Published online Jul 15, 2020. doi: 10.4251/wjgo.v12.i7.732
Peer-review started: December 30, 2019
First decision: April 2, 2020
Revised: May 28, 2020
Accepted: June 14, 2020
Article in press: June 14, 2020
Published online: July 15, 2020
Processing time: 197 Days and 14 Hours
Carcinomas of the anal canal are staged according to the size and extent of the disease; however, we propose including a novel ultrasound (US) staging system, based on depth of tumor invasion. In this study the clinical American Joint Committee on Cancer (AJCC) staging guidelines and the US classificationss in patients with anal cancer were compared.
To evaluate the prognostic role of the US staging system in patients with anal cancer.
The data of 48 patients with anal canal squamous cells carcinoma, observed at our University Hospital between 2007 and 2017, who underwent pre-treatment assessment with pelvic magnetic resonance imaging (MRI), total body computed tomography (CT) scan and endoanal US were retrospectively reviewed. Anal canal tumors were clinically staged according to AJCC, determined by MRI by measurement of the longest tumor diameter, and CT scan. Endoanal US was performed with a high multi-frequency (9-16 MHz), 360° rotational mechanical probe; US classification was based on depth of tumor penetration through the anal wall, according to Giovannini’s study. All patients were treated with definitive radiation combined with 5-fluorouracile and Mitomycin-C. After treatment patients were followed-up regularly.
At baseline there were 30 and 32 T1-2, 18 and 16 T3-4, 31 and 19 N+ patients classified according to the clinical AJCC and US staging system respectively. After a mean follow-up of 98 months, 38 patients (79.1%) are alive and 28 (58.3%) are disease free. During follow up 20 patients (41.6%) experienced recurrences. After univariate analysis, American Society of Anesthesiologists (ASA) score (P = 0.00000001) and US staging (P = 0.009) were significantly related to disease-free survival (DFS). When overall survival and DFS functions were compared, a statistically significant difference was observed for DFS survival when the US staging was applied with respect to the clinical AJCC staging. By combining the 2 significant prognostic variables, namely the US staging with the ASA score, four risks groups with different prognoses were identified.
Our findings suggest that US staging may be superior to traditional clinical staging, since it is significantly associated with DFS in anal cancer patients.
Core tip: In this paper the prognostic role of the ultrasound (US) staging system based on tumor penetration through the anal canal wall was examined and compared to the clinical American Joint Committee on Cancer staging system, in anal cancer patients. The results showed that US classification was significantly associated with disease-free survival. This classification could be introduced as one of the predictive clinical parameters to better stratify patients into risk categories.
- Citation: De Nardi P, Arru GG, Guarneri G, Vlasakov I, Massimino L. Prognostic role of ultrasonography staging in patients with anal cancer. World J Gastrointest Oncol 2020; 12(7): 732-740
- URL: https://www.wjgnet.com/1948-5204/full/v12/i7/732.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i7.732
Squamous cells carcinoma (SCC) of the anal canal is a rare disease, with a 2.2% increase in incidence every year on average over the last 10 years, according to the Surveillance, Epidemiology, and End Results database[1].
Since the introduction of the Nigro protocol in 1974, the standard of care for non-metastatic disease has shifted from surgery to radio-chemotherapy, consisting of 5-fluorouracile and mitomycin-C[2]. Since this treatment does not provide a surgical specimen, the staging of the tumor is solely based on clinical evaluation and radiological imaging[3]. Carcinomas of the anal canal are staged according to the size and extent of the disease. Primary tumor (T) stage is defined by tumor diameter from T1 to T3: T1 less than 2 cm, T2 between 2 cm and 5 cm, and T3 greater than 5 cm, while T4 is specific for a tumor invading other organs. In the early 2000’s, novel ultrasound (US) staging systems, based on depth of tumor invasion rather than dimension, have been proposed and have claimed to potentially affect initial treatment as well as prognosis[4,5].
The aim of this study was to compare the clinical American Joint Committee on Cancer (AJCC) staging and the US staging system in patients with anal cancer and to evaluate its prognostic role.
Patients with anal canal SCC, treated at San Raffaele Hospital between 2007 and 2017, were reviewed from our prospectively maintained database. Anal cancers were defined as cancers unable to be entirely visualized while gentle traction was placed on the buttocks[3]. Exclusion criteria included histology other than SCC, patients with previous pelvic radiotherapy, patients who did not undergo endo-anal US and patients with perianal tumors. AJCC clinical classification was determined by magnetic resonance imaging (MRI) and computed tomography (CT) scan; measurement of the longest tumor diameter was recorded, as well as presence of clinically involved lymph-nodes.
Patients underwent pre-treatment work-up consisting of endoscopy with biopsy, pelvic MRI, endo-anal ultrasound, and total-body CT scan; patients from 2012 and onward also underwent positron emission tomography/CT scan.
Endoanal ultrasounds were performed by the same examiner (De Nardi P). Patients were assessed in the left lateral position without bowel preparation or anesthesia. A high multi-frequency (9-16 MHz), 360° rotational mechanical probe (type 2052; BK Medical®, Herlev, Denmark) was employed. Details on US equipment and technique were previously described[6].
Based upon the sonography findings, tumors were classified according to Giovannini et al[5] as follows: (1) UST1: Involvement of the mucosa and submucosa without infiltration of the internal sphincter; (2) UST2: Involvement of the internal sphincter with sparing of the external sphincter; (3) UST3: Involvement of the external sphincter; (4) UST4: Involvement of a pelvic organ; (5) USN0: No suspicious perirectal lymph nodes; and (6) USN+: Suspicious perirectal lymph nodes.
All patients, non-dependent of tumor stage, were treated with definitive chemoradiation. Radiotherapy consisted of conventional radiation (2 or 4-field 3D photon therapy) until June 2008, afterwards step and shoot Intensity-modulated radiation therapy was employed. Concurrent chemotherapy included mitomycin-C 10 mg/m2 at day 1 and infusional 5-fluorouracil at 1000 mg/m2 from days 1 to 4 and from days 29 to 32.
After treatment the patients were evaluated clinically, by anoscopy, endoscopic ultrasound, and with radiologic investigations, every 3 mo for the first 2 years and every 6 mo thereafter.
Written informed consent was obtained by each patient and the study was performed in compliance with the Declaration of Helsinki.
Statistical analysis was performed with IBM statistical package for Social Science (version 18.0, SPSS inc. Chicago, IL, United States). A P value ≤ 0.05 was considered significant. Contingency analyses were performed with Pearson Chi-square and Fisher’s exact test. Survival and disease-free survival (DFS) functions were calculated with Kaplan-Meier statistics. Factor comparisons were performed with the Log Rank Mantle-Cox test. The study was reviewed by our expert biostatistician Luca Massimino.
Between 2007 and 2017, 63 patients, 21 males (33%) with SCC of the anal canal were observed. Endoscopic US was performed on 48 patients, 16 males (33%), with a median age of 59 years (IQR 53-68) and were included in the present analysis. Among these patients 13 (27.1%) were HIV positive, 42 (87%) Human Papilloma Virus positive, and 9 patients had an American Society of Anesthesiologists (ASA) score ≥ 3.
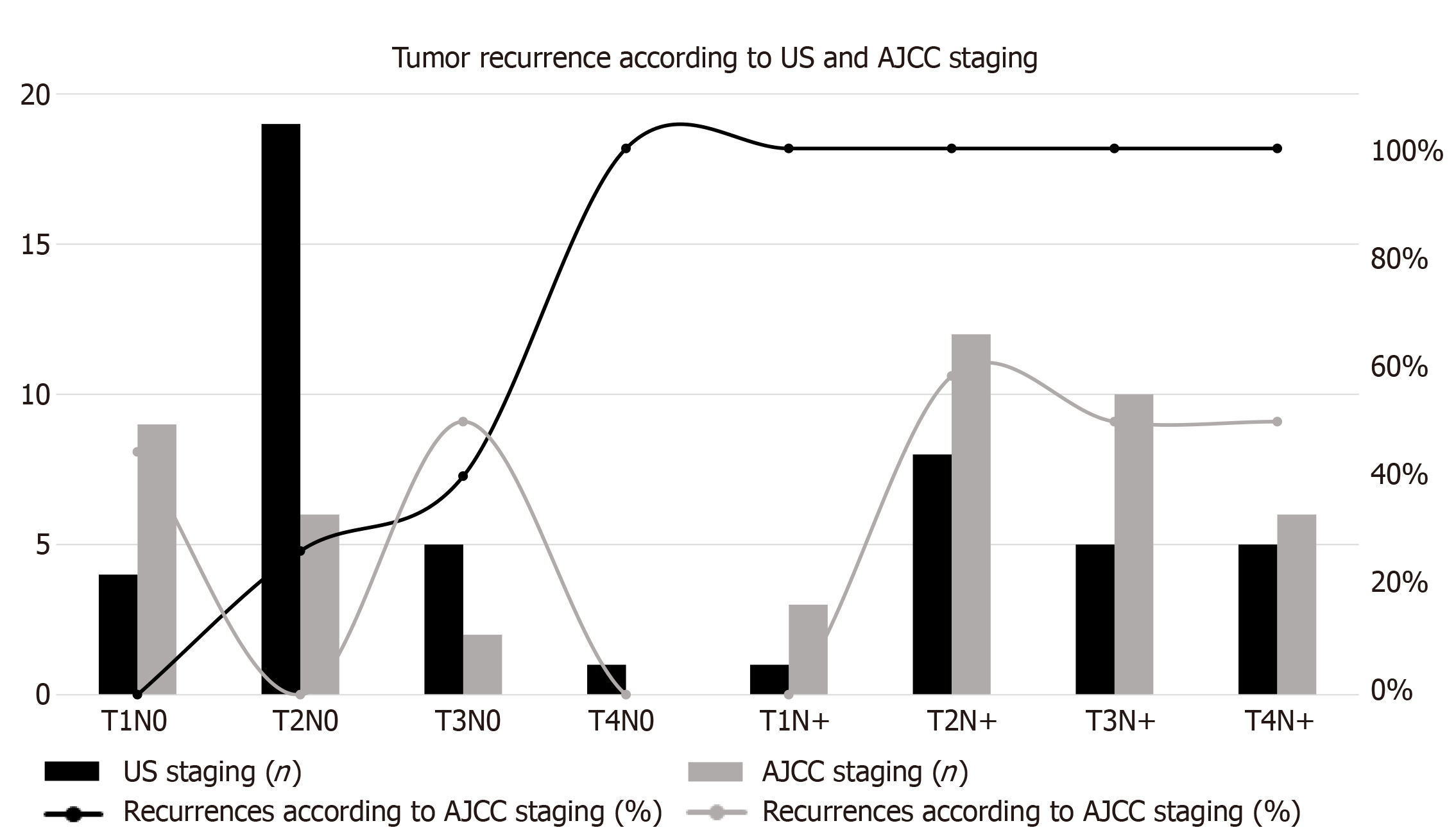
A comparison of the clinical AJCC and US staging of the 48 patients, together with factors potentially influencing tumor recurrence, are reported in Table 1.
| Ultrasonography staging | Clinical AJCC staging | |||||||||||
| n (%) | Recurrence n (%) | Age > 65, n (%) | Male, n (%) | HIV, n (%) | ASA score ≥ 3, n (%) | n (%) | Recurrence, n (%) | Age ≥ 65, n (%) | Male, n (%) | HIV, n (%) | ASA score ≥ 3, n (%) | |
| T1N0 | 4 (8) | 0 | 2 (50) | 3 (75) | 1 (25) | 1 (25) | 9 (19) | 4 (44) | 4 (44) | 2 (22) | 1 (11) | 3 (33) |
| T2N0 | 19 (40) | 5 (26) | 7 (37) | 5 (26) | 4 (21) | 4 (21) | 6 (12) | 0 | 4 (67) | 2 (33) | 1 (17) | 1 (17) |
| T3N0 | 5 (10) | 2 (20) | 1 (20) | 2 (40) | 3 (60) | 1 (20) | 2 (4) | 1 (50) | 0 | 2 (100) | 2 (100) | 1 (50) |
| T4N0 | 1 (2) | 1 (100) | 0 | 0 | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
| T1N+ | 1 (2) | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 3 (6) | 0 | 1 (33) | 2 (67) | 1 (33) | 1 (33) |
| T2N+ | 8 (17) | 8 (100) | 1 (13) | 4 (40) | 4 (50) | 2 (25) | 12 (25) | 7 (58) | 4 (33) | 4 (33) | 4 (33) | 4 (33) |
| T3N+ | 5 (10) | 5 (100) | 4 (80) | 1 (20) | 0 | 2 (40) | 10 (21) | 5 (50) | 2 (20) | 2 (20) | 3 (30) | 2 (20) |
| T4N+ | 5 (10) | 5 (100) | 0 | 1 (20) | 2 (40) | 2 (40) | 6 (13) | 3 (50) | 0 | 2 (33) | 2 (33) | 2 (33) |
Mean follow up was 98 mo and the median follow up was 108 mo. At last follow-up, 38 patients (79.1%) were alive, 28 (58.3%) of which were disease free. During follow up 20 patients (41.6%) experienced recurrences: 6 (12.5%) local, 5 in the inguinal nodes (10.4%), 5 in the lung (10.4%), 3 in the liver (6.2%) and one (2%) in the bone. In Figure 1 the recurrence rate according to the US and AJCC staging systems is reported.
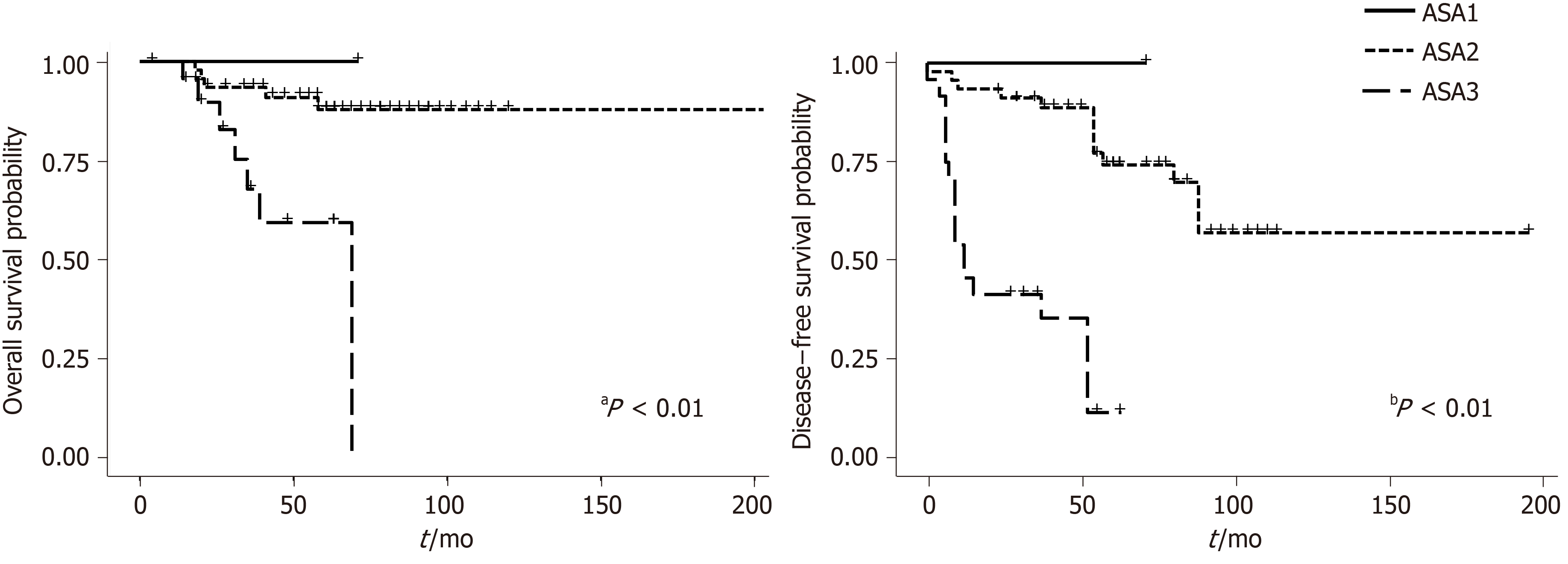
Among the factors possibly related to DFS only the ASA score and US staging were related, with a P = 0.00000001 and P = 0.009 respectively.
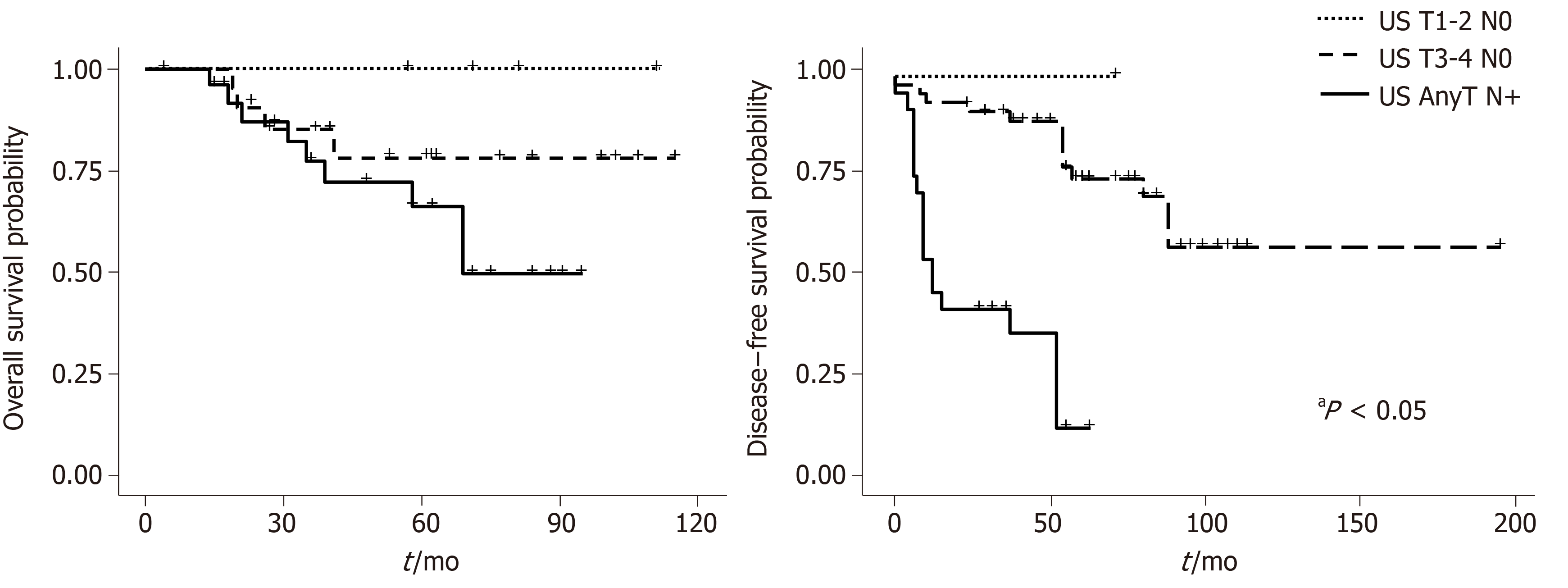
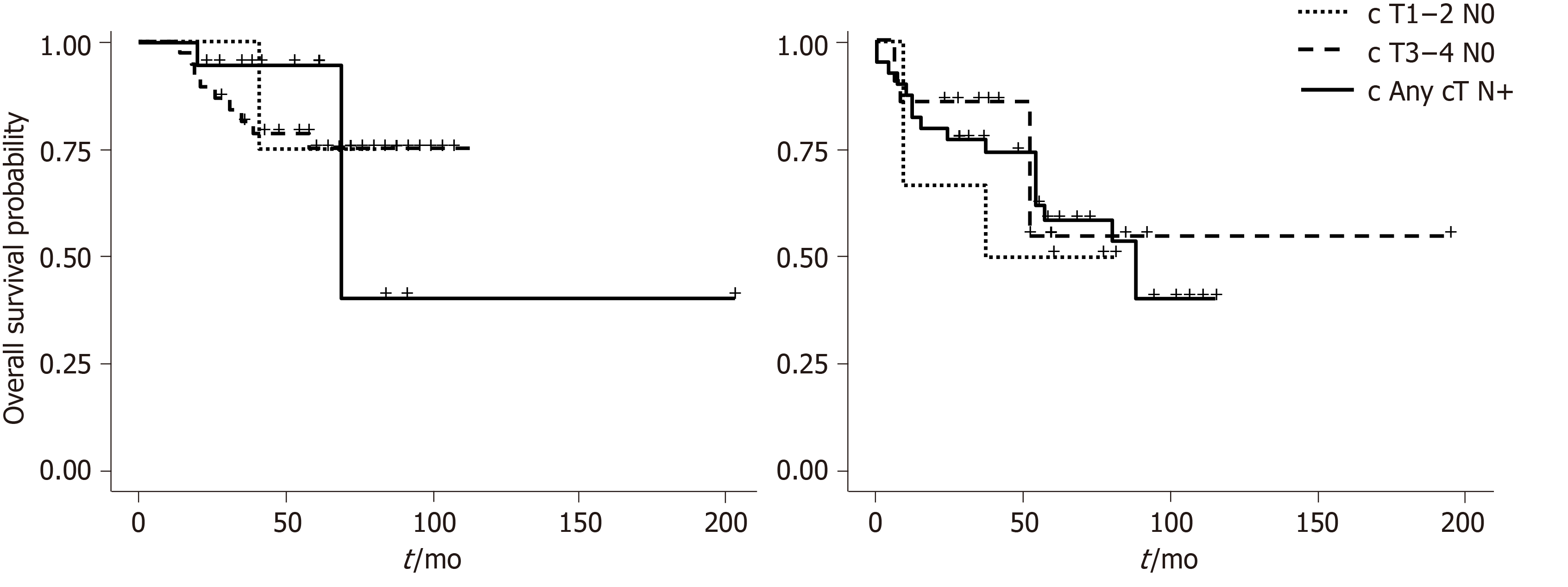
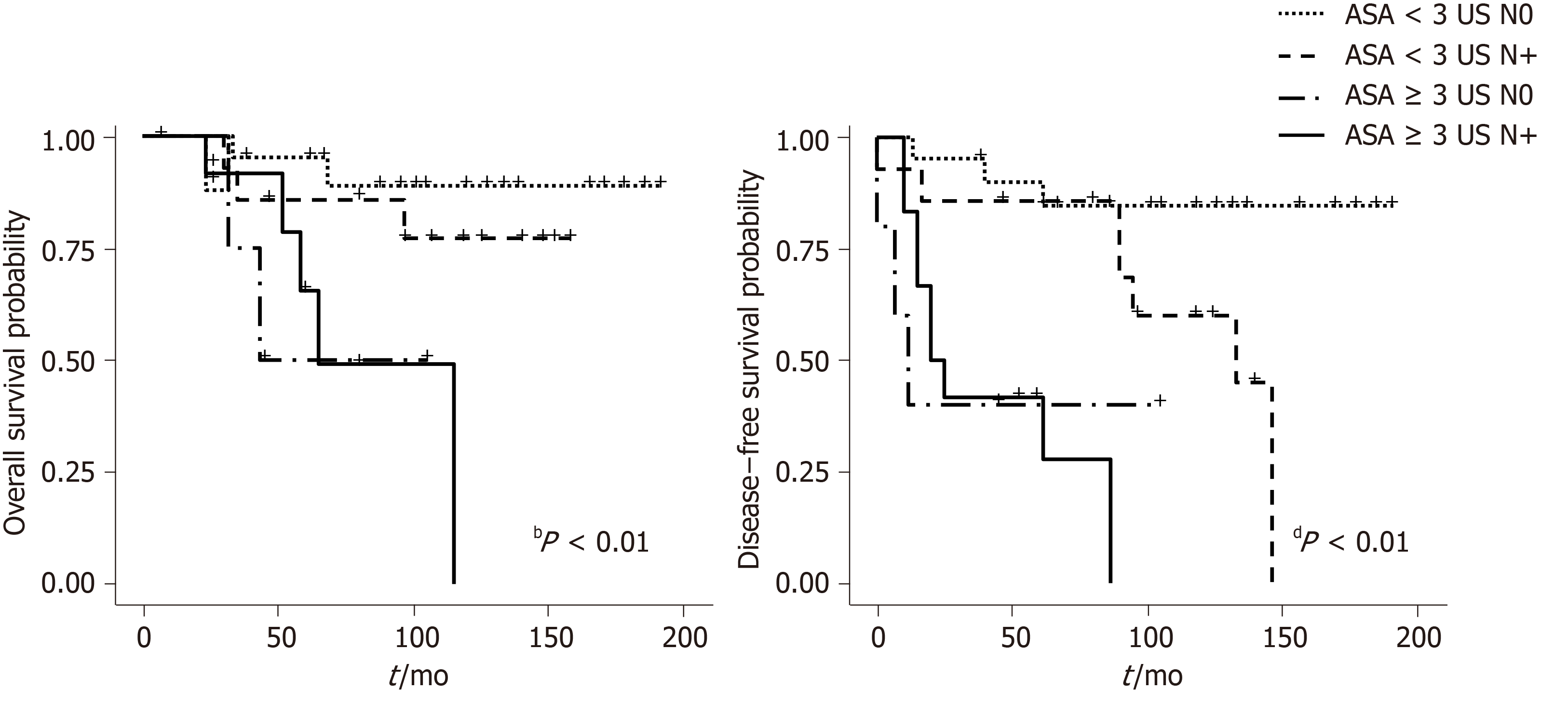
Figures 2 and 3 show overall survival (OS) and DFS according to the AJCC and US stage. A statistically significant difference was observed for DFS survival when the US staging was utilized (P = 0.009). ASA score was also associated with OS (P = 0.00003) (Figure 4).
The observation that US stage was predictive of DFS, together with the prognostic value of the ASA score, led us to combine the two categorizations, ultimately stratifying patients into four different groups, namely ASA < 3, any UST N0, ASA < 3, any UST N+, ASA ≥ 3 any UST N0 and ASA ≥ 3 any UST N+, whose median DFS was 62, 57.5, 13.5 and 7 mo respectively (P = 0.000007). Similar results were obtained when the prediction for OS was tested with the new joint variable, demonstrating a median OS of 64, 62, 33 and 26 mo respectively (P = 0.001) (Figure 5).
Our study compared the traditional AJCC tumor staging system based on tumor dimension, with an US staging system that emphasizes tumor depth, in 48 patients with SCC of the anal canal. The study demonstrated a moderate correlation between the two staging systems and proved that US staging is associated with DFS.
The most widely employed staging system for anal cancer is TNM staging. For tumors of the anal canal the T stage is based on tumor size, however for all the other tumors of the gastrointestinal tract the T stage is determined by tumor penetration through the different layers. T stage can be determined by digital anal examination or imaging techniques. In our study we rely on MRI, which can provide more objective measurements of tumor dimension than digital rectal examination.
T staging according to AJCC classification has been evaluated as a prognostic factor with conflicting results. Tumor diameter > than 5 cm is reported as an independent variable predicting DFS and OS by The Radiation Therapy Oncology Group 9811 analysis[7], but not by the European Organization for Research and Treatment of Cancer 22861 study[8,9]. Moreover, no difference in survival has been reported for T1 or T2 tumors.
The importance of T staging for overall and DFS has been underlined by the last 8th TNM edition in which stage II was sub-classified into stage IIA (T = 2-5 cm) and stage IIB (T > 5 cm)[3] and has been validated by two United States databases, the National Cancer Institute and the Surveillance, Epidemiology, and End Results[10]. These studies however only considered T2 and T3 tumors and did not include an analysis of T1 and T4.
In the early 2000’s several authors proposed their ultrasound-based classification of anal canal tumors; the principles of which were similar to those of rectal carcinoma and were based on depth of tumor invasion. With this classification the tumors confined to the mucosa, submucosa or invading the adjacent sphincters or perianal fat could be defined. This staging has claimed to provide information on very early tumors, amenable for local excision[4]. Giovannini et al[5] demonstrated that the classification based on tumor penetration through anal layers, was important in determining the response to radiotherapy, was predictive of local recurrence, and correlated with survival. In addition they did not find any correlation with local recurrence nor survival for the clinical staging system[5]. The present study confirms the superiority of US over AJCC clinical staging as a prognostic determinant since it was significantly associated with DFS.
Other imaging modalities are also employed for local staging of anal cancer. Among them MRI is routinely recommended for rectal and anal cancer, with a sensitivity for the identification of anal cancer approaching 90%-100% with a good assessment of tumor size, position, extent of the disease, infiltration of adjacent organs and response to treatment[11].
The accuracy of US and MRI was compared by Otto et al[12] who found a good concordance between the two diagnostic techniques, suggesting that US was superior for the detection of superficial tumors, while MRI was needed for N staging, since US cannot visualize inguinal or iliac lymph node that are outside of the field of vision[13]. In the present series, MRI detected more abnormal nodes than US, nevertheless the prognostic significance was more associated to the US staging. Additionally, US is less expensive, well tolerated and it can be performed by the physician during a clinical examination.
Since fifty percent of recurrences occurring within the first 2 years post treatment, are located around the primary site of disease, or as pelvic/inguinal lymph nodes, a loco regional staging system with a positive impact on prognosis is of paramount importance in order to stratify patients into appropriate risk categories and possibly tailor treatment plans[13,14].
The observation that ASA score was predictive of DFS has not been previously reported in anal cancer patients. The ASA score is generally employed to assess perioperative anesthetic risk and it is considered helpful in predicting short- and long-term outcome in surgical patients, however it has been rarely used for non-surgical treatments. The worse prognosis in patients with ASA score ≥ 3 possibly reflects a poorer general physical status related to co-morbidities and to the cancer itself, which could possibly lead to more treatment interruption or uncompleted chemoradiation, thus compromising outcome. Combining the US staging with the ASA score, allowed us to construct a prognostic model to assign patients into four subgroups with different prognoses with UST1-2N0-ASA < 3 being the best and UST3-4N+-ASA ≥ 3 the worst. Even if the most important difference in prognosis could be due to ASA score alone, the association of the two variables allowed for the identification of groups of patients with different risks of progression.
This study has several limits: It is retrospective, it is performed on a limited number of patients and reflects a single center experience. This is partly due to the low incidence of anal cancer and to the low diffusion of the use of US in the assessment of anal cancer patients. Moreover, there are several drawbacks related to the US technique itself. First of all, US is operator dependent and the results here described were not reviewed by a second examiner. Secondly, endoanal US can be hardly performed in patients bearing stenotic lesions. No such tumors have been found in our patient population; however, it is likely that in the case of a stenotic tumor, US cannot be performed. Nevertheless, with improvement of the technique and dedicated radiologists, MRI could also be employed for the evaluation of invasion depth in early tumors and the US staging system be performed with MRI.
In conclusions our study confirms that an US staging system is associated with a more accurate prognosis in anal cancer patients. Our results should be further validated on a larger scale and if proven advantageous, the use of the US system could be introduced as one of the predictive clinical parameters in the setting of anal cancer, in order to improve the prognostic accuracy and the possible implementation of a tailored therapeutic approach.
The primary tumor staging of anal canal carcinomas is based on tumor dimension which is clinically and radiologically assessed. Novel tumor staging, based on depth of tumor invasion assessed by endorectal ultrasonography (US), have been proposed and have claimed to potentially affect initial treatment as well as prognosis.
Several authors reported that the staging based on tumor diameter is not an independent prognostic variable. If a different staging system could more accurately reflect the prognosis, it could be used not only to better predict outcome, but also to tailor the treatment.
To evaluate the possible prognostic role of a staging system based on tumor penetration into the anal wall, in comparison with the traditional staging.
This is a retrospective evaluation of 48 patients with squamocellular carcinoma of the anal canal, who underwent endoscopic US as part of a pre-treatment assessment including endoscopy with biopsy, pelvic magnetic resonance imaging, and total body computed tomography scan. All the tumors were staged with the traditional anal cancer staging system and with a novel US staging. All the patients were treated with definitive chemoradiation and subsequent follow-up. Overall and disease-free survival (DFS), as well as factors influencing survival were analyzed.
Median follow up was 108 mo. American Society of Anesthesiologists score, and US based staging system were related with DFS. By combining these two prognostic variables 4 groups with different prognoses were identified.
A staging system based on tumor invasion is more similar to the staging of all other intestinal cancers and may better reflect a prognostic significance. By combining US staging with other prognostic variables, groups of patients with different prognoses can be determined. In the future the US staging could be introduced as one of the predictive clinical parameters in the setting of anal cancer, in order to improve the prognostic accuracy and possibly implement a tailored therapeutic approach.
Anal cancer is a rare disease and prospective studies are difficult to conduct in a single center. The usefulness of the US staging system, in addition to traditional staging, as prognostic determinants, should be further validated in larger studies in order to plan treatment strategies based on risk categories.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Colorectal Surgeons (Fellow); European Society of Coloproctology (Member); Italian Society of Colorectal Surgery (Member); Italian Society of Surgery (Member); and Italian Society of Surgical Oncology (Member).
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aktekin A, Chen Z, Govindarajan KK S-Editor: Dou Y L-Editor: A E-Editor: Liu MY
| 1. | Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2016. National Cancer Institute. Bethesda, MD, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. Available from: https://seer.cancer.gov/csr/1975_2016/. |
| 2. | Morton M, Melnitchouk N, Bleday R. Squamous cell carcinoma of the anal canal. Curr Probl Cancer. 2018;42:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. |
Welton ML, Steele SR, Goodman KA, Gunderson LL, Asare EA, Brierley JD, Compton CC, De Nardi P, Goldberg RM, Gress D, Washington MK, Jessup JM, Amin MB, ed. AJCC Cancer Staging Manual. 8th ed.
Chicago, IL: AJCC; 2017: |
| 4. | Tarantino D, Bernstein MA. Endoanal ultrasound in the staging and management of squamous-cell carcinoma of the anal canal: potential implications of a new ultrasound staging system. Dis Colon Rectum. 2002;45:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Giovannini M, Bardou VJ, Barclay R, Palazzo L, Roseau G, Helbert T, Burtin P, Bouché O, Pujol B, Favre O. Anal carcinoma: prognostic value of endorectal ultrasound (ERUS). Results of a prospective multicenter study. Endoscopy. 2001;33:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Dal Corso HM, D'Elia A, De Nardi P, Cavallari F, Favetta U, Pulvirenti D'Urso A, Ratto C, Santoro GA, Tricomi N, Piloni V. Anal endosonography: a survey of equipment, technique and diagnostic criteria adopted in nine Italian centers. Tech Coloproctol. 2007;11:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Doll CM, Moughan J, Klimowicz A, Ho CK, Kornaga EN, Lees-Miller SP, Ajani JA, Crane CH, Kachnic LA, Okawara GS, Berk LB, Roof KS, Becker MJ, Grisell DL, Ellis RJ, Sperduto PW, Marsa GW, Guha C, Magliocco AM. Significance of Co-expression of Epidermal Growth Factor Receptor and Ki67 on Clinical Outcome in Patients With Anal Cancer Treated With Chemoradiotherapy: An Analysis of NRG Oncology RTOG 9811. Int J Radiat Oncol Biol Phys. 2017;97:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M, Pierart M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 887] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 9. | Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, Arnold D; ESMO; ESSO; ESTRO. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol. 2014;111:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Goffredo P, Garancini M, Robinson TJ, Frakes J, Hoshi H, Hassan I. A National-Level Validation of the New American Joint Committee on Cancer 8th Edition Subclassification of Stage IIA and B Anal Squamous Cell Cancer. Ann Surg Oncol. 2018;25:1654-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Durot C, Dohan A, Boudiaf M, Servois V, Soyer P, Hoeffel C. Cancer of the Anal Canal: Diagnosis, Staging and Follow-Up with MRI. Korean J Radiol. 2017;18:946-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Otto SD, Lee L, Buhr HJ, Frericks B, Höcht S, Kroesen AJ. Staging anal cancer: prospective comparison of transanal endoscopic ultrasound and magnetic resonance imaging. J Gastrointest Surg. 2009;13:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Gourtsoyianni S, Goh V. MRI of anal cancer: assessing response to definitive chemoradiotherapy. Abdom Imaging. 2014;39:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Franco P, Ricardi U. Primary tumor size as a prognosticator in anal cancer patients. Ann Transl Med. 2019;7:157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |