Published online Dec 15, 2020. doi: 10.4251/wjgo.v12.i12.1416
Peer-review started: July 11, 2020
First decision: September 17, 2020
Revised: September 28, 2020
Accepted: October 28, 2020
Article in press: October 28, 2020
Published online: December 15, 2020
Processing time: 152 Days and 9.3 Hours
For the rarity of type 3 gastric neuroendocrine tumours (g-NETs), their clinicopathological characteristics and prognosis are not well illustrated.
To describe the clinicopathological features and outcome of type 3 g-NETs in the Chinese population.
Based on the 2019 WHO pathological classification, the clinicopathological characteristics and prognosis of patients with type 3 g-NETs in China were retrospectively analysed.
A total of 77 patients (55.8% of females) with type 3 g-NETs were analysed, with a median age of 48 years (range: 28-79 years). The tumours were mainly located in the gastric fundus/body (83.1%) and were mostly solitary (83.1%), with a median size of 1.5 cm (0.8-3.5 cm). Of these, there were 37 G1 tumours (48.1%), 31 G2 (40.3%), and 9 G3 (11.7%). Ten (13.0%) and 24 (31.2%) patients had lymph node and distant metastasis, respectively. In addition, type 3 g-NETs were heterogeneous. Compared with G1 NETs, G2 NETs had a higher lymph node metastasis rate, and G3 NETs had a higher distant metastasis rate. G1 and G2 NETs with stage I/II disease (33/68) received endoscopic treatment, and no tumour recurrence or tumour-related death was observed within a median follow-up time of 36 mo. Grade and distant metastasis were identified to be independent risk factors for prognosis in multivariable analysis.
Type 3 g-NETs are obviously heterogeneous, and the updated WHO 2019 pathological classification may be used to effectively evaluate their biological behaviors and prognosis. Also, endoscopic treatment should be considered for small (< 2 cm), low grade, superficial tumours.
Core Tip: Type 3 gastric neuroendocrine tumours (g-NETs) were heterogeneous based on the 2019 WHO pathological classification. Endoscopic treatment was safe and effective for patients with G1 NETs having tumours under 2 cm, confined to the mucosa or submucosa. The prognosis of type 3 g-NETs was related to stage and grade, which were its independent prognostic factors, and the 2019 WHO pathological classification was effective to predict the biological behaviors and prognosis of type 3 g-NETs.
- Citation: Li YL, Qiu XD, Chen J, Zhang Y, Li J, Xu JM, Wang C, Qi ZR, Luo J, Tan HY. Clinicopathological characteristics and prognosis of 77 cases with type 3 gastric neuroendocrine tumours. World J Gastrointest Oncol 2020; 12(12): 1416-1427
- URL: https://www.wjgnet.com/1948-5204/full/v12/i12/1416.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i12.1416
Gastric neuroendocrine neoplasms (g-NENs) are a group of rare tumours with strong heterogeneity originating from neuroendocrine cells. With the development of upper gastrointestinal endoscopy and imaging, an increasing number of patients with g-NENs have been found[1,2]. According to the Surveillance, Epidemiology, and End Results database, the age-adjusted incidence increased 6.4 times from 1973 (1.09/100000) to 2012 (6.98/100000). The digestive system was the most common site of NENs, and the stomach was ranked fourth, followed by the small intestine, rectum, and pancreas[3]. In South Korea, Austria, and Argentina, g-NENs accounted for 14.6%, 23%, and 6.9% of gastrointestinal and pancreatic neuroendocrine tumours, respectively[4-6].
According to differentiation, g-NENs can be divided into well-differentiated gastric neuroendocrine tumours (g-NETs) and poorly differentiated gastric neuroendocrine carcinomas (g-NECs). Well-differentiated g-NETs are classified as types 1 to 3 g-NETs[7-9]. Type 1 g-NETs are associated with autoimmune atrophic gastritis with elevated serum gastrin and gastric acid deficiency, while type 2 g-NETs with hypergastrinemia and high gastric acid secretion are related to gastrinoma or multiple endocrine neoplasia type 1 (MEN-1). Patients with type 3 g-NETs have normal serum gastrin, gastric acid secretion, and no related background disease. Types 1 and 2 g-NETs are generally considered indolent and have a low risk of malignancy, while type 3 g-NETs often show aggressive characteristics, including lymphatic invasion and tumour infiltration beyond the submucosa, and a poor prognosis[10,11]. Given the rarity, there are few related studies on type 3 g-NETs. Also, the World Health organization (WHO) pathological classification of gastroenteropancreatic neuroendocrine neoplasms was updated in 2019 and its role has not been well illustrated. Therefore, we are dedicated to exploring the clinicopathological characteristics and prognostic factors of this disease based on the 2019 WHO grading system.
We retrospectively analysed the clinicopathological features of 77 patients with type 3 g-NETs at four NET centres in China from July 2012 to December 2018 [China-Japan Friendship Hospital (n = 51), The First Affiliated Hospital of Sun Yat-sen University (n = 17), Peking University Cancer Hospital (n = 6), and The Fifth Medical Center, Chinese PLA General Hospital (n = 3)]. Patients with type 3 g-NETs met the inclusion criteria: (1) Histologically proven well-differentiated g-NETs; (2) Normal serum gastrin; and (3) No evidence of types 1 and 2 g-NETs. All pathological results were reviewed by an experienced pathologist. The study obtained the patients’ informed consent and was approved by the clinical research ethics committee of the China-Japan Friendship Hospital.
Endoscopy was used to find the lesions on the stomach and get biopsy, and endoscopic ultrasound (EUS) was performed to assess the infiltration of the gastric wall only when tumour size was larger than 1 cm. Computed tomography and magnetic resonance imaging were used to assess the condition of gastric lesions, the relationship between lesion and adjacent organs or tissues, the status of regional lymph nodes, and distant metastasis. Some patients may undergo somatostatin receptor scintigraphy or 68Ga DOTATATE positron emission tomography/computed tomography to assess the status of lymph node and distant metastases.
The 5th edition WHO classification (2019) of gastroenteropancreatic neuroendocrine neoplasms was used to determine the pathological grade[9], which is divided into G1 (Ki67 index < 3% or mitoses/2 mm2 < 2), G2 (3% ≤ Ki67 index ≤ 20% or 2 ≤ mitoses/2 mm2 ≤ 20), and G3 (Ki67 index > 20% or mitoses/2 mm2 > 20). Tumour staging was performed using the AJCC 8th Edition Gastric Neuroendocrine Tumour Staging[12].
Patients were followed by hospitalization, outpatient service, or telephone. The starting point was the time when the patient's histopathology yielded a diagnosis of g-NET. The deadline for follow-up was August 12, 2019. The end point of the follow-up was the time of tumour-specific death.
Categorical variables are reported as frequencies or percentages and continuous variables are expressed as medians (interquartile ranges). Comparisons between groups were performed using the Mann-Whitney U test or Kruskal-Wallis test. The Kaplan-Meier method was used for survival analysis, and comparisons were performed using the log-rank test. Multivariable survival analyses were also performed to rule out dependent variables using Cox proportional hazards regression models. When the two-tailed P value was less than 0.05, the difference was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics ver. 24 (IBM, Chicago IL, United States).
We analysed a total of 77 patients with type 3 g-NETs (Table 1), aged between 28 and 79 years old, with a median age of 48 years. There were 34 male patients (44.2%) and 43 female patients (55.8%). The tumours were mainly located in the gastric fundus/body (64/77, 83.1%), and most of them were single lesions (64/77, 83.1%), with a median tumour size of 1.5 cm (0.8-3.5 cm). Of the 73 patients with a known endoscopic appearance, 45 of the tumours were polypoid lesions, accounting for 61.6%, 17 were ulcers (23.3%), and 11 were bulges (15.1%). Among the 34 tumours showing gastric wall invasion, most were localized in the mucosa (M) and submucosa (22/34, 64.7%), five (14.7%) had invaded the muscularis propria (MP), and seven (20.6%) had invaded beyond the MP. The median Ki67 index of 77 patients was 3% (1%-10%), and there were 37 patients with G1 NETs (48.1%), 31 with G2 NETs (40.3%), and 9 with G3 NETs (11.7%). In terms of staging, there were 30 (39%) stage I tumours, 12 (15.6%) stage II, 11 (14.3%) stage III, and 24 (31.2%) stage IV, respectively. In terms of metastatic status, 10 patients (13.0%) had lymph node metastases, and 24 (31.2%) had distant metastases.
| Total, n (%) | G1 NETs, n (%) | G2 NETs, n (%) | G3 NETs, n (%) | P value | G2 vs G1 | G3 vs G1 | G3 vs G2 | |
| Number | 77 (100) | 37 (48.1) | 31 (40.3) | 9 (11.7) | ||||
| Age (range) | 48 (28-79) | 47 (29-79) | 47.5 (28-78) | 62 (33-68) | 0.048 | 1 | 0.077 | 0.049 |
| Gender | 0.016 | 1.000 | 0.011 | 0.039 | ||||
| Male | 34 (44.2) | 13 (35.1) | 13 (41.9) | 8 (88.9) | ||||
| Female | 43 (55.8) | 24 (64.9) | 18 (58.1) | 1 (11.1) | ||||
| Size (cm) | 1.5 (0.8-3.5) | 0.8 (0.5-1.2) | 3 (1.5-5) | 4 (2.5-5.25) | < 0.001 | < 0.001 | < 0.001 | 1.000 |
| Site | 0.122 | |||||||
| Cardia | 10 (13) | 2 (5.4) | 6 (19.4) | 2 (22.2) | ||||
| Fundus/body | 64 (83.1) | 34 (91.9) | 24 (77.4) | 6 (66.7) | ||||
| Antrum | 3 (3.9) | 1 (2.7) | 1 (3.2) | 1 (11.1) | ||||
| Number | 0.087 | |||||||
| 1 | 64 (83.1) | 27 (73) | 28 (90.3) | 9 (100) | ||||
| ≥ 2 | 13 (16.9) | 10 (27) | 3 (9.7) | 0 | ||||
| EA | 0.002 | 0.001 | 0.055 | 1.000 | ||||
| Polyp | 45 (61.6) | 29 (82.9) | 12 (41.4) | 4 (44.4) | ||||
| Bulge | 11 (15.1) | 4 (11.4) | 6 (20.7) | 1 (11.1) | ||||
| Ulcer | 17 (23.3) | 2 (5.7) | 11 (37.9) | 4 (44.4) | ||||
| Unknown | 4 | 2 | 2 | 0 | ||||
| Infiltration | 0.014 | 0.014 | 0.290 | 1.000 | ||||
| M/SM | 22 (64.7) | 17 (85.0) | 4 (36.4) | 1 (33.3) | ||||
| MP | 5 (14.7) | 2 (10) | 2 (18.2) | 1 (33.3) | ||||
| Beyond MP | 7 (20.6) | 1 (5.0) | 5 (45.5) | 1 (33.3) | ||||
| Unknown | 43 | 17 | 20 | 6 | ||||
| Ki67 (%) | 3 (1-10) | 1 (1-1) | 8 (4-10) | 30 (25-37.5) | < 0.001 | < 0.001 | < 0.001 | 0.047 |
| LNM only | 10 (13.0) | 2 (5.4) | 7 (22.6) | 1 (11.1) | < 0.001 | 0.027 | 0.439 | 1.000 |
| DM | 24 (31.2) | 2 (5.4) | 15 (48.4) | 7 (77.7) | < 0.001 | < 0.001 | < 0.001 | 0.288 |
| Stage | < 0.001 | < 0.001 | < 0.001 | 0.494 | ||||
| I | 30 (39.0) | 26 (70.3) | 4 (12.9) | 0 | ||||
| II | 12 (15.6) | 7 (18.9) | 5 (16.1) | 0 | ||||
| III | 11 (14.3) | 2 (5.4) | 7 (22.6) | 2 (22.2) | ||||
| IV | 24 (31.2) | 2 (5.4) | 15 (48.4) | 7 (77.8) | ||||
| Treatment | < 0.001 | < 0.001 | < 0.001 | 0.510 | ||||
| ER | 33 (42.9) | 29 (78.4) | 4 (12.9) | 0 | ||||
| Surgery | 17 (22.1) | 5 (13.5) | 10 (32.3) | 2 (22.2) | ||||
| SSA | 6 (7.8) | 1 (2.7) | 4 (12.9) | 1 (11.1) | ||||
| CBCT | 21 (27.3) | 2 (5.4) | 13 (41.9) | 6 (66.7) |
Among the 77 patients, 33 (42.9%) underwent endoscopic treatment, including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), 17 (22.1%) underwent surgical treatment, 6 (7.8%) received somatostatin analogs, and 21 (27.3%) received chemotherapy-based comprehensive treatment (CBCT). Treatments for G1 NETs were mainly endoscopic treatment (29/37, 78.4%), while G2 and G3 NETs were treated by surgery (12/40, 30%) and CBCT (19/40, 47.5%). It is worth noting that among the 33 patients treated by endoscopic resection (consisting of patients with G1 [29/33, 87.9%] and G2 NETs [4/33 12.1%]), no tumour recurrence or tumour-related death was observed within a median follow-up period of 36 mo (30-57 mo). The tumour size of these patients did not exceed 2 cm (range: 0.2-1.8 cm), and the median Ki67 index was 1% (1%-10%). Among the 17 patients with available data on gastric wall invasion, the tumours were limited to the mucosa (7/17, 41.2%) and submucosa (10/17, 58.8%). The 33 patients with endoscopic treatment were all in the early stage of the disease (28 in stage I and 5 in stage II), and no lymph node metastasis or distant metastasis was observed (Table 2).
| Endoscopic resection (n = 33), n (%) | |
| Size, median (25th–75th percentile), cm (range) | 0.7 (0.5-1.1); (0.2-1.8) |
| Infiltration | |
| M/SM | 17 (100%) |
| MP/beyond MP | 0 |
| Unknown | 16 |
| Ki67, median (25th–75th percentile), % | 1 (1-10) |
| Grade | |
| G1 | 29 (87.9) |
| G2 | 4 (12.1) |
| G3 | 0 |
| Stage | |
| I | 28 (84.8) |
| II | 5 (15.2) |
| III-IV | 0 |
| Recurrence or cancer-specific death | 0 |
As shown in Table 1, type 3 g-NETs had significant heterogeneity. Patients with G1, G2, and G3 NETs had their own clinicopathological characteristics. They was a significant difference in terms of age, gender, tumour size, endoscopic appearance, depth of gastric wall invasion, lymph node metastasis, distant metastasis, and treatment. The median tumour diameters of G2 and G3 NETs were 3 cm and 4 cm, respectively, which were significantly larger than that of G1 NETs (0.8 cm) (P < 0.001). In terms of endoscopic appearance, G2 and G3 NETs were mainly polypoid and ulcer-like lesions, while G1 NETs were mainly polypoid lesions (G2 vs G1 NETs, P = 0.001; G3 vs G1 NETs, P = 0.055). Compared with G1 NETs, G2 NETs had a higher proportion of invasion in the MP and beyond the MP (63.7% vs 15%, P = 0.014) and lymph node metastasis (22.6% vs 5.4%, P = 0.027). There were only two (5.4%) cases of G1 NETs with distant metastasis, while 15 (48.4%) and 7 (77.8%) cases of G2 and G3 NETs had distant metastasis (G2 vs G1 NETs, P < 0.001; G3 vs G1 NETs, P < 0.001). In terms of staging, the stage of G1 NETs patients was mostly early (stage I-II, 89.2%), while G2 and G3 NETs patients mostly had late stage (stage III-IV, 71% and 100%, respectively) (G2/G3 vs G1, P <0.001).
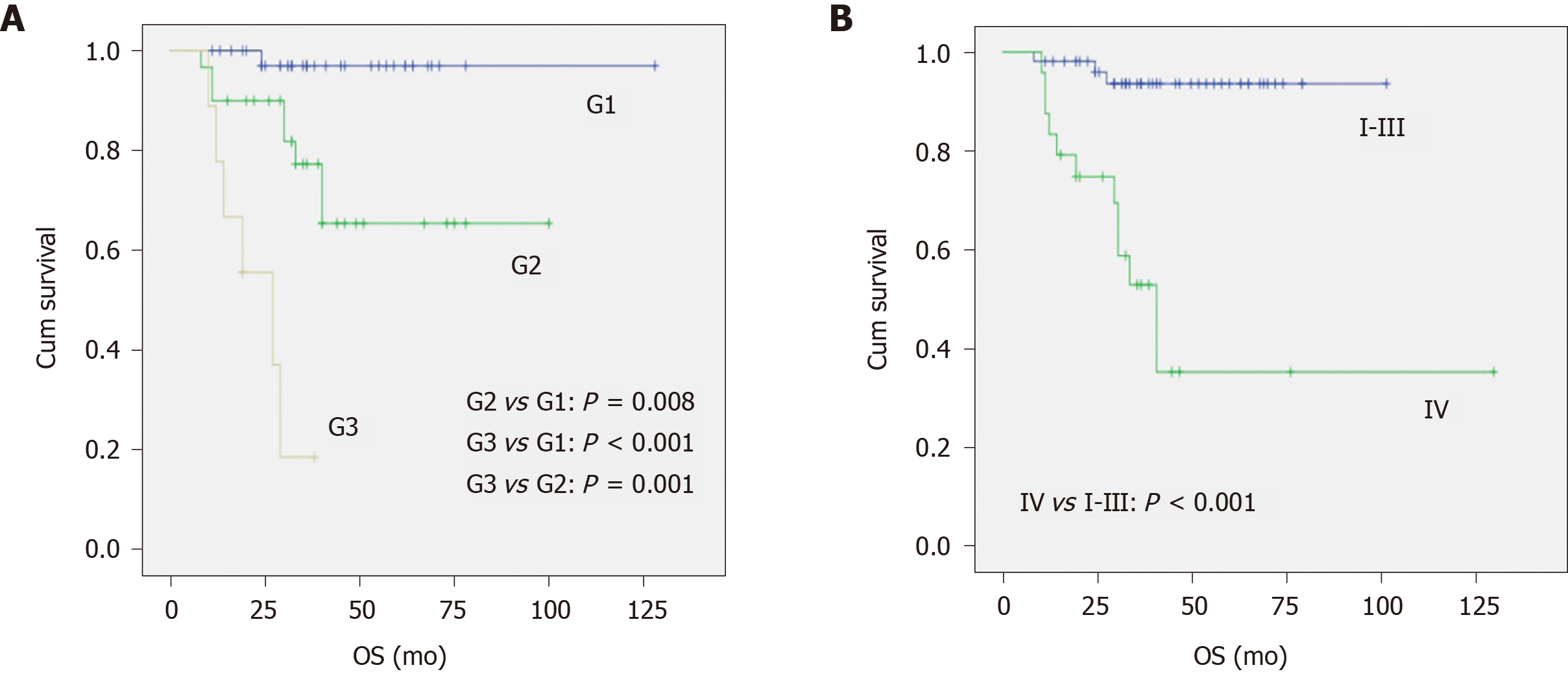
With a median follow-up period of 35 mo (24-52 mo), the 3-year tumour-specific survival of patients with type 3 g-NETs was 75%. From the univariable analysis (Table 3), we can see that tumour size, endoscopic appearance, gastric wall invasion, grade, clinical stage, and treatment were significantly related to prognosis. Pathological grade [G3 vs G1, hazard ratio (95% confidence interval): 20.58 (1.942-218.11), P = 0.012] and distant metastasis [stage IV vs I-III, hazard ratio (95% confidence interval): 4.51 (1.10-18.41), P = 0.036] were independent risk factors affecting prognosis (Table 4). As shown in Figure 1A, there was a significant difference in the survival of patients with G1, G2, and G3 NETs (P < 0.001), with 3-year tumour-specific survival rates of 96%, 66%, and 17%, respectively. In addition, the median survival time of patients with G3 NETs was 27 mo, while that of patients with G1 and G2 NETs was not reached. As shown in Figure 1B, the prognosis of patients with distant metastasis was significantly better than that of patients without (P < 0.001). The median survival of patients with distant metastasis was 40 mo, while the median survival of patients without distant metastasis was not reached.
| HR (95%CI) | P value | |
| Age | ||
| ≤ 45 years old | 1 | |
| > 45 years old | 1.92 (0.61-6.06) | 0.264 |
| Gender | ||
| Male | 1 | |
| Female | 0.87 (0.32-2.41) | 0.794 |
| Size | ||
| < 2 cm | 1 | |
| ≥ 2 cm | 10.07 (2.27-44.67) | 0.002 |
| Site | ||
| Cardia | 1 | |
| Fundus/body | 0.88 (0.20-3.98) | 0.871 |
| Antrum | 5.817 (0.81-42.01) | 0.081 |
| Number | ||
| 1 | 1 | |
| ≥2 | 0.33 (0.04-2.48) | 0.279 |
| EA | ||
| Polyp | 1 | |
| Bulge | 2.37 (0.45-12.54) | 0.312 |
| Ulcer | 4.20 (1.33-13.23) | 0.014 |
| Infiltration | ||
| M/SM | 1 | |
| MP | 8.57 (0.77-95.33) | 0.081 |
| Beyond MP | 3.02 (0.19-48.42) | 0.436 |
| Grade | ||
| G1 | 1 | |
| G2 | 9.77 (1.22-78.10) | 0.032 |
| G3 | 61.68 (7.01-542.62) | < 0.001 |
| Stage | ||
| I-III | 1 | |
| IV | 11.15 (3.13-39.66) | < 0.001 |
| Treatment | ||
| ER or surgery | 1 | |
| SSA | 4.65 (0.47-45.57) | 0.187 |
| CBCT | 11.38 (3.16-40.93) | < 0.001 |
| HR (95%CI) | P value | |
| Grade | ||
| G1 | 1 | |
| G2 | 4.923 (0.55-43.76) | 0.153 |
| G3 | 20.58 (1.942-218.11) | 0.012 |
| Stage | ||
| I-III | 1 | |
| IV | 4.51 (1.10-18.41) | 0.036 |
Type 3 g-NETs are a rare tumour with some clinicopathological characteristics and considered to be more aggressive[13-15]. With the increasing incidence of neuroendocrine neoplasms[1-3,16], it is gradually receiving attention from clinicians. The prognosis of type 3 g-NETs was worse with a 3-year survival rate of 75% in our study, while the survival rate of type 1 g-NETs was found to be almost 100%[17,18], followed by type 2 (60%-90%)[11].
There were several reasons for the malignant behavior and worse outcome of type 3 g-NETs. Unlike types 1 and 2 g-NETs, originating from enterochromaffin-like cells and stimulated by high serum gastrin with related background diseases, type 3 g-NETs were sporadic with no related diseases and normal gastrin levels. The origin of the cells in type 3 g-NETs are unclear yet, which may be derived from different neuroendocrine cells[9]. It may account for the poor prognosis of type 3 g-NETs. In addition, compared with types 1 and 2, type 3 g-NETs seemed to have a higher Ki67 index[19,20]. In our study, a higher Ki67 index was negatively correlated with the prognosis of type 3 g-NETs. Different molecular mechanisms have been described between well-differentiated NET and poorly differentiated NEC[21]. Patients with type 1 g-NETs have been found to have an association with mutations in ATP4A and PTH1R[22-24], while type 2 g-NETs has been associated with mutations in the MEN-1 gene[25]. However, there are fewer studies related to type 3 g-NETs. Some small sample studies have found that p53 gene expression exists in type 3 g-NETs and has a negative correlation with prognosis[26,27]. p53 was also shown to be one of the commonly mutated genes in gastric NEC[28,29], illustrating that type 3 g-NETs share some similarities to g-NEC, explaining the poor prognosis of type 3 g-NETs. However, more researches are required to support the above as the molecular mechanism of type 3 g-NETs.
Well-differentiated g-NETs are heterogeneous, but the heterogeneity of type 3 g-NETs has not been well described. And we did find well-differentiated G3 NETs and confirmed its unique feature among type 3 g-NETs. In our study, G1, G2, and G3 NETs had their own clinical pathological characteristics (Table 1), illuminating their heterogeneity based on the 2019 WHO pathological classification. Additionally, G1 NETs were common (37/77, 48.1%), not rare as previously reported[9]. Furthermore, G1 NETs showed a low metastasis rate [4 patients (10.8%) with lymph node metastases and 2 (5.4%) with distant metastases] and good prognosis (3-year tumour-specific survival rate: 96%). However, G2 and G3 NETs had higher lymph node metastasis rates and distant metastasis rates, and their prognoses were worse. Also, multivariable analysis also confirmed that G3 was an independent risk factor affecting prognosis. Well-differentiated G3 NETs were reported in gastroenteropancreatic NENs in several studies[30-32] and had their own morphological characteristics and pathways differing from NECs[21,30]. It seems wise to add G3 to well-differentiated NETs in the WHO 2019 neuroendocrine tumour grading system. This indicates that G3 NETs has more aggressive biological behavior than G1 and G2 NETs and pathological grade has an effective prognostic role on outcome for NETs. Surprisingly, as early as 2013, Chinese pathologists also proposed a similar classification[33].
Indeed, type 3 g-NETs showed more malignant biological behavior with a metastasis rate of 44.2%, which was similar to that reported in the literature[19,20], including 10 patients with regional lymph node metastasis (13.0%) and 24 with distant metastasis (31.2%). The 3-year tumour-specific survival with lymph node metastasis and distant metastasis was 70% and 35%, respectively, which was significantly associated with prognosis (P < 0.001). Additionally, distant metastasis was an independent risk factor affecting prognosis. Studies related to prognosis for type 3 g-NETs were scare, but several studies of gastroenteropancreatic NETs[34-36] had shown that distant metastasis was significantly associated with prognosis.
Treatment strategies for type 3 NETs are varied for heterogeneity[11,15] and the grade and stage need to be considered to make an optimal treatment for type 3 NETs. In our study, advanced G3 NET patients (6/7) received CBCT, while G1 and G2 NETs patients with early-stage disease underwent endoscopic treatment. Thirty-three patients treated endoscopically had no tumour recurrence or tumour-related death during a median follow-up period of 36 mo (Table 2). A South Korean study of 50 cases of endoscopic treatment of type 3 g-NETs found no evidence of tumour recurrence in the pathological complete resection group or incomplete resection group during a median follow-up period of 43.73 mo[37]. Another study involving 22 patients in South Korea reported that only one case of lymph node metastasis was found within a median follow-up period of 59 mo after endoscopic treatment[38]. Also, a retrospective multicentre study from Japan reported that of 48 patients treated by endoscopic resection alone, only one developed recurrence with a median follow-up period of 32 mo[39]. These studies suggested that endoscopic treatment was safe and effective for tumours smaller than 2 cm, 1.5 cm, and 1cm, respectively, confined to the mucosa and submucosa in type 3 gastric NETs. This may give us a clinical hint: For G1 and G2 NETs patients with a tumour size < 2 cm, confined to the mucosa and submucosa, endoscopic resection (EMR and ESD) should be considered.
This study also has several limitations. The patients in this study came from four NET centres, and the pathological diagnoses were made by different pathologists. Furthermore, the pathological grading system was updated in 2019. To solve this problem, all pathological diagnoses were reviewed by the same NET pathologist with more than 30 years of experience. Also, a few specimens obtained from endoscopic /EUS biopsy may be too small or deformed, which had an effect on assessment of Ki67 index and number of mitoses. In addition, missing data from some of the patients may have introduced some information bias, and suitable analyses were used to avoid it.
Type 3 g-NETs have a relatively malignant biological behavior with a poor prognosis and strong heterogeneity. G1, G2, and G3 NETs have their own clinicopathological characteristics and distinctive prognoses. The 2019 WHO pathological grade and distant metastasis are independent risk factors affecting prognosis. In addition, the 2019 WHO pathological classification is useful for assessing the biological behavior and prognosis of type 3 g-NETs. Treatment is related to the grade and stage of the tumours. Endoscopic treatment is safe and effective for G1 NETs patients with type 3 g-NETs having tumours smaller than 2 cm and limited to the mucosa and submucosa.
Given the rarity, type 3 gastric neuroendocrine tumours (g-NETs) have not been well described.
The pathological classification of gastroenteropancreatic neuroendocrine neoplasms was updated in 2019. Well-differentiated NET G3 was added, but its role has not been yet illustrated in type 3 g-NETs.
We dedicated to illustrate clinicopathological features and outcome of type 3 g-NETs. Also, we aimed to assess the role of the updated WHO pathological classification in type 3 g-NETs.
Data of patients with type 3 g-NETs from four NET centres in China were collected and analysed retrospectively.
Seventy-seven patients with type 3 g-NETs were enrolled. Of these, there were 37 G1 tumours (48.1%), 31 G2 (40.3%), and 9 G3 (11.7%). Compared with G1 NETs, G2 NETs had a higher lymph node metastasis rate, and G3 NETs had a higher distant metastasis rate. In terms of treatment, 33 patients (29 G1 and 4 G2 ) with stage I/II disease underwent endoscopic treatment, and no one had tumour recurrence or tumour-related death with a median follow-up period of 36 mo. Additionally, grade and distant metastasis were independent risk factors for prognosis in multivariable analysis.
Type 3 g-NETs is heterogeneous with unique clinicopathological features and the 2019 WHO pathological classification is effective to predict their biological behaviors and prognosis. Besides, endoscopic resection is safe and effective for G1 NETs with tumours under 2 cm and confined to the mucosa or submucosa.
Having a better understanding of the clinicopathological characteristics and outcome of type 3 g-NETs based on the 2019 WHO pathological classification, clinicians could offer an optimal treatment for patients. Grade and stage are related to outcome and should be considered before treatment. In addition, endoscopic treatment is effective and should be considered for small, low grade, superficial tumours.
We are grateful to the data collectors at each NET centre.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Balaban DV, Cunningham M, Kim DK, Nagai S, Skok P S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2489] [Article Influence: 311.1] [Reference Citation Analysis (4)] |
| 4. | Gastrointestinal Pathology Study Group of Korean Society of Pathologists, Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, Kim H, Kook MC, Park DY, Lee JH, Chang H, Jung ES, Kim HK, Jin SY, Choi JH, Gu MJ, Kim S, Kang MS, Cho CH, Park MI, Kang YK, Kim YW, Yoon SO, Bae HI, Joo M, Moon WS, Kang DY, Chang SJ. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat. 2012;44:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 322] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 6. | O'Connor JM, Marmissolle F, Bestani C, Pesce V, Belli S, Dominichini E, Mendez G, Price P, Giacomi N, Pairola A, Loria FS, Huertas E, Martin C, Patane K, Poleri C, Rosenberg M, Cabanne A, Kujaruk M, Caino A, Zamora V, Mariani J, Dioca M, Parma P, Podesta G, Andriani O, Gondolesi G, Roca E. Observational study of patients with gastroenteropancreatic and bronchial neuroendocrine tumors in Argentina: Results from the large database of a multidisciplinary group clinical multicenter study. Mol Clin Oncol. 2014;2:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 370] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Ruszniewski P, Delle Fave G, Cadiot G, Komminoth P, Chung D, Kos-Kudla B, Kianmanesh R, Hochhauser D, Arnold R, Ahlman H, Pauwels S, Kwekkeboom DJ, Rindi G; Frascati Consensus Conference; European neuroendocrine Tumor Society. Well-differentiated gastric tumors/carcinomas. Neuroendocrinology. 2006;84:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2439] [Article Influence: 487.8] [Reference Citation Analysis (3)] |
| 10. | Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther. 2014;39:1071-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 12. | Doescher J, Veit JA, Hoffmann TK. The 8th edition of the AJCC Cancer Staging Manual. HNO. 2017;65:956-961. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 13. | Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 279] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Rindi G, Azzoni C, La Rosa S, Klersy C, Paolotti D, Rappel S, Stolte M, Capella C, Bordi C, Solcia E. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 225] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Pfister D, Ang K, Brizel D. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. 2019. Available from: http://ci.nii.ac.jp/naid/10030762657. |
| 16. | Cao LL, Lu J, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Chen QY, Lin M, Tu RH, Huang CM. Incidence and survival trends for gastric neuroendocrine neoplasms: An analysis of 3523 patients in the SEER database. Eur J Surg Oncol. 2018;44:1628-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Chen WC, Warner RR, Ward SC, Harpaz N, Divino CM, Itzkowitz SH, Kim MK. Management and disease outcome of type I gastric neuroendocrine tumors: the Mount Sinai experience. Dig Dis Sci. 2015;60:996-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Sato Y, Imamura H, Kaizaki Y, Koizumi W, Ishido K, Kurahara K, Suzuki H, Fujisaki J, Hirakawa K, Hosokawa O, Ito M, Kaminishi M, Furuta T, Chiba T, Haruma K. Management and clinical outcomes of type I gastric carcinoid patients: retrospective, multicenter study in Japan. Dig Endosc. 2014;26:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Lee HE, Mounajjed T, Erickson LA, Wu TT. Sporadic Gastric Well-Differentiated Neuroendocrine Tumors Have a Higher Ki-67 Proliferative Index. Endocr Pathol. 2016;27:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Vanoli A, La Rosa S, Miceli E, Klersy C, Maragliano R, Capuano F, Persichella A, Martino M, Inzani F, Luinetti O, Di Sabatino A, Sessa F, Paulli M, Corazza GR, Rindi G, Bordi C, Capella C, Solcia E. Prognostic Evaluations Tailored to Specific Gastric Neuroendocrine Neoplasms: Analysis Of 200 Cases with Extended Follow-Up. Neuroendocrinology. 2018;107:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res. 2016;22:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 22. | Calvete O, Reyes J, Zuñiga S, Paumard-Hernández B, Fernández V, Bujanda L, Rodriguez-Pinilla MS, Palacios J, Heine-Suñer D, Banka S, Newman WG, Cañamero M, Pritchard DM, Benítez J. Exome sequencing identifies ATP4A gene as responsible of an atypical familial type I gastric neuroendocrine tumour. Hum Mol Genet. 2015;24:2914-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Calvete O, Herraiz M, Reyes J, Patiño A, Benitez J. A cumulative effect involving malfunction of the PTH1R and ATP4A genes explains a familial gastric neuroendocrine tumor with hypothyroidism and arthritis. Gastric Cancer. 2017;20:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Benítez J, Marra R, Reyes J, Calvete O. A genetic origin for acid-base imbalance triggers the mitochondrial damage that explains the autoimmune response and drives to gastric neuroendocrine tumours. Gastric Cancer. 2020;23:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Debelenko LV, Emmert-Buck MR, Zhuang Z, Epshteyn E, Moskaluk CA, Jensen RT, Liotta LA, Lubensky IA. The multiple endocrine neoplasia type I gene locus is involved in the pathogenesis of type II gastric carcinoids. Gastroenterology. 1997;113:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Peny MO, Donckier V, Gelin M, Haot J, Noel JC. Sporadic carcinoid of the stomach: a highly proliferative disease with a probable role for p53 protein dysregulation. Eur J Gastroenterol Hepatol. 1999;11:677-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Safatle-Ribeiro AV, Ribeiro U Jr, Corbett CE, Iriya K, Kobata CH, Sakai P, Yagi OK, Pinto PE Jr, Zilberstein B, Gama-Rodrigues J. Prognostic value of immunohistochemistry in gastric neuroendocrine (carcinoid) tumors. Eur J Gastroenterol Hepatol. 2007;19:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Makuuchi R, Terashima M, Kusuhara M, Nakajima T, Serizawa M, Hatakeyama K, Ohshima K, Urakami K, Yamaguchi K. Comprehensive analysis of gene mutation and expression profiles in neuroendocrine carcinomas of the stomach. Biomed Res. 2017;38:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | von Arx C, Capozzi M, López-Jiménez E, Ottaiano A, Tatangelo F, Di Mauro A, Nasti G, Tornesello ML, Tafuto S. Updates on the Role of Molecular Alterations and NOTCH Signalling in the Development of Neuroendocrine Neoplasms. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, Pisa E, Barberis M, Vanoli A, Buzzoni R, Pusceddu S, Concas L, Sessa F, Solcia E, Capella C, Fazio N, La Rosa S. The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories. Neuroendocrinology. 2017;104:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 31. | Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, Barriuso J, Pavel M, O'Toole D, Walter T; other Knowledge Network members. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 32. | Pellat A, Coriat R. Well Differentiated Grade 3 Neuroendocrine Tumors of the Digestive Tract: A Narrative Review. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Neuroendocrine Neoplasms Editorial Board. Neuroendocrine neoplasms 2013 Standardization in diagnosis of gastrointestinal and pancreatic neuroendocrine neoplasms: the Chinese consensus. Chin J Pathol. 2013;42:691-694. [DOI] [Full Text] |
| 34. | Zheng Z, Chen C, Jiang L, Zhou X, Dai X, Song Y, Li Y. Incidence and risk factors of gastrointestinal neuroendocrine neoplasm metastasis in liver, lung, bone, and brain: A population-based study. Cancer Med. 2019;8:7288-7298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Panzuto F, Pusceddu S, Faggiano A, Rinzivillo M, Brighi N, Prinzi N, Riccardi F, Iannicelli E, Maggio I, Femia D, Tafuto S, Manuzzi L, Di Sarno A, Annibale B, de Braud F, Campana D; Itanet (Italian Association for Neuroendocrine Tumours). Prognostic impact of tumour burden in stage IV neuroendocrine neoplasia: A comparison between pancreatic and gastrointestinal localizations. Pancreatology. 2019;19:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Wang Z, Li W, Chen T, Yang J, Luo L, Zhang L, Sun B, Liang R. Retrospective analysis of the clinicopathological characteristics of gastrointestinal neuroendocrine neoplasms. Exp Ther Med. 2015;10:1084-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Kwon YH, Jeon SW, Kim GH, Kim JI, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Choi KD, Moon JS. Long-term follow up of endoscopic resection for type 3 gastric NET. World J Gastroenterol. 2013;19:8703-8708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Min BH, Hong M, Lee JH, Rhee PL, Sohn TS, Kim S, Kim KM, Kim JJ. Clinicopathological features and outcome of type 3 gastric neuroendocrine tumours. Br J Surg. 2018;105:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Hirasawa T, Yamamoto N, Sano T. Is endoscopic resection appropriate for type 3 gastric neuroendocrine tumors? Dig Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |