Published online Dec 15, 2020. doi: 10.4251/wjgo.v12.i12.1428
Peer-review started: August 26, 2020
First decision: October 21, 2020
Revised: November 10, 2020
Accepted: November 17, 2020
Article in press: November 17, 2020
Published online: December 15, 2020
Processing time: 106 Days and 6.3 Hours
Patients with clinical T4 colorectal cancer (CRC) have a poor prognosis because of compromised surgical margins. Neoadjuvant therapy may be effective in downstaging tumors, thereby rendering possible radical resection with clear margins.
To evaluate tumor downsizing and resection with clear margins in T4 CRC patients undergoing neoadjuvant concurrent chemoradiotherapy followed by surgery.
This study retrospectively included 86 eligible patients with clinical T4 CRC who underwent neoadjuvant concurrent chemoradiotherapy followed by radical resection. Neoadjuvant therapy consisted of radiation therapy at a dose of 45-50.4 Gy and chemotherapy agents, either FOLFOX or capecitabine. A circumferential resection margin (CRM) of < 1 mm was considered to be a positive margin. We defined pathological complete response (pCR) as the absence of any malignant cells in a specimen, including the primary tumor and lymph nodes. A multivariate logistic regression model was used to identify independent predictive factors for pCR.
For 86 patients who underwent neoadjuvant chemoradiotherapy and surgery, the rate of pCR was 14%, and the R0 resection rate was 91.9%. Of the 61 patients with rectal cancer, 7 (11.5%) achieved pCR and 5 (8.2%) had positive CRMs. Of the 25 patients with colon cancer, 5 (20%) achieved pCR and 2 (8%) had positive CRMs. We observed that the FOLFOX regimen was an independent predictor of pCR (P = 0.046). After a median follow-up of 47 mo, the estimated 5-year overall survival (OS) and disease-free survival (DFS) rates were 70.8% and 61.4%, respectively. Multivariate analysis revealed that a tumor with a negative resection margin was associated with improved DFS (P = 0.014) and OS (P = 0.001). Patients who achieved pCR exhibited longer DFS (P = 0.042) and OS (P = 0.003) than those who did not.
Neoadjuvant concurrent chemoradiotherapy engenders favorable pCR and R0 resection rates among patients with T4 CRC. The R0 resection rate and pCR are independent prognostic factors for patients with T4 CRC.
Core Tip: Patients with clinical T4 colorectal cancer have a poor prognosis because of compromised surgical margins. This retrospective study demonstrated that neoadjuvant chemoradiotherapy resulted in high rates of pathological complete response and complete resection for patients with T4 colorectal cancer. An aggressive approach that entails implementing the FOLFOX regimen before, during, and after irradiation is safe and can improve pathological complete response rates. Negative resection margins and pathological complete response are significantly associated with survival.
- Citation: Huang CM, Huang CW, Ma CJ, Tsai HL, Su WC, Chang TK, Huang MY, Wang JY. Outcomes of neoadjuvant chemoradiotherapy followed by radical resection for T4 colorectal cancer. World J Gastrointest Oncol 2020; 12(12): 1428-1442
- URL: https://www.wjgnet.com/1948-5204/full/v12/i12/1428.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i12.1428
Colorectal cancer (CRC) is a major public health concern because of its high incidence and death rates in Western countries[1]. In Taiwan, CRC is the most commonly diagnosed cancer, with the number of patients with CRC increasing rapidly in recent years; CRC is also the third leading cause of cancer-related death in Taiwan[2]. Surgical resection with a free tumor margin (R0 resection) is a curative method for localized CRC. However, the resection of T4 CRC involves a high risk of positive surgical margins and local recurrence; therefore, patients with T4 CRC have relatively poor overall survival (OS) and disease-free survival (DFS)[3,4]. A study reported that patients with T4 CRC had a 5-year DFS rate of 75.4%, considerably lower than those for patients with T1-T3 tumors (T1: 98.8%; T2: 95.7; T3: 86.5%)[5].
R0 resection is a crucial prognostic factor in patients with CRC. Studies have reported that multivisceral resection (MVR) improves the prognosis of locally advanced CRC, but this is at the cost of increased morbidity and mortality[3,6]. Other studies have revealed that patients with locally advanced CRC who underwent MVR had R0 resection rates of 40%-90%[3,6,7]. Neoadjuvant concurrent chemoradiotherapy (CCRT) followed by surgical resection is the main treatment for locally advanced rectal cancer (LARC), and tumor downstaging may facilitate complete resection of T4 lesions[8,9]. However, administering neoadjuvant CCRT in patients with locally advanced colon cancer is controversial[10-12].
Patients achieving pathological complete response (pCR) experience more favorable oncologic outcomes compared with patients not[13,14]. Most studies have enrolled both patients with clinical T3 and those with T4 rectal cancer for the administration of neoadjuvant CCRT, but those with T4 disease usually exhibited more unfavorable responses to CCRT compared with those with T3 disease; this can be attributed to the extensive invasion of surrounding tissues and large tumor burden of T4 lesions that make radical resection difficult[15-17]. Research results or clinical evidence regarding pCR after the administration of neoadjuvant CCRT for clinical T4 (cT4) CRC is currently limited.
To address this gap in the literature, the present study was conducted to determine the oncologic results of neoadjuvant CCRT administration followed by radical resection and to identify predictive factors for pCR, DFS, and OS in patients with T4 CRC.
We retrieved records of consecutive patients with cT4 CRC and biopsy-proven adenocarcinoma who underwent neoadjuvant CCRT followed by radical resection between August 2010 and September 2018. A cT4 stage was defined as radiological evidence of tumor penetration into the surface of the visceral peritoneum (T4a) or direct tumor invasion or adhesion to nearby organs (T4b). Patients with distant metastases at diagnosis and previous or synchronous malignancies were excluded from this analysis. This study was approved by our institutional review board. Cancer staging was determined according to abdominal computed tomography (CT) scans for colon cancer and pelvic magnetic resonance imaging (MRI) for rectal cancer. For patients with locally advanced colon cancer, the imaging studies and treatment strategies were reviewed by a multidisciplinary cancer team.
Patients underwent one of two preoperative chemotherapy regimens, namely, capecitabine and FOLFOX regimens. A total of 13 patients with cT4 CRC received the capecitabine regimen; specifically, these patients received capecitabine (850 mg/m2) twice daily for 5 d/wk throughout the 5 wk of radiation therapy (RT). A total of 75 patients received the FOLFOX regimen, which entailed a biweekly schedule of FOLFOX. Each cycle of FOLFOX chemotherapy involved oxaliplatin (85 mg/m2) and folinic acid (400 mg/m2) infusion on day 1, followed by a 46-h infusion of 5-fluorouracil (2800 mg/m2) repeated every 2 wk. Patients in the FOLFOX group received one or two cycles of induction FOLFOX before CCRT, followed by two cycles of FOLFOX concomitantly administered during RT and an additional three or four cycles of consolidation FOLFOX after CCRT.
Target volumes were determined in accordance with the principles of the International Commission on Radiation Units and Measurements Reports 50 and 62. The gross tumor volume (GTV) was defined as the volume of the visible tumor and enlarged lymph nodes apparent on diagnostic CT or MRI images. A 1.5-2 cm clinical target volume (CTV) margin was added to the GTV. In addition to the CTV, we added a planning target margin of 1-1.5 cm. Irradiation was delivered at a total dose of 45-50.4 Gy with a daily fraction of 1.8 Gy or 2.0 Gy.
Patients underwent radical resection after completing neoadjuvant treatment. For colon cancer, hemicolectomy was performed, and for rectal cancer, total mesorectal excision was conducted. Partial organ resection procedures were conducted as necessary, and specimens were collected and sent to the pathology department to ascertain the status of the surgical margins. Two pathologists examined the specimens and evaluated the treatment response. In the event of a discrepancy between the evaluations of the two pathologists, we consulted a third pathologist to resolve the differences. The tumor response following CCRT was assessed according to the American Joint Committee on Cancer system[18]. A circumferential resection margin (CRM) of < 1 mm was considered to be a positive margin. We defined pCR as the absence of any malignant cells in a specimen, including the primary tumor and lymph nodes (ypT0N0).
Adjuvant chemotherapy (6 mo perioperative treatment) was suggested for patients with one of the following pathological parameters: Pathologic nodal metastases, positive resection margins, or pathologic T3-T4 tumors. An additional six cycles of adjuvant chemotherapy with FOLFOX were administered.
Toxicity was evaluated at each weekly visit, and postoperative follow-up was conducted at 3-mo intervals. Acute adverse events were recorded in accordance with the Common Terminology Criteria for Adverse Events, version 4.2.
Categorical variables are presented as percentages, and continuous variables are presented as median values and ranges. Categorical variables were compared using the χ2 test or Fisher’s exact test. We applied kappa statistics to quantify and confirm interobserver agreement. A multivariate logistic regression model was used to identify independent predictive factors for pCR.
Follow-up and survival periods were measured from the surgery date to the end points. The Kaplan-Meier method was used to estimate DFS and OS, and the log-rank test was used to measure differences between the groups. A multivariate Cox proportional hazards model was used to analyze the associated clinicopathologic factors. All statistical analyses were performed using JMP software (version 9.0, SAS Institute Inc., Cary, NC, United States).
Table 1 presents the characteristics of the patients. The sigmoid colon was the most common site of colon cancer (n = 14), followed by the ascending colon (n = 7), cecum (n = 2), and transverse colon (n = 2). The irradiation treatment modalities included three-dimensional conformal radiotherapy (n = 15), volumetric arc therapy (n = 49), and tomotherapy (n = 24). According to imaging studies, the following adjacent organs were involved: The bladder (n = 13), uterus (n = 9), vagina (n = 6), small intestine (n = 8), prostate (n = 5), ureter (n = 2), stomach (n = 1), and perineum (n = 1). After reviewing each surgical specimen, we determined that the bladder of three patients, the uterus of one patient, and the stomach of one patient were pathologically involved. In the remaining cases, dead tumor cells and fibrosis were found within the resected or biopsied adjacent organs.
| Characteristic | n = 88 |
| Age, median (yr, range) | 63 (34-93) |
| Sex | |
| Male | 42 (47.7) |
| Female | 46 (52.3) |
| Location | |
| Colon | 25 (28.4) |
| Rectum | 63 (71.6) |
| cT stage | |
| T4a | 44 (50) |
| T4b | 44 (50) |
| cN stage | |
| N0 | 7 (8) |
| N1 | 37 (42) |
| N2 | 44 (50) |
| cTNM stage | |
| II | 6 (6.8) |
| III | 82 (93.2) |
| Tumor grade | |
| Well differentiated | 4 (4.6) |
| Moderately differentiated | 72 (81.8) |
| Poorly differentiated | 12 (13.6) |
| Pretreatment CEA (ng/mL) | |
| ≤ 5 | 46 (52.3) |
| > 5 | 42 (47.7) |
| Preoperative chemotherapy | |
| FOLFOX | 75 (85.2) |
| Capecitabine | 13 (14.8) |
| Radiation technique | |
| Tomotherapy | 24 (27.9) |
| Volumetric arc therapy | 49 (57) |
| Conformal radiotherapy | 15 (15.1) |
| Radiation dose (Gy) | |
| < 50 | 26 (29.5) |
| ≥ 50 | 62 (70.5) |
| Radiation-surgery interval (wk, range) | 9 (5-40) |
Acute adverse events differed between the two chemotherapy groups. Overall, leukopenia (11.1%) was the most common grade 3 toxicity in the FOLFOX group, and diarrhea (14.2%) was the most common grade 3 toxicity in the capecitabine group. No grade 4 toxicity or treatment-related death was observed in the study participants.
All patients completed the suggested radiation dose. RT was interrupted for 1 wk because of grade 3 diarrhea (n = 2) in the FOLFOX group. In the FOLFOX group, three patients discontinued chemotherapy because of neutropenic fever (n = 2) and severe diarrhea (n = 1); no chemotherapy interruption occurred in the capecitabine group.
In this study, two patients did not undergo radical resection after completing CCRT. One patient was diagnosed as having sigmoid colon cancer invading the uterus and left ureter; therefore, the patient did not receive radical resection because tumor fixation to the common iliac artery was found during the operation. The patient continued chemotherapy with FOLFOX followed by FOLFIRI and exhibited a stable disease at the 18-month follow-up (the last follow-up). The other patient with ascending colon cancer developed peritoneal carcinomatosis after completing CCRT. Therefore, two cycles of FOLFIRI were administered, but the patient died of tumor progression 7 mo after diagnosis.
Table 2 lists the patients’ pathological results and tumor responses. For the 86 patients who underwent surgery, the pCR rate was 14%, and the R0 resection rate was 91.9%. Of the 61 patients with rectal cancer, 7 (11.5%) achieved pCR and 5 (8.2%) had positive CRMs. Of the 25 patients with colon cancer, 5 (20%) achieved pCR and 2 (8%) had positive CRMs. The κ value was 0.97, indicating excellent interobserver agreement in this study.
| n (%) | |
| ypT | |
| 0 | 13 (15) |
| 1 | 3 (3.8) |
| 2 | 12 (13.8) |
| 3 | 46 (53.4) |
| 4 | 12 (14) |
| ypN | |
| 0 | 72 (83.8) |
| 1 | 10 (11.6) |
| 2 | 4 (4.6) |
| Median number of resected nodes2 | 9 (2-26) |
| Median number of involved nodes2 | 0 (0-8) |
| Lymphovascular invasion | |
| Positive | 15 (17.4) |
| Negative | 71 (82.6) |
| Perineural invasion | |
| Positive | 22 (25.5) |
| Negative | 64 (74.5) |
| Resection margin | |
| Positive | 7 (8.1) |
| Negative | 79 (91.9) |
| Pathologic complete response | |
| Yes | 12 (14) |
| No | 74 (86) |
| Tumor regression grade | |
| 0 | 13 (15.1) |
| 1 | 30 (34.9) |
| 2 | 27 (31.4) |
| 3 | 16 (18.6) |
| Pathologic T stage | |
| Downstaging | 74 (86) |
| Stable | 112 (14) |
| Progressive | 0 (0) |
| Pathologic N stage | |
| Downstaging | 75 (87.3) |
| Stable | 7 (8.1) |
| Progressive | 4 (4.6) |
Table 3 presents a summary of the results of the univariate and multivariate analyses of clinical parameters used for pCR prediction. The univariate analysis indicated that FOLFOX-based CCRT was significantly associated with pCR occurrence (P = 0.037) and that a long radiation-surgery interval tended to improve pCR (P = 0.074). The multivariate analysis revealed that receiving the FOLFOX regimen was an independent predictor of pCR (odds ratio, 4.755; 95%CI, 2.118-88.203; P = 0.046).
| Variable | Univariate | Multivariate | |
| P value | OR (95%CI) | P value | |
| Age (< 60 yr vs ≥ 60 yr) | 0.471 | 0.556 (0.096-2.609) | 0.464 |
| Sex (female vs male) | 0.416 | 1.722 (0.327-9.531) | 0.515 |
| Location (colon vs rectum) | 0.107 | 2.615 (0.498-14.826) | 0.251 |
| cT stage (T4a vs T4b) | 0.247 | 1.221 (0.218-7.667) | 0.821 |
| cN stage (N0 vs N+) | 0.152 | 0.415 (0.104-1.337) | 0.145 |
| Tumor grade (WD/MD vs PD) | 0.509 | 3.071 (0.341-70.370) | 0.337 |
| CEA (≤ 5 vs > 5) | 0.894 | 0.611 (0.106-3.136) | 0.556 |
| Neoadjuvant chemotherapy (FOLFOX vs capecitabine) | 0.037a | 4.755 (2.118-88.203) | 0.046a |
| Radiation dose (< 50 Gy vs ≥ 50 Gy) | 0.265 | 0.507 (0.058-3.187) | 0.478 |
| Radiation-surgery interval (≤ 9 wk vs > 9 wk) | 0.074 | 0.836 (0.013-3.061) | 0.107 |
Table 4 lists tumor and nodal responses to neoadjuvant CCRT for each patient. Among all patients with cT4a CRC, 7 (8.1%) achieved pCR and 15 (17.4%) had tumor downstaging to ypT0-2. Moreover, among all patients with T4b disease, 6 (7%) achieved pCR and 13 (15.1%) had ypT0-2 after neoadjuvant CCRT. When all clinical factors were included in the analysis, the FOLFOX plus RT group had a higher number of patients with tumor downstaging to ypT0-2 than the capecitabine-based CCRT group (34.9% vs 7.1%; P = 0.022). Of the 42 patients with T4b disease, 13 underwent multivisceral resection and the remaining 29 underwent radical resection with the preservation of surrounding organs.
| Clinical staging | Pathologic T staging | ypN negative | ypN positive | Total | |||||
| ypT0 | ypT1 | ypT2 | ypT3 | ypT4a | ypT4b | ||||
| cT4a | 7 (8.1) | 3 (3.5) | 5 (5.8) | 24 (27.9) | 3 (3.5) | 2 (2.3) | 44 (51.2) | ||
| cT4b | 6 (7) | 0 (0) | 7 (8.1) | 22 (25.6) | 4 (4.6) | 3 (3.5) | 42 (48.8) | ||
| cN negative | 6 (7) | 1 (1.1) | 7 (8.1) | ||||||
| cN positive | 66 (76.8) | 13 (15.1) | 79 (91.9) | ||||||
| Total | 13 (15.1) | 3 (3.5) | 12 (13.9) | 46 (53.5) | 7 (8.1) | 5 (5.8) | 72 (83.8) | 14 (16.2) | 86 (100) |
No mortality was observed within 30 d after surgery. Two patients developed a wound abscess. One patient developed an intra-abdominal infection due to anastomotic leakage 1 mo after right hemicolectomy. Furthermore, three patients required surgical interventions because of adhesion ileus. Two patients developed rectovaginal fistulas. Of the 86 patients, 8 developed postoperative complications requiring intensive medical or surgical interventions (10.4%).
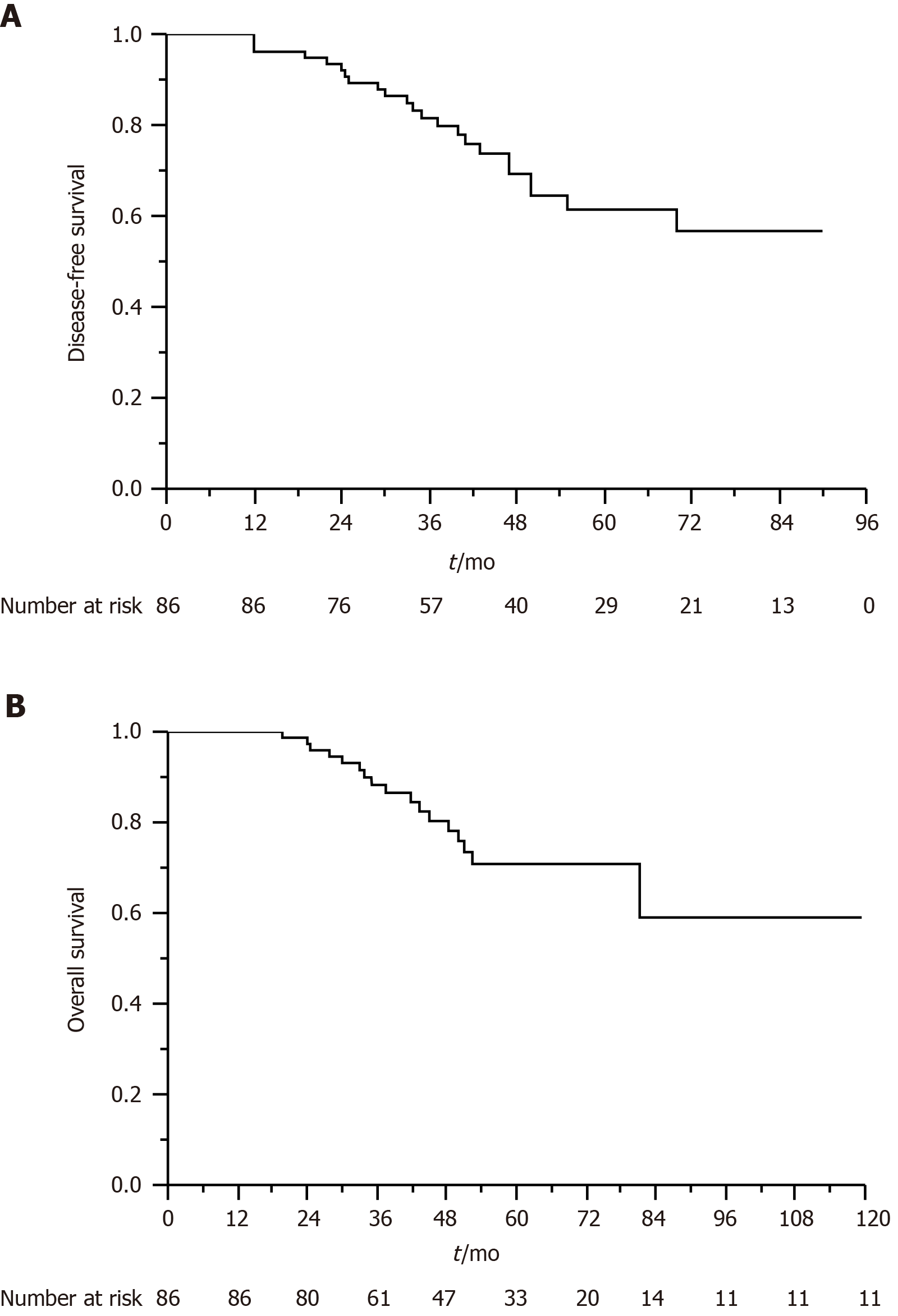
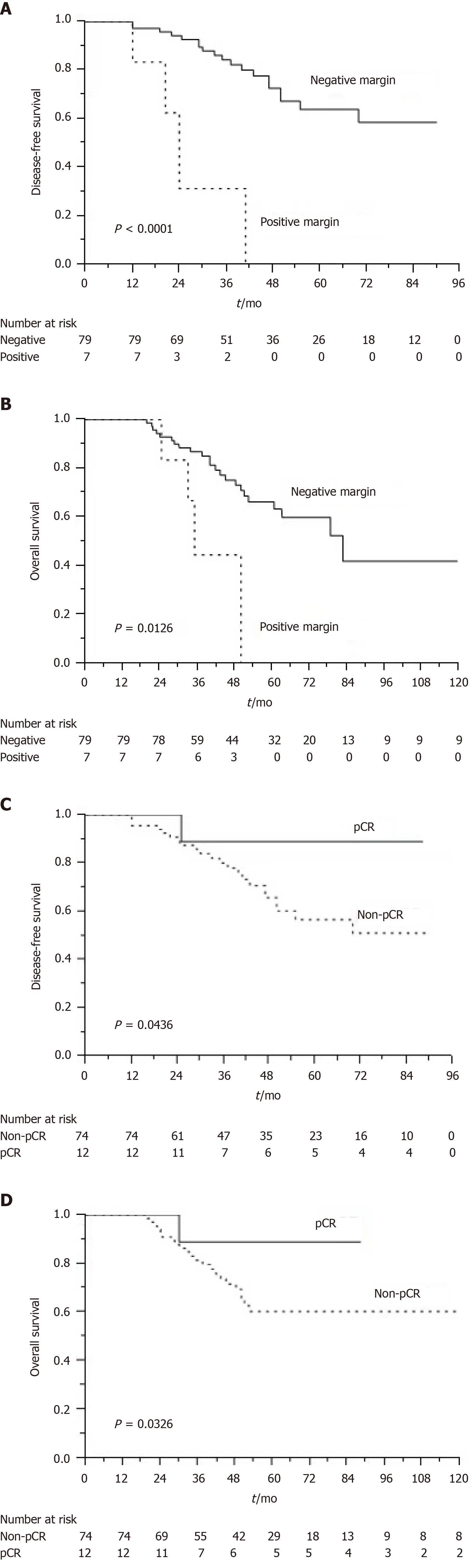
The median follow-up time was 47 mo (range, 17-120 mo). At the time of analysis, 37 patients had died. The estimated 5-year OS and DFS rates were 70.8% and 61.4%, respectively (Figure 1A and B). Table 5 presents the results of univariate and multivariate analyses for prognostic parameters used to predict DFS and OS rates. The multivariate analysis revealed that resection margin [hazard ratio (HR), 3.120; P = 0.014], ypN stage (HR, 3.549; P = 0.042), and pathological response (HR, 2.560; P = 0.017) were independent factors associated with DFS; moreover, resection margin (HR, 4.136; P = 0.001) and pathological response (HR, 2.977; P = 0.003) were independent factors associated with OS. The Kaplan-Meier method revealed that the number of patients with negative resection margins was significantly higher than that of those with involved resection margins (P < 0.001 and P = 0.012, respectively; Figure 2A and B). In addition, patients who achieved pCR had higher DFS and OS rates than those who did not (P = 0.043 and P = 0.032, respectively; Figure 2C and D).
| Variable | Disease-free survival | Overall survival | ||||
| Univariate P value | Multivariate | Univariate P value | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |||
| Age (< 60 vs ≥ 60 yrs) | 0.492 | 0.770 (0.255-1.618) | 0.492 | 0.729 | 0.621 (0.314-1.245) | 0.177 |
| Sex (female vs male) | 0.493 | 0.773 (0.375-1.626) | 0.493 | 0.678 | 0.906 (0.442-1.812) | 0.783 |
| Location (colon vs rectum) | 0.353 | 0.216 (0.102-1.231) | 0.153 | 0.346 | 0.291 (0.054-1.154) | 0.082 |
| cT stage (T4a vs T4b) | 0.127 | 2.423 (0.604-9.836) | 0.258 | 0.206 | 2.611 (0.752-9.054) | 0.128 |
| cN stage (N0 vs N+) | 0.127 | 0.690 (0.139-3.014) | 0.157 | 0.102 | 0.336 (0.094-1.087) | 0.078 |
| Tumor grade (WD/MD vs PD) | 0.335 | 0.503 (0.098-1.936) | 0.413 | 0.423 | 0.840 (0.247-2.500) | 0.765 |
| CEA (≤ 5 ng/mL vs > 5 ng/mL) | 0.418 | 1.422 (0.614-3.391) | 0.383 | 0.528 | 1.219 (0.581-2.622) | 0.602 |
| Neoadjuvant chemotherapy (FOLFOX vs Capecitabine) | 0.142 | 3.549 (0.944-7.467) | 0.082 | 0.117 | 2.846 (0.860-8.715) | 0.158 |
| Radiation dose (< 50 Gy vs ≥ 50 Gy) | 0.351 | 0.291 (0.054-1.154) | 0.182 | 0.327 | 2.525 (0.347-5.843) | 0.221 |
| Radiation-surgery interval (≤ 9 wk vs > 9 wk) | 0.086 | 1.236 (0.792-5.276) | 0.097 | 0.106 | 2.064 (0.589-7.062) | 0.167 |
| ypT stage (ypT3-4 vs ypT0-2) | 0.237 | 2.484 (0.744-5.691) | 0.636 | 0.097 | 2.150 (0.820-6.123) | 0.121 |
| ypN stage (ypN+ vs ypN0) | 0.005 | 3.120 (1.245-8.357) | 0.017a | 0.073 | 1.771 (0.435-6.255) | 0.405 |
| Lymphovascular invasion (positive vs negative) | 0.363 | 2.503 (0.724-7.788) | 0.141 | 0.175 | 3.046 (0.961-9.081) | 0.069 |
| Perineural invasion (positive vs negative) | 0.072 | 2.649 (0.869-8.976) | 0.087 | 0.091 | 1.222 (0.323-4.078) | 0.757 |
| Resection margin (positive vs negative) | 0.001 | 3.549 (1.004-12.747) | 0.014a | 0.013 | 4.136 (1.675-10.829) | 0.001a |
| Pathological response (non-pCR vs pCR) | 0.045 | 2.560 (1.186-6.013) | 0.042a | 0.031 | 2.977 (1.420-6.369) | 0.003a |
The failure patterns, according to the tumor location and clinical tumor stage, are summarized in Table 6. For patients with rectal cancer, cT4b disease resulted in a recurrence rate of 32.3%, which was higher than that (26.6%) among patients with cT4a disease. Among the patients with colon cancer, the recurrence rates in patients with cT4b and cT4a were 38.5% and 25%, respectively. A total of 19 patients (22.1%) developed distant metastases: The lung was the most common first site of distant metastasis (n = 9), followed by the liver (n = 5), bone (n = 2), para-aortic lymph nodes (n = 2), and peritoneal carcinomatosis (n = 1). Local recurrence was observed in 13 patients (15.1%). Only one patient with pCR developed peritoneal carcinomatosis and bone metastases 11 mo after surgery; she died of tumor progression 2 mo after developing distant metastases. No patient experienced local failure in the pCR group.
| Recurrence | Colon | Rectum | ||
| cT4a (%) | cT4b (%) | cT4a (%) | cT4b (%) | |
| Local/regional only | 2 (16.7) | 1 (7.7) | 1 (3.3) | 3 (9.7) |
| Distant only | 1 (8.3) | 2 (15.4) | 5 (16.7) | 5 (16.1) |
| Local/regional/distant | 0 (0) | 2 (15.4) | 2 (6.6) | 2 (6.5) |
| No recurrence | 9 (75) | 8 (61.5) | 22 (73.4) | 21 (67.7) |
| Total | 12 (100) | 13 (100) | 30 (100) | 31 (100) |
In general, cT4 CRC requires MVR to improve local control and survival. However, several studies have demonstrated that MVR leads to considerably high morbidity and mortality rates[3,19,20]. Therefore, in a population-based study of patients selected from the SEER registry, only 33.3% of 8380 patients with locally advanced adherent T4 CRC eventually underwent MVR, and the delivery of neoadjuvant RT was associated with decreased cases of MVR[21]. Accordingly, we evaluated the oncologic outcomes of patients with T4 CRC undergoing neoadjuvant CCRT and subsequent surgery.
Neoadjuvant CCRT followed by surgical resection is the main treatment for LARC[8,9]. To enhance the effects of CCRT in tumor downsizing, oxaliplatin is added to the fluoropyrimidine-based regimen during RT. Several phase III randomized trials have failed to demonstrate the superiority of the oxaliplatin-based regimen over fluoropyrimidine-based therapy, with only the German CAO/ARO/AIO-04 trial demonstrating a positive impact of FOLFOX-based CCRT on pCR[22-24]. However, some studies have extended the delivery of FOLFOX after CCRT and revealed that extending the oxaliplatin regimen resulted in higher rates of pCR and major regression compared with the delivery of FOLFOX only during RT (as done in the aforementioned phase III trials)[25,26]. To summarize, despite the disappointing results of concurrently administering oxaliplatin during RT, some studies have demonstrated that implementing a more intense neoadjuvant chemotherapy regimen either before radiation[27,28] or concurrently with radiation[29,30] or extending administration of chemotherapy to the resting period between RT and surgery[31-33] resulted in improved oncological outcomes. In our study, we delivered FOLFOX prior to, concurrently with, and following RT for most patients with cT4 CRC (86%) in an attempt to maximize the effects of CCRT on tumor regression for those with locally advanced adherent CRC. The remaining patients with cT4 disease received capecitabine only during RT because the neoadjuvant FOLFOX regimen plus RT was unavailable at that time.
Radiation-induced tumor regression is time dependent[17,31]. In this study, a long interval between radiation and surgery tended to be associated with high pCR rates (P = 0.074), possibly because we included only locally advanced T4 CRC for analysis; advanced tumors require high-intensity treatment. Garcia et al[26] reported that adding cycles of mFOLFOX6 during the radiation-surgery interval and prolonging the interval between radiation and surgery could increase pCR rates. Liang et al[34] observed that the addition of chemotherapy during the resting period, with a long interval between radiation and surgery, resulted in improved pCR and DFS rates compared with the nonaddition of consolidation chemotherapy.
The pCR rate in the current study was 14%, which is lower than those reported in other studies[23-26]. For rectal cancer treatment, neoadjuvant CCRT resulted in varying pCR rates, ranging from 13% to 38%[9,27-29]. Numerous clinical predictors of pCR have been identified, and advanced clinical T stage has been associated with a relatively low pCR rate[15-17]. Because our study focused on T4 disease, we expected to observe a relatively low pCR rate.
MVR has a high R0 resection rate for patients with locally advanced T4 CRC[19,21,35]. Such aggressive surgery yields improved outcomes, but at the cost of increases in postoperative morbidity and mortality rates. Studies have reported such surgery to be associated with morbidity rates of 11%-49% and mortality rates of 0%-9%[19-21,35]. The tumor downstaging of T4 disease facilitates complete tumor resection; this may thus prevent complications of MVR. In our study, only 13 of 42 patients (31%) with cT4b disease required MVR after neoadjuvant CCRT. Qiu et al[36] revealed that MVR was required in only seven patients (33.3%) with locally advanced colon cancer. Therefore, neoadjuvant CCRT could diminish tumor infiltration and the necessity of MVR, which may subsequently reduce the occurrence of postoperative complications.
The response to neoadjuvant CCRT varies among patients. Numerous studies have reported that patients who achieve pCR tend to exhibit excellent tumor control and survival[13,32,37]. Therefore, many researchers have identified some predictors of pCR, including cT3/4 and N+[15,17,32]. However, few studies have evaluated the response of cT4 CRC to CCRT or the predictors of pCR in patients with cT4 CRC undergoing neoadjuvant CCRT. In the current study, patients who underwent an intensified neoadjuvant therapy and received the FOLFOX regimen before, during, and after RT had higher chances of achieving pCR than those who received capecitabine-based CCRT. The Chinese FOWARC trial demonstrated that mFOLFOX6-based preoperative CCRT had a higher pCR rate than fluorouracil-based treatment[25]. Our preoperative intensified regimen was similar to the regimens used in the Chinese FOWARC study[33]. Therefore, our results seem to accord with the Chinese FOWARC study.
The current study revealed that R0 resection was associated with favorable DFS and OS, which accords with the results of other studies[3,6]. R0 resection rates have been reported to range from 40% to 100% in patients with locally advanced T4 CRC who underwent radical resection with or without neoadjuvant therapy[10,35,36]. Cukier et al[10] analyzed 33 patients, all of whom underwent R0 resection after CCRT, with locally adherent colon cancer patients who received neoadjuvant CCRT and MVR. Qiu et al[36] studied 21 patients with locally advanced sigmoid colon cancer who underwent preoperative CCRT followed by surgery, and they observed an R0 resection rate of 95.2%. The published R0 resection rates (generally > 90%) in patients who underwent CCRT followed by surgery were higher than those in patients who underwent surgery first (range: 40%-90%).
The benefits of neoadjuvant CCRT for locally advanced colon cancer remain controversial. Two single-arm cohort studies have evaluated the role of neoadjuvant CCRT in locally advanced colon cancer, and both studies have reported high R0 resection rates (100% and 95.2%, respectively)[10,36]. Zhou et al[12] compared the oncological results of patients (n = 58) with locally advanced colon cancer who underwent neoadjuvant FOLFOX-based CCRT followed by surgery with those of patients (n = 44) with the same disease who received surgery without neoadjuvant CCRT; they determined that neoadjuvant CCRT improved the pCR and resection rates, in addition to improving 3-year DFS rates.
We acknowledge some limitations of the current study. First, the sample size was relatively small, and the follow-up time was short. Consequently, long-term oncological outcomes and adverse events could not be adequately investigated. Second, this was a retrospective study; therefore, selection bias was possible. Third, chemotherapeutic regimens, radiation doses, and radiation techniques were not identical among all enrolled patients.
Neoadjuvant CCRT results in high pCR and complete resection rates for patients with T4 CRC. The aggressive approach involving the administration of the FOLFOX regimen before, during, and after RT proves to be safe and capable of improving pCR in patients with cT4 CRC. Negative resection margins and pCR are significantly associated with survival. Further prospective randomized studies are warranted to validate our results.
Patients diagnosed with clinical T4 colorectal cancer are at high risk of recurrence because of difficulty in achieving free surgical margins. Multi-visceral resection is needed for the complete resection of the disease.
Patients diagnosed with clinical T4 colorectal cancer are at high risk of recurrence because of difficulty in achieving free surgical margins. Multi-visceral resection is needed for the complete resection of the disease.
Patients diagnosed with clinical T4 colorectal cancer are at high risk of recurrence because of difficulty in achieving free surgical margins. Multi-visceral resection is needed for the complete resection of the disease.
We retrospectively reviewed colorectal cancer (CRC) patients from the database of The Kaohsiung Medical University Hospital from August 2010 to September 2018. Eighty-six patients who completed neoadjuvant chemoradiation and radical resection were enrolled for analysis. The neoadjuvant regimens in this study were capecitabine plus radiotherapy, and FOLFOX plus radiotherapy. The radiation dose was 45 to 50.4 Gy with a daily fraction of 1.8 or 2 Gy. We used multivariate logistic regression analysis to identify independent predictors of pathological complete response (pCR). Using Kaplan-Meier method and log-rank test, we measured the disease-free survival (DFS) and overall survival (OS) between groups, where multivariate Cox proportional hazard models were used to analyze the impact of pCR and resection margins as prognostic factors.
The rates of pCR and R0 resection were 14% and 91.9%, respectively. Nineteen patients (22.1%) developed distant metastases and local recurrence was found in 13 patients (15.1%). Patients who underwent FOLFOX plus radiotherapy were more likely to achieve pCR compared to those who received capecitabine plus radiotherapy (P = 0.046). Multivariate analysis revealed that an R0 resection was associated with favorable DFS (P = 0.014) and OS (P = 0.001), and the pCR group obtained better DFS (P = 0.042) and OS (P = 0.003) than the non-pCR group.
Neoadjuvant chemoradiation results in high rates of pCR and complete resection for patients with T4 CRC. R0 resection and pCR are significant predictors of favorable survival.
Neoadjuvant chemoradiation should be considered as one of the treatment options in T4 colon and rectal cancer. Further prospective randomized studies are warranted to validate our results.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ieni A, Sergi C, Yeh H S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Li JH
| 1. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2068] [Article Influence: 188.0] [Reference Citation Analysis (0)] |
| 2. | Ministry of Health and Welfare, the Executive Yuan, Republic of China. Health and Vital Statistics. Available from: http://www.mohw.gov.tw/CHT/Ministry/. |
| 3. | Lehnert T, Methner M, Pollok A, Schaible A, Hinz U, Herfarth C. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg. 2002;235:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Govindarajan A, Fraser N, Cranford V, Wirtzfeld D, Gallinger S, Law CH, Smith AJ, Gagliardi AR. Predictors of multivisceral resection in patients with locally advanced colorectal cancer. Ann Surg Oncol. 2008;15:1923-1930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Ueno H, Mochizuki H, Akagi Y, Kusumi T, Yamada K, Ikegami M, Kawachi H, Kameoka S, Ohkura Y, Masaki T, Kushima R, Takahashi K, Ajioka Y, Hase K, Ochiai A, Wada R, Iwaya K, Shimazaki H, Nakamura T, Sugihara K. Optimal colorectal cancer staging criteria in TNM classification. J Clin Oncol. 2012;30:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Croner RS, Merkel S, Papadopoulos T, Schellerer V, Hohenberger W, Goehl J. Multivisceral resection for colon carcinoma. Dis Colon Rectum. 2009;52:1381-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Gebhardt C, Meyer W, Ruckriegel S, Meier U. Multivisceral resection of advanced colorectal carcinoma. Langenbecks Arch Surg. 1999;384:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4448] [Article Influence: 211.8] [Reference Citation Analysis (1)] |
| 9. | Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ, Wolmark N. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-5130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 699] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 10. | Cukier M, Smith AJ, Milot L, Chu W, Chung H, Fenech D, Herschorn S, Ko Y, Rowsell C, Soliman H, Ung YC, Wong CS. Neoadjuvant chemoradiotherapy and multivisceral resection for primary locally advanced adherent colon cancer: a single institution experience. Eur J Surg Oncol. 2012;38:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Huang CM, Huang MY, Ma CJ, Yeh Y-, Tsai HL, Huang CW, Huang CJ, Wang JY. Neoadjuvant FOLFOX chemotherapy combined with radiotherapy followed by radical resection in patients with locally advanced colon cancer. Radiat Oncol. 2017;12:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Zhou J, Guo Z, Yu W, Li S, Qiao W. Clinical Evaluation of Preoperative Radiotherapy Combined with FOLFOX Chemotherapy on Patients with Locally Advanced Colon Cancer. Am Surg. 2019;85:313-320. [PubMed] |
| 13. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 949] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 14. | Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, Khanduja KS. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 15. | Huh JW, Kim HR, Kim YJ. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum. 2013;56:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Peng H, Wang C, Xiao W, Lin X, You K, Dong J, Wang Z, Yu X, Zeng Z, Zhou T, Gao Y, Wen B. Analysis of Clinical characteristics to predict pathologic complete response for patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. J Cancer. 2018;9:2687-2692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Al-Sukhni E, Attwood K, Mattson DM, Gabriel E, Nurkin SJ. Predictors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Ann Surg Oncol. 2016;23:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Mace AG, Pai RK, Stocchi L, Kalady MF. American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum. 2015;58:32-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 19. | Park S, Lee YS. Analysis of the prognostic effectiveness of a multivisceral resection for locally advanced colorectal cancer. J Korean Soc Coloproctol. 2011;27:21-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Nakafusa Y, Tanaka T, Tanaka M, Kitajima Y, Sato S, Miyazaki K. Comparison of multivisceral resection and standard operation for locally advanced colorectal cancer: analysis of prognostic factors for short-term and long-term outcome. Dis Colon Rectum. 2004;47:2055-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Govindarajan A, Coburn NG, Kiss A, Rabeneck L, Smith AJ, Law CH. Population-based assessment of the surgical management of locally advanced colorectal cancer. J Natl Cancer Inst. 2006;98:1474-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P, Bonetti A, Negru ME, Tronconi MC, Luppi G, Silvano G, Corsi DC, Bochicchio AM, Chiaulon G, Gallo M, Boni L. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 23. | Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O, Mirabel X, Denis B, Mineur L, Berdah JF, Mahé MA, Bécouarn Y, Dupuis O, Lledo G, Montoto-Grillot C, Conroy T. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 575] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 24. | O'Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, Pitot HC, Shields AF, Landry JC, Ryan DP, Parda DS, Mohiuddin M, Arora A, Evans LS, Bahary N, Soori GS, Eakle J, Robertson JM, Moore DF Jr, Mullane MR, Marchello BT, Ward PJ, Wozniak TF, Roh MS, Yothers G, Wolmark N. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32:1927-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 328] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 25. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34:3300-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 26. | Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 27. | Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, Tebbutt N, Hill M, Ross PJ, Massey A, Oates J. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 28. | Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E, Mengual JL, Saigi E, Estevan R, Mira M, Polo S, Hernandez A, Gallen M, Arias F, Serra J, Alonso V. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 29. | Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R; German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 500] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 30. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC; EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2034] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 31. | Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM; Timing of Rectal Cancer Response to Chemoradiation Consortium. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254:97-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 32. | Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, São Julião GP, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52:1927-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 33. | Huang CM, Huang MY, Tsai HL, Huang CW, Ma CJ, Yeh YS, Juo SH, Huang CJ, Wang JY. An observational study of extending FOLFOX chemotherapy, lengthening the interval between radiotherapy and surgery, and enhancing pathological complete response rates in rectal cancer patients following preoperative chemoradiotherapy. Therap Adv Gastroenterol. 2016;9:702-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Liang HQ, Dong ZY, Liu ZJ, Luo J, Zeng Q, Liao PY, Wu DH. Efficacy and safety of consolidation chemotherapy during the resting period in patients with local advanced rectal cancer. Oncol Lett. 2019;17:1655-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Eveno C, Lefevre JH, Svrcek M, Bennis M, Chafai N, Tiret E, Parc Y. Oncologic results after multivisceral resection of clinical T4 tumors. Surgery. 2014;156:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Qiu B, Ding PR, Cai L, Xiao WW, Zeng ZF, Chen G, Lu ZH, Li LR, Wu XJ, Mirimanoff RO, Pan ZZ, Xu RH, Gao YH. Outcomes of preoperative chemoradiotherapy followed by surgery in patients with unresectable locally advanced sigmoid colon cancer. Chin J Cancer. 2016;35:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Huang CM, Huang CW, Ma CJ, Yeh YS, Su WC, Chang TK, Tsai HL, Juo SH, Huang MY, Wang JY. Predictive Value of FOLFOX-Based Regimen, Long Interval, Hemoglobin Levels and Clinical Negative Nodal Status, and Postchemoradiotherapy CEA Levels for Pathological Complete Response in Patients with Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy. J Oncol. 2020;2020:9437684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |