Published online Mar 15, 2020. doi: 10.4251/wjgo.v12.i3.332
Peer-review started: August 24, 2019
First decision: October 18, 2019
Revised: December 26, 2019
Accepted: January 14, 2020
Article in press: January 14, 2020
Published online: March 15, 2020
Processing time: 200 Days and 19 Hours
FOLFIRINOX regimen is the first-line reference chemotherapy (L1) in advanced pancreatic ductal adenocarcinoma (aPDAC). FOLFOXIRI, a schedule with a lower dose of irinotecan and no bolus 5-fluorouracil, has demonstrated efficacy and feasibility in colorectal cancer.
To investigate the potential clinical value of FOLFOXIRI in patients with aPDAC in routine clinical practice.
Analyses were derived from all consecutive aPDAC patients treated in L1 between January 2011 and December 2017 in two French institutions, with either FOLFOXIRI (n = 165) or FOLFIRINOX (n = 124) regimens. FOLFOXIRI consisted of irinotecan (165 mg/m2), oxaliplatin (85 mg/m2), leucovorin (200 mg/m2) and 5-fluorouracil (3200 mg/m2 as a 48-h continuous infusion) every 2 wk. Ninety-six pairs of patients were selected through propensity score matching, and clinical outcomes of the two treatment regimens were compared.
Median overall survival was 11.1 mo in the FOLFOXIRI and 11.6 mo in the FOLFIRINOX cohorts, respectively. After propensity score matching, survival rates remained similar between the two regimens in terms of overall survival (hazard ratio = 1.22; P = 0.219) and progression-free survival (hazard ratio = 1.27; P = 0.120). The objective response rate was 37.1% in the FOLFOXIRI group vs 47.8% in the FOLFIRINOX group (P = 0.187). Grade 3/4 toxicities occurred in 28.7% of patients in the FOLFOXIRI cohort vs 19.5% in the FOLFIRINOX cohort (P = 0.079). FOLFOXIRI was associated with a higher incidence of grade 3/4 digestive adverse events. Hematopoietic growth factors were used after each chemotherapy cycle and the low hematological toxicity rates were below 5% with both regimens.
FOLFOXIRI is feasible in L1 in patients with aPDAC but does not confer any therapeutic benefit as compared with FOLFIRINOX. The low hematological toxicity rates strengthened the relevance of primary prophylaxis with hematopoietic growth factors.
Core tip: This is the first study to compare FOLFOXIRI and FOLFIRINOX regimens head-to-head, to assess whether FOLFOXIRI contributes to a better balance in the toxicity/efficacy ratio in advanced pancreatic ductal adenocarcinoma. These findings do not suggest any therapeutic benefit of FOLFOXIRI compared to FOLFIRINOX in first-line chemotherapy. These results show that additional evaluation is not warranted in future clinical trials. FOLFIRINOX chemotherapy remains the standard of care first-line therapy in metastatic pancreatic ductal adenocarcinoma. Interestingly, the low hematological toxicity rates in both regimens underscore the relevance of prophylactic administration of hematopoietic growth factors in routine use after each polychemotherapy cycle.
- Citation: Vienot A, Chevalier H, Bolognini C, Gherga E, Klajer E, Meurisse A, Jary M, Kim S, d’Engremont C, Nguyen T, Calcagno F, Almotlak H, Fein F, Nasri M, Abdeljaoued S, Turpin A, Borg C, Vernerey D. FOLFOXIRI vs FOLFIRINOX as first-line chemotherapy in patients with advanced pancreatic cancer: A population-based cohort study. World J Gastrointest Oncol 2020; 12(3): 332-346
- URL: https://www.wjgnet.com/1948-5204/full/v12/i3/332.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i3.332
Pancreatic ductal adenocarcinoma (PDAC) carries a poor prognosis, with a 5-year overall survival (OS) rate of only 8%-9% for all stages taken together[1]. Pancreatic cancer is expected to become the second leading cause of cancer death in the United States and Europe by 2030[2,3]. This poor prognosis is mainly due to late diagnosis, with only 20% of patients with PDAC eligible for surgery. Complete surgical resection of localized PDAC followed by 6 mo of adjuvant chemotherapy is the only recognized standard of care that has been shown to improve patient survival, with a median OS up to 54.4 mo with modified FOLFIRINOX [5-fluorouracil (5-FU), irinotecan, and oxaliplatin][4]. Furthermore, more than 80% of cases are diagnosed at an advanced, unresectable stage, with almost 50% of patients presenting with metastatic disease and almost 30% with locoregional extension[1]. In addition, it has been shown that most patients who undergo surgery develop further tumor recurrence[4].
Advanced or recurrent PDAC remains a challenging, non-curable disease, for which therapeutic options are still limited and mainly rely on supportive care and systemic chemotherapy to improve patient OS and health-related quality of life (HRQoL)[5]. Up to 2010, gemcitabine was the only standard of care as first-line chemotherapy (L1) in patients with metastatic PDAC[6]. Over the last decade, incremental progress has been achieved in the landscape of advanced PDAC (aPDAC) management with the approval of two active cytotoxic combinations, namely FOLFIRINOX, and gemcitabine plus nab-paclitaxel regimens[7,8]. FOLFIRINOX polychemotherapy became a reference regimen in this setting, based on the results of the PRODIGE 4/ACCORD 11 phase III trial[7]. This study demonstrated the superiority of FOLFIRINOX over gemcitabine monotherapy in terms of OS (median: 11.1 vs 6.8 mo; P < 0.001) and progression-free survival (PFS; median: 6.4 mo vs 3.3 mo; P < 0.001) in 342 selected patients with metastatic PDAC, age < 76 years, Eastern Cooperative Oncology Group performance status 0-1, and normal bilirubin level (< 1.5 times the upper limit of normal). The objective response rate for FOLFIRINOX was 31.6% vs 9.4% for gemcitabine (P < 0.001). FOLFIRINOX consisted of oxaliplatin (85 mg/m2), irinotecan (180 mg/m2), leucovorin (400 mg/m2), and 5-FU (400 mg/m2 administered by intravenous bolus, followed by 2400 mg/m2 given as a 46-h continuous infusion), every 2 wk[7].
The HRQoL of patients was significantly better with FOLFIRINOX as compared with gemcitabine, except for diarrhea[9]. A higher incidence of adverse events was observed with the FOLFIRINOX group, including grade 3 or 4 neutropenia (45.7%), febrile neutropenia (5.4%) and diarrhea (12.7%)[7]. A retrospective study evaluated a “modified FOLFIRINOX” regimen without the bolus of 5-FU, and administration of hematopoietic growth factors to all patients with aPDAC[10]. This study showed a better safety profile (grade 3 or 4 neutropenia 3%), and maintained efficacy, with a response rate of 30% and a median OS of 16.4 mo (9 mo for metastatic disease). However, the incidence of severe diarrhea remained high, at 13%[10].
FOLFOXIRI, another modified schedule was developed based on the experience of the Gruppo Oncologico Nord Ovest (GONO) in metastatic colorectal cancers[11] to limit digestive and hematological toxicities. This regimen consisted of a lower dose of irinotecan (165 mg/m2), no bolus of 5-FU, and an increase in continuous intravenous 5-FU infusion at 3200 mg/m2, while oxaliplatin and leucovorin remained unchanged. To the best of our knowledge, to date, the FOLFOXIRI and FOLFIRINOX regimens have never been compared head-to-head. In this exploratory population-based cohort study, we aimed to compare clinical outcomes, in terms of safety and efficacy, between the FOLFOXIRI and FOLFIRINOX regimens, in patients with aPDAC in routine clinical practice.
All consecutive patients with histologically proven aPDAC (i.e., metastatic, locally advanced, or recurrent after surgery) who were treated in L1 in two French institutions were included. Patients treated with FOLFOXIRI were enrolled at Besancon University Hospital, between January 2011 and December 2015, whereas, the FOLFIRINOX group comprised patients who received this standard regimen at Lille University Hospital, between January 2011 and December 2017. Patients were prospectively identified through the chemotherapy prescribing software used at Besancon (Bonnes Pratiques de la Chimiothérapie - BPC®, SQLI) and Lille University Hospitals (CHIMIO®, Computer Engineering). Patients with early postoperative tumor relapse (i.e., within 6 mo after the last administration of the adjuvant chemotherapy) were excluded. All therapeutic decisions were discussed and validated during digestive oncology-dedicated multidisciplinary meetings. Computed tomography-scan assessment was performed every 3 mo.
The database was registered and declared to the National French Commission for bioinformatics data and patient liberty (CNIL; No. of CNIL declaration: 1906173 v 0). The study followed standard procedures in France, with approval by the relevant institutional review boards. All patients with cancer signed a general informed consent at the time of their first visit to both Medical Oncology Departments. This consent allows the use of their clinical and biological data in the cohort study. No additional specific informed consent for this study was deemed necessary. Demographics, cancer history, pathological, clinical, biological, and radiological parameters at chemotherapy initiation, as well as treatment outcomes, were retrospectively collected from medical records. The database was locked on April 23, 2019.
FOLFIRINOX was administered according to the standard schedule validated by the PRODIGE 4/ACCORD 11 study. This regimen consisted of a combination of oxaliplatin (85 mg/m2, over 2 h), followed by leucovorin (400 mg/m2, over 2 h), with the addition through a Y-connector, after 30 min, of irinotecan (180 mg/m2, over 90 min), followed by 5-FU (400 mg/m2) by intravenous bolus, on Day 1. Then, a continuous intravenous infusion of 5-FU (2400 mg/m2) was administered over 46 h starting on Day 1[7]. FOLFOXIRI consisted of the same molecules with a reduced dose of irinotecan and no bolus 5-FU, according to the GONO regimen used in metastatic colorectal cancer: Irinotecan (165 mg/m2, over 1 h), followed by oxaliplatin (85 mg/m2) and leucovorin (200 mg/m2) concomitantly over 2 h through a Y‐connector, on Day 1; and followed by a continuous intravenous infusion of 5-FU (3200 mg/m2) over 48 h starting on Day 1[11]. These two treatments were administered every 2 wk until disease progression or unacceptable toxicity. Hematopoietic growth factors were systematically used after each chemotherapy cycle in at least 80% of cases in the FOLFIRINOX group and for all patients enrolled in the FOLFOXIRI group.
Median value (interquartile range) and frequency (percentage) were provided for the description of continuous and categorical variables, respectively. Medians and proportions were compared between the FOLFIRINOX and FOLFOXIRI groups using Wilcoxon–Mann–Whitney and chi-square tests (or Fisher’s exact test, if appropriate), respectively. OS was calculated from the date of the first administration of L1 to the date of death from any cause. Survival data were censored at the last follow-up. PFS was calculated from the date of the first administration of L1 to the date of progression or death from any cause, or the date of the last follow-up, at which point data were censored. OS and PFS were estimated using the Kaplan-Meier method and described using median or rate at specific time points with 95% confidence intervals (CIs), and compared using the log-rank test. Follow-up time was calculated using a reverse Kaplan-Meier estimation when feasible[12]. Objective tumor response was determined according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 criteria[13]. Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria[14].
The primary analysis was conducted using data from the total population, and compared characteristics and outcomes between the FOLFIRINOX and FOLFOXIRI groups. A propensity score approach was then applied to deal with potential heterogeneity in baseline characteristics between the two administered regimens in L1. Two methods were used to address the potential confounding effect of the unbalanced factors: First, the inverse probability of treatment weighting (IPTW) and second, propensity score matching[15]. Propensity score construction was based on probability estimation with a non‐parsimonious multivariable logistic regression model including the main parameters distributed unequally between the FOLFIRINOX and FOLFOXIRI groups. Hazard ratios (HRs) and 95%CIs were estimated using the IPTW Cox proportional hazards model. Accuracy of the model was verified by testing discrimination and calibration. Discrimination of the IPTW Cox model was assessed by the area under the curve, and calibration by the Hosmer–Lemeshow goodness-of-fit test. Propensity score matching, based on the caliper method with a ratio of 1:1, was performed to generate two samples with well-balanced characteristics. Sensitivity analysis to determine the reliability and the robustness of the primary analysis was performed in the subgroup of patients with metastatic disease. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary NC, USA). P < 0.05 were considered statistically significant, and all tests were two-sided.
A total of 289 patients with aPDAC treated in L1 were included in this study. Of these patients, 124 received the FOLFIRINOX regimen and 165 received FOLFOXIRI chemotherapy. Patient characteristics of the two treatment groups are described and compared in Table 1. The two cohorts displayed similar characteristics, except for primary tumor site (located in the pancreatic head in 43.1% in the FOLFIRINOX group vs 56.7% in the FOLFOXIRI group; P = 0.022), histological grade, stage at chemotherapy initiation (88.7% had metastatic stage in the FOLFIRINOX group vs 63.6% in the FOLFOXIRI group; P < 0.001), and pain (corresponding to the prescription of morphine). Of note, patients in the FOLFIRINOX group had a significantly higher albumin level (39.1 g/L vs 35.0 g/L; P < 0.001), and an increased number of metastatic sites (P < 0.001) than those in the FOLFOXIRI group (Table 1).
| Characteristics | FOLFIRINOX (n = 124) | FOLFOXIRI (n = 165) | P value |
| Demographic parameters | |||
| Age, median [IQR], yr | 60.2 [53.0-65.9] | 62.5 [54.6-67.5] | 0.174 |
| Missing | 1 | 0 | |
| Gender | 0.989 | ||
| Male | 73 (58.9) | 97 (58.8) | |
| Female | 51 (41.1) | 68 (41.2) | |
| Familial history of cancer | 0.195 | ||
| No | 32 (45.1) | 89 (54.3) | |
| Yes | 39 (54.9) | 75 (45.7) | |
| Missing | 53 | 1 | |
| Personal history of cancer | 0.219 | ||
| No | 110 (90.2) | 139 (85.3) | |
| Yes | 12 (9.8) | 24 (14.7) | |
| Missing | 2 | 2 | |
| Pathological parameters | |||
| Stage at diagnosis | < 0.001 | ||
| Localized | 20 (16.1) | 13 (7.9) | |
| Locally advanced | 18 (14.5) | 60 (36.3) | |
| Metastatic | 86 (69.4) | 92 (55.8) | |
| Primary tumor site | 0.022 | ||
| Head | 53 (43.1) | 93 (56.7) | |
| Body and/or tail | 70 (56.9) | 71 (43.3) | |
| Missing | 1 | 1 | |
| Histological grade | 0.014 | ||
| Well or moderately differentiated | 45 (83.3) | 39 (62.9) | |
| Poorly differentiated or undifferentiated | 9 (16.7) | 23 (37.1) | |
| Missing | 70 | 103 | |
| Tumor extension | |||
| Stage at chemotherapy initiation | < 0.001 | ||
| Locally advanced | 14 (11.3) | 60 (36.4) | |
| Metastatic | 110 (88.7) | 105 (63.6) | |
| Number of metastatic sites | < 0.001 | ||
| 0 | 14 (11.3) | 60 (36.6) | |
| 1 | 69 (55.7) | 74 (44.9) | |
| ≥ 2 | 41 (33.0) | 31 (18.8) | |
| Lymph node metastases | < 0.001 | ||
| No | 95 (76.6) | 153 (92.7) | |
| Yes | 29 (23.4) | 12 (7.3) | |
| Liver metastases | < 0.001 | ||
| No | 37 (29.8) | 84 (50.9) | |
| Yes | 87 (70.2) | 81 (49.1) | |
| Peritoneal metastases | 0.124 | ||
| No | 109 (87.9) | 134 (81.2) | |
| Yes | 15 (12.1) | 31 (18.8) | |
| Lung metastases | 0.133 | ||
| No | 102 (82.3) | 146 (88.5) | |
| Yes | 22 (17.7) | 19 (11.5) | |
| Other metastases | 0.060 | ||
| No | 116 (93.6) | 162 (98.2) | |
| Yes | 8 (6.4) | 3 (1.8) | |
| Clinical parameters | |||
| Performance status (WHO) | 0.185 | ||
| 0 | 42 (35.0) | 54 (32.7) | |
| 1 | 66 (55.0) | 103 (62.4) | |
| ≥ 2 | 12 (10.0) | 8 (4.9) | |
| Missing | 4 | 0 | |
| Body mass index, kg/m2 | 23.9 [20.8-27.4] | 23.0 [20.8-25.6] | 0.114 |
| Missing | 9 | 0 | |
| Pain | < 0.001 | ||
| No | 90 (73.8) | 83 (50.9) | |
| Yes | 32 (26.2) | 80 (49.1) | |
| Missing | 2 | 2 | |
| Jaundice | 0.266 | ||
| No | 114 (93.4) | 148 (89.7) | |
| Yes | 8 (6.6) | 17 (10.3) | |
| Missing | 2 | 0 | |
| Ascites | 1.000 | ||
| No | 117 (95.9) | 157 (96.3) | |
| Yes | 5 (4.1) | 6 (3.7) | |
| Missing | 2 | 2 | |
| Biological parameters | |||
| Albumin, median [IQR], g/L | 39.1 [37.0-43.0] | 35.0 [29.0-39.0] | < 0.001 |
| Missing | 48 | 80 | |
| Lymphocytes, median [IQR], mm3 | 0.408 | ||
| < 1000 | 10 (15.9) | 12 (11.4) | |
| ≥ 1000 | 53 (84.1) | 93 (88.6) | |
| Missing | 19 | 102 | |
| Neutrophil-to-lymphocyte ratio, median [IQR] | 0.103 | ||
| < 5 | 82 (78.1) | 42 (66.7) | |
| ≥ 5 | 23 (21.9) | 21 (33.3) | |
| Missing | 19 | 102 | |
| CA19-9, median [IQR], UI/mL | 885.0 [79.0-4756.0] | 650.0 [138.0-5300.0] | 0.669 |
| Missing | 3 | 34 | |
| Previous treatment | |||
| Primary tumor resection | 0.277 | ||
| Yes | 18 (14.5) | 17 (10.3) | |
| No | 106 (85.5) | 148 (89.7) | |
| Adjuvant chemotherapy | 0.865 | ||
| Yes | 12 (9.7) | 15 (9.1) | |
| No | 112 (90.3) | 150 (90.9) | |
| Median follow-up time (95%CI), mo | 30.8 [23.0-NA] | 61.4 [43.2-87.9] | |
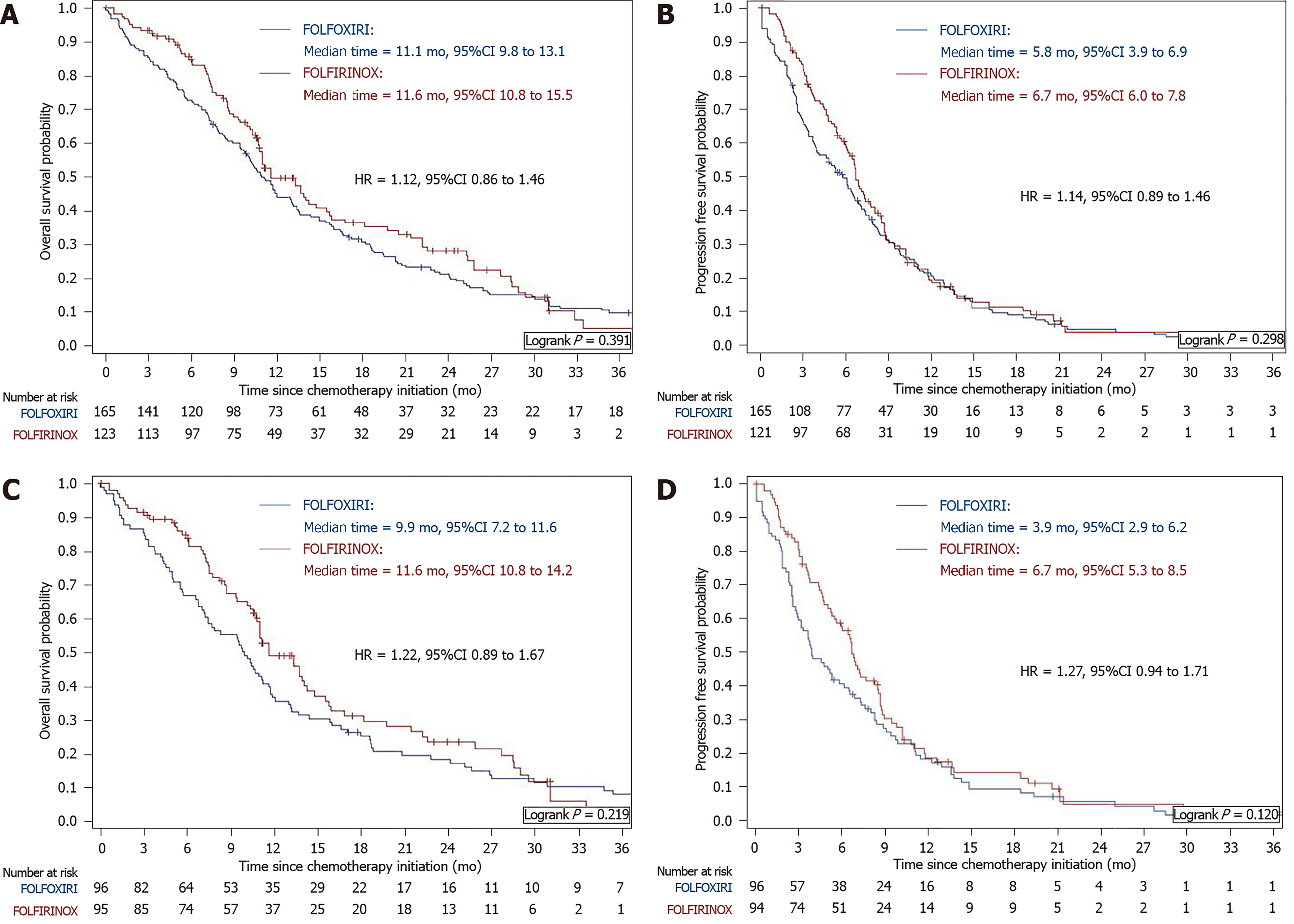
After a median follow-up of 30.8 mo (95%CI: 23.0-NA) and 61.4 mo (95%CI: 43.2-87.9), median OS was 11.6 mo (95%CI: 10.8-15.5) and 11.1 mo (95%CI: 9.8-13.1) in the FOLFIRINOX and FOLFOXIRI groups, respectively (HR = 1.12; 95%CI: 0.86-1.46; P = 0.391; Figure 1A). Median PFS was 5.8 mo (95%CI: 3.9-6.9) in the FOLFOXIRI group and 6.7 mo (95%CI: 6.0-7.8) in the FOLFIRINOX group (HR = 1.14; 95%CI: 0.89 to 1.46; P = 0.298; Figure 1B). OS rates at 12, 18, and 24 mo were 49.6%, 36.4%, and 28.3%, respectively, in the FOLFIRINOX group as compared with 45.1%, 30.9%, and 21.2%, respectively, in the FOLFOXIRI group.
Detailed outcomes data are summarized in Table 2. The objective response rate was 47.8% in the FOLFIRINOX group, compared to 37.1% in the FOLFOXIRI group (P = 0.187), while disease-control rates were 75.7% and 66.7%, respectively (P = 0.124). The number of cycles was significantly higher in the FOLFIRINOX group (11.0 vs 7.0 cycles; P = 0.027). Maintenance chemotherapy was administered in 45.2% of patients in the FOLFIRINOX group and in 37.6% of those in the FOLFOXIRI group (P = 0.194). The median maintenance time was 2.8 mo (95%CI: 1.0-4.4) in the FOLFIRINOX group vs 2.7 mo (95%CI: 1.9-5.8) in the FOLFOXIRI group (P = 0.421). Second-line chemotherapy was administered to 91 (73.4%) patients in the FOLFIRINOX group and 117 (70.9%) patients in the FOLFOXIRI group (P = 0.643). No treatment-related deaths were observed. Grade 3 or 4 toxicities occurred in 19.5% of patients in the FOLFIRINOX group as compared with 28.7% in the FOLFOXIRI group, but this difference did not reach statistical significance (P = 0.079). In the FOLFOXIRI group, grade 3 or 4 hematological adverse events of any type were observed in 3.1% of patients. FOLFOXIRI was associated with a higher incidence of grade 3 or 4 digestive adverse events as compared to the FOLFIRINOX group (12.8% vs 4.2%, respectively).
| Outcomes | Overall population | Propensity score-matched population | ||||
| FOLFIRINOX (n = 124) | FOLFOXIRI (n = 165) | P value | FOLFIRINOX (n = 96) | FOLFOXIRI (n = 96) | P value | |
| Number of cycles, median [IQR] | 11.0 [6.0-13.0] | 7.0 [4.0-13.0] | 0.027 | 11.0 [6.0-14.0] | 7.0 [4.0-14.5] | 0.164 |
| Missing | 1 | 0 | 1 | 0 | ||
| RECIST best response | 0.187 | 0.079 | ||||
| Complete or partial response | 53 (47.8) | 49 (37.1) | 41 (47.7) | 25 (32.5) | ||
| Stability | 31 (27.9) | 39 (29.6) | 24 (27.9) | 22 (28.5) | ||
| Progression | 27 (24.3) | 44 (33.3) | 21 (24.4) | 30 (39.0) | ||
| Missing | 13 | 33 | 108 | 19 | ||
| Toxicity of grade 3 or 4 | 0.079 | 0.148 | ||||
| No | 95 (80.5) | 117 (71.3) | 72 (80.0) | 68 (70.8) | ||
| Yes | 23 (19.5) | 47 (28.7) | 18 (20.0) | 28 (29.2) | ||
| Digestive | 5 (4.2) | 21 (12.8) | 5 (5.6) | 9 (9.4) | ||
| Hematology | 1 (0.9) | 5 (3.1) | 1 (1.0) | 4 (4.2) | ||
| Neurology | 9 (7.6) | 14 (8.5) | 6 (6.7) | 9 (9.4) | ||
| Other | 8 (6.8) | 7 (4.3) | 6 (6.7) | 6 (6.2) | ||
| Missing | 6 | 1 | 6 | 0 | ||
| Reason for discontinuation | 0.291 | 0.441 | ||||
| Progression | 83 (68.0) | 108 (65.5) | 63 (67.0) | 67 (69.8) | ||
| Toxicity | 12 (9.9) | 10 (6.0) | 12 (12.8) | 7 (7.3) | ||
| Other | 27 (22.1) | 47 (28.5) | 19 (20.2) | 22 (22.9) | ||
| Missing | 2 | 0 | 2 | 0 | ||
| Maintenance | 0.194 | 0.553 | ||||
| Yes | 56 (45.2) | 62 (37.6) | 39 (40.6) | 35 (36.5) | ||
| No | 68 (54.8) | 103 (64.4) | 57 (59.4) | 61 (63.5) | ||
| Second-line chemotherapy administration | 0.643 | 0.636 | ||||
| Yes | 91 (73.4) | 117 (70.9) | 69 (71.9) | 66 (68.8) | ||
| No | 33 (26.6) | 48 (29.1) | 27 (28.1) | 30 (31.2) | ||
Potential biases identified during the description of the total cohort were minimized by the use of a propensity score estimated by an unconditional multivariable logistic regression model. Histological grade and albumin level were not selected in the propensity score process due to the high rate of missing data (Supplementary Table 1). Thus, primary tumor site, stage at diagnosis, stage at chemotherapy initiation, number of metastatic sites, lymph node and liver metastases, and pain were included in the propensity score (Supplementary Table 2). The model exhibited excellent discrimination with an area under the curve of 0.73 (Supplementary Figure 1) and a good calibration (P = 0.840, Hosmer–Lemeshow goodness‐of‐fit test). For each patient, a propensity score value was then calculated based on the multivariable model (Supplementary Figure 2). In the IPTW analysis, the L1 regimen was not significantly associated with either OS (282 patients, 236 events; HR = 1.19; 95%CI: 0.91-1.54; P = 0.202) or PFS (281 patients, 256 events; HR = 1.25; 95%CI: 0.98-1.60; P = 0.077). Patients treated with the FOLFOXIRI regimen were then matched considering their nearest neighbor, with a caliper of 0.10 and a ratio of 1:1, with patients in the FOLFIRINOX group.
After propensity score matching, 96 patients in each group (79.0% and 59.3% in the FOLFIRINOX and FOLFOXIRI groups, respectively) were successfully matched. There were no statistically significant differences between the two matched groups in baseline characteristics: Primary tumor site (P = 0.385), stage at chemotherapy initiation (P = 0.439), number of metastatic sites (P = 0.724), lymph node metastases (P = 0.817), liver metastases (P = 0.385), and pain (P = 0.877). Patients in the FOLFIRINOX group were characterized by tumors with a more differentiated histological grade (P = 0.011) and a higher albumin level (P = 0.001) (Table 3). After a median follow-up of 43.2 mo (95%CI: 31.0-61.4) in the matched groups, survival rates for patients remained similar between the two regimens in terms of OS (HR= 1.22; 95%CI: 0.89-1.67; P = 0.219; Figure 1C) and PFS (HR = 1.27; 95%CI: 0.94-1.71; P = 0.120; Figure 1D). There was no statistically significant difference in objective response (P = 0.079), maintenance chemotherapy (P = 0.553), or second-line administration rates (P = 0.636). Grade 3 or 4 toxicities remained unchanged between the two treatments (20% in the FOLFIRINOX group vs 29.2% in the FOLFOXIRI group, P = 0.148). The incidence of grade 3 or 4 digestive adverse events remained higher in the FOLFOXIRI group (9.4% vs 5.6%) (Table 2).
| Characteristics | FOLFIRINOX (n = 96) | FOLFOXIRI (n = 96) | P value |
| Demographic parameters | |||
| Age, median [IQR], years | 59.9 [53.0-66.9] | 63.1 [55.2-67.1] | 0.221 |
| Missing | 1 | 0 | |
| Gender | 0.661 | ||
| Male | 54 (56.3) | 57 (59.4) | |
| Female | 42 (43.7) | 39 (40.6) | |
| Familial history of cancer | 0.806 | ||
| No | 31 (51.7) | 51 (53.7) | |
| Yes | 29 (48.3) | 44 (46.3) | |
| Missing | 36 | 1 | |
| Personal history of cancer | 0.824 | ||
| No | 83 (88.3) | 82 (87.2) | |
| Yes | 11 (11.7) | 12 (12.8) | |
| Missing | 2 | 2 | |
| Pathologic parameters | |||
| Stage at diagnosis | 0.598 | ||
| Localized | 14 (14.6) | 12 (12.5) | |
| Locally advanced | 13 (13.5) | 18 (18.87 | |
| Metastatic | 69 (71.9) | 66 (68.8) | |
| Primary tumor site | 0.385 | ||
| Head | 41 (42.7) | 47 (49.0) | |
| Body and/or tail | 55 (57.3) | 49 (51.0) | |
| Histological grade | 0.011 | ||
| Well or moderately differentiated | 36 (83.7) | 24 (58.5) | |
| Poorly differentiated or undifferentiated | 7 (16.3) | 17 (41.5) | |
| Missing | |||
| Tumor extension | |||
| Stage at chemotherapy initiation | 0.439 | ||
| Locally advanced | 14 (14.6) | 18 (18.2) | |
| Metastatic | 82 (85.4) | 78 (81.3) | |
| Number of metastatic sites | 0.724 | ||
| 0 | 14 (14.6) | 18 (18.8) | |
| 1 | 57 (59.4) | 53 (55.2) | |
| ≥ 2 | 25 (26.0) | 25 (26.0) | |
| Lymph node metastases | 0.817 | ||
| No | 86 (89.6) | 85 (88.5) | |
| Yes | 10 (10.4) | 11 (11.5) | |
| Liver metastases | 0.274 | ||
| No | 26 (27.1) | 33 (34.4) | |
| Yes | 70 (72.9) | 63 (65.6) | |
| Peritoneal metastases | 0.059 | ||
| No | 84 (87.5) | 74 (77.1) | |
| Yes | 12 (12.5) | 22 (22.9) | |
| Lung metastases | 0.845 | ||
| No | 81 (84.4) | 80 (83.3) | |
| Yes | 15 (15.6) | 16 (16.7) | |
| Other metastases | 0.279 | ||
| No | 90 (93.8) | 94 (97.9) | |
| Yes | 6 (6.2) | 2 (2.1) | |
| Clinical parameters | |||
| Performance status (WHO) | 0.165 | ||
| 0 | 36 (39.1) | 35 (36.5) | |
| 1 | 49 (53.3) | 59 (61.5) | |
| ≥ 2 | 7 (7.6) | 2 (2.0) | |
| Missing | 4 | 0 | |
| Body mass index, kg/m2 | 23.9 [20.7-27.1] | 23.0 [21.2-25.0] | 0.285 |
| Missing | 5 | 0 | |
| Pain | 0.877 | ||
| No | 65 (67.7) | 66 (68.8) | |
| Yes | 31 (32.3) | 30 (31.2) | |
| Jaundice | 0.637 | ||
| No | 87 (91.6) | 86 (89.6) | |
| Yes | 8 (8.4) | 10 (10.4) | |
| Missing | 1 | 0 | |
| Ascites | 1.000 | ||
| No | 92 (96.8) | 92 (95.8) | |
| Yes | 3 (3.2) | 4 (4.2) | |
| Missing | 1 | 0 | |
| Biological parameters | |||
| Albumin, median [IQR], g/L | 39.6 [37.0-43.0] | 34.6 [29.0-40.0] | 0.001 |
| Missing | 40 | 51 | |
| Lymphocytes, median [IQR], mm3 | 0.157 | ||
| < 1000 | 9 (11.2) | 8 (21.0) | |
| ≥ 1000 | 71 (88.8) | 30 (79.0) | |
| Missing | 16 | 58 | |
| Neutrophil-to-lymphocyte ratio, median [IQR] | 0.072 | ||
| < 5 | 63 (78.8) | 24 (63.2) | |
| ≥ 5 | 17 (21.2) | 14 (36.8) | |
| Missing | 16 | 58 | |
| CA19-9, median [IQR], UI/mL | 857.5 [69.0-6210.0] | 726.5 [152.0-5507.0] | 0.500 |
| Missing | 2 | 20 | |
| Previous treatment | |||
| Primary tumor resection | 0.284 | ||
| Yes | 10 (10.4) | 15 (15.6) | |
| No | 86 (89.6) | 81 (84.4) | |
| Adjuvant chemotherapy | 0.091 | ||
| Yes | 6 (6.2) | 13 (13.5) | |
| No | 90 (93.8) | 83 (86.5) |
Sensitivity analysis was performed exclusively including the metastatic population from the two treatment groups, corresponding to 110 patients in the FOLFIRINOX group and 105 patients in the FOLFOXIRI group. Patient characteristics are detailed in Supplementary Table 3. As observed for the overall population, characteristics were similar, except for histological grade, lymph node metastases, pain, and albumin level (39 g/L in the FOLFIRINOX group vs 34.6 g/L in the FOLFOXIRI group, P < 0.001). Patients in the FOLFIRINOX group displayed significantly more peritoneal metastases (86.4% vs 70.5%, P = 0.005) and a lower neutrophil-to-lymphocyte ratio (P = 0.010), compared to those in the FOLFOXIRI group (Supplementary Table 3).
The median duration of follow-up was 26.7 mo (95%CI: 23.0-31.1) in the FOLFIRINOX group compared to 44.2 mo (95%CI: 36.7-71.5) in the FOLFOXIRI group. Median OS was significantly longer in the FOLFIRINOX group (13.3 mo; 95%CI: 10.7-15.5) compared to the FOLFOXIRI group (8.5 mo; 95%CI: 6.7-10.2) (HR= 1.44; 95%CI 1.07-1.94; P = 0.017; Supplementary Figure 3A). Similarly, patients treated with the FOLFIRINOX regimen had more favorable PFS (6.7 mo; 95%CI: 5.7-7.8; vs 3.9 mo; 95%CI: 2.9-6.1, respectively), but the difference was not statistically significant (HR= 1.30; 95%CI: 0.97-1.72; P = 0.073; Supplementary Figure 3B). Both cohorts exhibited other similarities in outcomes, which are summarized in Supplementary Table 4.
Furthermore, propensity score analysis was performed in metastatic patients (Supplementary Tables 5 and 6, Supplementary Figures 4 and 5). In the IPTW analysis, the L1 regimen was not significantly associated with either OS (122 patients, 100 events; HR = 1.08; 95%CI: 0.73-1.60; P = 0.703) or PFS (122 patients, 113 events; HR = 1.06; 95%CI: 0.73-1.55; P = 0.746). After propensity score matching (Supplementary Table 7), survival rates for patients were similar between the two regimens in terms of OS (HR = 0.94; 95%CI: 0.54-1.61; P = 0.810; Supplementary Figure 6A) and PFS (HR = 0.94; 95%CI: 0.55-1.61; P = 0.827; Supplementary Figure 6B). Moreover, no difference in objective response (P = 0.317), maintenance chemotherapy (P = 1.000), or second-line administration rates (P = 1.000) was observed. Treatment-related grade 3 or 4 adverse events, including digestive and hematological adverse events, were similar between the two propensity score-matched treatment groups (P = 0.362) (Supplementary Table 8).
This is the first study to show, in a head-to-head comparison, that FOLFOXIRI is feasible as L1 in patients with aPDAC but does not confer any therapeutic benefit as compared with FOLFIRINOX. PDAC is a highly aggressive cancer, and chemotherapy remains the cornerstone of advanced disease therapy. Preclinical studies showed synergistic activity between oxaliplatin, irinotecan and 5-FU[16-18], and phase II/III trials confirmed the antitumor activity of the FOLFIRINOX combination in metastatic pancreatic cancers[7,19]. Although FOLFIRINOX is a first-line option for patients with metastatic PDAC, the significant adverse event rate limits its administration in full doses. However, its substantial benefit in terms of survival rates and HRQoL encourages the assessment of a modified schedule that will likely yield a much-needed improvement in the balance of toxicity vs efficacy in this setting.
FOLFOXIRI, a triplet-chemotherapy regimen with a lower dose of irinotecan and no bolus of 5-FU, has already demonstrated its efficacy and good tolerance and is validated in metastatic colorectal cancer[11,20]. Vivaldi et al[21] evaluated FOLFOXIRI in pancreatic cancer in an observational cohort study of 137 patients, of whom 59.1% had metastatic disease. They reported that FOLFOXIRI improved patient survival rates with a median OS of 12 mo for the overall population and 10.8 mo for the metastatic patients. The objective response rate was 38.6% in the whole population and 35.8% in patients with metastatic PDAC. Moreover, the schedule showed a good tolerance profile with the occurrence of grade 3 diarrhea in only 8%, and febrile neutropenia (grade 3 or 4 neutropenia 35.7%) in < 1%. These results are in line with those observed in our study. Nevertheless, FOLFOXIRI was never compared head-to-head to the standard FOLFIRINOX in this setting.
In our study, the two regimens were compared for the first time in routine clinical practice, taking into account a large number of variables. Due to the design of this exploratory study, patients were included in an observational cohort and the treatment regimens were not randomized. In addition to its retrospective nature, further limitations of the present study warrant discussion. Patients were treated in two centers, although both were high-volume units with similar clinical practices. Of note, patients with a different tumor extension were included, with locally advanced and metastatic stages. Thus, sensitivity analyses were performed exclusively in the metastatic population. Computed tomography-scan assessment of tumor response according to RECIST criteria was not performed centrally. This bias could explain a trend towards better tumor response in the FOLFIRINOX group. Additional variables, particularly febrile neutropenia, biological or HRQoL data, could not be evaluated in our study due to the retrospective design of the data collection, with a high rate of missing patient information.
To overcome these limitations, FOLFOXIRI and FOLFIRINOX were compared from a large population-based cohort of prospectively included patients with aPDAC. Most importantly, we used a rigorous methodological framework and applied a propensity score approach to take into account the potential heterogeneity in baseline characteristics between the two populations. Moreover, two different methods, namely the IPTW Cox model and propensity score matching, demonstrated the satisfactory performance and validity of the analysis. The reproducibility obtained with the sensitivity analysis in the metastatic cohort strengthened the observed results.
The present study showed that the FOLFOXIRI and FOLFIRINOX L1 regimens were similar in terms of efficacy. Median OS and PFS were comparable between the two schedules, and similar to survival rates reported by Conroy et al[7] in FOLFIRINOX-treated patients. The objective response rate with the FOLFOXIRI regimen observed in our cohort (37.1%) was similar to that reported in the GONO study in FOLFOXIRI-treated patients (38.6%)[21]. No differences in response rates between the FOLFOXIRI and FOLFIRINOX regimens in our unselected population were detected. Nevertheless, they were higher than those reported in the randomized phase III trial (31.6%)[7]. The methodological approach used in our study showed that FOLFOXIRI does not provide an improved efficacy compared to FOLFIRINOX.
The FOLFOXIRI regimen was associated with an increased risk of the occurrence of grade 3 or 4 digestive toxicities (12.8%), including diarrhea, nausea/vomiting, and stomatitis. Of note, this incidence was similar to the gastrointestinal safety profile reported by Vivaldi et al[21] with FOLFOXIRI chemotherapy. Interestingly, hematological toxicities were very low in both regimens in our study, compared to FOLFIRINOX in the PRODIGE 4/ACCORD 11 study[7]. A primary prophylactic administration of hematopoietic growth factors contributed to the reduction of grade 3 or 4 neutropenia[22,23], and thus, routine use after each polychemotherapy cycle has been adopted in some institutions.
Many combinations with “modified FOLFIRINOX” chemotherapy have been evaluated, and the schedules used to deliver this polychemotherapy are heterogeneous[24,25]. Dose reductions of single or multiple agents differ among studies compared to the standard schedule[24]. An optimal relative dose intensity for FOLFIRINOX was determined by Lee et al[23] to balance toxicity and efficacy, suggesting that a decrease of 30% in chemotherapy dosages preserve tumor response. In addition, two meta-analyses suggested that dosage attenuation improves tolerance while preserving survival benefits (overall response rates: 32% with “modified FOLFIRINOX” vs 33% with full doses; P = 0.879)[24,25]. A modified-dose regimen decreased the frequency of hematological and digestive adverse events and cycles reported, while making it possible to maintain dose-dense chemotherapy and treatment activity[26]. In the adjuvant setting, a modified FOLFIRINOX with no bolus of 5-FU and irinotecan at a dose of 150 mg/m2 significantly increased survival compared to gemcitabine for PDAC[4]. These dose adjustments were also effective in the neoadjuvant setting for patients with locally advanced or borderline PDAC. Of note, resection was performed in more than half of the patients, and with R0 resection in 86.4% of cases[27].
In previous retrospective and single-arm phase II studies, the bolus of 5-FU was more frequently discontinued in the “modified FOLFIRINOX” combination[10,28-31]. Infusion of 5-FU is preferred for the treatment of colorectal cancer over bolus 5-FU. In this setting, the omission of the bolus of 5-FU has been shown to improve the safety profile, while significantly decreasing hematological toxicity[32]. A dose reduction of irinotecan (130-135 mg/m2, 150 mg/m2, or 165 mg/m2) was evaluated in previous studies[28-31,33-35]. The addition of irinotecan in FOLFOXIRI chemotherapy increased digestive toxicity occurrence, notably nausea/vomiting and diarrhea, compared to the doublet-chemotherapy (FOLFOX) in metastatic colorectal cancer[36]. In aPDAC, a 25% reduction of irinotecan compared to full dose has been associated with a decrease in diarrhea (3.1% vs 12.5%, respectively) and vomiting (0% vs 23.5%, respectively)[35].
Dihydropyrimidine dehydrogenase and uridine diphosphate glucurono-syltransferase (UGT) 1A1 are two key enzymes involved in the catabolic pathways of 5-FU and irinotecan, respectively. Their deficiency related to genetic polymorphisms, leads to increased exposure to the cytotoxic agents with a higher risk of adverse events. Indeed, variants of UGT1A1 have been reported to increase the risk of grade 3 or 4 hematological toxicity and diarrhea[37,38]. A study that evaluated the FOLFIRINOX regimen in pancreatic cancer, reported a significantly higher incidence of diarrhea among patients with UGT1A1 heterozygous type (UGT1A1 −/*6 and UGT1A1 −/*28) compared to those with UGT1A1 wild-type (−/−). However, for patients who received the “modified FOLFIRINOX”, there was no observed difference in the frequency of adverse events due to UGT1A1 status[39]. Furthermore, studies have indicated an association between polymorphisms of the dihydropyrimidine dehydrogenase gene encoding (DPYD) and 5-FU-induced toxicity[40]. Currently, DPYD genotype or phenotype-based dose reduction improves the safety of patients receiving fluoropyrimidine treatment and is recommended[41,42]. Preemptive screening of DPYD and UGT1A1 variants could identify patients at risk of clinically relevant adverse events, to improve FOLFIRINOX administration[43]. In an era of personalized medicine, a “genotype-guided” approach could help to individualize the dose to optimize efficacy, limit toxicity and guarantee HRQoL.
In conclusion, FOLFOXIRI is feasible in L1 in patients with aPDAC, but does not appear to confer any therapeutic benefit as compared with the FOLFIRINOX regimen. FOLFOXIRI was associated with a higher incidence of grade 3 or 4 digestive adverse events compared to FOLFIRINOX. A major difference in hematological toxicities was observed between our cohort and the PRODIGE 4/ACCORD 11 trial[7], underlining the relevance of prophylactic administration of hematopoietic growth factors in routine clinical practice. These results show that additional evaluation is not warranted in future clinical trials. FOLFIRINOX chemotherapy remains the standard of care in L1 in metastatic PDAC.
The FOLFIRINOX regimen is the first-line reference chemotherapy (L1) in advanced pancreatic ductal adenocarcinoma (PDAC). FOLFOXIRI might contribute to a better balance in the toxicity/efficacy ratio in this setting.
FOLFOXIRI has demonstrated efficacy and feasibility in colorectal cancer.
To investigate the potential clinical value of FOLFOXIRI in patients with advanced PDAC (aPDAC) in routine clinical practice.
This exploratory study compared clinical outcomes between the two treatments in the overall population and after propensity score matching.
All consecutive aPDAC patients treated in L1 with FOLFOXIRI (n = 165) or FOLFIRINOX (n = 124) regimens were included. Median overall survival was 11.1 mo in the FOLFOXIRI cohort and 11.6 mo in the FOLFIRINOX cohort. After propensity score matching, survival rates remained similar between the regimens in terms of overall survival and progression-free survival. FOLFOXIRI was associated with a higher incidence of grade 3/4 digestive adverse events. The low hematological toxicity rates in both regimens underline the relevance of primary prophylaxis with hematopoietic growth factors.
FOLFOXIRI is feasible in L1 in patients with aPDAC but does not confer any therapeutic benefit as compared with FOLFIRINOX.
These results suggest that further evaluation of FOLFOXIRI in future clinical trials is not warranted. FOLFIRINOX chemotherapy remains the standard of care in L1 in metastatic PDAC.
We thank Fiona Ecarnot for English writing assistance.
Manuscript source: Unsolicited Manuscript
Specialty type: Oncology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sun XT, Rungsakulkij N S-Editor: Wang YQ L-Editor: Webster JR E-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15469] [Article Influence: 2578.2] [Reference Citation Analysis (2)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5134] [Article Influence: 466.7] [Reference Citation Analysis (0)] |
| 3. | Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016;55:1158-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 312] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 4. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1942] [Article Influence: 277.4] [Reference Citation Analysis (0)] |
| 5. | Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, Arnold D; ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56-v68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 928] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 6. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4351] [Cited by in RCA: 4176] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 7. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5634] [Article Influence: 402.4] [Reference Citation Analysis (1)] |
| 8. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4882] [Article Influence: 406.8] [Reference Citation Analysis (0)] |
| 9. | Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Boige V, Bérille J, Conroy T. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 10. | Mahaseth H, Brutcher E, Kauh J, Hawk N, Kim S, Chen Z, Kooby DA, Maithel SK, Landry J, El-Rayes BF. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G; Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 887] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 12. | Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 2146] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 13. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21598] [Article Influence: 1349.9] [Reference Citation Analysis (1)] |
| 14. | National Cancer Institute. National Cancer Institute Common Terminology Criteria. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. |
| 15. | Austin PC, Schuster T. The performance of different propensity score methods for estimating absolute effects of treatments on survival outcomes: A simulation study. Stat Methods Med Res. 2016;25:2214-2237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Pavillard V, Formento P, Rostagno P, Formento JL, Fischel JL, Francoual M, Etienne MC, Milano G. Combination of irinotecan (CPT11) and 5-fluorouracil with an analysis of cellular determinants of drug activity. Biochem Pharmacol. 1998;56:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Ducreux M, Mitry E, Ould-Kaci M, Boige V, Seitz JF, Bugat R, Breau JL, Bouché O, Etienne PL, Tigaud JM, Morvan F, Cvitkovic E, Rougier P. Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5-FU, and infusional 5-FU alone in advanced pancreatic carcinoma patients. Ann Oncol. 2004;15:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Zeghari-Squalli N, Raymond E, Cvitkovic E, Goldwasser F. Cellular pharmacology of the combination of the DNA topoisomerase I inhibitor SN-38 and the diaminocyclohexane platinum derivative oxaliplatin. Clin Cancer Res. 1999;5:1189-1196. [PubMed] |
| 19. | Conroy T, Paillot B, François E, Bugat R, Jacob JH, Stein U, Nasca S, Metges JP, Rixe O, Michel P, Magherini E, Hua A, Deplanque G. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23:1228-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, Athanasiadis A, Kakolyris S, Tsousis S, Kouroussis Ch, Vamvakas L, Kalykaki A, Samonis G, Mavroudis D, Georgoulias V. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br J Cancer. 2006;94:798-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Vivaldi C, Caparello C, Musettini G, Pasquini G, Catanese S, Fornaro L, Lencioni M, Falcone A, Vasile E. First-line treatment with FOLFOXIRI for advanced pancreatic cancer in clinical practice: Patients' outcome and analysis of prognostic factors. Int J Cancer. 2016;139:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Terazawa T, Goto M, Miyamoto T, Asaishi K, Shimamoto F, Kuwakado S, Nishitani H, Kii T, Higuchi K. Efficacy of Prophylactic G-CSF in Patients Receiving FOLFIRINOX: A Preliminary Retrospective Study. Intern Med. 2015;54:2969-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lee JC, Kim JW, Ahn S, Kim HW, Lee J, Kim YH, Paik KH, Kim J, Hwang JH. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: Using cumulative relative dose intensity. Eur J Cancer. 2017;76:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Usón Junior PLS, Rother ET, Maluf FC, Bugano DDG. Meta-analysis of Modified FOLFIRINOX Regimens for Patients With Metastatic Pancreatic Cancer. Clin Colorectal Cancer. 2018;17:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Tong H, Fan Z, Liu B, Lu T. The benefits of modified FOLFIRINOX for advanced pancreatic cancer and its induced adverse events: a systematic review and meta-analysis. Sci Rep. 2018;8:8666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393:1440-1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 27. | Blazer M, Wu C, Goldberg RM, Phillips G, Schmidt C, Muscarella P, Wuthrick E, Williams TM, Reardon J, Ellison EC, Bloomston M, Bekaii-Saab T. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Li X, Ma T, Zhang Q, Chen YG, Guo CX, Shen YN, Sun PW, Li GG, Gao SL, Que RS, Lou JY, Yu RS, Yuan Y, Wei QC, Wei SM, Zhang Y, Zheng L, Bai XL, Liang TB. Modified-FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer Lett. 2017;406:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Yoshida K, Iwashita T, Uemura S, Maruta A, Okuno M, Ando N, Iwata K, Kawaguchi J, Mukai T, Shimizu M. A multicenter prospective phase II study of first-line modified FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget. 2017;8:111346-111355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Wang ZQ, Zhang F, Deng T, Zhang L, Feng F, Wang FH, Wang W, Wang DS, Luo HY, Xu RH, Ba Y, Li YH. The efficacy and safety of modified FOLFIRINOX as first-line chemotherapy for Chinese patients with metastatic pancreatic cancer. Cancer Commun (Lond). 2019;39:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K, Sata N, Miyashita K, Mizuno N, Tsuji K, Okusaka T, Furuse J. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2018;81:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 32. | Tezuka T, Hamada C, Ishida H, Ooshiro M, Matsuoka H, Kawasaki S, Mishima H, Maeda K, Sakamoto J, Koda K. Phase II clinical study of modified FOLFOX7 (intermittent oxaliplatin administration) plus bevacizumab in patients with unresectable metastatic colorectal cancer-CRAFT study. Invest New Drugs. 2013;31:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, Staugaard C, Indukala D, Boustani AM, Patel V, Cha CH, Salem RR, Chang B, Hochster HS, Lacy J. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 34. | Ghorani E, Wong HH, Hewitt C, Calder J, Corrie P, Basu B. Safety and Efficacy of Modified FOLFIRINOX for Advanced Pancreatic Adenocarcinoma: A UK Single-Centre Experience. Oncology. 2015;89:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Cavanna L, Stroppa EM, Citterio C, Mordenti P, Di Nunzio C, Peveri S, Orlandi E, Vecchia S. Modified FOLFIRINOX for unresectable locally advanced/metastatic pancreatic cancer. A real-world comparison of an attenuated with a full dose in a single center experience. Onco Targets Ther. 2019;12:3077-3085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 37. | Okusaka T, Ikeda M, Fukutomi A, Ioka T, Furuse J, Ohkawa S, Isayama H, Boku N. Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014;105:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 38. | Rouits E, Boisdron-Celle M, Dumont A, Guérin O, Morel A, Gamelin E. Relevance of different UGT1A1 polymorphisms in irinotecan-induced toxicity: a molecular and clinical study of 75 patients. Clin Cancer Res. 2004;10:5151-5159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 39. | Shirasu H, Todaka A, Omae K, Fujii H, Mizuno N, Ozaka M, Ueno H, Kobayashi S, Uesugi K, Kobayashi N, Hayashi H, Sudo K, Okano N, Horita Y, Kamei K, Yukisawa S, Kobayashi M, Fukutomi A. Impact of UGT1A1 genetic polymorphism on toxicity in unresectable pancreatic cancer patients undergoing FOLFIRINOX. Cancer Sci. 2019;110:707-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Amstutz U, Froehlich TK, Largiadèr CR. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics. 2011;12:1321-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 41. | Henricks LM, Lunenburg CATC, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E, Creemers GJ, Baars A, Dezentjé VO, Imholz ALT, Jeurissen FJF, Portielje JEA, Jansen RLH, Hamberg P, Ten Tije AJ, Droogendijk HJ, Koopman M, Nieboer P, van de Poel MHW, Mandigers CMPW, Rosing H, Beijnen JH, Werkhoven EV, van Kuilenburg ABP, van Schaik RHN, Mathijssen RHJ, Swen JJ, Gelderblom H, Cats A, Guchelaar HJ, Schellens JHM. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 2018;19:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 280] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 42. | Meulendijks D, Henricks LM, Jacobs BAW, Aliev A, Deenen MJ, de Vries N, Rosing H, van Werkhoven E, de Boer A, Beijnen JH, Mandigers CMPW, Soesan M, Cats A, Schellens JHM. Pretreatment serum uracil concentration as a predictor of severe and fatal fluoropyrimidine-associated toxicity. Br J Cancer. 2017;116:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | Cremolini C, Del Re M, Antoniotti C, Lonardi S, Bergamo F, Loupakis F, Borelli B, Marmorino F, Citi V, Cortesi E, Moretto R, Ronzoni M, Tomasello G, Zaniboni A, Racca P, Buonadonna A, Allegrini G, Ricci V, Di Donato S, Zagonel V, Boni L, Falcone A, Danesi R. DPYD and UGT1A1 genotyping to predict adverse events during first-line FOLFIRI or FOLFOXIRI plus bevacizumab in metastatic colorectal cancer. Oncotarget. 2018;9:7859-7866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |