Published online Dec 15, 2020. doi: 10.4251/wjgo.v12.i12.1456
Peer-review started: October 13, 2020
First decision: October 23, 2020
Revised: November 1, 2020
Accepted: November 10, 2020
Article in press: November 10, 2020
Published online: December 15, 2020
Processing time: 57 Days and 19.8 Hours
Pancreatic mucinous cystadenocarcinoma (MCAC) is a rare malignancy with a poor prognosis when it presents metastases at diagnosis. Due to its very low incidence, there are no clear recommendations for the treatment of advanced disease. Olaparib (an oral PARP inhibitor) has been approved for the maintenance treatment of patients with metastatic pancreatic adenocarcinoma harbouring germline BRCA1/2 mutations. Herein, we report the first case of a germline BRCA1 mutated unresectable MCAC which was effectively treated with olaparib.
A 41-year-old woman, without personal or family history of cancer, was diagnosed with ovarian and peritoneal metastases of MCAC. She underwent 12 cycles of gemcitabine plus oxaliplatin (GEMOX) obtaining a partial response and allowing radical surgery. One year later, local recurrence was documented, and other 12 cycles of GEMOX were administered obtaining a complete response. Seven years later, another local recurrence, not amenable to surgical resection, was diagnosed. She started FOLFIRINOX (oxaliplatin, irinotecan, leucovorin and fluorouracil), obtaining a partial response after 8 cycles. Given the excellent response to platinum-based chemotherapy, BRCA testing was performed, and a BRCA1 germline mutation was detected. She was switched to maintenance olaparib due to chemotherapy-related toxicities and achieved an almost complete metabolic response, with a reduction in the diameter of the lesion, after three months of therapy.
The current case suggests the beneficial effect of olaparib in BRCA mutated MCAC. However, further studies are required.
Core Tip: Pancreatic mucinous cystadenocarcinoma (MCAC) is a rare malignancy with a poor prognosis when it presents metastases at diagnosis. Due to its very low incidence, there are no clear recommendations for the treatment of advanced disease. Olaparib (an oral PARP inhibitor) has remarkable curative effects in BRCA mutated pancreatic ductal adenocarcinoma, breast and ovarian cancers. However, there are few data on its efficacy in the treatment of other types of pancreatic malignancies. Herein, we report the first case of a germline BRCA1 mutated MCAC which was effectively treated with olaparib. We also provide a brief overview of the most relevant clinical features of MCAC.
- Citation: Di Marco M, Carloni R, De Lorenzo S, Mosconi C, Palloni A, Grassi E, Filippini DM, Ricci AD, Rizzo A, Di Federico A, Santini D, Turchetti D, Ricci C, Ingaldi C, Alberici L, Minni F, Golfieri R, Brandi G, Casadei R. Pancreatic mucinous cystadenocarcinoma in a patient harbouring BRCA1 germline mutation effectively treated with olaparib: A case report. World J Gastrointest Oncol 2020; 12(12): 1456-1463
- URL: https://www.wjgnet.com/1948-5204/full/v12/i12/1456.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i12.1456
Mucinous cystic neoplasms (MCNs) of the pancreas range from benign mucinous cystadenoma to malignant cystadenocarcinoma, and they represent 23% of all resected pancreatic cysts[1]. Lou et al[2] estimated that pancreatic mucinous cystadenocarcinoma (MCAC) accounts for approximately 0.5% of all pancreatic malignancies, using the Surveillance, Epidemiology and End Results program database. Surgery represents the first choice of treatment for patients with resectable disease. The prognosis of resectable MCAC seems to be significantly more favourable compared to that of pancreatic ductal adenocarcinoma (PDAC)[3]. However, locally advanced and metastatic MCACs have a poor prognosis. Due to the very low incidence of MCAC, both controlled prospective studies and clear recommendations for the treatment of advanced disease are lacking[4].
BRCA1 and BRCA2 genes code for proteins that are involved in homologous recombination repair of double-strand DNA breaks, and their mutations are associated with an increased risk of several types of cancer[5]. In pancreatic adenocarcinoma, the prevalence of germline BRCA1/2 mutations among patients with apparently sporadic forms of the disease ranges from 1.8% to 4.6%[6,7]. Recently, maintenance olaparib (an oral PARP inhibitor) has been approved for the treatment of patients with metastatic pancreatic adenocarcinoma harbouring germline BRCA1/2 mutations based on the results of the POLO trial[8]. Nevertheless, the prevalence of germline BRCA1/2 mutations in pancreatic MCAC is unclear, and there are no data on the efficacy of PARP inhibitors in the treatment of BRCA1/2 mutated MCAC.
A 41-year-old Caucasian woman was referred to our centre. She had a history of nausea, constipation and epigastric pain for several months.
The patient had no relevant events in her medical history.
The patient had no relevant events in her medical history.
No significant personal or family history.
Unremarkable.
The serum cancer antigen (CA) 125 level was 150 IU/mL (reference range 0.00–35.00 IU/mL), while serum CA19-9 level was 354 IU/mL (reference range 0.00–37.00 IU/mL) and serum carcinoembryonic antigen (CEA) level was 13.4 ng/mL (reference range 0.00–5.00 ng/mL for non-smokers).
She underwent computed tomography (CT) which revealed two bilateral ovarian masses 145 mm and 71 mm in diameter, respectively, and a lesion measuring 40 mm in the body of the pancreas.
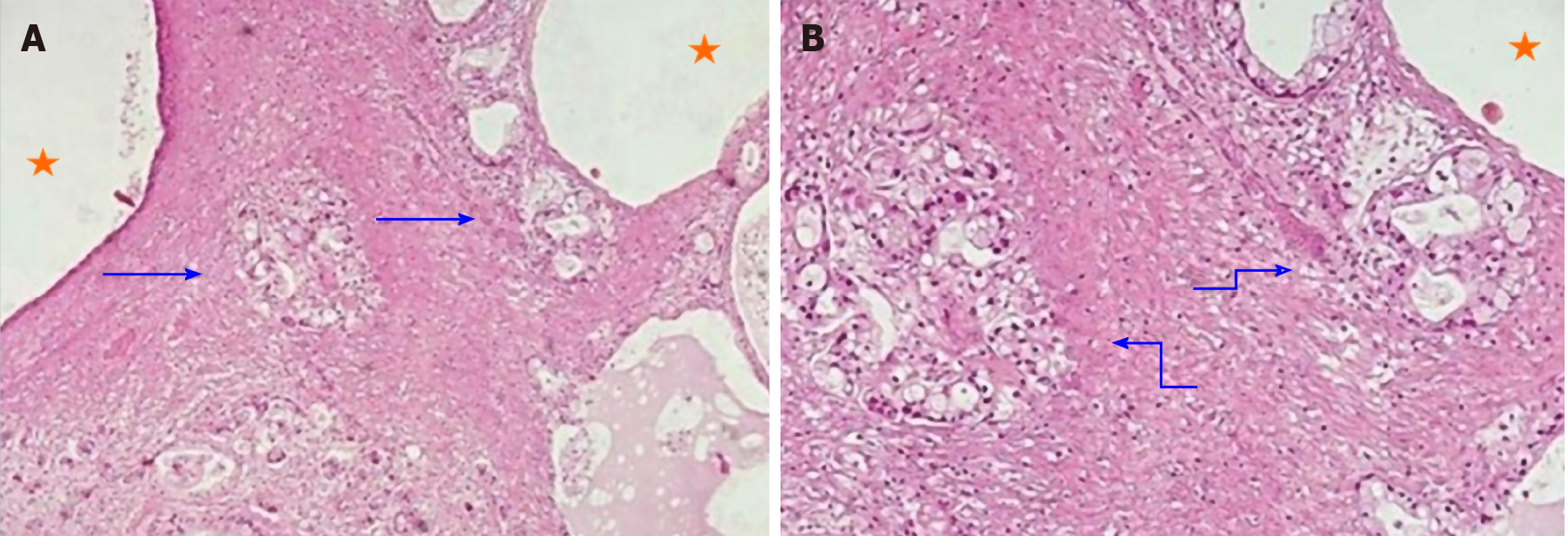
Pathology revealed ovarian and peritoneal metastases of pancreatic MCAC.
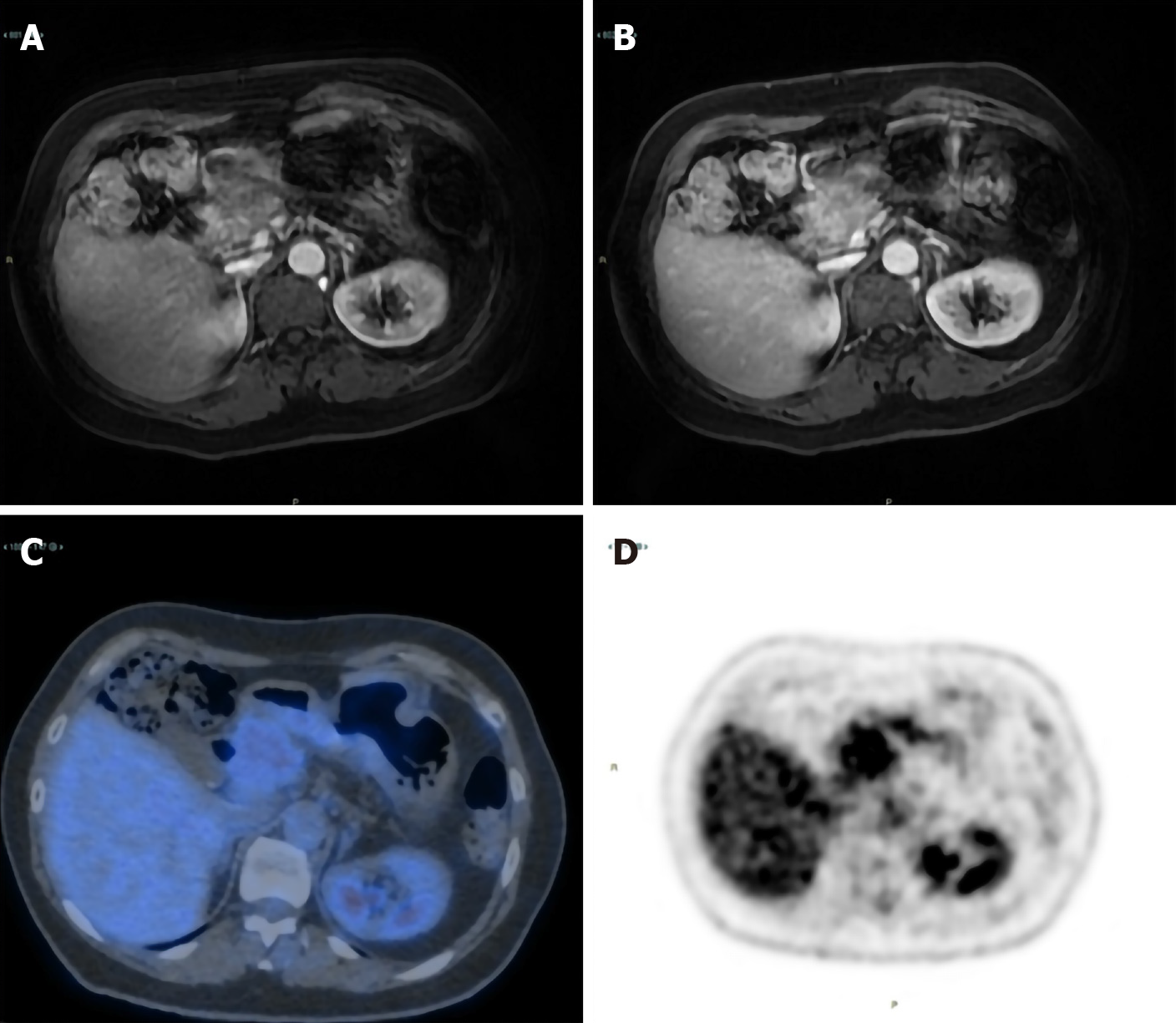
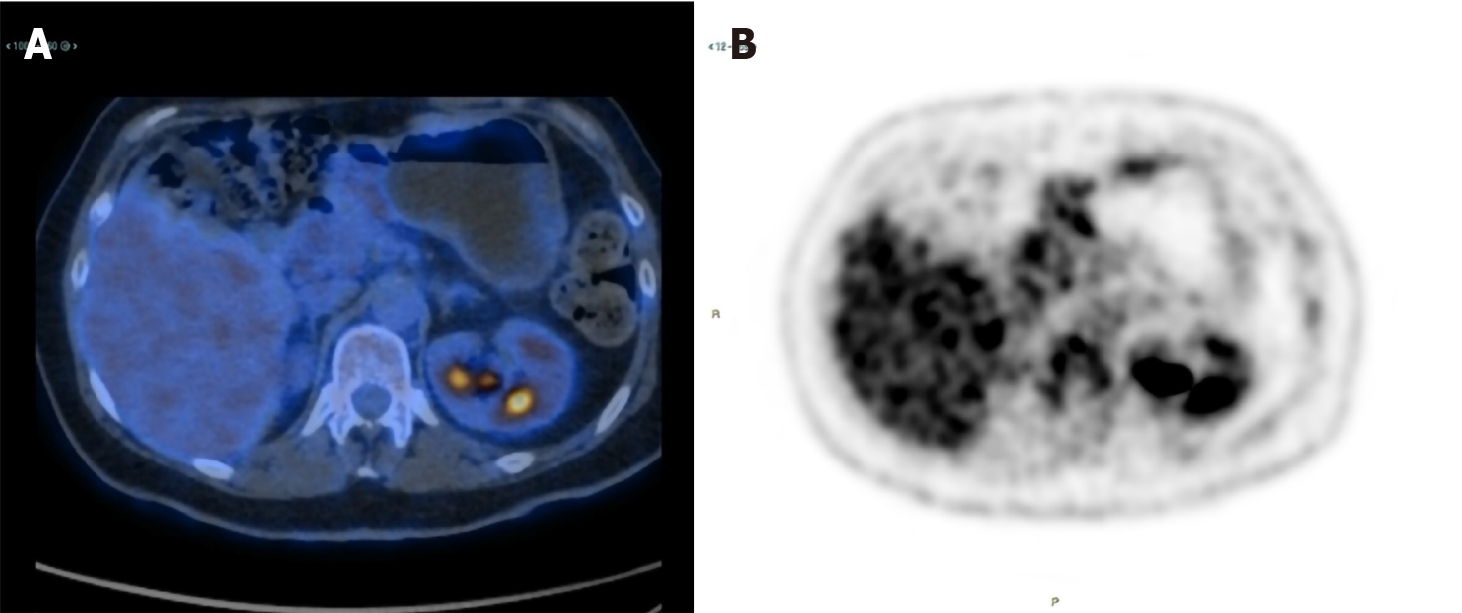
Suspecting two different tumours, she underwent surgery with radical intent in November 2008. The surgeons immediately detected a previously unrecognized peritoneal carcinosis during the exploratory laparotomy. Nonetheless, they still performed a hysterectomy and a bilateral salpingo-oophorectomy to resolve the associated symptoms caused by these masses. The surgical specimen included the uterus and ovaries, which were macroscopically infiltrated by two cystic lesions measuring 65 mm × 60 mm × 40 mm and 100 mm × 90 mm × 50 mm, respectively. Pathology revealed ovarian and peritoneal metastases of pancreatic MCAC (Figure 1). One month later, she started chemotherapy with gemcitabine plus oxaliplatin (GEMOX, gemcitabine 1000 mg/sm/d1 and oxaliplatin 100 mg/sm/d2 every two weeks) obtaining a partial response after 12 cycles. Given the excellent response to chemotherapy, we performed a second exploratory laparotomy in October 2009 during which no peritoneal carcinosis was found. She underwent distal pan-createctomy and pathological examination confirmed the initial diagnosis of pancreatic MCAC. The patient remained disease-free until September 2011, when both CT and 18F-fluoro-D-glucose positron emission tomography/X-ray computed tomography (18F-FDG PET-CT) showed a local recurrence and GEMOX was restarted obtaining a complete response after 12 cycles (confirmed both by CT and 18-FDG PET-CT). In June 2019, during the follow-up program, laboratory tests documented an increase of serum CEA (14.2 ng/mL), while serum CA125 and CA19-9 were normal. We performed magnetic resonance imaging and 18F-FDG PET-CT (Figure 2) which showed a local recurrence not amenable to surgical resection (invasion of the hepatic artery and superior mesenteric vein). The diagnosis was confirmed with an endoscopic ultrasound-guided biopsy. She started FOLFIRINOX (oxaliplatin 85 mg/sm, irinotecan 180 mg/sm, leucovorin 400 mg/sm and fluorouracil 400 mg/sm given as a bolus followed by 2400 mg/sm as a 46 h continuous infusion, every two weeks) obtaining a significant response, with a decrease of 18F-FDG uptake (SUV = 3.9 vs 6) and reduction in CEA level (2.78 ng/mL) after 8 cycles (Figure 3). However, toxicities were observed and included febrile neutropenia and grade 3 vomiting. Considering the excellent radiological response to platinum-based chemotherapy, we performed BRCA testing. Constitutional DNA of the patient was analyzed for BRCA1 and BRCA2 variants using Next Generation Sequencing (ION Torrent S5) and the variant c.4117G>T (p.Glu1373Ter) was detected in the BRCA1 gene. In January 2020 she was switched to maintenance olaparib tablets (300 mg twice daily) with good tolerance, except for anaemia G2 and fatigue G1.
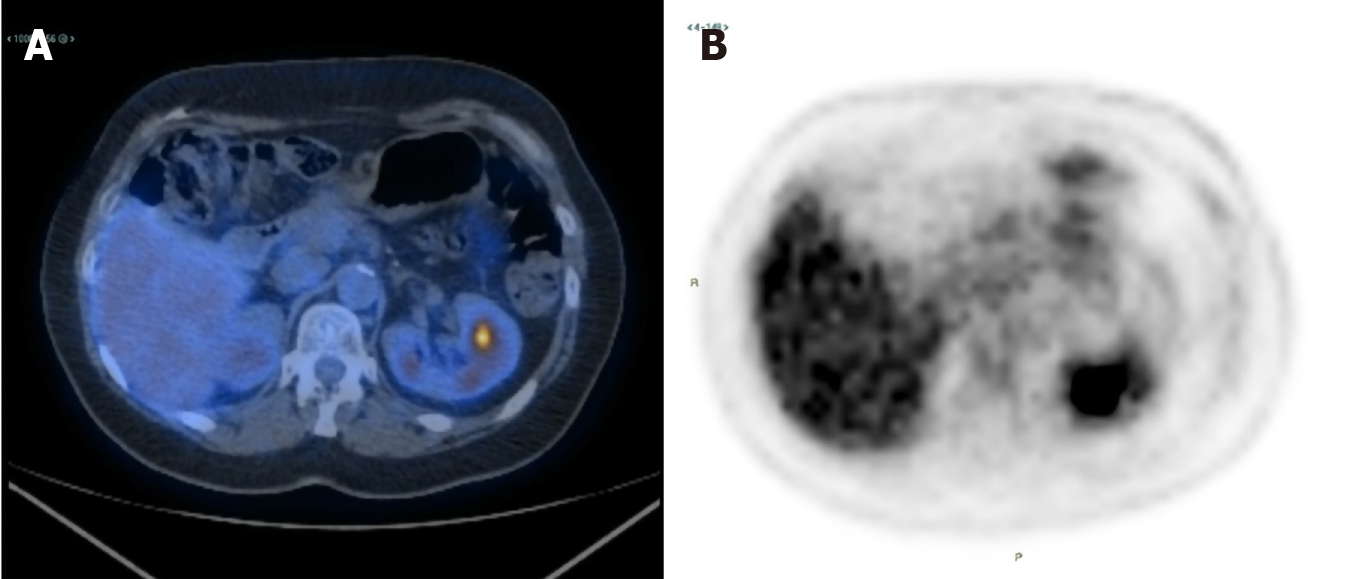
An 18F-FDG PET-CT performed in May 2020 documented an almost complete metabolic response (SUV = 2.1) with a reduction in the diameter of the lesion (Figure 4). Over the next three weeks, treatment was discontinued due to drug shortage related to the COVID-19 pandemic. In June 2020, treatment was restarted and another 18F-FDG PET-CT performed in September 2020 revealed 18F-FDG uptake similar to that shown in May 2020 (SUV = 2.3), while CEA level was 1.45 ng/mL. The patient is still well and receiving olaparib.
Mucin-producing cystic tumours of the pancreas are classified into two different entities: Intraductal papillary mucinous neoplasms (IPMNs) and MCNs, which are defined by the presence of ovarian-type stroma and do not communicate with the ductal system, unlike IPMNs[9-13]. MCNs represent 23% of all resected pancreatic cysts with a mean age at diagnosis of 51 years[1], whereas the mean age at diagnosis for MCAC is higher with a difference of more than ten years (mean 64 years)[2]. MCAC represents the malignant form of a mucinous cystic neoplasm; approximately 70% of all patients are female presenting with a relatively large tumour (median size 5.5 cm) which is mainly located in the body/tail of the pancreas[14]. MCAC is usually symptomatic at the time of diagnosis with pain (59%) and jaundice (32%) being the two most frequent symptoms. Also, serum CA19-9 level is often increased[15]. The prognosis of resectable MCAC seems to be significantly more favourable compared to pancreatic ductal adenocarcinoma[2,3]. In a retrospective study involving 507 patients with MCAC, the median observed survival for patients with resectable disease was 111.0 mo vs 14.0 mo and 4.0 mo for those with regional and metastatic disease, respectively[14]. Interestingly, the performance of curative-intent surgery and the tumour stage were independent predictors of survival. Instead, tumour size seemed to have an insignificant impact on the disease-specific survival of patients with localized MCAC, different to pancreatic ductal adenocarcinoma[16]. Due to the very low incidence of this malignancy, there are no prospective studies focusing on chemotherapy for MCAC, and data are available from only case series and case reports. Thus, it is unclear what is the most appropriate therapeutic approach for advanced disease[4,17,18]. This fact is reflected in the poor prognosis of advanced MCAC, especially when compared with that of resectable disease.
BRCA1 and BRCA2 proteins play a crucial role in repairing double-strand DNA breaks via the homologous DNA repair mechanism. Deleterious mutations within these genes are associated with an increased risk of breast, ovarian and prostate cancer[19,20]. In addition, germline BRCA1 and BRCA2 mutations are associated with a relative risk of 2.26 and 3.51 for developing pancreatic adenocarcinoma, respectively[21,22]. PARP inhibitors interfere with base excision repair, a crucial pathway in the repair of DNA single-strand breaks, causing an accumulation of single-strand DNA breaks which result into DNA double-strand breaks. Inactivation of BRCA1 or BRCA2 genes sensitizes cells to the inhibition of PARP, causing cell cycle arrest and apoptosis when cells are exposed to PARP inhibitors[23]. Olaparib (an oral PARP inhibitor) has recently been approved for the maintenance treatment of patients with metastatic pancreatic adenocarcinoma that had not progressed during first-line platinum-based chemotherapy[8]. To date, there are no reports of BRCA-mutated MCAC treated with PARP inhibitors.
At the time of diagnosis, our patient had metastatic disease, but an aggressive surgical approach associated with a marked response to platinum-based chemotherapy allowed a complete response. Although her family history was negative for cancer, given the impressive response to platinum-based chemotherapy, we investigated and detected a BRCA1 germline mutation. Historically, screening for BRCA mutations was offered only to those patients with a family history highly suspicious for a genetic predisposition syndrome[24]. However, this strategy fails to identify a relevant proportion of patients with germline BRCA1/2 mutations[24]. Given the results of the POLO trial, and emerging evidence on the efficacy of platinum-based chemotherapy in BRCA mutated PDAC, National Comprehensive Cancer Network guidelines now recommend germline testing for all patients with PDAC[25-27].
To our knowledge, this is the first report on the efficacy of a PARP inhibitor in a MCAC patient harbouring a germline BRCA mutation. After three months of treatment with maintenance olaparib, we documented a deep metabolic response with a reduction in the diameter of the lesion and after seven months of therapy 18F-FDG PET-CT still shows an almost complete metabolic response. Treatment was well tolerated except for anaemia G2 and fatigue G1, and it was discontinued for only one week due to fatigue. Reported toxicities in our case are in line with previous studies, with fatigue (60%), nausea (45%) and anaemia (27%) being the three most common adverse events reported in the POLO trial[8].
Increased use of BRCA testing, not based only on personal or family history of cancer, may help to identify a higher number of patients which could benefit from target therapies. As previously demonstrated in the treatment of patients with other types of cancers harbouring a germline BRCA mutation, we suggest that maintenance olaparib should also be considered for MCAC patients as it is safe and effective. However, further studies are needed to confirm our results.
We would like to thank the Centro Interdipartimentale di Ricerca sul Cancro “Giorgio Prodi” for the support.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe H S-Editor: Zhang H L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 276] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 2. | Luo G, Fan Z, Gong Y, Jin K, Yang C, Cheng H, Huang D, Ni Q, Liu C, Yu X. Characteristics and Outcomes of Pancreatic Cancer by Histological Subtypes. Pancreas. 2019;48:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Ridder GJ, Maschek H, Klempnauer J. Favourable prognosis of cystadeno- over adenocarcinoma of the pancreas after curative resection. Eur J Surg Oncol. 1996;22:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Brunetti O, Aprile G, Marchetti P, Vasile E, Casadei Gardini A, Scartozzi M, Barni S, Delfanti S, De Vita F, Di Costanzo F, Milella M, Cella CA, Berardi R, Cataldo I, Scarpa A, Basile D, Mazzuca F, Graziano G, Argentiero A, Santini D, Reni M, Cascinu S, Silvestris N. Systemic Chemotherapy for Advanced Rare Pancreatic Histotype Tumors: A Retrospective Multicenter Analysis. Pancreas. 2018;47:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 5. | Walsh CS. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, Siddiqui A, Witmer PD, Tamura K, Song TJ, Navarro Almario JA, Brant A, Borges M, Ford M, Barkley T, He J, Weiss MJ, Wolfgang CL, Roberts NJ, Hruban RH, Klein AP, Goggins M. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J Clin Oncol. 2017;35:3382-3390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 7. | Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, Dhani N, Narod S, Akbari M, Moore M, Gallinger S. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J Clin Oncol. 2015;33:3124-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 8. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1614] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 9. | Klöppel G, Solcia E, Longnecker DS, Capella C, SOBIN L. Histological typing of tumors of the exocrine pancreas. World Health Organization. Geneva, Switzerland: Springer; 1996. Available from: https://www.springer.com/gp/book/9783540602804. |
| 10. | Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic Cyst Disease: A Review. JAMA. 2016;315:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 11. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1612] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 12. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1440] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 13. | Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, Farnell MB, Sarr MG, Chari ST. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Doulamis IP, Mylonas KS, Kalfountzos CE, Mou D, Haj-Ibrahim H, Nasioudis D. Pancreatic mucinous cystadenocarcinoma: Epidemiology and outcomes. Int J Surg. 2016;35:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Park H, An S, Eo SH, Song KB, Park JH, Kim KP, Lee SS, Cho H, Seo DW, Kim SC, Yu E, Hong SM. Survival effect of tumor size and extrapancreatic extension in surgically resected pancreatic cancer: proposal for improved T classification. Hum Pathol. 2014;45:2341-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Björk Werner J, Sturesson C, Dawiskiba S, Andersson R. Mucinous cystadenocarcinoma of the pancreas - outcome following different modes of treatment. Ann Gastroenterol. 2011;24:213-217. [PubMed] |
| 18. | Shimada K, Iwase K, Aono T, Takeda S, Yoshida H, Koma M, Nomura M, Nishikawa K, Tamagawa H, Matsuda C, Fushimi H, Tanaka Y. A case of advanced mucinous cystadenocarcinoma of the pancreas with peritoneal dissemination responding to gemcitabine. Gan To Kagaku Ryoho. 2009;36:995-998. [PubMed] |
| 19. | Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348:2339-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 390] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 20. | Castro E, Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl. 2012;14:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1110] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 22. | Thompson D, Easton DF; Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 765] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 23. | Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4316] [Cited by in RCA: 4859] [Article Influence: 243.0] [Reference Citation Analysis (0)] |
| 24. | Wong W, Raufi AG, Safyan RA, Bates SE, Manji GA. BRCA Mutations in Pancreas Cancer: Spectrum, Current Management, Challenges and Future Prospects. Cancer Manag Res. 2020;12:2731-2742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 25. | Wattenberg MM, Asch D, Yu S, O'Dwyer PJ, Domchek SM, Nathanson KL, Rosen MA, Beatty GL, Siegelman ES, Reiss KA. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020;122:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 26. | Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 27. | National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 3. 2019). Available from: https://www.nccn.org/. |