Published online Mar 15, 2020. doi: 10.4251/wjgo.v12.i3.267
Peer-review started: August 1, 2019
First decision: August 27, 2019
Revised: January 12, 2020
Accepted: February 7, 2020
Article in press: February 7, 2020
Published online: March 15, 2020
Processing time: 224 Days and 7.2 Hours
The extracellular matrix is the main component of the tumor microenvironment. Extracellular matrix remodels with the oncogenesis and development of tumors. Previous studies usually focused on the changes of proteins in normal colorectal tissues and colorectal cancers. Little is known about the changes in the extracellular matrix in different stages of colorectal cancer and the effects of these changes on the development of this cancer.
To test the changes of type I collagen, type IV collagen, matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), and tissue inhibitor of metalloproteinase-3 (TIMP-3) in different stages of colorectal cancer and the effects of these changes on the proliferation of cancer cells.
The extracellular matrix from various stages of colorectal cancer and normal colon tissue was obtained by using acellular technology. We used proteomics to detect the differential expression of proteins between normal colon tissues and colorectal cancer tissues, and then we used Western blot to observe their expression in each stage of colorectal cancer and in normal colon tissue. By co-culturing the extracellular matrix and HT29 colon cancer cells in vivo and in vitro, we tested the cancer cell proliferation rate in vitro by methyl thiazolyl tetrazolium (MTT) assay and in vivo by measuring the tumor volume.
The expression of type I collagen and MMP-2 increased with increased tumor stage. The expression of MMP-9 was higher in colorectal cancer tissues and was highest in stage III cancer. The expression of type IV collagen and TIMP-3 decreased with increased tumor stage. The proliferation rate of cancer cells in the extracellular matrix of colorectal cancer was higher than that in the extracellular matrix of the normal colon.
These data suggest that the extracellular matrix structure and composition become disorganized during the development of tumors, which is more conducive for the growth of cancer cells.
Core tip: The extracellular matrix remodels during the occurrence and development of tumor. In order to study the changes of extracellular matrix, we obtained the extracellular matrix of colorectal cancer by acellular technology. We found that type I collagen, MMP-2, and MMP-9 increased in the colorectal cancer tissue, while type IV collagen and TIMP-3 decreased in the colorectal cancer tissue. Furthermore, we co-cultured the extracellular matrix and HT 29 cancer cells in vivo and in vitro, and found that the cancer extracellular matrix was more conducive for the growth of cancer cells than the normal tissue extracellular matrix.
- Citation: Li ZL, Wang ZJ, Wei GH, Yang Y, Wang XW. Changes in extracellular matrix in different stages of colorectal cancer and their effects on proliferation of cancer cells. World J Gastrointest Oncol 2020; 12(3): 267-275
- URL: https://www.wjgnet.com/1948-5204/full/v12/i3/267.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i3.267
With the development of the “soil-seed” theory, the effect of the tumor microenvironment on tumor cells has received more attention[1]. The tumor microenvironment is a complex system consisting of the extracellular matrix (ECM), many types of cells, and bioactive factors, which control the complex interactions between tumor cells and stromal cells and between cells and the ECM[2]. During tumorigenesis, the ECM plays a role of “double-edged sword” in the process of tumor proliferation and invasion[3]. On the one hand, the ECM controls the proliferation, differentiation, and metastasis of tumor cells, and acts as a natural barrier. On the other hand, the remodeled ECM provides a loose “soil” for tumor cells to promote the occurrence and development of tumors[4,5]. This process of remodeling occurs at the same time with tumor formation, as shown by changes in the molecular composition, amount, and structure of the ECM[6].
The research on cell function is either in two-dimensional (2D) environment or in three-dimensional (3D) environment. Studies have shown that there are differences in cell proliferation, gene expression, and cell migration in 2D vs 3D cultures[6-8]. The 3D in vitro experiments were better than 2D cultures in imitating tumor cell microenvironment in vivo. The in vitro 3D culture refers to tumor cells cultured in collagen, Matrigel, or fibrin[9]. However, none of them can capture the complexity of the native matrix. The tumor ECM obtained by decellularization technology can contain almost all the proteins and their ratios in the natural tissue, which can more realistically simulate the tumor environment in which tumor cells live.
Previous studies have demonstrated that the composition of the ECM changes during cancer formation[10,11]. However, the relationship between the stages of colorectal cancer (CRC) and the changes in the ECM and the proliferation of cancer cells is unclear. Therefore, we obtained the tumor ECM by decellularization and aimed to observe the changes in the ECM during different stages of CRC and the effects of these changes on the proliferation of cancer cells.
Tissues were removed from 60 patients with CRC during surgery at Beijing Chaoyang Hospital, Capital Medical University. All procedures in this study were approved by the Medical Ethics Committee of Beijing Chaoyang Hospital, and all patients provided written informed consent. Tumor stage was classified according to the 8th edition of the American Joint Committee on Cancer TNM staging system for CRC. The patients included 34 males and 26 females and none had received preoperative chemoradiotherapy. Of these patients, 10 were classified with stage I disease, 22 with stage II, 19 with stage III, and 9 with stage IV. Ten cases with a normal colon were selected as a control group. All samples were put into the digestive solution composed of 0.25% trypsin and 0.02% EDTA and were continuously oscillated at 37 °C for 24 h at 130 rounds per minute. After that, the tissues were put into 0.5% Triton X-100 buffer and continuously oscillated at 150 rounds per minute for 24 h. Then, the ECM was obtained. After freeze-drying, the ECM samples were sterilized by Co-60 radiation and stored at -20 °C.
Western blot assays were performed to detect the protein level. The protein concentrations were tested with a BCA Protein Assay Kit (Pierce, United States). Equal amounts of protein (20 μg) were loaded. Type I collagen, type IV collagen,MMP-2, MMP-9, and TIMP-3 antibodies were purchased from Beijing Biosynthesis Biotechnology Co, LTD. All primary antibodies were used at a 1:1000 dilution. The enhanced chemiluminescence reaction was used to detect the protein bands.
The sterilized ECM was cut into 3 mm × 3 mm × 3 mm pieces under aseptic conditions and placed in a 96-well culture plate. One hundred microliter of colon cancer HT29 cells at a density of 1 × 106 cells/mL (1 × 105 cells) was slowly added vertically into the ECM, and then cultured in an incubator containing 5% CO2.
The culture medium in the 96-well plate was removed and treated with methyl thiazolyl tetrazolium (MTT) solution for 4 h. After removing the supernatant, 150 μL of DMSO was added to dissolve the tetrazolium salt and measure the optical density using a Multiskan Spectrum Microplate Reader (Thermo Labsystems, Milan, Italy) at 570 nm. The experiment was repeated three times.
Six-week-old male nude BALB/c mice were randomly divided into five groups with 10 mice in each group. The density of colon cancer HT29 cells was adjusted to 1 × 106 cells/mL. The ECM from each group was cut into 3 mm × 3 mm × 3 mm pieces under aseptic conditions and placed in a 96-well plate. In each well, 50 μL of the above cell suspension (5 × 104 cells) was slowly added and allowed to stand for 1 h. Abdominal anesthesia was performed with 10% chloral hydrate (0.01 g/mL), and the right forearm underarm skin in each mouse was cut under aseptic conditions, and the ECM and cancer cell complex were embedded subcutaneously. The animals were killed on the 30th day, and the long diameter (a) and short diameter (b) of the tumor were measured with a Vernier caliper. Approximate tumor volume was obtained using the following equation: V = a × b × b/2.
All animals were housed under a 12/12 h light/dark cycle at 22 °C and 40%-60% relative humidity conditions. They were given free access to water and food. All animal experimental protocols were done according to the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals, published by the National Science Council, China.
Statistical analyses were carried out using the Statistical Package for the Social Sciences version 22.0 and figures were made by GraphPad Prism 6.0. All data are expressed as the mean ± SD. Statistical analyses were performed by means of t-tests when two groups were compared or one-way ANOVA when more than two groups. Statistical significance was set at P < 0.05.
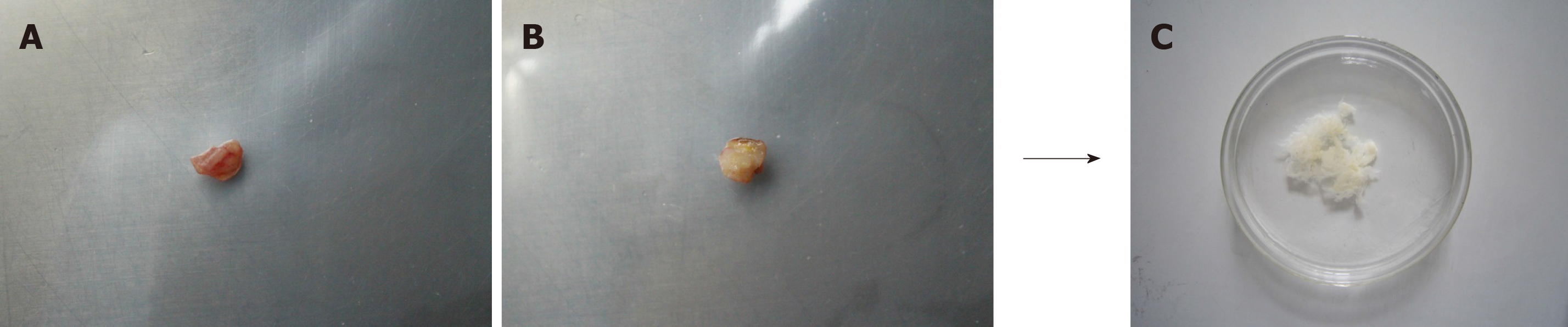
An overview of ECM preparation is provided in Figure 1. The normal tissue ECM and CRC ECM were obtained by decellularization. From outward appearance, there was no obvious difference in normal tissue ECM and CRC ECM. All of them presented milk white, sticky surface and soft texture.
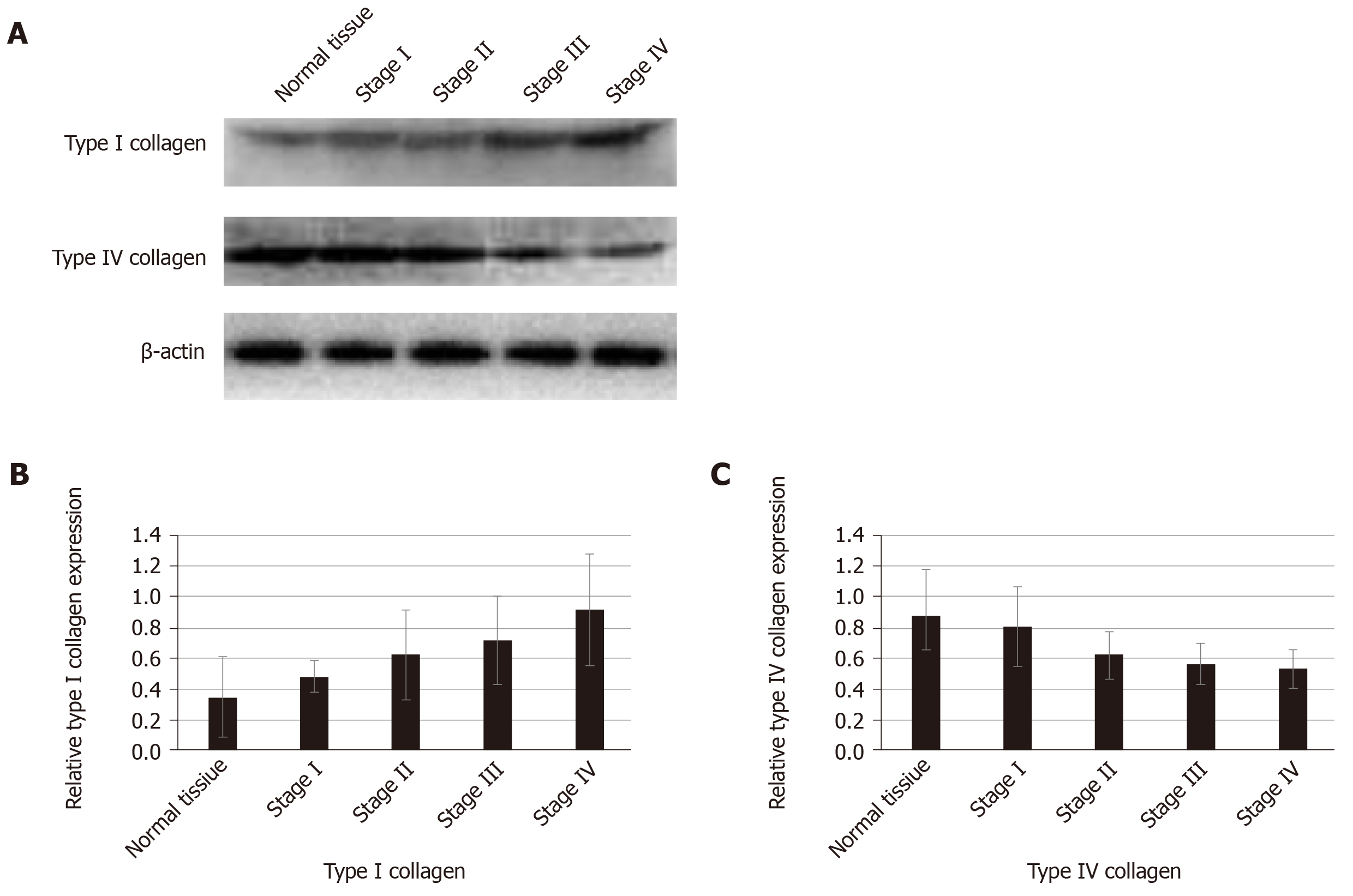
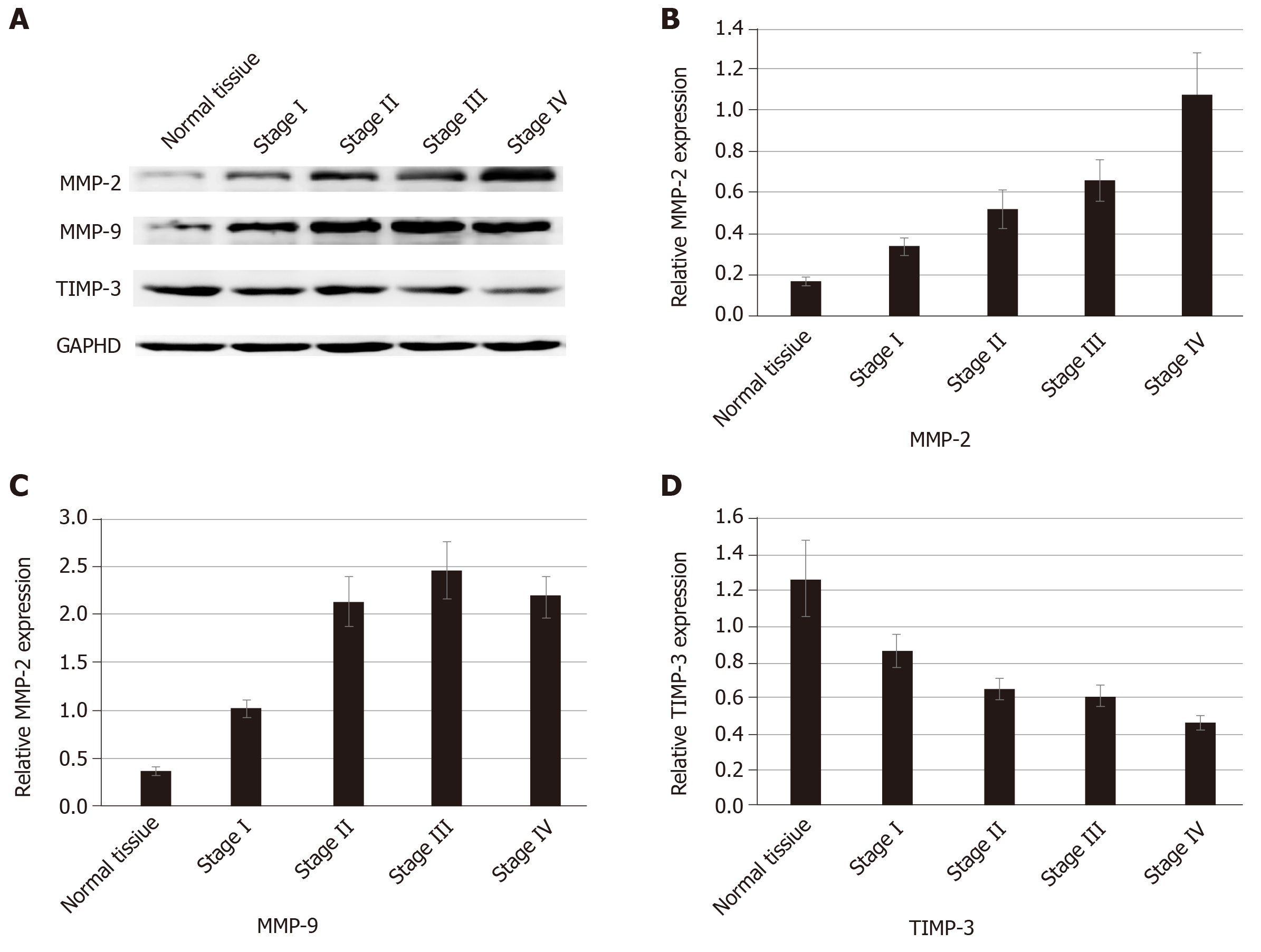
We used proteomics to analyze the differential expression of proteins in normal colorectal tissue and colorectal cancer tissue, and some of them were selected for analysis in each stage of colorectal cancer (Table 1). The expression of type I collagen was highest in stage III and stage IV and lowest in normal tissue and stage I. Spearman correlation analysis showed that the expression of type I collagen was positively correlated with the stage of CRC (Figure 2). The expression of MMP-2 was higher in the colorectal cancer tissues and it increased with the increased tumor stage. The expression of MMP-9 was higher in the colorectal cancer tissue, but it was highest in the stage III CRC (Figure 3). However, the expression of type IV collagen and TIMP-3 gradually decreased with increased CRC stage. Spearman correlation analysis showed that type IV collagen was negatively correlated with the stage of CRC (Figures 2 and 3).
| Protein | Description | T vs N fold-change | Regulated type | P value |
| A0A024R6R4 | MMP2 | 1.2683 | Up-regulated | 0.042 |
| P14780 | MMP9 | 1.3930 | Up-regulated | 0.031 |
| P35625 | TIMP3 | 0.5057 | Down-regulated | 0.026 |
| P02462 | Collagen IV | 0.4551 | Down-regulated | 0.049 |
| P02452 | Collagen I | 1.9724 | Up-regulated | 0.018 |
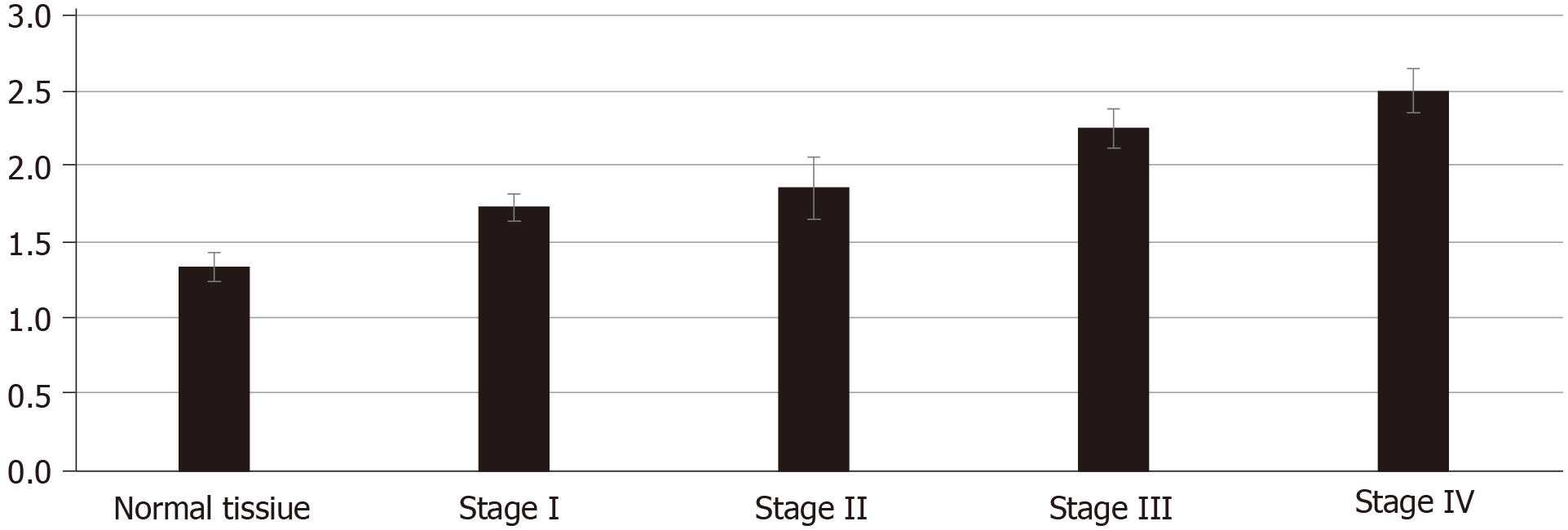
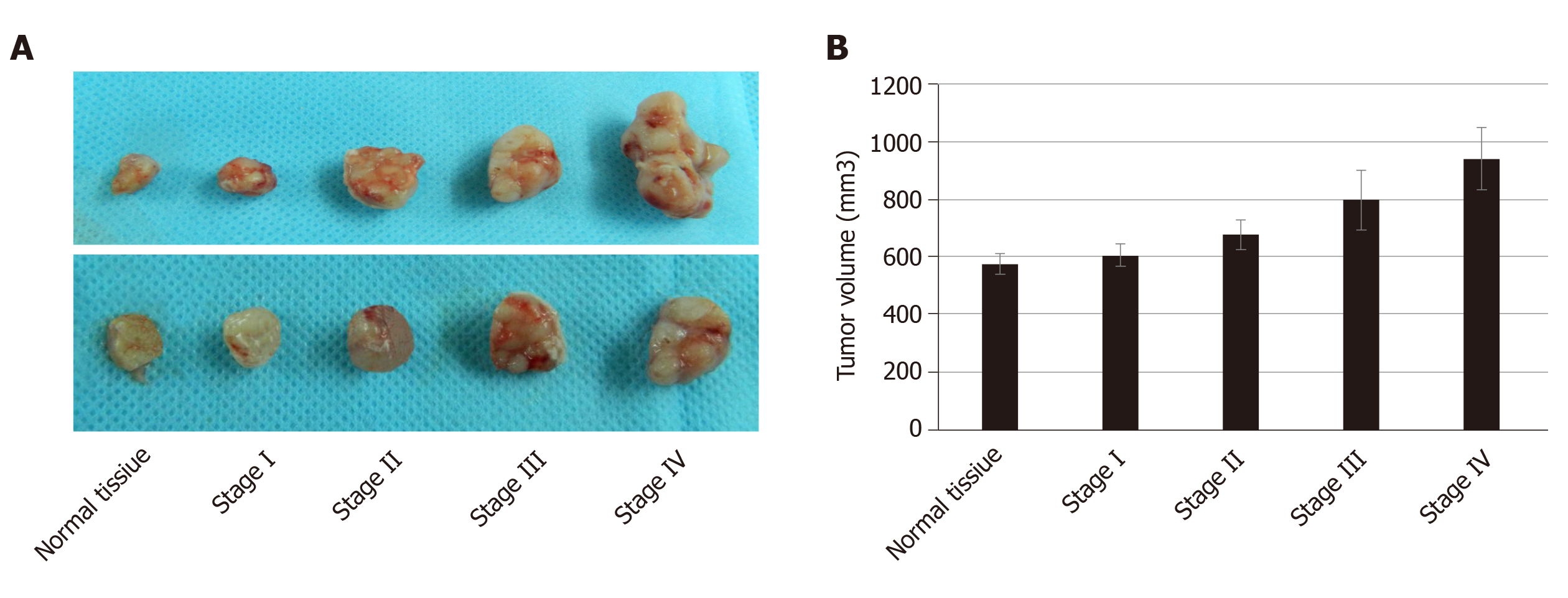
To study the growth of cancer cells in each group, we co-cultured cancer cells and ECM in vivo and in vitro. The proliferation of cancer cells was determined in vitro by the MTT assay. We found that cancer cells co-cultured with CRC ECM grew significantly better than cancer cells with normal tissue ECM (Figure 4). In vivo tumor volume in each group was larger and was greatest in stage IV CRC ECM. Compared to the normal tissue ECM, the CRC ECM was more conducive to the proliferation of cancer cells (Figure 5).
The occurrence of malignant tumors is a complex process of interactions between cancer cells and the tumor microenvironment[12,13]. Paget described the relationship between cancer cells and the tumor microenvironment as the seed and soil, indicating that the tumor microenvironment is very important for tumorigenesis and tumor progression[14]. The tumor microenvironment is a unique environment that emerges during the course of tumor progression[12,15]. The tumor microenvironment is composed of ECM, cells, and interstitial fluids, and the ECM is the major component[16]. During cancer progression, the structure and composition of the ECM become disorganized, and this change can promote cellular transformation and metastasis[4,16,17].
Collagen is an important component of the ECM and is considered a structural barrier against tumor invasion[18,19]. Paradoxically, increased expression of collagen is associated with an elevated incidence of proliferation and invasion[20,21]. Abnormal expression of collagens and pathological collagen crosslinking ultimately resulted in increased tissue stiffness and altered tissue homeostasis[6]. The stiff ECM affects many aspects of the cell, such as motility, proliferation, and chemotherapeutic drug efficiency[22,23]. In our study, we found increased expression of type I collagen and decreased expression of type IV collagen in the ECM of CRC. The imbalance of ECM composition could result in an increase in ECM stiffness, which provides enough traction for cell proliferation and migration[24].
MMP-2 and MMP-9 are members of the MMP family, and they play an important role in the degradation and remodeling of the ECM[25]. TIMP-3 exists only in the ECM and could inactivate the MMPs by binding to MMPs[26]. Thus, reaching a balance between TIMPs and MMPs is conducive to the stability of the ECM. The remodeled ECM affects the motility and proliferation of cancer cells[22]. In our study, we found that the expression of MMP-2 and MMP-9 was higher in CRC tissue than in the normal tissue. The expression of MMP-2 increased with increased tumor stage. The expression of MMP9 was highest in stage III and we speculated that this is associated with the deactivation of MMP9.
In conclusion, our study showed that the expression of type I collagen, MMP-2, and MMP-9 increases in CRC while the expression of type IV collagen and TIMP-3 decreases in this malignancy. The changes in the composition of the ECM are conducive to cell proliferation. Thus, these findings will provide a new platform for the future design of anticancer drugs based on the biophysical properties of the tumor microenvironment.
The extracellular matrix is not only the substantial support for tumor cells but also promotes the occurrence and development of tumors.
The extracellular matrix changes in the structure and composition during the process of oncogenesis and development of tumors. However, little is known about the changes of the extracellular matrix in different stages of colorectal cancers and the effect of these changes on the development of colorectal cancer. The answer to this may provide a new platform for the future design of anticancer drugs.
In this study, the authors aimed to study the changes of the extracellular matrix in different stages of colorectal cancer and the relationship between the changes of the extracellular matrix with the proliferation of cancer cells.
The extracellular matrix was obtained by acellular technology from 60 colorectal cancer patients. Type I collagen, type IV collagen, MMP-2, MMP-9, and TIMP-3 were analyzed by Western blot. Besides, the extracellular matrix and the cancer cells were co-cultured in vivo and in vitro to study the effect of the extracellular matrix on the cancer cell proliferation.
The expression of type I collagen, MMP-2, and MMP-9 increased with increased tumor stage. The expression of type IV collagen and TIMP-3 decreased with increased tumor stage. The changed extracellular matrix promotes the cancer cell proliferation.
This study showed that the extracellular matrix plays an important role in the development of tumor and this provides a certain theoretical basis for anti-tumor therapy.
The tumor microenvironment is a complex system. The extracellular matrix obtained by decellularization provides an ideal tumor model to study the occurrence and development of tumor.
We gratefully acknowledge Mrs. Gu Bei from the Chinese Academy of Medical Sciences for her technical help during the cell culture. We would also like to thank Mr. Li Lei-Lei for helping us in the data analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hoensch HP, Hamaguchi M, Leung E S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Faurobert E, Bouin AP, Albiges-Rizo C. Microenvironment, tumor cell plasticity, and cancer. Curr Opin Oncol. 2015;27:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Noguera R, Nieto OA, Tadeo I, Fariñas F, Alvaro T. Extracellular matrix, biotensegrity and tumor microenvironment. An update and overview. Histol Histopathol. 2012;27:693-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 3. | Fang M, Yuan J, Peng C, Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014;35:2871-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 409] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 4. | Kaushik N, Kim S, Suh Y, Lee SJ. Proinvasive extracellular matrix remodeling for tumor progression. Arch Pharm Res. 2019;42:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol. 2013;23:522-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Brauchle E, Kasper J, Daum R, Schierbaum N, Falch C, Kirschniak A, Schäffer TE, Schenke-Layland K. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018;68-69:180-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 2009;28:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Zaman MH. The role of engineering approaches in analysing cancer invasion and metastasis. Nat Rev Cancer. 2013;13:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Nyga A, Loizidou M, Emberton M, Cheema U. A novel tissue engineered three-dimensional in vitro colorectal cancer model. Acta Biomater. 2013;9:7917-7926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 3017] [Article Influence: 301.7] [Reference Citation Analysis (0)] |
| 11. | Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1094] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 12. | Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904-5912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1725] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 13. | Najafi M, Goradel NH, Farhood B, Salehi E, Solhjoo S, Toolee H, Kharazinejad E, Mortezaee K. Tumor microenvironment: Interactions and therapy. J Cell Physiol. 2019;234:5700-5721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 14. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 15. | Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR, Crawford S, Fujii H, Georgakilas AG, Guha G, Halicka D, Helferich WG, Heneberg P, Honoki K, Keith WN, Kerkar SP, Mohammed SI, Niccolai E, Nowsheen S, Vasantha Rupasinghe HP, Samadi A, Singh N, Talib WH, Venkateswaran V, Whelan RL, Yang X, Felsher DW. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35 Suppl:S199-S223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 16. | Crotti S, Piccoli M, Rizzolio F, Giordano A, Nitti D, Agostini M. Extracellular Matrix and Colorectal Cancer: How Surrounding Microenvironment Affects Cancer Cell Behavior? J Cell Physiol. 2017;232:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 466] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 18. | Walker C, Mojares E, Del Río Hernández A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 724] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 19. | Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris). 2005;53:430-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2671] [Cited by in RCA: 3069] [Article Influence: 191.8] [Reference Citation Analysis (0)] |
| 21. | Li A, Zhou T, Guo L, Si J. Collagen type I regulates beta-catenin tyrosine phosphorylation and nuclear translocation to promote migration and proliferation of gastric carcinoma cells. Oncol Rep. 2010;23:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Simi AK, Pang MF, Nelson CM. Extracellular Matrix Stiffness Exists in a Feedback Loop that Drives Tumor Progression. Adv Exp Med Biol. 2018;1092:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Raavé R, van Kuppevelt TH, Daamen WF. Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J Control Release. 2018;274:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Xu J, Sun M, Tan Y, Wang H, Wang H, Li P, Xu Z, Xia Y, Li L, Li Y. Effect of matrix stiffness on the proliferation and differentiation of umbilical cord mesenchymal stem cells. Differentiation. 2017;96:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Bodey B, Bodey B, Siegel SE, Kaiser HE. Prognostic significance of matrix metalloproteinase expression in colorectal carcinomas. In Vivo. 2000;14:659-666. [PubMed] |
| 26. | Yu WH, Yu S, Meng Q, Brew K, Woessner JF. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226-31232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 259] [Article Influence: 10.4] [Reference Citation Analysis (0)] |