Published online Mar 15, 2020. doi: 10.4251/wjgo.v12.i3.347
Peer-review started: October 21, 2019
First decision: December 5, 2019
Revised: January 4, 2020
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: March 15, 2020
Processing time: 142 Days and 16.1 Hours
Duodenal adenocarcinoma (DA) and intestinal-type papilla of Vater adenocarcinoma (it-PVA) are rare malignancies of the gastrointestinal tract. Current therapeutic options are translated nowadays from treatment strategies for patients with colorectal cancer due to histopathological similarities.
To retrospectively investigate the clinical outcome of patients with DA and it-PVA.
All patients with DA and it-PVA diagnosed between 2000 and 2017 were included at two academic centers in the Netherlands. All patients with histopathologically-confirmed DA or it-PVA were eligible for inclusion. Clinical outcome was compared between DA and it-PVA per disease stage. In the subgroup of stage IV disease, survival after local treatment of oligometastases was compared with systemic therapy or supportive care.
In total, 155 patients with DA and it-PVA were included. Patients with it-PVA more often presented with stage I disease, while DA was more often diagnosed at stage IV (P < 0.001). Of all patients, 79% were treated with curative intent. The median survival was 39 mo, and no difference in survival was found for patients with DA and it-PVA after stratification for disease stage. Seven (23%) of 31 patients with synchronous stage IV disease underwent resection of the primary tumor, combined with local treatment of oligometastases. Local treatment of metastases was associated with an overall survival of 37 mo, compared to 14 and 6 mo for systemic therapy and supportive care, respectively.
Survival of patients with DA and it-PVA is comparable per disease stage. These results suggest a potential benefit for local treatment strategies in selected patients with oligometastases, although additional prospective studies are needed.
Core tip: This study demonstrates the clinical outcome for duodenal adenocarcinoma and intestinal-type papilla of Vater adenocarcinoma, which are rare tumor types of the gastrointestinal tract. The overall survival is comparable per disease stage, resulting in a median survival of 39 mo. Most patients (79%) are treated with curative intent by surgical resection of the tumor. For patients with metastatic disease, local treatment of metastases was associated with a better overall survival compared to systemic treatment or supportive care. Future prospective studies are needed to confirm this survival benefit.
- Citation: Meijer LL, Strijker M, de Bakker JK, Toennaer JG, Zonderhuis BM, van der Vliet HJ, Wilmink H, Verheij J, Daams F, Busch OR, van Grieken NC, Besselink MG, Kazemier G. Clinical outcomes of patients with duodenal adenocarcinoma and intestinal-type papilla of Vater adenocarcinoma. World J Gastrointest Oncol 2020; 12(3): 347-357
- URL: https://www.wjgnet.com/1948-5204/full/v12/i3/347.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i3.347
Duodenal adenocarcinoma (DA) is a rare malignancy of the gastrointestinal tract with an incidence of approximately 0.5 per 100000 persons, and it constitutes less than 5% of all gastrointestinal tumors[1-3]. Despite its rarity, the incidence of DA has increased over the last years[3,4]. Papilla of Vater adenocarcinoma (PVA) also develops as a primary tumor in the duodenal wall[5]. The papilla of Vater has an ambiguous position in the duodenum, since tumors originating from the papilla can be classified as either an intestinal- or pancreaticobiliary-type based on their histological differentiation[6]. While the outcome of pancreaticobiliary-type PVA resembles pancreatic or bile duct cancer, the clinical outcome of patients with intestinal-type PVA (it-PVA) is comparable to DA[7,8]. Interestingly, it-PVA carcinomas and DA show considerable overlap in molecular features and clinical behavior, underlining the rationale for similar treatment strategies[9,10]. However, comparisons between clinical characteristics and outcome of these tumor types are sparse.
Surgical resection of the primary tumor is the preferred treatment option for patients with localized tumors[11,12]. However, no practical guidelines exist for patients with metastatic disease stages. Recent studies have identified histopathological and molecular biological similarities between small bowel adenocarcinomas, including DA, it-PVA and colorectal cancer (CRC)[2,13]. Therefore, treatment protocols for patients with DA and it-PVA are increasingly based on combined multimodality therapy regimens that are already established and validated for patients with CRC[14,15].
Optimal curative therapy for patients with metastatic CRC comprises local treatment of oligometastases, alone or combined with (induction) chemotherapy[16-18]. The benefit of these approaches has not been investigated for patients with DA and it-PVA. Based on the comparability with CRC, our clinical practice was changed towards local treatment for all consecutive patients with DA and it-PVA with oligometastases of the liver and lymph nodes. These patients were considered for resection of the primary tumor, combined with local treatment of oligometastases, according to the guidelines for patients with CRC. This study aims to investigate the clinical outcomes of patients with DA and it-PVA, and evaluate the effect of the introduction of this new way of treatment in these selected patients with metastatic disease by comparing this regimen to systemic treatment and supportive care.
This retrospective case series analysis included all consecutive patients with DA or PVA treated at one of the locations of the Cancer Center Amsterdam, Amsterdam UMC (VUMC and AMC, Amsterdam) from 2000 to 2017. To assess all eligible patients, a systematic search was performed in the automated pathology database (PALGA), in which all histopathologically-confirmed diagnoses by either biopsy or resection are documented. All patients ≥ 18 years with histopathologically-confirmed intestinal-type adenocarcinoma of the duodenum or intestinal-type PVA were eligible for inclusion. Histopathological confirmation was based on the written pathology report, and no additional staining was performed if histological differentiation was unspecified, omitting these patients for further analysis[19,20]. Patients with tumors other than intestinal-type adenocarcinoma or metastases from other primary tumors were excluded, as well as patients diagnosed with a malignancy within 5 years prior to or after diagnosis. The medical ethics committees of both medical centers approved of this retrospective multicenter study (#2017.215 and #W17_399), in accordance with the Declaration of Helsinki.
At location VUMC, the clinical treatment of patients with oligometastases of DA or it-PVA changed into applying the local treatment of metastases. Consecutive patients presenting at location VUMC with synchronous liver or lymph node metastases were discussed at a multidisciplinary meeting, with experience in the management of hepatobiliary disease, for consideration of the resection of the primary tumor combined with local treatment of metastases, i.e., resection or ablation. Patients were considered if they had sufficient performance status, and in case local therapy was technically feasible, according to the guidelines for local treatment of CRC liver metastases[21]. Briefly, the following criteria were applied to select candidate patients for the local treatment of oligometastases: (1) Patients with synchronous oligometastases of the liver, with the following criteria: (a) The aim of liver resection was to achieve a complete resection with negative resection margins, and leave sufficient liver function; (b) Patients were considered for ablative therapy if deemed unfit for surgery, or if unfavorable location of the metastases for surgery was present, or insufficient liver remnants was expected after resection[22]; and (2) Patients with synchronous lymph node metastases for which resection of the metastases was feasible during resection of the primary tumor. When repeat metastases occurred after the local treatment of liver metastases, local treatment was reconsidered in one patient.
Consecutive patients presenting at the AMC location were also discussed at a dedicated multidisciplinary meeting, and received standard of care for metastatic disease, i.e., systemic therapy or supportive care. Patients treated with local therapy of metastases were compared to the standard of care for metastatic disease applied at the AMC location, and a historic cohort concerning all patients treated before the introduction of this novel strategy at the VUMC location. To optimally compare the effect of local metastases treatment, a subgroup analysis was performed on patients with synchronous metastases confined to the liver. Patients with synchronous liver metastases who received systemic treatment or supportive care were retrospectively reviewed by a surgeon, who had been blinded, for the outcome of previous feasibility of local therapy application based on location, number, and size of metastases.
All relevant data were retrospectively collected, including age, sex, ASA-score, site of primary tumor, disease stage (AJCC staging system, 8th edition)[23], tumor size, histopathological subtype, location and number of metastases, and treatment, including type of surgical resection, local treatment of metastases and systemic therapy, including specified treatment regimens. The involvement of lymph nodes was either classified as regional (N1/2) or distant (M1), depending on the location of lymph node involvement according to the AJCC staging system. Follow-up of patients included clinical assessment and diagnostic imaging every 6 mo, or based on clinical symptoms. Overall survival was calculated from the date of diagnosis until the date of death, or date of last follow-up. Surgery-related deaths, defined as patient death within 30 d after resection of the primary tumor or a palliative bypass procedure, were excluded for survival analysis.
Descriptive statistics were reported for demographics and clinicopathological characteristics. Continuous variables are presented as median [interquartile range (IQR)], and comparisons between groups were analyzed using the Student’s t test or one-way ANOVA, as appropriate. Categorical variables are presented as frequencies (percentages), and were analyzed using the χ2-test. Overall survival was calculated using the Kaplan-Meier method, and statistical significance for survival was assessed using the log-rank test. P < 0.05 was considered statistically significant. Statistical analyses were computed using SPSS® version 25 (IBM, New York, United States).
A total of 155 consecutive patients were identified; 99 consecutive patients with DA and 56 patients with it-PVA. Patient characteristics are summarized in Table 1. Baseline characteristics were comparable between DA and it-PVA. Patients with it-PVA presented more often with stage I disease compared to patients with DA (32% vs 7%, respectively), while DA was more often diagnosed at stage IV disease (29% vs 9% for it-PVA, P < 0.001). Treatment with curative intent was performed in 122 (79%) of the included patients. Among these, 90% of the patients with it-PVA were treated with surgery alone. Curative resection combined with either (neo) adjuvant therapy (28%) or local treatment of metastasis (11%) was more common in patients with DA compared to it-PVA (P = 0.003). In patients with metastases, the use of palliative chemotherapy did not significantly differ between patients with DA (90%) and it-PVA (100%, P = 0.848).
| Total, n = 155 | Duodenum, n = 99 | Papilla, n = 56 | P value | |
| Age | 0.237 | |||
| Age, median [IQR] | 64 [58-73] | 63 [56-72] | 66 [58-74] | |
| Sex (%) | 0.742 | |||
| Male | 94 (60.6) | 61 (61.6) | 33 (58.9) | |
| ASA (%) | 0.852 | |||
| ASA I | 19 (12.3) | 12 (12.1) | 7 (12.5) | |
| ASA II | 83 (53.5) | 49 (49.5) | 34 (60.7) | |
| ASA III | 31 (20.0) | 19 (19.2) | 12 (21.4) | |
| ASA IV | 1 (0.6) | 1 (1.0) | 0 (0.0) | |
| NR | 21 (13.5) | 18 (18.2) | 3 (5.4) | |
| TNM stage (%) | < 0.001 | |||
| Stage I | 25 (16.1) | 7 (7.1) | 18 (32.1) | |
| Stage II | 26 (16.8) | 20 (20.2) | 6 (10.7) | |
| Stage III | 65 (41.9) | 39 (39.4) | 26 (46.4) | |
| Stage IV | 34 (21.9) | 29 (29.3) | 5 (8.9) | |
| NR | 5 (3.2) | 4 (4.0) | 1 (1.8) | |
| Treatment (%) | ||||
| Curative intent | 122 (78.7) | 71 (71.7) | 51 (91.1) | 0.003 |
| Surgery | 89 (73.0) | 43 (60.6) | 46 (90.2) | |
| Neoadjuvant + surgery | 3 (2.5) | 3 (4.2) | 0 (0.0) | |
| Surgery + adjuvant therapy | 21 (17.2) | 17 (23.9) | 4 (7.8) | |
| Primary resection + treatment metastasis | 9 (7.4) | 8 (11.3) | 1 (2.0) | |
| Palliative treatment | 23 (14.8) | 20 (20.2) | 3 (5.4) | 0.848 |
| Chemotherapy | 21 (91.3) | 18 (90.0) | 3 (100.0) | |
| Radiotherapy | 1 (4.3) | 1 (5.0) | 0 (0.0) | |
| Chemotherapy + radiotherapy | 1 (4.3) | 1 (5.0) | 0 (0.0) | |
| Best-supportive care | 8 (5.2) | 7 (7.1) | 1 (1.8) | |
| NR | 2 (1.3) | 1 (1.0) | 1 (1.8) | |
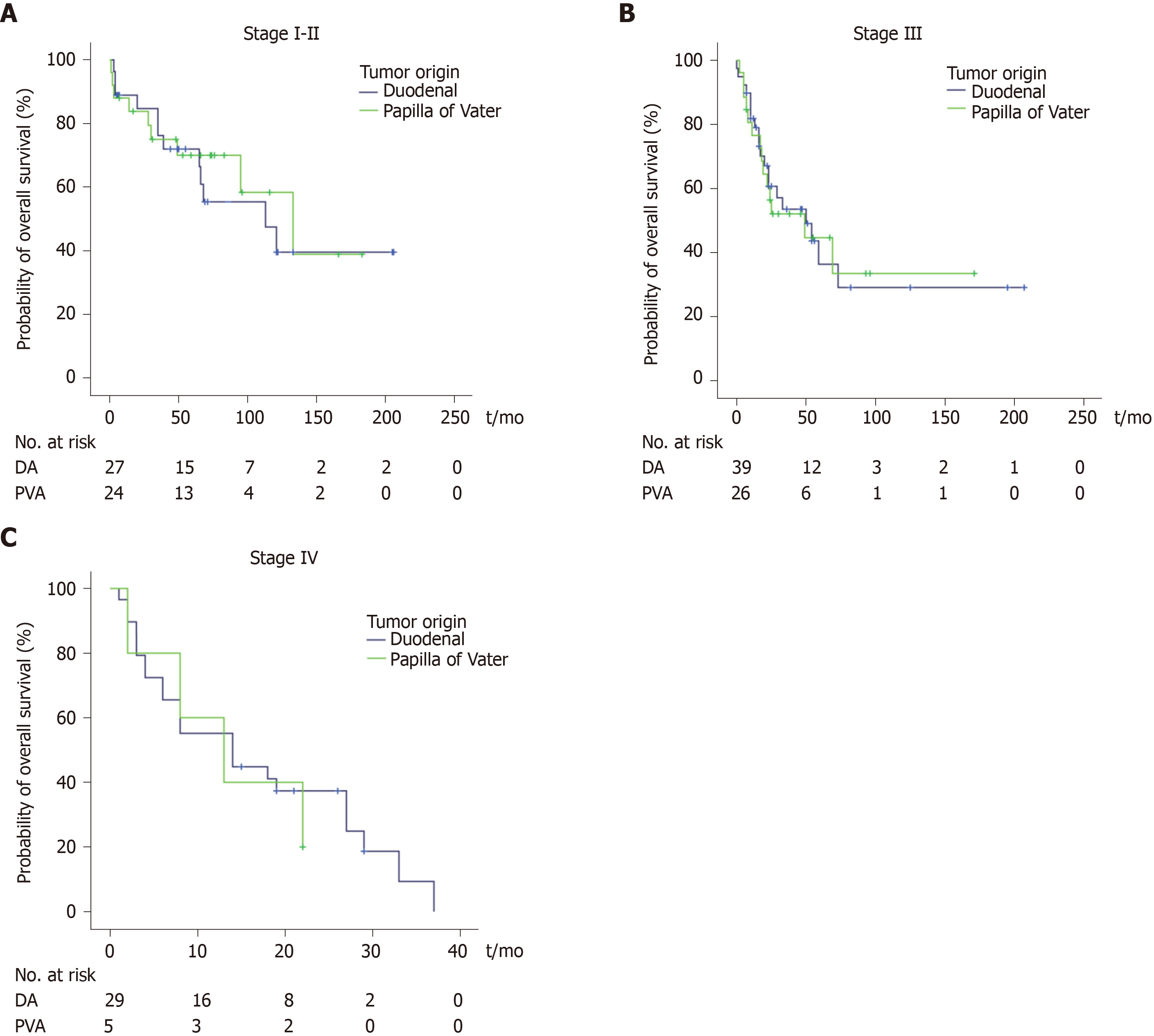
Of all patients, 86 (55%) were followed until death, and the median follow-up period was 55 mo (IQR 22-82 mo) for patients still alive at the end of follow-up. Median survival for the entire cohort was 39 mo, and the 1-year, 3-year and 5-year overall survival (OS) rates were 72%, 38% and 23%, respectively. There was no significant difference in OS between patients with DA and it-PVA after adjusting for disease stage (Figure 1). The median OS for stage I-II was 113 mo and 133 mo (P = 0.841), OS for stage III was 50 mo and 49 mo (P = 0.927) and OS for stage IV was 14 mo and 13 mo (P = 0.676) for DA and it-PVA, respectively.
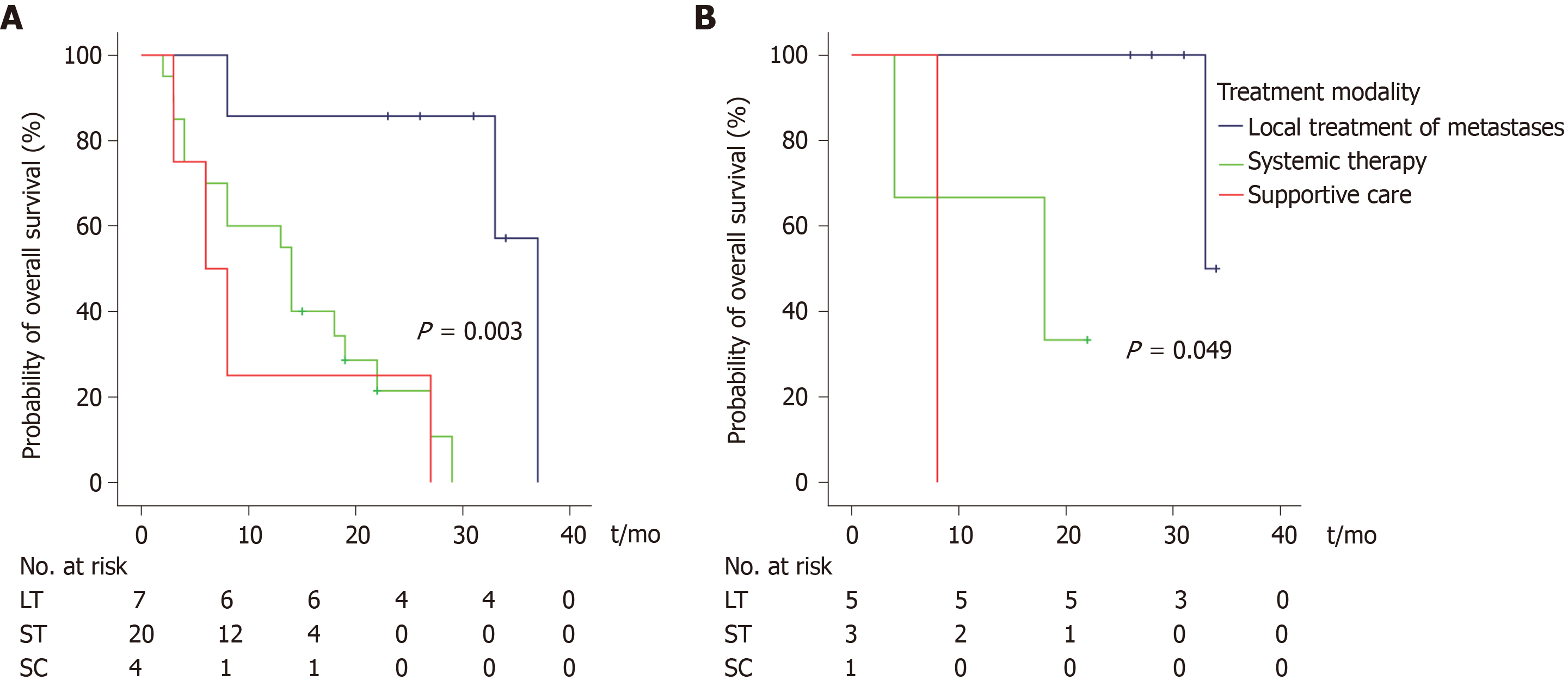
To investigate the outcomes of patients with metastatic disease, 34 patients with synchronous metastatic DA and it-PVA at initial presentation were eligible for analyses. Three patients were excluded due to surgery-related deaths, which included two patients after resection of the primary tumor and one patient after a palliative bypass procedure. Subsequently, 31 patients were divided retrospectively into three treatment modality groups: local treatment of oligometastases (n = 7), systemic treatment (n = 20), or supportive care (n = 4, Table 2). All patients selected for local treatment presented with synchronous oligometastases, and they all underwent resection of the primary tumor, while none of the patients received induction chemotherapy prior to resection or adjuvant therapy. In addition, these patients underwent synchronous metastasectomy (n = 5), ablation of liver metastases (n = 1), or a combination of metastasectomy and ablation (n = 1). One patient in this group underwent an additional resection of metachronous liver metastases, and three patients received palliative chemotherapy for recurrence of metastases. In all stage IV patients, capecitabine combined with oxaliplatin (CAPOX) was most commonly administered as systemic therapy (n = 13). Other administrated regimens are reported in Table 2. One patient also received radiotherapy in addition to systemic therapy. The supportive care group was defined as patients who did not receive any type of treatment for the primary tumor or metastases. A palliative bypass procedure was performed in 11 patients of the systemic treatment group, and in three patients of the supportive care group. The median OS was 37 mo in the patient group receiving local treatment of metastases, vs 14 mo for the patient group receiving systemic treatment and 6 mo for patients receiving supportive care, after a median follow-up of 23 mo (IQR 15–34 mo) for patients who were still alive. The local treatment of metastases was associated with a better survival, with five of seven patients still living after 2 years (Figure 2A). Subgroup analysis of all patients, with metastases confined to the liver and retrospectively deemed eligible for local treatment, confirmed these results, including four patients who remained alive after almost 2 years following the local treatment of metastases (Figure 2B).
| Total, n = 31 | Local treatment of metastases, n = 7 | Systemic treatment, n = 20 | Supportive care, n = 4 | |
| Age | ||||
| Age, median [IQR] | 63 [58-71] | 69 [59-73] | 62 [52-73] | 63 [58-67] |
| Sex | ||||
| Male | 17 | 5 | 11 | 1 |
| Origin | ||||
| Duodenum | 27 | 7 | 17 | 3 |
| Papilla (intestinal-type) | 4 | 0 | 3 | 1 |
| ASA | ||||
| ASA I | 3 | 1 | 2 | 0 |
| ASA II | 13 | 4 | 8 | 1 |
| ASA III | 4 | 1 | 1 | 2 |
| NR | 11 | 1 | 9 | 1 |
| Metastatic site | ||||
| Liver | 13 | 5 | 7 | 1 |
| Lymphatic | 4 | 2 | 2 | 0 |
| Lung | 2 | 0 | 2 | 0 |
| Peritoneal | 9 | 0 | 6 | 3 |
| Lung + liver or lymphatic | 3 | 0 | 3 | 0 |
| Number of liver oligometastases1 (%) | ||||
| Number, median [IQR] | 2 [1-7] | 2 [1-3] | 4 [1-15] | 3 |
| Local treatment of liver metastases | ||||
| Metastasectomy | 5 (14.7) | 5 | N/A | N/A |
| Ablation | 1 (2.9) | 1 | N/A | N/A |
| Metastasectomy + ablation | 1 (2.9) | 1 | N/A | N/A |
| Systemic therapy | ||||
| 5-FU-LV | 1 (2.9) | 0 | 1 | 0 |
| Capecitabine | 5 (14.7) | 0 | 5 | 0 |
| CAPOX | 13 (41.9) | 2 | 11 | 0 |
| EOX | 1 (2.9) | 0 | 1 | 0 |
| FOLFOX | 1 (2.9) | 1 | 0 | 0 |
| FOLFOX + radiotherapy | 1 (2.9) | 0 | 1 | 0 |
| No chemotherapy | 6 (19.4) | 3 | 0 | 3 |
| Unspecified chemotherapy | 1 (2.9) | 0 | 1 | 0 |
| NR | 2 (6.5) | 1 | 0 | 1 |
This study demonstrates a comparable outcome for patients with DA and it-PVA per disease stage, with a median survival of 39 mo. A potential benefit was found for the local treatment of oligometastases in selected patients with liver or lymph node metastases in DA. The survival of patients who received local treatment of metastases was longer than in patients receiving systemic therapy or supportive care. This was also true after subgroup analysis of patients with synchronous metastases confined to the liver. Eventually, only patients with DA received local treatment of metastases in this study, hampering translation of these results to patients with it-PVA. However, since molecular biology and survival per disease stage have been shown to be comparable for patients with DA and it-PVA, further investigation of local treatment strategies in patients with it-PVA is justifiable.
The current study provides the largest reported series comparing OS for DA and it-PVA. Both tumor types demonstrated comparable survival rates after stratification for disease stage, consistent with previous studies[7,8,24]. Of note, patients with it-PVA were more often diagnosed at early disease stages, which is likely due to earlier clinical presentation with tumor-related symptoms, such as jaundice[25]. Clinical presentation of patients with DA might be less specific, as 25%-43% of patients may be asymptomatic at diagnosis[12,26,27]. Patients with DA were more frequently diagnosed with metastatic disease. This might not represent the true incidence of stage IV it-PVA, since histopathological differentiation has often been inconclusive. Moreover, these tumors might easily be mistaken for other tumors of the periampullary area, especially when no surgical resection specimens are available[28]. This could have resulted in an underestimation of the incidence of stage IV it-PVA. The resemblance of DA and it-PVA based on biological similarity and clinical behavior underlines the importance of accurate histopathological classification and corresponding treatment approaches[29,30].
A recent study investigating the outcomes of patients with small bowel adenocarcinomas, including DA, reported similar results of enhanced survival after the local treatment of metastases[26]. However, the current study expands this knowledge, with a unique focus on DA and it-PVA. The small bowel adenocar-cinomas also include tumors originating from the jejunum and ileum. Controversy exists regarding the association between primary tumor location within the small bowel and prognosis. Previously, a higher incidence and worse outcome for DA compared to jejunal tumors has been reported, although this was not confirmed by others[4,31-34]. The duodenum might be more susceptible to carcinogenesis, due to its proximity near the ampulla, and pancreaticobiliary excretions[34]. In the current study, only patients with DA and it-PVA were included to minimize the possible bias of tumor location. Several clinically-relevant factors have not been sufficiently considered in previous studies, such as the number of metastases, specifications of local treatment regimens, and the type of chemotherapy administered to patients, making the results presented in this study more transparent[26].
Molecular characterization and histopathology demonstrated the resemblance of DA to CRC[9,13]. Therefore, resection of oligometastases in patients with DA and it-PVA is an attractive therapeutic option to explore. Interestingly, the benefit of local treatment of liver metastases in CRC is solely based on historical cohort studies, which demonstrated enhanced survival benefit. Furthermore, local treatment of oligometastases confined to the liver is currently the first choice of treatment in CRC[35-38]. Although many therapeutic options remain to be fully explored in carefully selected patient groups, the results of this first study demonstrate the feasibility of applying local treatment of oligometastases in selected patients with DA. Nevertheless, patient-related factors, such as the physical condition of the patient, tumor size, tumor location, and extent of metastases, could still withhold aggressive treatment interventions.
The limitations of this study include those intrinsic to the retrospective design and the small sample size, which restricted statistical analysis, although this is one of the largest series to report outcomes for DA and it-PVA[11]. Ultimately, multicenter, prospective studies that include larger numbers of patients could provide more insight into the outcome specified per treatment modality (e.g., resection, ablation, and chemotherapeutic regimen). The small number of patients impeded adequate stratification for clinicopathological and prognostic factors, such as tumor markers, tumor grade, and distribution of metastases[26,31,39]. Thus, the presented results are based on selected patients who may have a favorable prognostic biology, and these results hold an overall bias.
Despite the small study size and limited evidence, we advocate further studies to investigate the true merits of more aggressive and intensified treatment modalities in selected patients with oligometastases from DA and it-PVA[40]. In the future, the selection of patients could also be based on novel insights into tumor biology, the biological behavior of the tumor, and response to induction chemotherapy. However, based on evidence from this study, the local treatment of oligometastases deserves consideration by a dedicated multidisciplinary team in an attempt to optimally utilize available treatment possibilities, and may help to further enhance survival in patients with DA.
Duodenal adenocarcinoma (DA) and intestinal-type papilla of Vater adenocarcinoma (it-PVA) are rare malignancies of the gastrointestinal tract. No practical guidelines exist for patients with metastatic disease stages. Current treatment protocols are increasingly based on treatment strategies for patients with colorectal cancer.
The clinical outcomes of patients with DA and it-PVA are unclear. In addition, the benefit of local treatment of oligometastases, alone or combined with chemotherapy, has not been investigated for these patients.
This study aims to investigate the clinical outcomes of patients with DA and it-PVA, specified per disease stage. The outcome after treatment of oligometastases in selected patients with DA and it-PVA is evaluated.
All patients with DA and it-PVA diagnosed between 2000 and 2017 were included. All patients with histopathologically-confirmed DA or it-PVA were eligible for inclusion. Clinical outcome was compared between DA and it-PVA per disease stage. In the subgroup of stage IV disease, survival after the local treatment of oligometastases was compared with systemic therapy or supportive care.
No difference in survival was found for patients with DA and it-PVA stratified for disease stage. Seven (23%) of 31 patients with synchronous stage IV disease underwent resection of the primary tumor, combined with local treatment of oligometastases. Local treatment of metastases was associated with an overall survival of 37 mo, compared to 14 and 6 mo for systemic therapy and supportive care, respectively.
Survival of patients with DA and it-PVA is comparable per disease stage. A potential benefit of local treatment strategies in selected patients with oligometastases was found.
Multicenter, prospective studies, including larger numbers of patients, are needed to provide more insight into the outcome specified per treatment modality, and the true merits of more aggressive and intensified treatment modalities in selected patients with oligometastases.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xie HP, Yang ZH, Liu XF, Dambrauskas Z S-Editor: Wang YQ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Coupland VH, Kocher HM, Berry DP, Allum W, Linklater KM, Konfortion J, Møller H, Davies EA. Incidence and survival for hepatic, pancreatic and biliary cancers in England between 1998 and 2007. Cancer Epidemiol. 2012;36:e207-e214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Overman MJ, Hu CY, Kopetz S, Abbruzzese JL, Wolff RA, Chang GJ. A population-based comparison of adenocarcinoma of the large and small intestine: insights into a rare disease. Ann Surg Oncol. 2012;19:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Lu Y, Fröbom R, Lagergren J. Incidence patterns of small bowel cancer in a population-based study in Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol. 2012;36:e158-e163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Legué LM, Bernards N, Gerritse SL, van Oudheusden TR, de Hingh IH, Creemers GM, Ten Tije AJ, Lemmens VE. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in The Netherlands. Acta Oncol. 2016;55:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Brian A. Tumors of the Gallbladder, Extrahepatic Bile Ducts, and Ampulla of Vater. Atlas of Tumor Pathology, Third Series, Fascicle 27. In: Journal of Clinical Gastroenterology. Netherlands: Lippincott Williams & Wilkins, 2000; 33: 347. |
| 7. | Westgaard A, Pomianowska E, Clausen OP, Gladhaug IP. Intestinal-type and pancreatobiliary-type adenocarcinomas: how does ampullary carcinoma differ from other periampullary malignancies? Ann Surg Oncol. 2013;20:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, Clausen OP, Gladhaug IP. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Yachida S, Wood LD, Suzuki M, Takai E, Totoki Y, Kato M, Luchini C, Arai Y, Nakamura H, Hama N, Elzawahry A, Hosoda F, Shirota T, Morimoto N, Hori K, Funazaki J, Tanaka H, Morizane C, Okusaka T, Nara S, Shimada K, Hiraoka N, Taniguchi H, Higuchi R, Oshima M, Okano K, Hirono S, Mizuma M, Arihiro K, Yamamoto M, Unno M, Yamaue H, Weiss MJ, Wolfgang CL, Furukawa T, Nakagama H, Vogelstein B, Kiyono T, Hruban RH, Shibata T. Genomic Sequencing Identifies ELF3 as a Driver of Ampullary Carcinoma. Cancer Cell. 2016;29:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Gingras MC, Covington KR, Chang DK, Donehower LA, Gill AJ, Ittmann MM, Creighton CJ, Johns AL, Shinbrot E, Dewal N, Fisher WE; Australian Pancreatic Cancer Genome Initiative, Pilarsky C, Grützmann R, Overman MJ, Jamieson NB, Van Buren G 2nd, Drummond J, Walker K, Hampton OA, Xi L, Muzny DM, Doddapaneni H, Lee SL, Bellair M, Hu J, Han Y, Dinh HH, Dahdouli M, Samra JS, Bailey P, Waddell N, Pearson JV, Harliwong I, Wang H, Aust D, Oien KA, Hruban RH, Hodges SE, McElhany A, Saengboonmee C, Duthie FR, Grimmond SM, Biankin AV, Wheeler DA, Gibbs RA. Ampullary Cancers Harbor ELF3 Tumor Suppressor Gene Mutations and Exhibit Frequent WNT Dysregulation. Cell Rep. 2016;14:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Meijer LL, Alberga AJ, de Bakker JK, van der Vliet HJ, Le Large TYS, van Grieken NCT, de Vries R, Daams F, Zonderhuis BM, Kazemier G. Outcomes and Treatment Options for Duodenal Adenocarcinoma: A Systematic Review and Meta-Analysis. Ann Surg Oncol. 2018;25:2681-2692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Solaini L, Jamieson NB, Metcalfe M, Abu Hilal M, Soonawalla Z, Davidson BR, McKay C, Kocher HM; UK Duodenal Cancer Study Group. Outcome after surgical resection for duodenal adenocarcinoma in the UK. Br J Surg. 2015;102:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Haan JC, Buffart TE, Eijk PP, van de Wiel MA, van Wieringen WN, Howdle PD, Mulder CJ, van de Velde CJ, Quirke P, Nagtegaal ID, van Grieken NC, Grabsch H, Meijer GA, Ylstra B. Small bowel adenocarcinoma copy number profiles are more closely related to colorectal than to gastric cancers. Ann Oncol. 2012;23:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL, Wolff RA. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, Schöffski P, Sobrero A, Van Cutsem E, Díaz-Rubio E. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:2084-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 372] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 16. | Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 809] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 17. | Imai K, Allard MA, Castro Benitez C, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Baba H, Adam R. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg. 2017;104:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay D, Bismuth H, Castaing D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26:3672-3680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Ang DC, Shia J, Tang LH, Katabi N, Klimstra DS. The utility of immunohistochemistry in subtyping adenocarcinoma of the ampulla of vater. Am J Surg Pathol. 2014;38:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Kumari N, Prabha K, Singh RK, Baitha DK, Krishnani N. Intestinal and pancreatobiliary differentiation in periampullary carcinoma: the role of immunohistochemistry. Hum Pathol. 2013;44:2213-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, Primrose JN, Parks RW. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55 Suppl 3:iii1-iii8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Meijerink MR, Puijk RS, van Tilborg AAJM, Henningsen KH, Fernandez LG, Neyt M, Heymans J, Frankema JS, de Jong KP, Richel DJ, Prevoo W, Vlayen J. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2018;41:1189-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 23. | Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. New Jersey: John Wiley Sons, 2016. |
| 24. | Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, Pinese M, Humphris JL, Jones MD, Toon C, Nagrial AM, Chantrill LA, Chin VT, Pinho AV, Rooman I, Cowley MJ, Wu J, Mead RS, Colvin EK, Samra JS, Corbo V, Bassi C, Falconi M, Lawlor RT, Crippa S, Sperandio N, Bersani S, Dickson EJ, Mohamed MA, Oien KA, Foulis AK, Musgrove EA, Sutherland RL, Kench JG, Carter CR, Gill AJ, Scarpa A, McKay CJ, Biankin AV. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Onkendi EO, Boostrom SY, Sarr MG, Farnell MB, Nagorney DM, Donohue JH, Kendrick ML, Reid-Lombardo KM, Harmsen WS, Que FG. 15-year experience with surgical treatment of duodenal carcinoma: a comparison of periampullary and extra-ampullary duodenal carcinomas. J Gastrointest Surg. 2012;16:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Sakae H, Kanzaki H, Nasu J, Akimoto Y, Matsueda K, Yoshioka M, Nakagawa M, Hori S, Inoue M, Inaba T, Imagawa A, Takatani M, Takenaka R, Suzuki S, Fujiwara T, Okada H. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer. 2017;117:1607-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Ushiku T, Arnason T, Fukayama M, Lauwers GY. Extra-ampullary duodenal adenocarcinoma. Am J Surg Pathol. 2014;38:1484-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Pomianowska E, Grzyb K, Westgaard A, Clausen OP, Gladhaug IP. Reclassification of tumour origin in resected periampullary adenocarcinomas reveals underestimation of distal bile duct cancer. Eur J Surg Oncol. 2012;38:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Schirmacher P, Büchler MW. Ampullary adenocarcinoma - differentiation matters. BMC Cancer. 2008;8:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Chandrasegaram MD, Gill AJ, Samra J, Price T, Chen J, Fawcett J, Merrett ND. Ampullary cancer of intestinal origin and duodenal cancer - A logical clinical and therapeutic subgroup in periampullary cancer. World J Gastrointest Oncol. 2017;9:407-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (3)] |
| 31. | Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:5374-5382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol. 2009;19:58-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Agrawal S, McCarron EC, Gibbs JF, Nava HR, Wilding GE, Rajput A. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol. 2007;14:2263-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 466] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 35. | Nordlinger B, Rougier P. Liver metastases from colorectal cancer: the turning point. J Clin Oncol. 2002;20:1442-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 825] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 37. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 38. | Ruers T, Punt C, Van Coevorden F, Pierie JP, Borel-Rinkes I, Ledermann JA, Poston G, Bechstein W, Lentz MA, Mauer M, Van Cutsem E, Lutz MP, Nordlinger B; EORTC Gastro-Intestinal Tract Cancer Group, Arbeitsgruppe Lebermetastasen und—tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO) and the National Cancer Research Institute Colorectal Clinical Study Group (NCRI CCSG). Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23:2619-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 39. | Raghav K, Overman MJ. Small bowel adenocarcinomas--existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Meijer LL, Kazemier G. ASO Author Reflections: Current Treatment Options for Duodenal Adenocarcinoma-A Call for Collaborative Studies. Ann Surg Oncol. 2018;25:737-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |