | 44 | 10 | > 0.05 | 15 | 117 | 23 | 0.05 |
| Distal and antrum | 95 | 17 | 34 | 41 | 3 | 6 | 84 | 5 | |||
| Diffuse localization | 16 | 1 | 11 | 0 | 4 | 0 | 0 | 16 | |||
| Microscopy | G1+2 | 75 | 10 | 41 | 22 | 2 | 0.01 | 5 | 65 | 5 | > 0.05 |
| G3 | 76 | 16 | 42 | 14 | 4 | 8 | 54 | 14 | |||
| Papillary | 10 | 0 | 5 | 5 | 0 | 0 | 10 | 0 | |||
| Poorly cohesive | 53 | 10 | 19 | 20 | 4 | 8 | 35 | 10 | |||
| Signet ring cell | 25 | 2 | 1 | 18 | 4 | 0 | 16 | 9 | |||
| Micro-glandular | 27 | 1 | 17 | 6 | 3 | 0 | 21 | 6 | |||
| Budding degree (Dyscohesivity) | Nodular | 34 | 5 | 20 | 9 | 0 | < 0.05 | 0 | 34 | 0 | > 0.05 |
| ≤ 5 buds/HPF | 17 | 1 | 10 | 5 | 1 | 0 | 14 | 3 | |||
| 5-9 buds/HPF | 50 | 7 | 25 | 15 | 3 | 4 | 41 | 5 | |||
| ≥ 10 buds/HPF | 60 | 18 | 27 | 13 | 2 | 7 | 40 | 13 | |||
| Diffuse | 105 | 8 | 43 | 43 | 11 | 10 | 72 | 23 | |||
| pT stage | pT1,2 | 37 | 3 | 21 | 10 | 3 | > 0.05 | 0 | 21 | 16 | > 0.05 |
| pT3,4 | 229 | 36 | 104 | 75 | 14 | 21 | 180 | 28 | |||
| pN stage | pN0 | 53 | 9 | 19 | 20 | 5 | > 0.05 | 3 | 43 | 7 | > 0.05 |
| pN1-3 | 213 | 30 | 106 | 65 | 12 | 18 | 158 | 37 | |||
| Dukes-MAC-like stage | 1+3 - T1,2N0 | 17 | 3 | 11 | 3 | 0 | > 0.05 | 0 | 17 | 0 | > 0.05 |
| 2+4 - T1,2N1-3 | 20 | 0 | 12 | 5 | 3 | 0 | 17 | 3 | |||
| 5+7 – T3,4N0 | 36 | 6 | 12 | 15 | 3 | 3 | 24 | 9 | |||
| 6+8 – T3,4N1-3 | 193 | 30 | 90 | 62 | 11 | 18 | 143 | 32 | |||
The most interesting correlation was Maspin subcellular expression in the invasion front. On the one hand, all 21 Maspin negative cases in both the core and front (Table 1) showed mutations in exon 7 of the TP53 gene (C>T and G>A); all of them had high-grade budding (2,3) adenocarcinomas, without associated intestinal metaplasia, which expressed p53 in over 50% of tumor cells and were diagnosed in stages T3,4N1-3. On the other hand, all of the 44 cases with nuclear Maspin in the invasion area were wt TP53. At the same time, 18/39 Maspin negative cases, in the tumor core, which gained nuclear positivity in the invasion area, did not have TP53 gene mutations (Table 5, Figure 1). All of the cases (n = 44) with pure nuclear Maspin in the invasion area (Figures 1 and 2) were wt TP53.
| Immunohistochemical parameter | Number (n = 266) | aP53 nuclear expression (n = 266) | aP value | bTP53 gene status (n = 266) | bP value | |||||
| Negative (n = 40) | 10%-50% (n = 69) | > 50% (n = 157) | Wild-type (n = 176) | Mutated (n = 90) | ||||||
| Maspin expression - tumor core | Negative | 39 | 5 | 7 | 27 | > 0.05 | 26 (14.77) | 13 (14.44) | 0.05 | |
| Cytoplasm | 125 | 19 | 34 | 72 | 74 (42.05) | 51 (56.67) | ||||
| Nucleus | 17 | 7 | 5 | 5 | 15 (8.52) | 2 (2.22) | ||||
| Mixed | 85 | 9 | 23 | 53 | 61 (34.66) | 24 (26.67) | ||||
| Maspin expression - invasion front | Negative | 21 | 9 | 3 | 9 | > 0.05 | 8 (4.55) | 13 (14.44) | < 0.01 | |
| Nucleus | 44 | 14 | 16 | 14 | 35 (19.88) | 9 (10) | ||||
| Mixed | 201 | 17 | 50 | 134 | 133 (75.57) | 68 (75.56) | ||||
| p53– nuclear expression | Negative | 40 | - | - | - | 30 (17.04) | 10 (11.11) | > 0.05 | ||
| 5%-50% | 69 | 47 (26.71) | 22 (24.44) | |||||||
| > 50% | 157 | 99 (56.25) | 58 (64.45) | |||||||
No statistical correlations were found between p53 immunostaining (nuclear or cytoplasm) and TP53 gene status (Table 5). From the 40 cases with p53 negativity, 11 (27.50%) showed mutations in exon 3 to exon 8; in two cases double mutations were seen in exon 3 + 4 and 5 + 6, respectively. At the same time, 21 of the 69 cases with low p53 positivity (30.43%) had mutations in exons 2, 5, 6, 7, 8, 10, and 11; in one case both exons 5 and 6 were involved. Finally, 15 of 157 cases with p53 positivity in over 50% of tumor cells (36.31%) in exons 3, 4, 5, 6, 7, 8, 10, and 11; double or triple mutations were seen in five of the cases involving exons 3 + 4, 4 + 5, 4 + 8, 5 + 6, and 6 + 7 + 8 (Table 3). No mutations in exon 9 were identified.
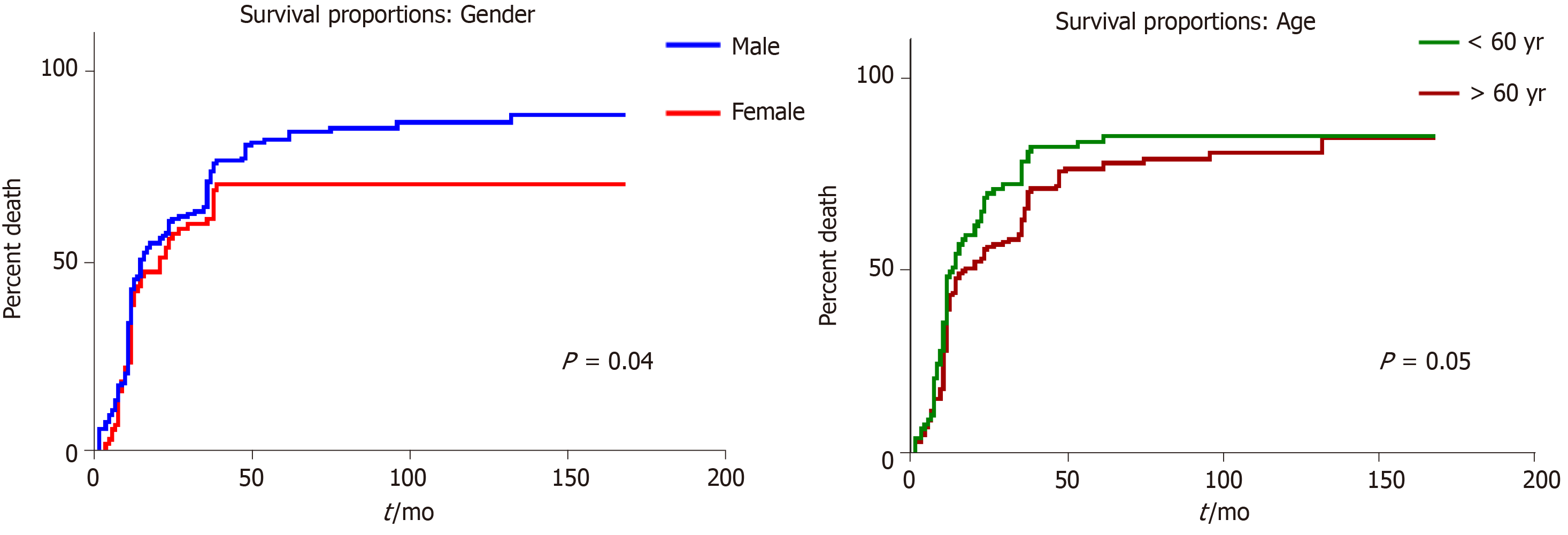
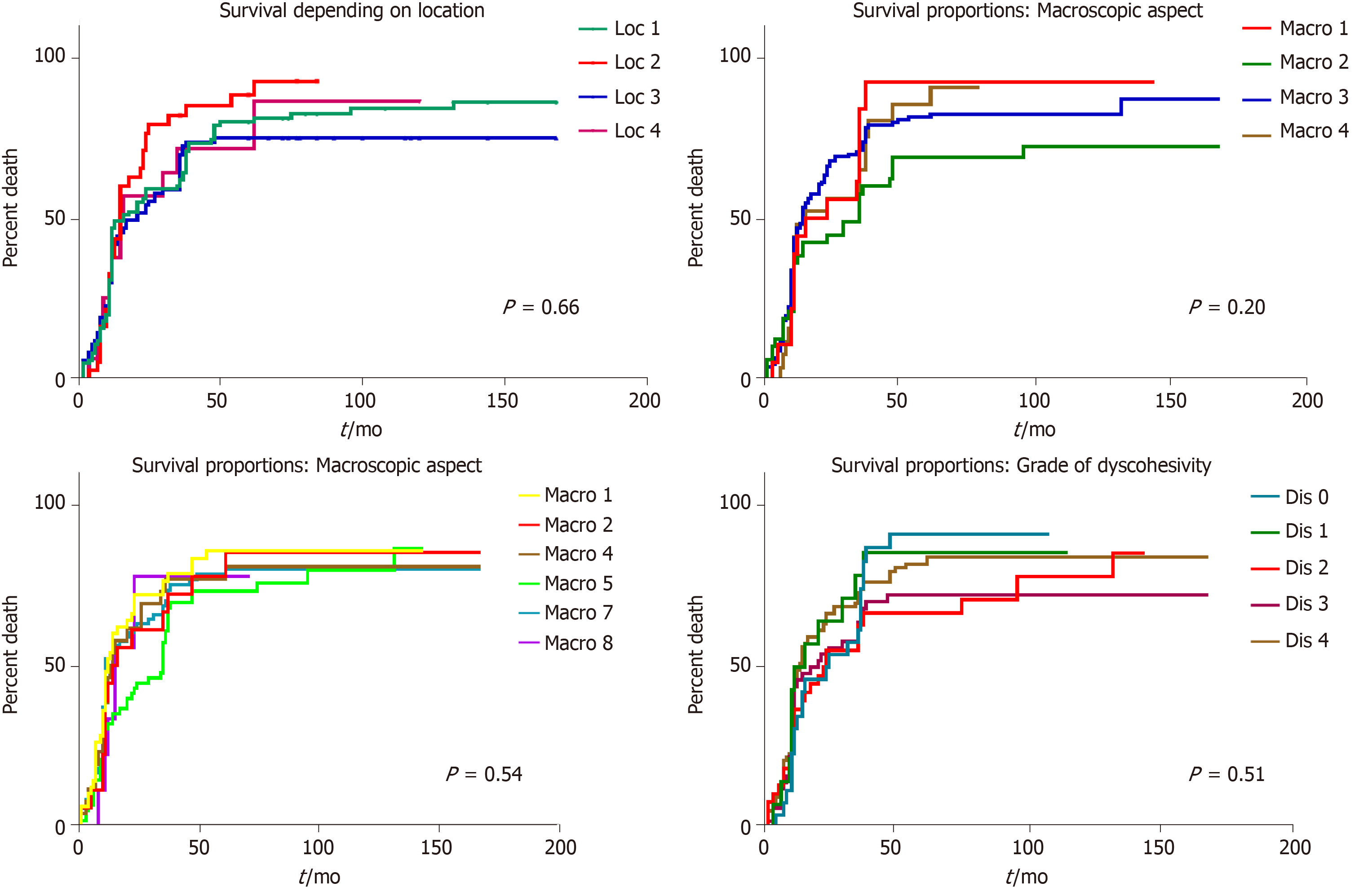
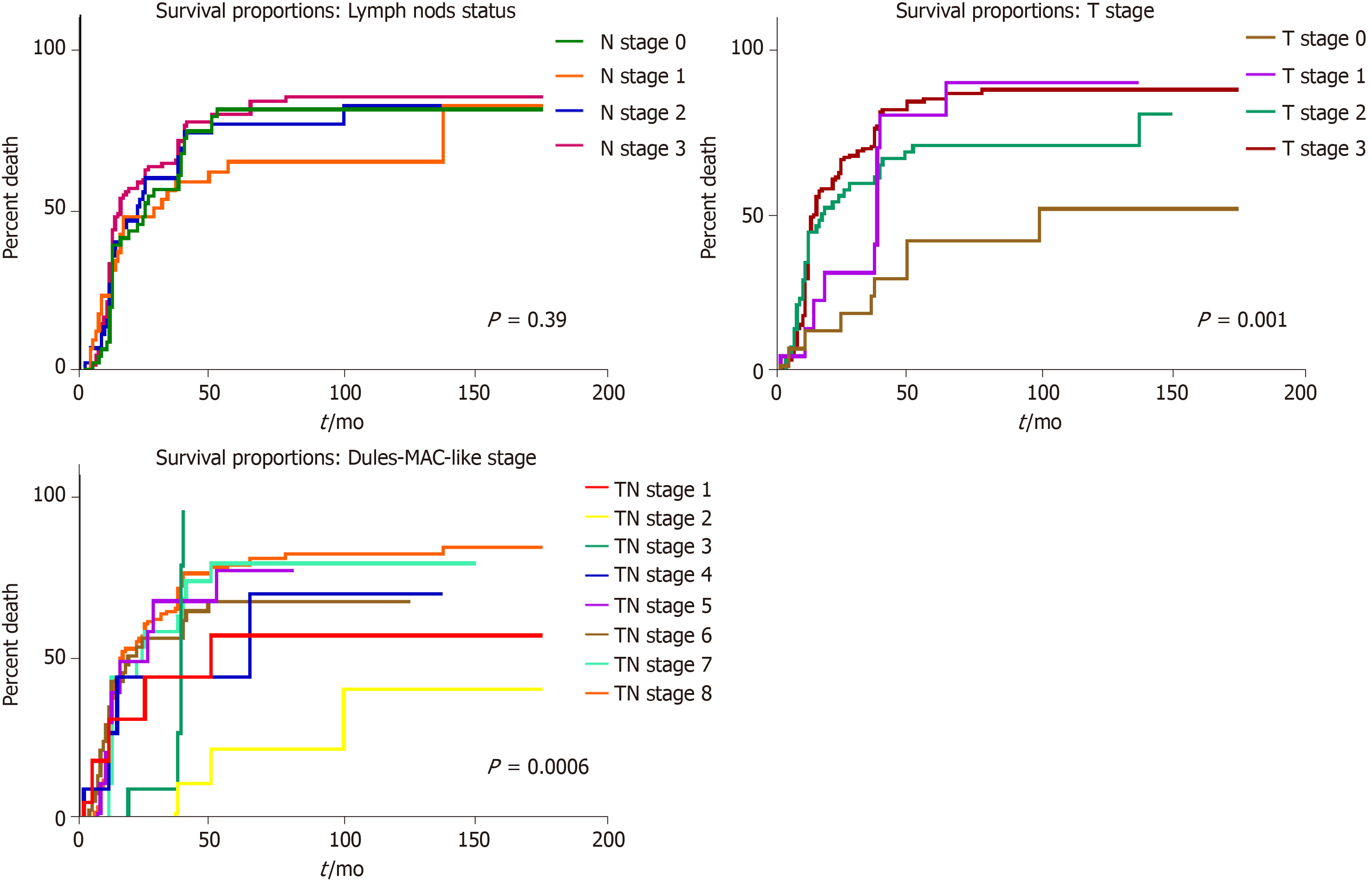
In univariate survival analysis, any of the following clinicopathological parameters, proved to have independent prognostic value (P > 0.05): Tumor localization, macroscopic- or microscopic aspect, associated metaplasia, lymph node status, and budding degree. A slightly longer survival was seen in males younger than 60 years (Figures 3 and 4).
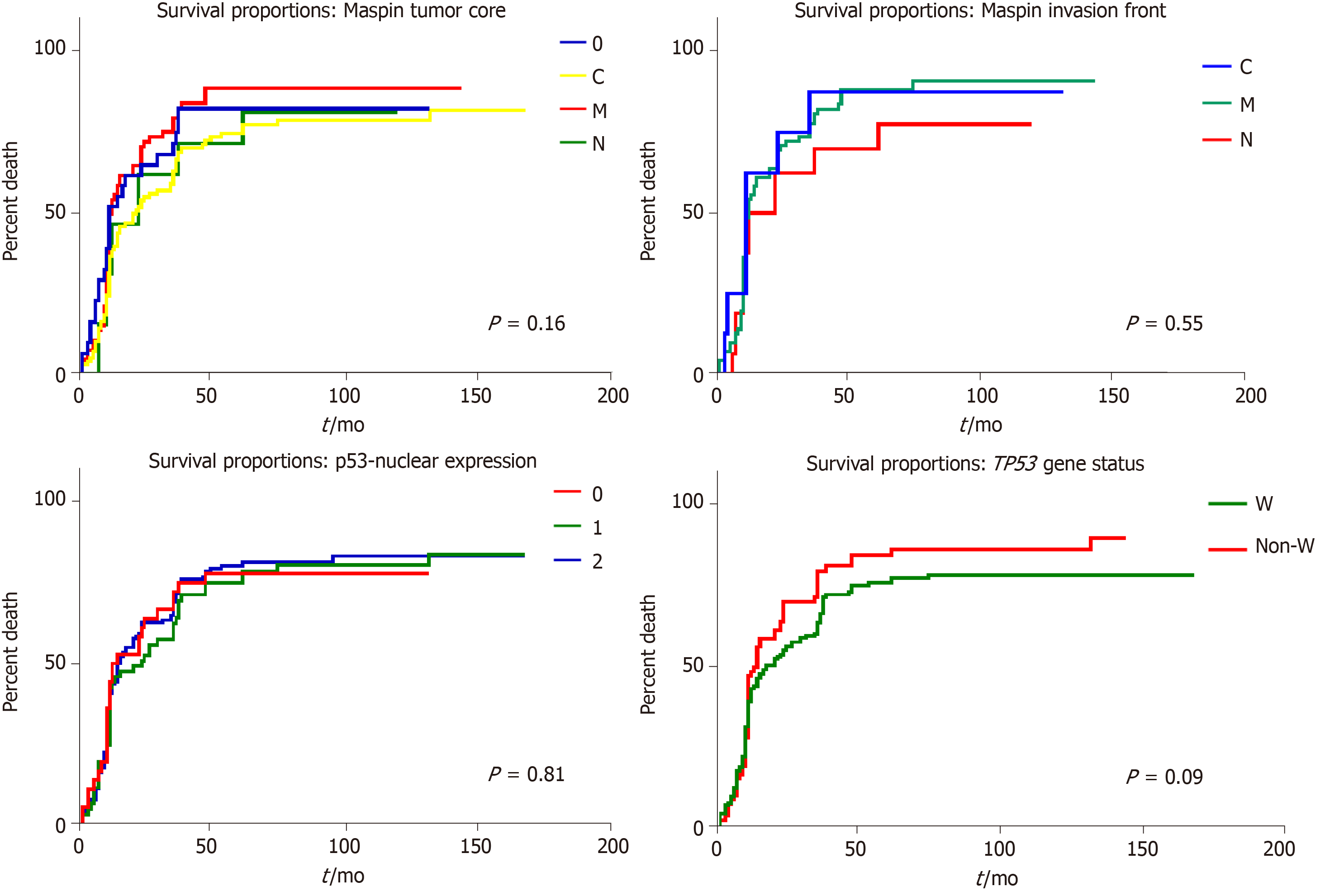
The most significant independent prognostic parameters proved to be tumor depth of infiltration–pT stage (P < 0.01) and Dukes-MAC-like stage (P < 0.01) (Figure 5). A longer survival was found in pT1 cases, with/without lymph node metastases (TN1 or TN2 Dukes-MAC-like stage); 59.09% of the patients diagnosed in stages TN1+TN2 (13/22) survived for over 60 mo after surgery. In contrast, in pT2 cases, even in the absence of lymph node metastases (TN3 stage), all of these seven patients died at a median period of 34.57 ± 7.32 mo after surgical intervention (Figure 5). No prognostic value was found for IHC expression of Maspin in the tumor core or invasion front, even for p53 expression (P > 0.05) (Figure 6).
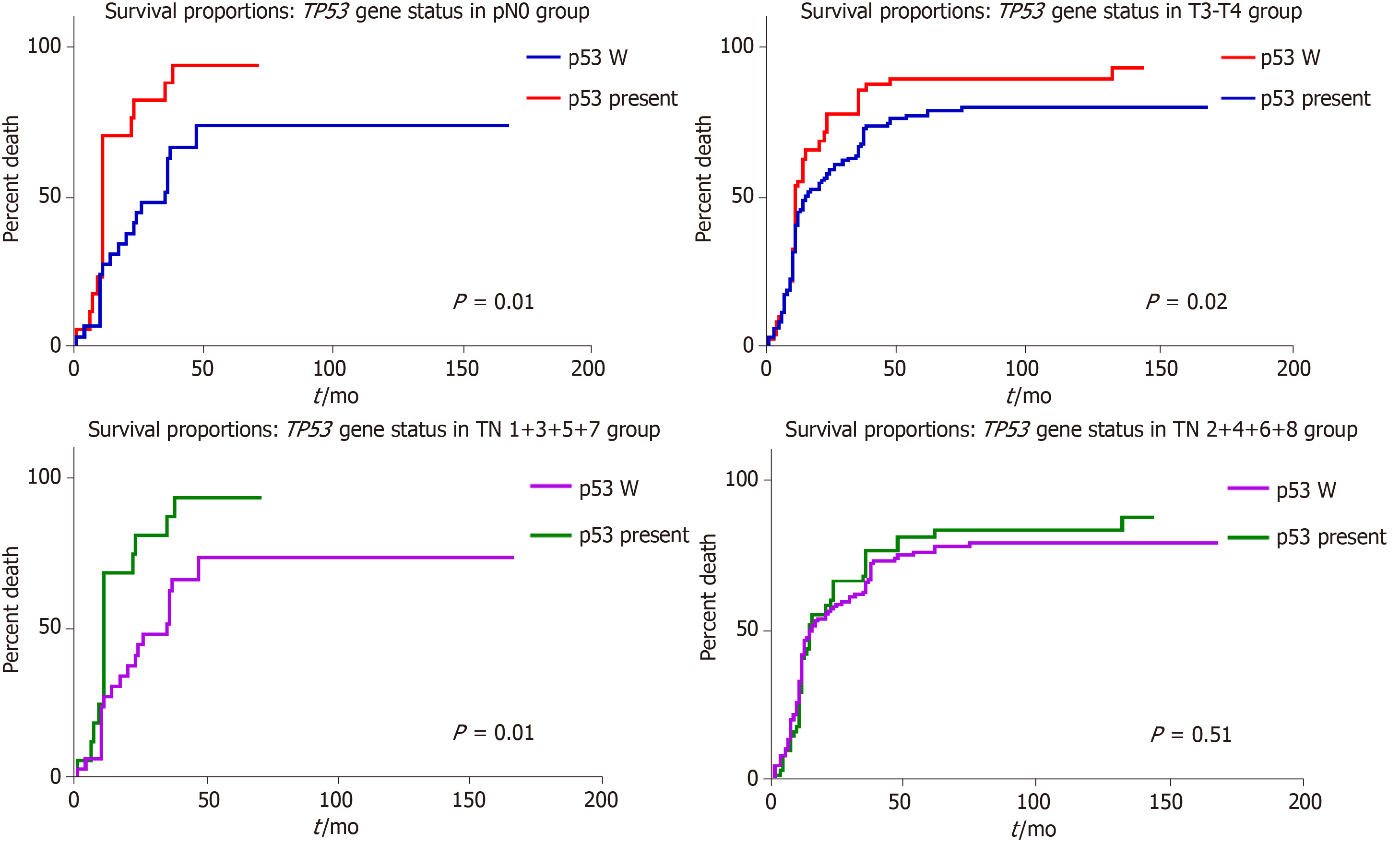
As TP53 gene status was found to have independent prognostic value but was not statistically significant (P < 0.05), we adjusted it for pTNM and Dukes-MAC like stage (Figures 6 and 7). Regarding the depth of infiltration, in locally advanced stages (pT3-4), wt cases showed a significant longer survival than those with TP53 gene mutations (P = 0.01); the difference was not significant in the group of patients diagnosed in the early stages, respectively, pT1-2 (P > 0.05). Within the group of patients without lymph node metastases (pN0 and Dukes-Mac like stage 1 + 3 + 5 + 7), longer survival was also seen in wt cases, compared with those with TP53 gene mutations (P = 0.01). This was not observed in patients with lymph node metastases, and when examined independently the pT stage parameter or combined TN (Dukes-Mac like stage 1 + 3 + 5 + 7) (Figure 7). Multivariate correlation indicated that a negative prognostic value was associated with TP53 gene mutations, especially in patients without lymph node metastases (Figure 7).
As overall survival varies in patients with GC within the same stage, the most recent papers have shown that the currently used TNM staging system is not sufficient for prognosis estimation[1]. The present study confirmed the utility of the newest Dukes-MAC-like staging system[9], for a proper estimation of long-time survival, and confirmed the independent prognostic role of IHC expression of Maspin and p53, in patients with GC. Despite the intention of including new biomarkers with prognostic potential, the depth of invasion, combined with lymph node status (pTN stage) remain the strongest predictors of outcome in patients with GC[8,11].
This study confirmed the age-related characteristics[11], of an increasing number of distally-located GCs, especially adenocarcinomas developed on the background of intestinal metaplasia; however, the clinicopathological features, including grade of dyscohesivity (budding degree), did not have independent prognostic value.
In previously published studies, the presence of TP53 gene mutation was not proved to be an independent prognostic factor for patients with advanced GCs[1,12], or it was correlated with a shorter survival[13,14]. Our study confirmed the longer survival of wt cases, compared to those with TP53 gene mutation, especially in patients without lymph node metastases.
The TP53 mutation rate was proved to be dependent by various parameters, including geographic characteristics known to be induced by interactions between Helicobacter pylori infection and other environmental, molecular and genetic factors[13,15,16]. In our study, which included Romanian patients, the mutation rate was 33.83%, similar to American Caucasian (40%) and Hispanic patients (43%), whereas Asian and African American patients, similar to patients in Bangladesh, proved to have significantly elevated mutations rates of 56%, 89%, and 73%, respectively[16,37].
The literature shows the prevalence of TP53 somatic mutations (over 95%) in exons 5-8, independent of carcinoma localization[12,14]. As no correlation was seen between the mutation status of IHC p53 protein expression, this study confirmed that p53 negativity cannot be used as an indicator of wt status[1]. This aspect is, however, controversial. There are authors who maintain that certain TP53 somatic mutation types might be associated with p53 negativity, and as wt p53 protein has a very short half-life, it cannot be detected properly using IHC stains and false negative results can be obtained[12,17]. On the other hand, the clone DO7, also used in the present study, can detect only truncated mutations in exons 9 and 10[12,17]. Interaction between p53 and Maspin, which was previously proved for colorectal, prostate and bladder cancer[10,18,19], was partially confirmed in this study, for GC.
In colorectal cancer, experimental studies showed that although Maspin nuclear positivity might be an indicator of aggressive behavior, it also indicates the possibility of responding to 5-Fluorouracil (5-FLU)-based chemotherapy. Most of the colorectal carcinoma cells displaying cytoplasmic and carcinomas with microsatellite instability and nuclear Maspin, were found to be p53 negative[4,20]. On the other hand, Maspin negative/p53 positive colorectal carcinomas are 5-FLU resistant and have a risk of distant metastases[4,10,18,20]. The best prognosis was proved for p53 negative cases that displayed cytoplasmic positivity for Maspin, whereas Maspin nuclear staining associated with a p53 index over 50% was an indicator of poor prognosis[4].
In bladder and prostate cancer, p53 was found to upregulate Maspin expression and stimulate cisplatin-induced apoptosis[19]. Knockdown of Maspin in p53 wt carcinoma cells stimulates tumor cell proliferation[19].
Although it was shown that two p53 binding sites are responsible for promoting the human Maspin gene: GGCATGTTGGAGGCCTTTG and GGACAAGCTGCCAAGAGGCTTGAGT[2,19,21], no relevant data on the Maspin-p53 interaction have been published for GC. The present study suggests that knockdown of Maspin might be induced by G:C→A:T transition in exon 7 of the TP53 gene. As Maspin negative GCs have a high risk of distant metastases[3], the role of exon 7 in GC behavior, especially in cases without lymph node metastases, should be more extensively examined.
Another unusual finding is the absence of TP53 gene mutations in cases with nuclear Maspin positivity in the tumor front, known to be a risk for local relapse and lymph node metastases[3]. Nuclear positivity was present in the invasion front and tumor core in only 17/44 positive cases and in over 46% of negative cases, in the core, and Maspin nuclear expression was found in the invasion area only. In cell cultures, wt TP53 induced chromatin changes and even partial Maspin restoration, approaching basal levels in non-tumorigenic cells[2].
The previous results obtained by our team and other publications show that in GC, Maspin is downregulated in carcinoma cells, compared with normal mucosa, and silencing of Maspin increases metastatic behavior[2,3]. The present study showed that Maspin silencing occurs more frequently in cases with mutations in exon 7 of the TP53 gene, but wt p53 may induce changes in chromatin architecture and reactivate nuclear Maspin, in the invasion front, decreasing the risk of distant metastases. The small number of cases does not allow interpretation of the relationship between p53 and mixed Maspin in which, probably, partial Maspin restoration is only obtained.
An in-depth examination of TP53 gene status, which is one of the most frequently mutated tumor suppressor genes, was proved to influence GC behavior. Similar to other studies, TP53 mutations, independently of the involved exon, were observed to increase the capacity of tumor invasion[1].
Maspin is a serine protease that has been extensively studied by our team using immunostaining in gastric cancer (GC) and colorectal cancer samples. Our previous data, which are in line with literature data, showed that the role of Maspin is strongly dependent on its subcellular expression. In GC cells, Maspin downregulation increases the metastatic potential, cytoplasmic localization induces a better prognosis and nuclear staining is correlated with a higher local invasion.
As data on the correlation between Maspin and p53 are scarce in GC, the aim of this study was to determine the particular features of this possible interaction.
We compared immunohistochemical (IHC) staining of p53 and a p53 gene-related protein Maspin in GCs, and correlated the results with the TP53 gene expression profile. The independent prognostic values of the examined parameters were also determined.
In 266 consecutive GC samples, we performed IHC staining with Maspin and p53 antibodies, and performed sequencing (from paraffin-embedded tissues), to determine TP53 gene mutations in exons 2 to 11.
In the examined cohort, the TP53 gene mutation rate was 33.83%, and no correlation with the immunoexpression of p53 protein was observed. Wild-type cases, especially those without lymph node metastases, had a better survival rate. The most significant independent prognostic parameter proved to be the Dukes-MAC-like tumor stage. Statistical correlations proved that Maspin nuclear restoration, in the invasion front, can be obtained in TP53 wild-type cases, whereas mutations in exon 7 of the TP53 gene induce Maspin negativity, in both the tumor core and invasion area.
Despite several prognostic parameters proposed for GC, the survival rate is better predicted by the classic TN stage. Downregulated Maspin might be induced by mutations in exon 7 of the TP53 gene but wild-type p53 can partially restore nuclear Maspin expression.
As the possible role of mutations in exon 7 of the TP53 gene was proved for the first time in the present study, following downregulation of Maspin, further investigations are necessary to elucidate the possible therapeutic role of anti-Maspin chemical derivates.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koseoglu RD, Tanabe S S-Editor: Wang J L-Editor: Webster JR E-Editor: Liu MY
| 1. | Shin YJ, Kim Y, Wen X, Cho NY, Lee S, Kim WH, Kang GH. Prognostic implications and interaction of L1 methylation and p53 expression statuses in advanced gastric cancer. Clin Epigenetics. 2019;11:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Oshiro MM, Watts GS, Wozniak RJ, Junk DJ, Munoz-Rodriguez JL, Domann FE, Futscher BW. Mutant p53 and aberrant cytosine methylation cooperate to silence gene expression. Oncogene. 2003;22:3624-3634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Gurzu S, Kadar Z, Sugimura H, Orlowska J, Bara T, Bara T, Szederjesi J, Jung I. Maspin-related Orchestration of Aggressiveness of Gastric Cancer. Appl Immunohistochem Mol Morphol. 2016;24:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Gurzu S, Szentirmay Z, Jung I. Molecular classification of colorectal cancer: a dream that can become a reality. Rom J Morphol Embryol. 2013;54:241-245. [PubMed] |
| 5. | Banias L, Jung I, Bara T, Fulop Z, Simu P, Simu I, Satala C, Gurzu S. Immunohistochemical-based molecular subtyping of colorectal carcinoma using maspin and markers of epithelial-mesenchymal transition. Oncol Lett. 2020;19:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Ajani JA, In H, Sano T, Gaspar LE, Erasmus JJ, Tang LH, Washington MK, Gerdes H, Wittekind CW, Mansfield PF, Rimmer C, Hofstetter WL, Kelsen D. Amin MB, editor-in-chief. American Joint Committee on Cancer. Cancer Staging Manual, Eighth Edition. New York: Springer 2017; 203-219. [DOI] [Full Text] |
| 7. | Banias L, Gurzu S, Kovacs Z, Bara T, Bara T, Jung I. Nuclear maspin expression: A biomarker for budding assessment in colorectal cancer specimens. Pathol Res Pract. 2017;213:1227-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Ulase D, Heckl S, Behrens HM, Krüger S, Röcken C. Prognostic significance of tumour budding assessed in gastric carcinoma according to the criteria of the International Tumour Budding Consensus Conference. Histopathology. 2020;76:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Gurzu S, Sugimura H, Orlowska J, Szederjesi J, Szentirmay Z, Bara T, Bara T, Fetyko A, Jung I. Proposal of a Dukes-MAC-like staging system for gastric cancer. J Investig Med. 2017;65:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Gurzu S, Szentirmay Z, Popa D, Jung I. Practical value of the new system for Maspin assessment, in colorectal cancer. Neoplasma. 2013;60:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Gurzu S, Kadar Z, Sugimura H, Bara T, Bara T, Halmaciu I, Jung I. Gastric cancer in young vs old Romanian patients: immunoprofile with emphasis on maspin and mena protein reactivity. APMIS. 2015;123:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Ando K, Oki E, Saeki H, Yan Z, Tsuda Y, Hidaka G, Kasagi Y, Otsu H, Kawano H, Kitao H, Morita M, Maehara Y. Discrimination of p53 immunohistochemistry-positive tumors by its staining pattern in gastric cancer. Cancer Med. 2015;4:75-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | van Beek EJAH, Hernandez JM, Goldman DA, Davis JL, McLaughlin K, Ripley RT, Kim TS, Tang LH, Hechtman JF, Zheng J, Capanu M, Schultz N, Hyman DM, Ladanyi M, Berger MF, Solit DB, Janjigian YY, Strong VE. Rates of TP53 Mutation are Significantly Elevated in African American Patients with Gastric Cancer. Ann Surg Oncol. 2018;25:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 685] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 15. | Kadar Z, Jung I, Orlowska J, Szentirmay Z, Sugimura H, Turdean S, Simona G. Geographic particularities in incidence and etiopathogenesis of sporadic gastric cancer. Pol J Pathol. 2015;66:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Rahman MM, Sarker MAK, Hossain MM, Alam MS, Islam MM, Shirin L, Sultana R, Sultana GNN. Association of p53 Gene Mutation With Helicobacter pylori Infection in Gastric Cancer Patients and Its Correlation With Clinicopathological and Environmental Factors. World J Oncol. 2019;10:46-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Shahin MS, Hughes JH, Sood AK, Buller RE. The prognostic significance of p53 tumor suppressor gene alterations in ovarian carcinoma. Cancer. 2000;89:2006-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Gurzu S, Szentirmay Z, Toth E, Jung I. Possible predictive value of maspin expression in colorectal cancer. Recent Pat Anticancer Drug Discov. 2013;8:183-190. [PubMed] |
| 19. | Lin YH, Tsui KH, Chang KS, Hou CP, Feng TH, Juang HH. Maspin is a PTEN-Upregulated and p53-Upregulated Tumor Suppressor Gene and Acts as an HDAC1 Inhibitor in Human Bladder Cancer. Cancers (Basel). 2019;12:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Murnyák B, Hortobágyi T. Immunohistochemical correlates of TP53 somatic mutations in cancer. Oncotarget. 2016;7:64910-64920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Tsui KH, Lin YH, Chang KS, Hou CP, Chen PJ, Feng TH, Juang HH. Transgelin, a p53 and PTEN-Upregulated Gene, Inhibits the Cell Proliferation and Invasion of Human Bladder Carcinoma Cells in Vitro and in Vivo. Int J Mol Sci. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |