Published online Dec 15, 2020. doi: 10.4251/wjgo.v12.i12.1381
Peer-review started: July 4, 2020
First decision: September 18, 2020
Revised: September 26, 2020
Accepted: October 30, 2020
Article in press: October 30, 2020
Published online: December 15, 2020
Processing time: 159 Days and 7.3 Hours
Cholangiocarcinoma is a disease with a high mortality rate. Our previous study revealed that cholelithiasis patients who undergo endoscopic sphincterotomy (ES)/endoscopic papillary balloon dilatation are at a higher risk for subsequent cholangiocarcinoma than cholelithiasis patients who undergo cholecystectomy.
To clarify the relationship between recurrent biliary events and subsequent cholangiocarcinoma risk in choledocholithiasis patients.
From one million random cases in the Taiwan National Health Insurance Research Database 2004–2011, we selected symptomatic choledocholithiasis patients older than 18 years who were admitted from January 2005 to December 2009 (study group). Cases for a control group were defined as individuals who had never been diagnosed with cholelithiasis, matched by sex and age in a 1:3 ratio. The study group was further divided into ES/endoscopic papillary balloon dilatation, both ES/endoscopic papillary balloon dilatation and cholecystectomy, and no intervention groups.
We included 2096 choledocholithiasis patients without previous intervention or cholangiocarcinoma. A total of 12 (2.35%), 11 (0.74%), and 1 (1.00%) subsequent cholangiocarcinoma cases were diagnosed among 511 ES/endoscopic papillary balloon dilatation patients, 1485 patients with no intervention, and 100 ES/endoscopic papillary balloon dilatation and cholecystectomy patients, respectively. The incidence rates of recurrent biliary event were 527.79/1000 person-years and 286.69/1000 person-years in the subsequent cholangiocarcinoma and no cholangiocarcinoma group, showing a high correlation between subsequent cholangiocarcinoma risk and recurrent biliary events.
Choledocholithiasis patients who undergo further cholecystectomy after ES/endoscopic papillary balloon dilatation have decreased subsequent cholangiocarcinoma risk due to reduced recurrent biliary events.
Core Tip: Choledocholithiasis patients who undergo further cholecystectomy after endoscopic sphincterotomy/endoscopic papillary balloon dilatation have a decreased subsequent cholangiocarcinoma risk. The relationship between the incidence of recurrent biliary events and that of subsequent cholangiocarcinoma is statistically meaningful.
- Citation: Wang CC, Tseng MH, Wu SW, Yang TW, Chen HY, Sung WW, Su CC, Wang YT, Lin CC, Tsai MC. Cholecystectomy reduces subsequent cholangiocarcinoma risk in choledocholithiasis patients undergoing endoscopic intervention. World J Gastrointest Oncol 2020; 12(12): 1381-1393
- URL: https://www.wjgnet.com/1948-5204/full/v12/i12/1381.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i12.1381
Cholangiocarcinoma, which carries a medium incidence and high mortality rate[1], has been under re-evaluation in Asia-Pacific countries[2,3]. Over the past two decades, the incidence of intra-hepatic cholangiocarcinoma (ICC) has increased, while the incidence of extra-hepatic cholangiocarcinoma has declined internationally[4-7]. The most important factor for survival in cholangiocarcinoma patients is R0 resection[8], and this means that a surveillance program that can lead to early detection is the key to survival.
There are many historical risk factors for cholangiocarcinoma that have been discussed in the previous literature, like primary sclerosing cholangitis[9-11], choledochal cyst disease[12,13], parasite infection[14], cholelithiasis[15,16], diabetes mellitus[17,18], and Helicobacter pylori infection[19,20]. The impact of liver cirrhosis[21,22] and chronic hepatitis C[23,24] and B[21,22,25] virus infections has also been confirmed in recent studies. Because the etiology of cholangiocarcinoma is not fully understood, several hypotheses were proposed to explain its cause, including chronic inflammation of the bile duct or the destruction of the integrity of the bile duct[26], such as from endoscopic retrograde cholangiopancreatography. The topic of endoscopic sphincterotomy and endoscopic papillary balloon dilatation (EPBD) and the late complication, like cholangiocarcinoma, is still a matter of debate[27-31], and the current evidence is insufficient for a conclusion to be drawn. Because cholelithiasis itself is an important risk factor for cholangiocarcinoma, the impact on the incidence of subsequent cholangiocarcinoma from advanced bile duct management is difficult to evaluate.
Although previous studies have shown differing results regarding cholecystectomy (CCY) due to cholelithiasis and subsequent ICC[32] or extra-hepatic cholangio-carcinoma[33,34], our prior study revealed that cholelithiasis patients who undergo endoscopic sphincterotomy/endoscopic papillary balloon dilatation (ES/EPBD) are at a greater risk for subsequent cholangiocarcinoma, while cholelithiasis patients who undergo CCY have reduced risk for subsequent cholangiocarcinoma[3]. These results can be explained by different inflammation sites or by CCY reducing recurrent biliary events (RBEs)[35] and further decreasing future cholangiocarcinoma rates.
Because of the inconsistent results of the previous evidence, we performed this study using the National Health Insurance Research Database (NHIRD) 2004–2011 of Taiwan to clarify the risk of cholangiocarcinoma after ES/EPBD, ES/EPBD and CCY, and supportive care with no invasive intervention in patients who were admitted to the hospital because of choledocholithiasis.
This study was a population-based retrospective cohort study based on Taiwan’s NHIRD 2004-2011, and the study methods of the NHIRD have been described in detail in previous studies[3,35-37]. This study was approved by the Institutional Review Board (IRB) of Chung Shan Medical University Hospital, Taiwan. All methods were performed in accordance with the relevant guidelines and regulations and under surveillance by the IRB of Chung Shan Medical University Hospital. All authors declare they have no any conflict of interest to report.
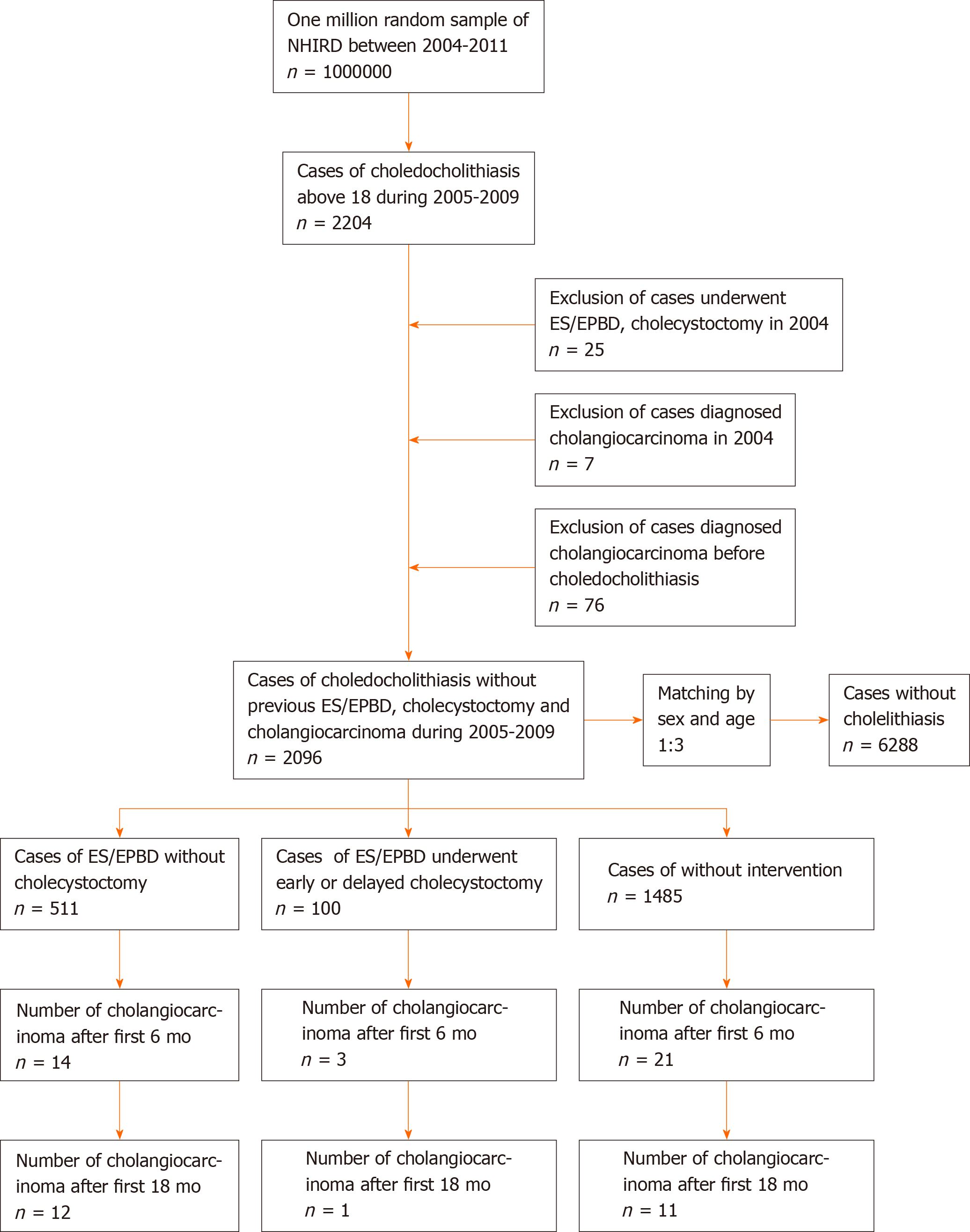
Symptomatic choledocholithiasis cases in patients over 18 years old were obtained from among one million random samples of the NHIRD between January 2005 and December 2009 by the codes of the International Statistical Classification of Diseases and Related Health Problems, ninth revision (ICD-9), which were registered when patients were admitted to hospital. Then, we excluded patients who had already undergone ES/EPBD or CCY in 2004, were diagnosed with cholangiocarcinoma before 2005, or who had been diagnosed with cholangiocarcinoma prior to being diagnosed with choledocholithiasis. After the study group was selected, we built a control group, matched by sex and age in a 1:3 ratio. The cases for the control group were defined as individuals who had never been diagnosed with cholelithiasis or had never undergone a related medical procedure prior to 2005. The study group was further divided into ES/EPBD, ES/EPBD and CCY, and no intervention groups. Because cholangio-carcinoma patients in Taiwan use a catastrophic illness card to wave medical expenses when the patients seek for medical help using ICD-9 registration of cholangio-carcinoma, we believe that a 1-year exclusion period is enough. Variables such as economic status, place of residence, follow-up time, and historical common risk factors, including chronic hepatitis B (CHB), chronic hepatitis C, Helicobacter pylori infection, diabetes mellitus, end-stage renal disease on dialysis, congenital cystic disease of the liver, Clonorchis/Opisthorchis infection, and inflammatory bowel disease, were compared among the ES/EPBD, ES/EPBD and CCY, and no intervention groups. We calculated the number and rates for cholangiocarcinoma just after the procedure and then again 6, 12, and 18 mo after the procedure. The study flow chart is shown in Figure 1. The ICD-9 codes for the above diseases and procedure codes are listed in Supplementary Table 1.
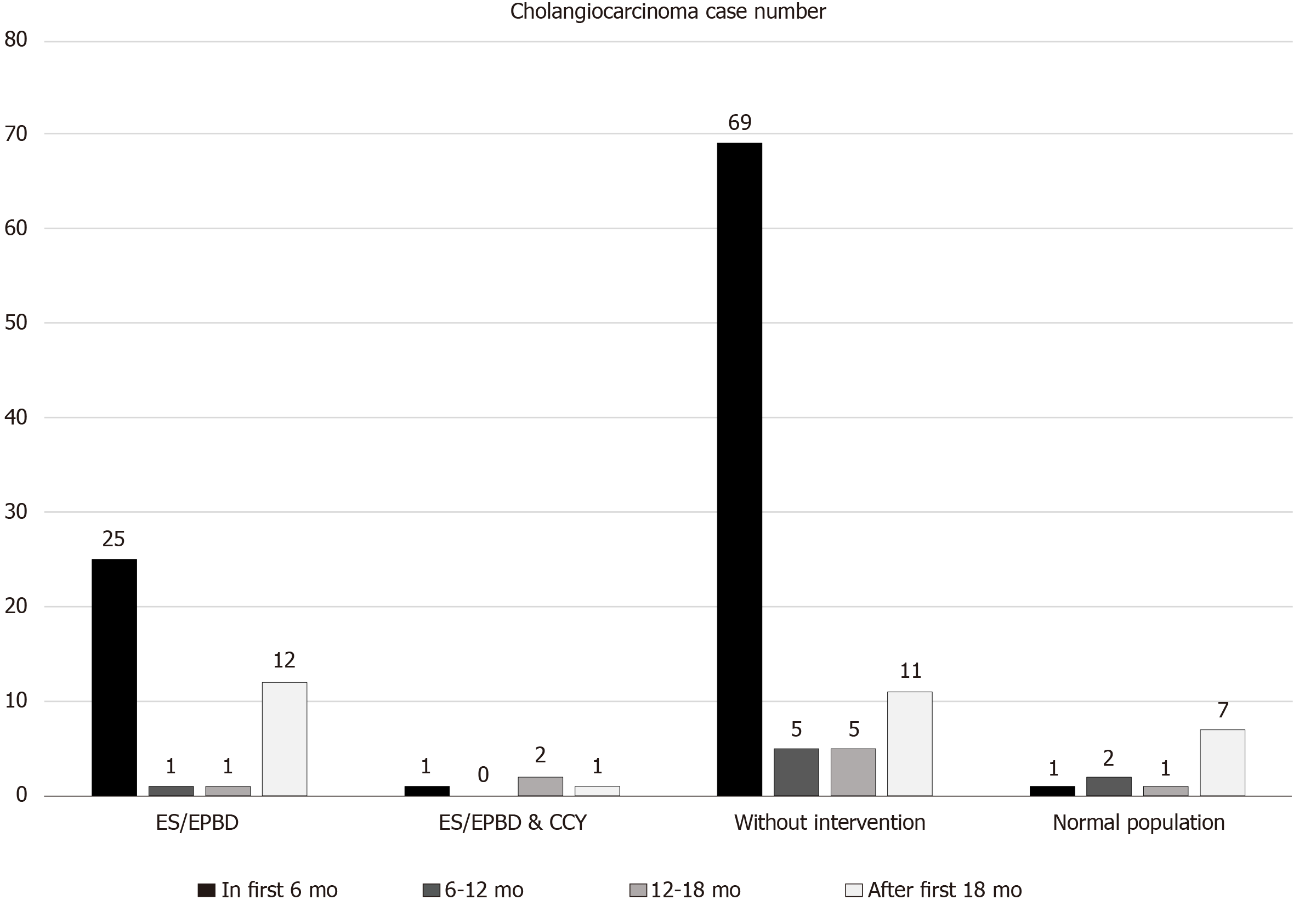
Furthermore, we chose cholangiocarcinoma that occurred after the first 18 mo post-ES/EPBD or biliary event to be considered as subsequent cholangiocarcinoma because the incidence of cholangiocarcinoma during the first 18 mo were obviously higher than that in the following years (Figure 2) and this condition was most obvious in the no intervention group. We believed that these cases should be considered as concurrent malignancies rather than subsequent cholangiocarcinoma. The incidence of subsequent cholangiocarcinoma in patients in the ES/EPBD, ES/EPBD and CCY, or no intervention group was compared with that of the normal population. The time cumulative risk for subsequent cholangiocarcinoma in the different groups was calculated. The definition of RBEs was further ER visits or admission course due to cholangitis, cholecystitis, or biliary pancreatitis. The incidence of RBEs and that of subsequent cholangiocarcinoma were compared in different groups to identify their relationships.
The NHIRD was managed by employing the Microsoft SQL Server 2008 R2 (Microsoft Corporation, Redmond, WA, United States), and the data query and data processing procedures were done using the SQL programming language. Statistical analyses was performed using OpenEpi: Open Source Epidemiologic Statistics for Public Health, version 3.01[38]. Kaplan-Meier survival analyses were completed using SPSS version 19. Person-time analyses were done using OpenEpi version 3.01.
Data obtained from the study were compared by a chi-square (χ2) test for the categorical variables, t-test or one-way ANOVA (analysis of variance) for the continuous variables, and log-rank (Mantel-Cox) test for the survival curves. A Cox regression model was used for analysis of the hazard ratio of subsequent cholangio-carcinoma. A two-tailed P value of 0.05 was considered statistically significant in this study.
In total, 2204 adult symptomatic choledocholithiasis admission cases were obtained from one million random samples of the NHIRD between January 2005 and December 2009. After excluding 25 cases who had undergone ES/EPBD or CCY in 2004 and 7 cases with confirmed cholangiocarcinoma in 2004, we excluded another 76 cases who had a diagnosis of cholangiocarcinoma prior to a diagnosis of choledocholithiasis. We finally selected 2096 choledocholithiasis patients without prior ES/EPBD, CCY, or cholangiocarcinoma between January 2005 and December 2009. The control group, built with cases without cholelithiasis by matching by age and sex, included 6288 cases. The mean age was 66.18 years in both the study and control groups.
There were 511 patients who had undergone ES/EPBD without CCY, while 100 patients had ES/EPBD with further CCY. At the same time, there were 1485 patients with choledocholithiasis who accepted supportive care without any further intervention during admission because of either disease severity or baseline health condition. Regarding the demographic data, the average age was 67.44 ± 15.23 years in the ES/EPBD group, 59.96 ± 16.87 years in the ES/EPBD and CCY group and 66.16 ± 16.02 years in the no intervention group.
We found that only age, age distribution, and follow-up time had statistically significant differences among the three groups in the demographic data, which also included gender, economic status, place of residence, and comorbidities. Patients who underwent ES/EPBD and CCY were younger than those in the ES/EPBD and no intervention groups, and they had the longest follow-up time, with 47.91 mo vs 42.77 mo and 36.14 mo, respectively. Meanwhile, the proportion of historical risk factors for cholangiocarcinoma, such as CHB, chronic hepatitis C, DM, end-stage renal disease on dialysis, cystic disease of the liver, Clonorchis/Opisthorchis infection, and inflammatory bowel disease, were similar in these three groups except that there was a non-significantly lower CHB proportion in the ES/EPBD and CCY group (3% vs 9.78% and 10.37%) in Table 1.
| ES/EPBD, n = 511, n (SD; %) | ES/EPBD and CCY, n = 100, n (SD; %) | Without intervention, n = 1485, n (SD; %) | P value | |
| Age, mean (SD) | 67.44 (15.23) | 59.96 (16.87) | 66.16 (16.02) | < 0.001 |
| Age, yr | ||||
| 18-49 | 40.08 (6.91) | 39.13 (8.43) | 39.48 (6.76) | |
| 50-69 | 60.48 (5.43) | 58.21 (5.88) | 60.14 (5.55) | |
| > 70 | 79.45 (6.02) | 77.58 (4.71) | 79.89 (6.43) | |
| Gender | 0.450 | |||
| Male | 282 (55.19) | 49 (49.00) | 824 (55.49) | |
| Female | 229 (44.81) | 51 (51.00) | 661 (44.51) | |
| Follow-up time (mo), mean (SD) | 42.77 (22.20) | 47.91 (20.86) | 36.14 (24.87) | < 0.001 |
| Economic status | 0.445 | |||
| MBS | 292 (57.14) | 54 (54.00) | 867 (58.38) | |
| 1-3 times MBS | 189 (36.99) | 36 (36.00) | 536 (36.09) | |
| Above 3 times MBS | 29 (5.68) | 10 (10.00) | 82 (5.52) | |
| Place of residence | 0.154 | |||
| City | 288 (56.36) | 68 (68.00) | 900 (60.61) | |
| Countryside | 213 (41.68) | 31 (31.00) | 551 (37.10) | |
| Remote village | 9 (1.76) | 1 (1.00) | 33 (2.22) | |
| Comorbidity | ||||
| CHB | 50 (9.78) | 3 (3.00) | 154 (10.37) | 0.057 |
| CHC | 23 (4.50) | 5 (5.00) | 103 (6.94) | 0.127 |
| DM | 172 (33.66) | 29 (29.00) | 504 (33.94) | 0.599 |
| ESRD | 8 (1.57) | 1 (1.00) | 32 (2.15) | 0.552 |
| CCDL | 12 (2.35) | 2 (2.00) | 20 (1.35) | 0.289 |
| CO | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| IBD | 8 (1.57) | 0 (0.00) | 32 (2.15) | 0.253 |
| Cholangiocarcinoma | ||||
| Number of CC | 39 (7.63) | 4 (4.00) | 90 (6.06) | 0.279 |
| Number of CC after first 18 mo | 12 (2.35) | 1 (1.00) | 11 (0.74) | 0.013 |
| Hazard ratio | 3.45 (1.52-7.82) | 1.31 (0.17-10.13) | 1.00 | 0.012 |
| Time to diagnosis of CC (excluding case in first 18 mo), month | 39.71 (18.93) | 25.12 (0.00) | 43.27 (17.98) | 0.624 |
| Overall mortality rate | 146 (28.57) | 12 (12.00) | 596 (40.13) | < 0.001 |
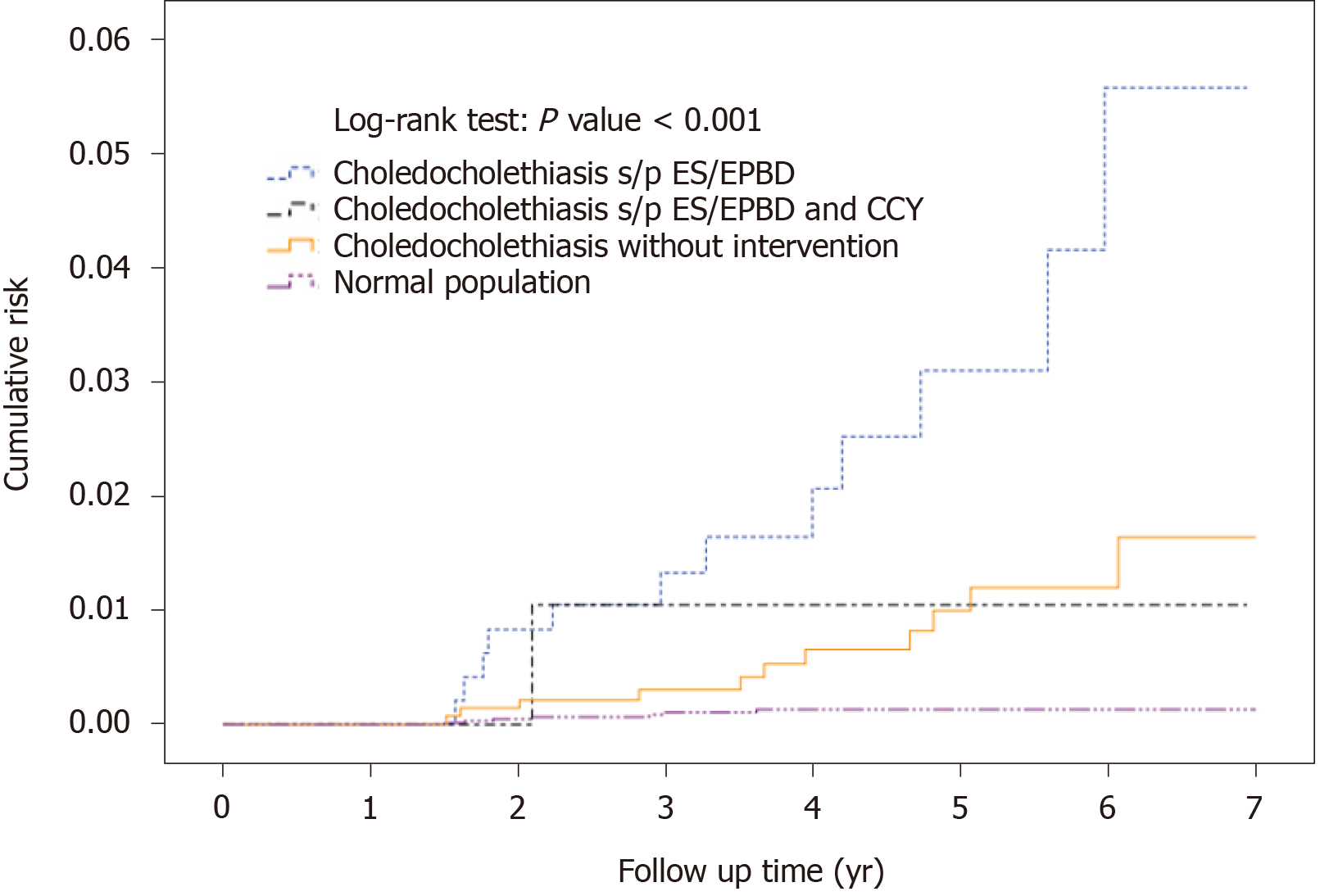
Thirty-nine (7.63%) patients were diagnosed with cholangiocarcinoma in the ES/EPBD group, while 90 cholangiocarcinoma (6.06%) patients were found in the no intervention group and four (4.00%) were diagnosed in the ES/EPBD and CCY group. Because the incidence of cholangiocarcinoma during the first 6 mo was extremely high in all groups and abnormally high during the first 18 mo in the no intervention group, we excluded the cholangiocarcinoma cases that were diagnosed during the first 18 mo after index admission to build the definition of subsequent cholangiocarcinoma. The detailed information is revealed in Figure 2. After the exclusion, 12 (2.35%), 11 (0.74%), and 1 (1.00%) subsequent cholangiocarcinoma cases were diagnosed in the ES/EPBD, no intervention, and ES/EPBD and CCY groups, respectively. The hazard ratio for subsequent cholangiocarcinoma was 3.45 in the ES/EPBD group and 1.31 in the ES/EPBD and CCY group when compared with the no intervention group. The results were similar in that the no intervention group had the lowest subsequent cholangio-carcinoma rate if we excluded the cholangiocarcinoma cases diagnosed within 6 mo or 1 year after index admission. The cumulative cholangiocarcinoma rates in the ES/EPBD, ES/EPBD and CCY, no intervention, and control groups during the 7-year follow-up period revealed that choledocholithiasis patients who needed an ES/EPBD intervention initially had the highest subsequent cholangiocarcinoma rate. The subsequent cholangiocarcinoma rate in the no intervention group exceeded that in the ES/EPBD and CCY group after the 5-year follow-up period. The cumulative subsequent cholangiocarcinoma rates in the ES/EPBD, ES/EPBD and CCY, no intervention, and control groups are shown in Figure 3.
As for the comparisons between the patients who had undergone ES/EPBD, ES/EPBD and CCY, and no intervention for choledocholithiasis and the normal population, the incidence of cholangiocarcinoma after the first 18 mo was compared using incidence rate/1000 person-years. In the ES/EPBD group, the incidence of subsequent cholangiocarcinoma was 7.23 (3.92–12.29) per 1000 person-years, which is more than 25 times the incidence in the normal population. The incidence of subsequent cholangiocarcinoma in the ES/EPBD group was higher especially in females (10.39/1000 person-years) and patients above 70 years old (8.42/1000 person-years).
In the ES/EPBD and CCY group, the incidence of subsequent cholangiocarcinoma was 2.54 (0.13–12.55) per 1000 person-years, which was higher than that of the normal population. As for choledocholithiasis patients without any intervention, the incidence of subsequent cholangiocarcinoma, which was 2.52 (1.35–4.38) per 1000 person-years, was similar to that in the ES/EPBD and CCY group. The incidence of subsequent cholangiocarcinoma was the highest in the ES/EPBD group, and it was almost equal in the ES/EPBD and CCY and no intervention groups. Meanwhile, the incidence of subsequent cholangiocarcinoma was significantly higher for all choledocholithiasis patients as compared to the normal population. The detailed information is shown in Table 2.
| Variable | Person-years at risk in study cohort | Person-years at risk in control cohort | No. of observed cases of cholangiocarcinoma in study cohort | No. of observed cases of cholangiocarcinoma in control cohort | Incidence rate/1000 person-years (95%CI) in study cohort | Incidence rate/1000 person-years (95%CI) in control cohort | P value |
| ES/EPBD | |||||||
| Total | 1659.70 | 25360.07 | 12 | 7 | 7.23 (3.92-12.29) | 0.28 (0.12-0.55) | < 0.001 |
| Gender | |||||||
| Male | 889.95 | 13822.88 | 4 | 3 | 4.50 (1.43-10.84) | 0.22 (0.06-0.59) | < 0.001 |
| Female | 769.76 | 11537.20 | 8 | 4 | 10.39 (4.83-19.74) | 0.35 (0.11-0.84) | < 0.001 |
| Age, years | |||||||
| 18-49 | 253.84 | 4392.03 | 1 | 0 | 3.94 (0.20-19.43) | 0.00 (0.00-0.00) | < 0.001 |
| 50-69 | 574.78 | 8631.76 | 4 | 5 | 6.96 (2.21-16.79) | 0.58 (0.21-1.28) | < 0.001 |
| > 70 | 831.08 | 12336.28 | 7 | 2 | 8.42 (3.68-16.66) | 0.16 (0.03-0.54) | < 0.001 |
| ES/EPBD and CCY | |||||||
| Total | 393.11 | 25360.07 | 1 | 7 | 2.54 (0.13-12.55) | 0.28 (0.12-0.55) | 0.011 |
| Gender | |||||||
| Male | 184.36 | 13822.88 | 0 | 3 | 0.00 (0.00-0.00) | 0.22 (0.06-0.59) | 0.842 |
| Female | 208.75 | 11537.20 | 1 | 4 | 4.79 (0.24-23.63) | 0.35 (0.11-0.84) | 0.002 |
| Age, yr | |||||||
| 18-49 | 113.01 | 4392.03 | 0 | 0 | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | NA |
| 50-69 | 143.37 | 8631.76 | 1 | 5 | 6.98 (0.35-34.40) | 0.58 (0.21-1.28) | 0.004 |
| > 70 | 136.73 | 12336.28 | 0 | 2 | 0.00 (0.00-0.00) | 0.16 (0.03-0.54) | 0.882 |
| Without intervention | |||||||
| Total | 4364.45 | 25360.07 | 11 | 7 | 2.52 (1.35-4.38) | 0.28 (0.12-0.55) | < 0.001 |
| Gender | |||||||
| Male | 2345.22 | 13822.88 | 6 | 3 | 2.56 (1.04-5.32) | 0.22 (0.06-0.59) | < 0.001 |
| Female | 2019.23 | 11537.20 | 5 | 4 | 2.48 (0.91-5.49) | 0.35 (0.11-0.84) | < 0.001 |
| Age, yr | |||||||
| 18-49 | 871.87 | 4392.03 | 0 | 0 | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | NA |
| 50-69 | 1528.74 | 8631.76 | 6 | 5 | 3.93 (1.601-8.16) | 0.58 (0.21-1.28) | < 0.001 |
| > 70 | 1963.84 | 12336.28 | 5 | 2 | 2.55 (0.93-5.64) | 0.16 (0.03-0.54) | < 0.001 |
We further analyzed the relationship between RBEs and the incidence of subsequent cholangiocarcinoma. Using the RBE incidence rate/1000 person-years of the no intervention group as a reference, the RBE incidence rate/1000 person-years of the ES/EPBD group was 350.06, which was significantly higher than that of the ES/EPBD and CCY and no intervention groups. Meanwhile, the incidence of subsequent cholangiocarcinoma was highest in the ES/EPBD group at 7.23/1000 person-years. Based on the idea that frequent RBEs may increase the subsequent cholangiocarcinoma rate, we separated the entire study group into two groups: Patients diagnosed with subsequent cholangiocarcinoma and patients without cholangiocarcinoma. The RBE incidence rates/1000 person-years were 527.79 and 286.69 in the subsequent cholangiocarcinoma group and the without cholangiocarcinoma group, respectively. The RBE event rate and the proportion of RBEs in patients with confirmed subsequent cholangiocarcinoma were significantly higher in our results.
Southeast Asia has the highest incidence of cholangiocarcinoma worldwide. Its annual incidence ranges from 0.1/100000 to 71.3/100000[39], and our reports came from this endemic area. Although the most famous risk factor for cholangiocarcinoma in the Western world is primary sclerosing cholangitis[10], this rarely occurs in Asian countries except in Japan. At the same time, choledochal cyst disease contributes to a more than 30-fold higher cholangiocarcinoma risk[40] over that of the normal population, but this kind of disease is as rare as Clonorchis/Opisthorchis infection[14] in patients in modern Taiwan. Because hepatolithiasis is associated with a 6- to 50-fold increased risk for ICC[40] and there has been an increase in worldwide incidence of cholelithiasis in recent years[41,42], we analyzed the relationship between RBEs and the incidence of subsequent cholangiocarcinoma in choledocholithiasis patients who required admission for medical management.
Because we used only age and sex to find three times as many in the normal population without a cholelithiasis diagnosis to be our control group, other risk factors for cholangiocarcinoma may confound the results in our three study groups. Meanwhile, we can observe the distribution of these well-known risk factors for cholangiocarcinoma in the different groups.
We noticed that the subsequent and overall cholangiocarcinoma risks of choledocholithiasis patients were statistically higher than those of the normal population (Table 2 and Figure 3). These results are compatible with the previous literature in proving that cholelithiasis is an important risk factor for cholangio-carcinoma[15,16].
The comparison among the choledocholithiasis patients who had undergone ES/EPBD, ES/EPBD and CCY, and no intervention showed no statistically significant differences regarding previous risk factors for cholangiocarcinoma. Because the cholangiocarcinoma incidence was obviously higher during the first 6 mo after index admission in the ES/EPBD and ES/EPBD and CCY groups, we considered these events as initial misdiagnoses or concurrent cholangiocarcinoma events. Interestingly, we noticed that the incidence of cholangiocarcinoma in the choledocholithiasis patients who were admitted without any further endoscopic or surgical intervention was still abnormally higher during the 6–12 mo and 12–18 mo after index admission. Because there were no further advanced interventions in this group, we chose to exclude cholangiocarcinoma that occurred within 18 mo after index admission to build up the subsequent cholangiocarcinoma definition in our database. The subsequent cholangiocarcinoma cumulative risk was highest in the ES/EPBD group, and the cumulative risk for subsequent cholangiocarcinoma in the no intervention group gradually exceeded that of the ES/EPBD and CCY group as the observation time went by. The overall subsequent cholangiocarcinoma hazard ratios were 3.45, 1.31, and 1.0 in the ES/EPBD, ES/EPBD and CCY, and no intervention groups, respectively. This phenomenon can be explained by the fact that the greater the severity of the inflammation in the bile duct, the more advanced the interventions performed. Meanwhile, the frequency or severity of bile duct inflammatory events may increase the risk of subsequent cholangiocarcinoma. We believe that the lowest subsequent cholangiocarcinoma risk in the no intervention group comes from having fewer inflammatory events than the other two groups. We further hypothesize that more RBEs result in a higher subsequent cholangiocarcinoma rate. The comparisons between RBE incidence and subsequent cholangiocarcinoma incidence among the ES/EPBD, ES/EPBD and CCY, and no intervention groups showed a strong correlation: The incidence of RBEs was much higher in the choledocholithiasis patients who were later diagnosed with cholangiocarcinoma than in those who were not, which is shown in Table 3. These results prove that RBEs can increase the subsequent cholangiocarcinoma rate.
| Variable | Number of RBE events | Number of RBE patients | RBE per person (SD) | Incidence proportion (person) | Person-years | Cholangiocarcinoma incidence rate/1000 person-years | RBEs incidence rate/1000 person-years | P value |
| ES/EPBD, n = 511 | 581 | 126 | 1.14 (2.28) | 24.66% | 1659.70 | 7.23 | 350.06 | < 0.0011 |
| ES/EPBD and CCY, n = 100 | 70 | 14 | 0.70 (1.36) | 14.00% | 393.11 | 2.54 | 178.07 | < 0.0011 |
| Choledocholithiasis without intervention, n = 1485 | 1213 | 286 | 0.82 (1.52) | 19.26% | 4364.45 | 2.52 | 277.93 | Reference |
| Patients had cholangiocarcinoma after 18 mo, n = 24 | 43 | 10 | 1.79 (2.06) | 41.67% | 81.47 | NA | 527.79 | < 0.001 |
| Patients without cholangiocarcinoma, n = 1963 | 1812 | 414 | 0.92 (1.76) | 21.09% | 6320.31 | NA | 286.69 | Reference |
Second, we attempted to prove that early or delayed CCY can decrease subsequent cholangiocarcinoma risk by reducing future RBEs, and it has already been proved in the previous literature that early or delayed CCY can reduce future RBEs[35,43]. In our study, the risk for subsequent cholangiocarcinoma in the no intervention group was initially low but gradually increased and exceeded that of the ES/EPBD and CCY group, starting with the fifth year after index admission. We believe that this result gives us a clue that CCY after ES/EPBD for choledocholithiasis management maybe reduces long-term subsequent cholangiocarcinoma risk by decreasing RBEs.
There are two major limitations to our study. First, this is a retrospective database cohort study that showed no laboratory data results or clinical images, and this makes concurrent cholangiocarcinoma or misdiagnoses initially hard to identify. That is why we excluded cholangiocarcinoma during the first 18 mo after index admission in our study design. Second, although this study used a representative database sample of one million patients, the incidence of cholangiocarcinoma was low, and we could only find 12, 1, and 11 cases in the ES/EPBD, ES/EPBD, and CCY and no intervention groups, respectively. However, the relationship between the incidence of RBEs and that of subsequent cholangiocarcinoma was statistically meaningful. Further large-scale retrospective or prospective studies are needed on this topic. We have already started to initiate a prospective hospital-based cohort study of cholelithiasis patients who have undergone therapeutic endoscopic or surgical interventions to clarify the results that we found in this retrospective cohort study.
In conclusion, choledocholithiasis patients who have undergone ES/EPBD intervention without further CCY have a 25–26-fold higher subsequent cholangiocarcinoma risk than the normal population. Further CCY in ES/EPBD patients decreases subsequent cholangiocarcinoma risk by reducing RBEs.
Our previous study revealed that cholelithiasis patients who undergo endoscopic sphincterotomy/endoscopic papillary balloon dilatation (ES/EPBD) are at a greater risk for subsequent cholangiocarcinoma, while cholelithiasis patients who undergo cholecystectomy (CCY) have a much lower risk for subsequent cholangiocarcinoma.
Because of problems in prior study’s design, we could not identify whether different inflammation sites or CCY-reduced recurrent biliary events (RBEs) influenced the subsequent cholangiocarcinoma risk in cholelithiasis patients.
To evaluate the relationships of recurrent biliary events with subsequent cholangiocarcinoma risk.
We selected symptomatic choledocholithiasis patients older than 18 years who were admitted from January 2005 to December 2009 from one million random samples in the National Health Insurance Research Database of Taiwan.
This study showed that choledocholithiasis patients who underwent further CCY after ES/EPBD can reduce subsequent cholangiocarcinoma risk from 2.35% to 1%. And the subsequent cholangiocarcinoma risks had a good correlation with RBEs in our study. Choledocholithiasis patients who have undergone an ES/EPBD intervention without further CCY had a 25–26-fold higher subsequent cholangiocarcinoma risk than the normal population.
Further CCY in ES/EPBD patients decreases subsequent cholangiocarcinoma risk by reducing RBEs.
This study not only showed different subsequent cholangiocarcinoma risks in the ES/EPBD, ES/EPBD and CCY, and no intervention groups of symptomatic choledocholithiasis patients, but also the significant relationship between the incidence of RBEs and that of subsequent cholangiocarcinoma.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: The Gastroenterological Society of Taiwan.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kawabata H, Kitamura K S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li JH
| 1. | Chaiteerakij R, Pan-Ngum W, Poovorawan K, Soonthornworasiri N, Treeprasertsuk S, Phaosawasdi K. Characteristics and outcomes of cholangiocarcinoma by region in Thailand: A nationwide study. World J Gastroenterol. 2017;23:7160-7167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 2. | Treeprasertsuk S, Poovorawan K, Soonthornworasiri N, Chaiteerakij R, Thanapirom K, Mairiang P, Sawadpanich K, Sonsiri K, Mahachai V, Phaosawasdi K. A significant cancer burden and high mortality of intrahepatic cholangiocarcinoma in Thailand: a nationwide database study. BMC Gastroenterol. 2017;17:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Wang CC, Tsai MC, Sung WW, Yang TW, Chen HY, Wang YT, Su CC, Tseng MH, Lin CC. Risk of cholangiocarcinoma in patients undergoing therapeutic endoscopic retrograde cholangiopancreatography or cholecystectomy: A population based study. World J Gastrointest Oncol. 2019;11:238-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 797] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 8. | Zhou Y, Liu S, Wu L, Wan T. Survival after surgical resection of distal cholangiocarcinoma: A systematic review and meta-analysis of prognostic factors. Asian J Surg. 2017;40:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, Almer S, Granath F, Broomé U. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 469] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Scott J, Shousha S, Thomas HC, Sherlock S. Bile duct carcinoma: a late complication of congenital hepatic fibrosis. Case report and review of literature. Am J Gastroenterol. 1980;73:113-119. [PubMed] |
| 13. | Lipsett PA, Pitt HA, Colombani PM, Boitnott JK, Cameron JL. Choledochal cyst disease. A changing pattern of presentation. Ann Surg. 1994;220:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 222] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Watanapa P, Watanapa WB. Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002;89:962-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J, Fraumeni JF Jr. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Jing W, Jin G, Zhou X, Zhou Y, Zhang Y, Shao C, Liu R, Hu X. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev. 2012;21:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 18. | Zhang LF, Zhao HX. Diabetes mellitus and increased risk of extrahepatic cholangiocarcinoma: a meta-analysis. Hepatogastroenterology. 2013;60:684-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Chang JS, Tsai CR, Chen LT. Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One. 2013;8:e69981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Murphy G, Michel A, Taylor PR, Albanes D, Weinstein SJ, Virtamo J, Parisi D, Snyder K, Butt J, McGlynn KA, Koshiol J, Pawlita M, Lai GY, Abnet CC, Dawsey SM, Freedman ND. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology. 2014;60:1963-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? J Hepatol. 2012;57:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 22. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1378] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 23. | El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, Ahn YO, Shigemastu T. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914-3918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Kurumado K, Nagai T, Kondo Y, Abe H. Long-term observations on morphological changes of choledochal epithelium after choledochoenterostomy in rats. Dig Dis Sci. 1994;39:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Kalaitzis J, Vezakis A, Fragulidis G, Anagnostopoulou I, Rizos S, Papalambros E, Polydorou A. Effects of endoscopic sphincterotomy on biliary epithelium: a case-control study. World J Gastroenterol. 2012;18:794-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Oliveira-Cunha M, Dennison AR, Garcea G. Late Complications After Endoscopic Sphincterotomy. Surg Laparosc Endosc Percutan Tech. 2016;26:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Peng YC, Lin CL, Hsu WY, Chow WK, Lee SW, Yeh HZ, Chang CS, Kao CH. Association of Endoscopic Sphincterotomy or Papillary Balloon Dilatation and Biliary Cancer: A Population-Based Cohort Study. Medicine (Baltimore). 2015;94:e926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Langerth A, Sandblom G, Karlson BM. Long-term risk for acute pancreatitis, cholangitis, and malignancy more than 15 years after endoscopic sphincterotomy: a population-based study. Endoscopy. 2015;47:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Strömberg C, Böckelman C, Song H, Ye W, Pukkala E, Haglund C, Nilsson M. Endoscopic sphincterotomy and risk of cholangiocarcinoma: a population-based cohort study in Finland and Sweden. Endosc Int Open. 2016;4:E1096-E1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Guo L, Mao J, Li Y, Jiao Z, Guo J, Zhang J, Zhao J. Cholelithiasis, cholecystectomy and risk of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther. 2014;10:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Tao LY, He XD, Qu Q, Cai L, Liu W, Zhou L, Zhang SM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Nordenstedt H, Mattsson F, El-Serag H, Lagergren J. Gallstones and cholecystectomy in relation to risk of intra- and extrahepatic cholangiocarcinoma. Br J Cancer. 2012;106:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Wang CC, Tsai MC, Wang YT, Yang TW, Chen HY, Sung WW, Huang SM, Tseng MH, Lin CC. Role of Cholecystectomy in Choledocholithiasis Patients Underwent Endoscopic Retrograde Cholangiopancreatography. Sci Rep. 2019;9:2168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 2009; 137: 1641-8. e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 37. | Wu CY, Chan FK, Wu MS, Kuo KN, Wang CB, Tsao CR, Lin JT. Histamine2-receptor antagonists are an alternative to proton pump inhibitor in patients receiving clopidogrel. Gastroenterology. 2010;139:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. Available from: https://www.scienceopen.com/document?vid=61cdd360-9883-4330-8c18-3f0341b0f715. |
| 39. | Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver. 2017;11:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 40. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 690] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 41. | Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 42. | Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 486] [Article Influence: 25.6] [Reference Citation Analysis (6)] |
| 43. | Huang RJ, Barakat MT, Girotra M, Banerjee S. Practice Patterns for Cholecystectomy After Endoscopic Retrograde Cholangiopancreatography for Patients With Choledocholithiasis. Gastroenterology 2017; 153: 762-771. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |