Published online Nov 15, 2020. doi: 10.4251/wjgo.v12.i11.1372
Peer-review started: August 12, 2020
First decision: September 16, 2020
Revised: September 29, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: November 15, 2020
Processing time: 91 Days and 14.4 Hours
Cutaneous metastases originating from pancreatic cancer are relatively rare. The most common reported site of metastasis is the umbilicus, and this manifestation is known as the Sister Mary Joseph’s nodule. Non-umbilical cutaneous metastases are far less common, with only a few cases reported in the literature. Our case is the first case report, to our knowledge, on metastasis involving the labia majora and flat papules.
A 49-year-old Chinese female patient presented with a number of red, swollen papules on the vulva for 2 mo. Histological examination of the labia majora lesion revealed metastatic adenocarcinoma. The serum levels of tumor biomarkers CA199, CA242, and CA125 were significantly elevated. B-mode ultrasound-guided needle biopsy of the pancreas demonstrated moderately and poorly differentiated adenocarcinoma. The patient finally declined treatment for financial reasons and died 3 mo later.
Metastatic cutaneous lesions could indicate pancreatic cancer. Serum levels of tumor biomarkers may aid in diagnosing metastatic pancreatic adenocarcinoma.
Core Tip: Cutaneous metastasis from pancreatic cancer is uncommon. The most common site of the skin lesion is the umbilicus. The majority of skin lesions are singular, particularly in patients exhibiting the Sister Mary Joseph’s nodule. We describe an unusual case of flat papules on the labia majora that metastasized from pancreatic cancer. The lesion was the first sign of metastatic disease, and serum levels of CA199, CA242, and CA125 were also elevated. We report this case to improve the understanding of cutaneous metastasis of pancreatic cancer and emphasize the importance of serum levels of CA199, CA242, and CA125 in diagnosing pancreatic cancer.
- Citation: Shi Y, Li SS, Liu DY, Yu Y. Cutaneous metastases of pancreatic carcinoma to the labia majora: A case report and review of literature. World J Gastrointest Oncol 2020; 12(11): 1372-1380
- URL: https://www.wjgnet.com/1948-5204/full/v12/i11/1372.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i11.1372
Pancreatic cancer is one of the most lethal human malignancies and is often diagnosed late in the course of the disease. Cutaneous metastases originating from pancreatic cancer are relatively rare. The most common reported site of metastasis is the umbilicus, and this manifestation is known as the Sister Mary Joseph’s nodule. Non-umbilical cutaneous metastases are far less common, with only a few cases reported in the literature. To our knowledge, there are no previous reports on metastasis involving the labia majora and flat papules. This report describes a case of cutaneous pancreatic metastases on the labia majora in a Chinese woman, and a review of previously reported non-umbilical cutaneous pancreatic carcinoma metastases (by conducting a detailed PubMed search). Furthermore, an analysis of 34 reported cases of non-umbilical cutaneous metastasis from pancreatic cancer was conducted with regard to the clinical characteristics.
A 49-year-old female patient with a number of red, swollen papules on the vulva for 2 mo was referred to our department.
The patient had developed red, swollen papules on the vulva 3 mo previously. She attended a local hospital, where she was diagnosed with contact dermatitis and took regular anti-allergy treatment. There was no obvious improvement in her symptoms. Erythema gradually affected the abdomen and lower extremities. The patient had experienced intermittent shortness of breath and coughed up white sputum during this period.
The patient had experienced hypertension for 2 years and had a history of sulfonamide allergy.
There was not a personal or family history of pancreatic cancer.
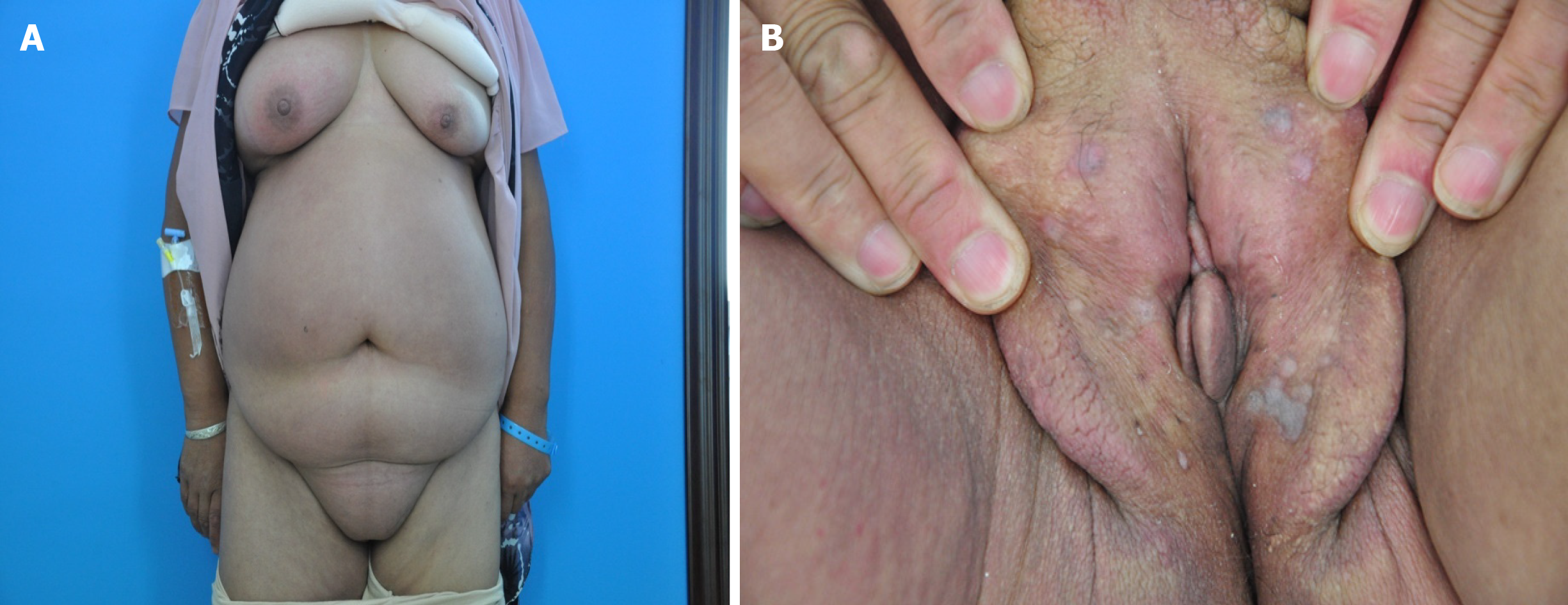
Physical examination revealed diffuse erythema and swelling on the chest, abdomen, and vulva; right leg edema; and a number of flat skin-colored or gray papules on the labia majora (Figure 1).
Routine laboratory testing revealed that the patient was human immunodeficiency virus (HIV) negative; the results of blood and urine tests and levels of renal and hepatic markers and antinuclear antibodies were within the normal ranges. There was a remarkable elevation in the serum concentrations of tumor markers, comprising cytokeratin 19 (8.98 g/L; normal: < 5 µg/L), CA242 (> 500 U/mL; normal: < 20 U/mL), CA125 (52.41 U/mL; normal: < 35 U/mL), and CA199 (726.54 U/mL; normal: < 37 U/mL).
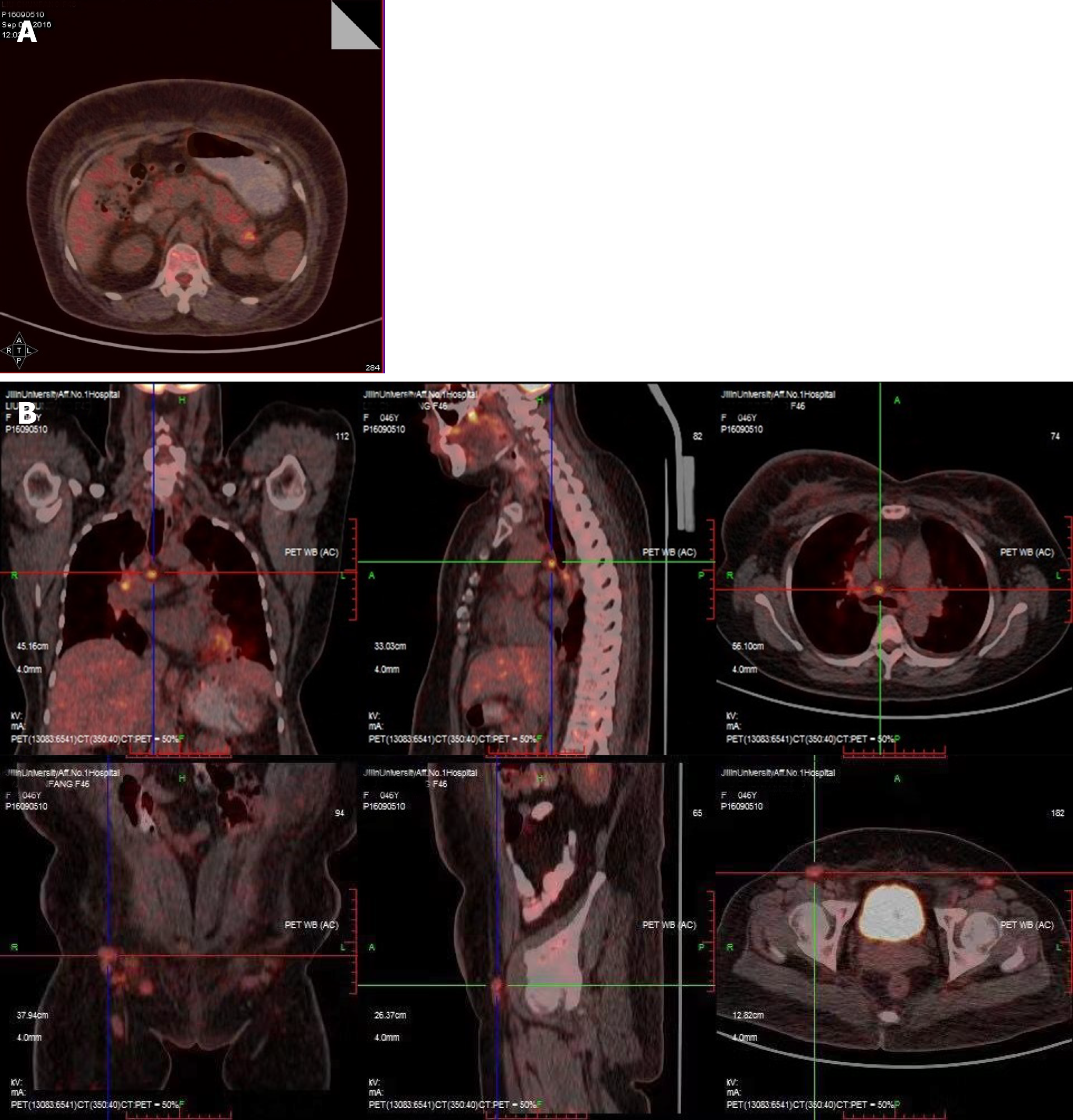
An ultrasound scan showed bilateral mammary gland hyperplasia, no other gynecological abnormalities, and right leg lymphedema. A chest computerized tomography (CT) scan revealed inflammation, with patterns of interstitial change scattered across both lungs and a little pleural effusion in the left lung. An abdominal CT scan demonstrated many enlarged lymph nodes, associated with the abdominal aorta, bilateral iliac artery, and bilateral inguinal region, as well as changes in the pancreatic and peripancreatic morphology. A positron emission tomography (PET)-CT scan showed increased metabolic activity in the tail of the pancreas and multiple enlarged lymph nodes throughout the body, as well as subcutaneous tissue edema (Figure 2).
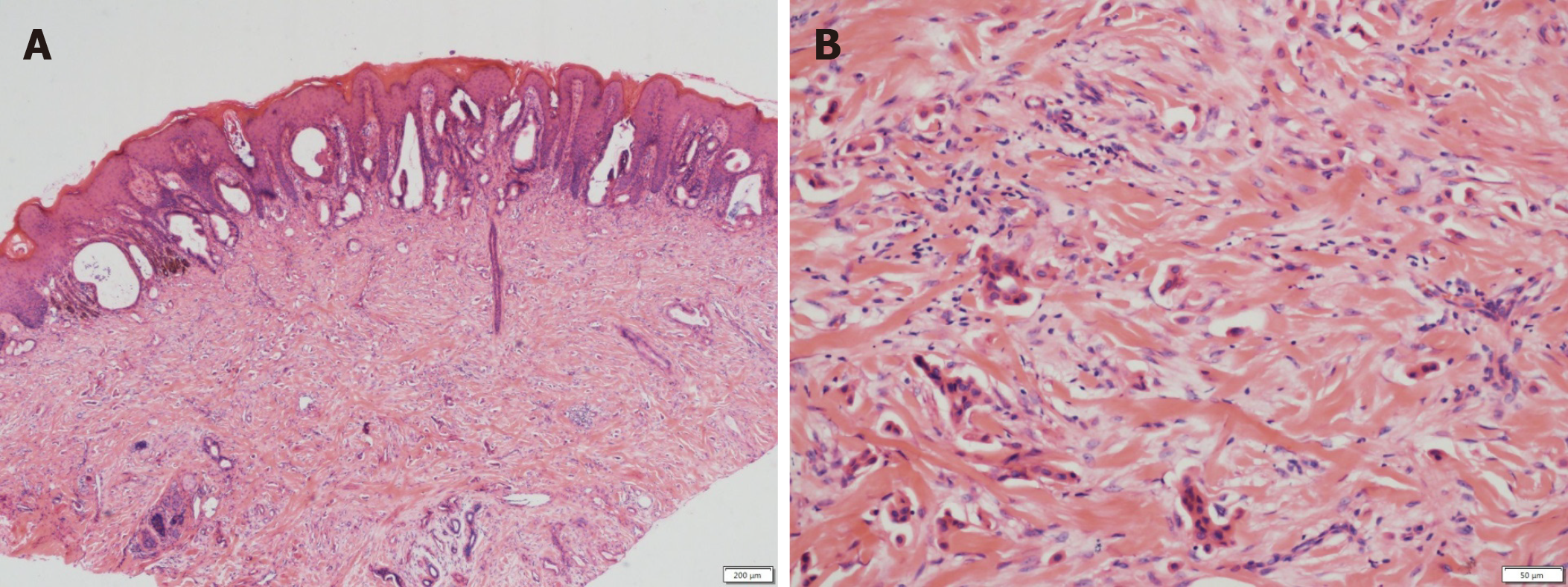
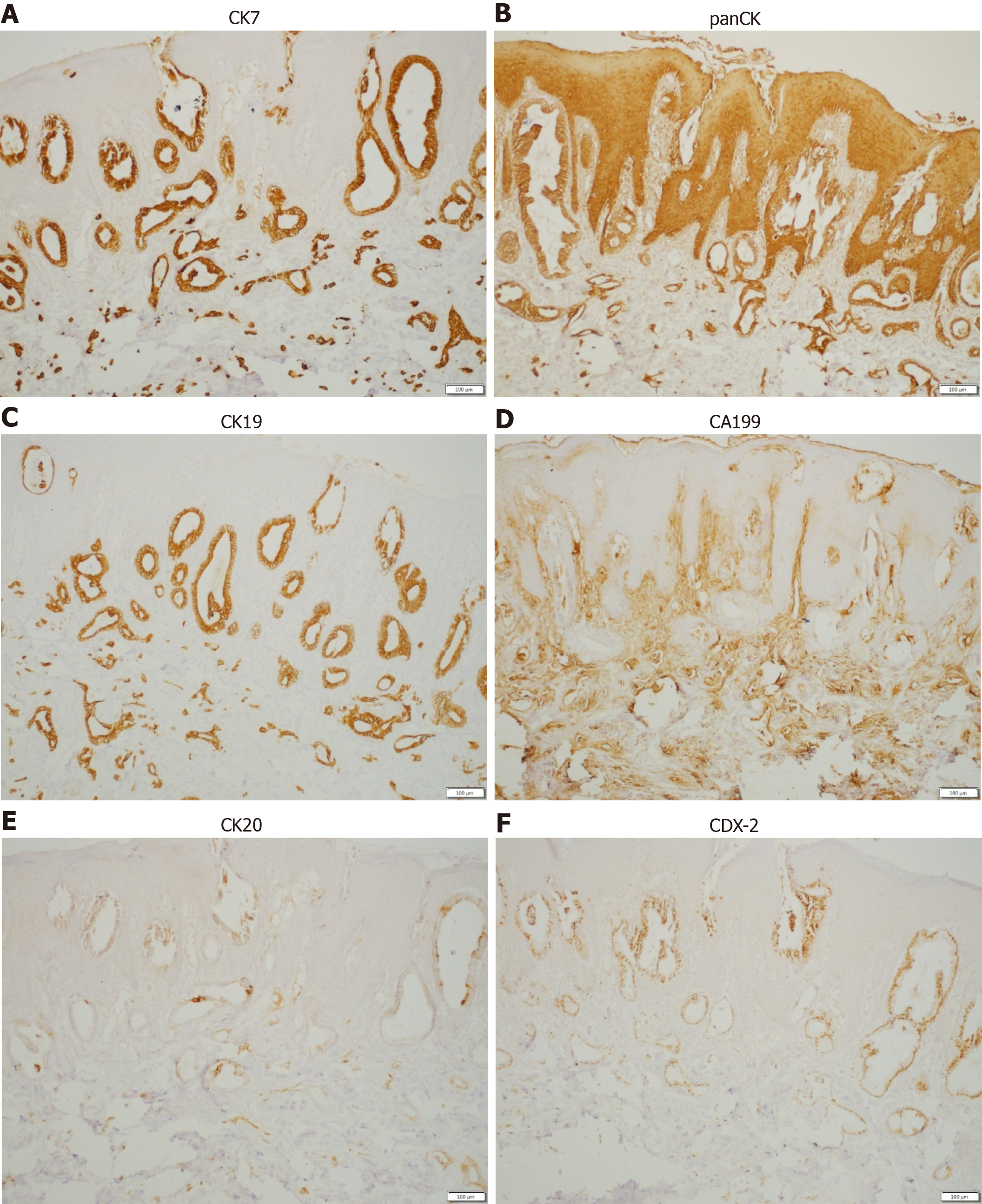
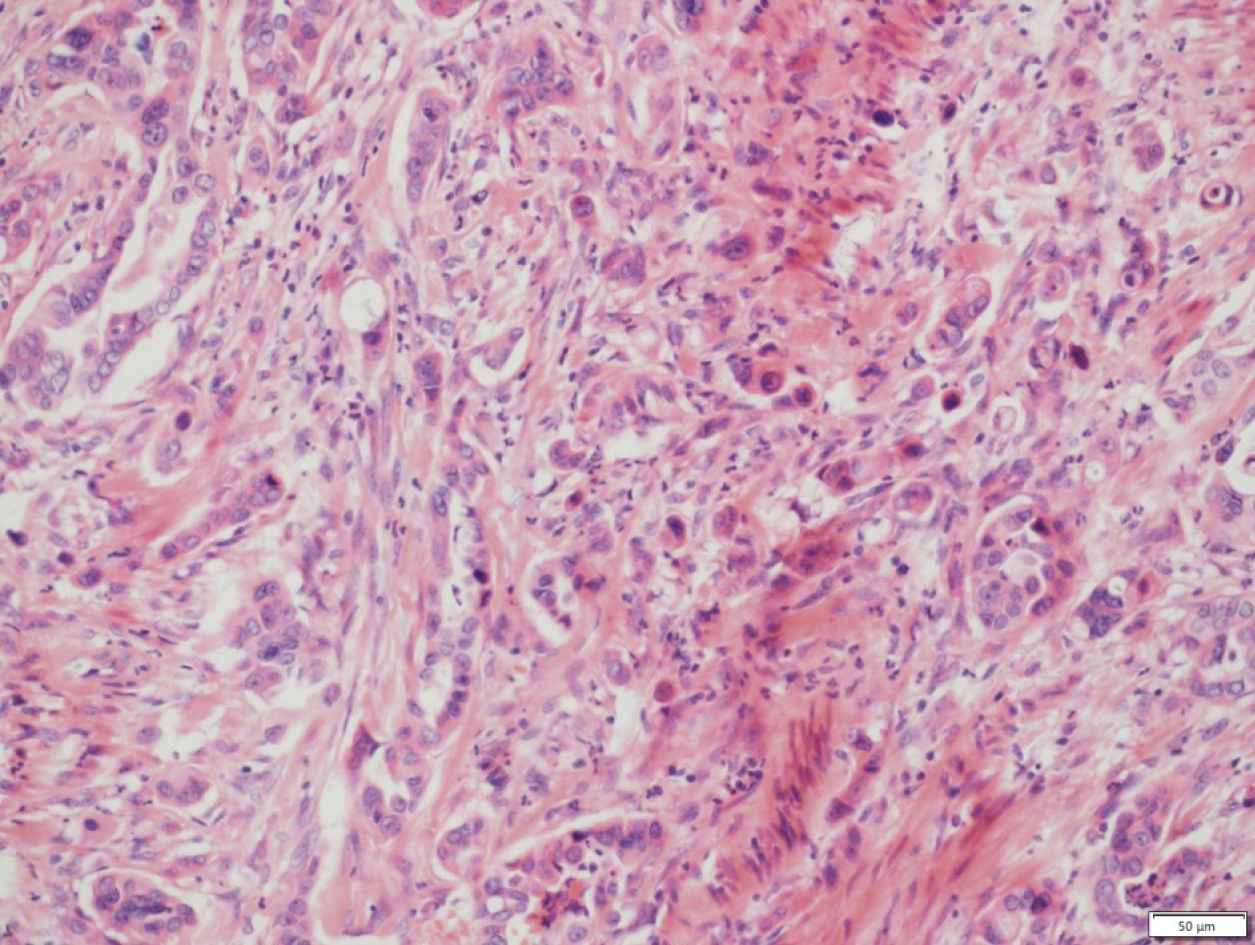
A punch biopsy was performed on the abdomen and labia majora lesions. The abdominal epidermis was normal, and there were dilated small blood vessels and lymph vessels in the dermis, and small quantities of infiltrating lymphocytes. Histological examination of the labia majora papules showed nests of moderately differentiated atypical cells partly forming adenomatous structures in the collagen bundles of the dermis and lymphangiectasia (pathologic dilation of the lymph vessels) in the dermis (Figure 3). The immunohistochemical staining results (Figure 4) were as follows: CK7 (+), panCK (+), CK19 (+), CA19-9 (+), CDX-2 (+), CK20 (+), D2-40 (-), and P63 (-), which is consistent with a possible pancreatic source of the metastasis.
In addition, a large amount of milky fluid was drained from the skin biopsy sites. While the serum triglyceride level was normal, the level in the fluid was 1.55 mmol/L, indicating lymphocytosis. Neither bacteria (including Mycobacterium tuberculosis) nor fungi were found in the fluid. According to the laboratory and histopathologic findings, a diagnosis of chylous reflux fluid was made.
Thereafter, B-mode ultrasound-guided needle biopsy of the pancreas demonstrated moderately and poorly differentiated adenocarcinoma (Figure 5).
According to the clinical manifestations and histopathologic findings, a diagnosis of metastatic pancreatic carcinoma was established.
The patient declined treatment for financial reasons.
The patient died 3 mo later.
Cutaneous metastases are rare, occurring in 0.7%-9.0% of all patients with cancer[1]. Breast, lung, and colon cancer are the most frequent origins of cutaneous metastases.
Pancreatic cancer is one of the most lethal cancers, with a 5 year survival rate of 5%. Owing to a lack of early clinical features and the fulminant disease course, early diagnosis is difficult and the metastasis rate is high, with a median overall survival of 5 mo[2]. Pancreatic cancer is often known to metastasize via the lymphatic system, with direct invasion at an early stage and hematogenous dissemination at a later stage. The incidence of cutaneous metastases is significantly more frequent for cancers of the pancreatic body and tail than for pancreatic head cancer[3].
The most frequent organs involved in the metastasis are the liver, peritoneum, lungs, bones, and brain[2]. Indeed, only 2% of the first metastases from pancreatic cancer are cutaneous metastases, making cutaneous metastases relatively rare[4]. The most common site of cutaneous metastasis from the pancreas is the umbilicus, and this manifestation is known as Sister Mary Joseph’s nodule[5]. Horino et al[6] reviewed 42 reported cases of pancreatic metastasis from 1950 to 2011, and included only 14 cases of non-umbilical cutaneous metastasis, involving the head, neck, and truncal region. The patients with pancreatic metastasis had such symptoms as subcutaneous nodules (in 26 patients) and inflammatory erythema (in 3 patients).
We collected all case reports of non-umbilical metastatic skin cancer from 1950 to 2020 on the electronic PubMed database (www.ncbi.nlm.nih.gov/pubmed; up to July 2020) by searching using Medical Subject Headings (www.nlm.nih. gov/mesh) and keywords, and by limiting the search to human studies (Table 1)[1-4,6-30]. The terms pancreatic cancer, pancreatic neoplasm, pancreatic carcinoma, cutaneous metastasis, and skin metastasis were used. The abstracts were reviewed, and articles that were associated with umbilical metastasis were excluded. Duplicate references as well as repeated publications were discarded. All of the studies that were considered to be eligible were retrieved and the final selection was based on the full article.
| Ref. | Age (yr) | Sex | Appearance | Metastatic site | Primary | Year | |
| 1 | Edelstein et al[10] | 60 | M | Cellulitis | Face, neck | NA | 1950 |
| 2 | Sakai et al | 47 | M | Herpes zoster-like | No details | Head | 1969 |
| 3 | Sironi et al[12] | 72 | M | Nodule | Right thigh | Head | 1991 |
| 4 | Lookingbill et al[13] | No details | No details | Nodule | Abdomen | No details | 1993 |
| 5 | Taniguchi et al[4] | 63 | F | Erythematousplaques, nodule | Left axilla, chest | No details | 1994 |
| 6 | Ohashi et al | 79 | M | Nodule | Neck, chest, abdomen | No details | 1995 |
| 7 | Ohashi et al | 65 | M | Nodule | Back | No details | 1995 |
| 8 | Fukui et al | 49 | M | Nodule | Face, chest | No details | 1995 |
| 9 | Puri et al[14] | 45 | M | Nodule | Scalp, face, neck, back | No details | 1995 |
| 10 | Nakano et al[15] | 80 | M | Nodule | Occipital scalp | Tail | 1996 |
| 11 | Miyahara et al[16] | 43 | M | Nodule | Scalp | Uncus | 1996 |
| 12 | Miyahara et al[16] | 65 | M | Nodule | Mentum | Uncus | 1996 |
| 13 | Horino et al[17] | 65 | F | Nodule | Chest wall | Head | 1999 |
| 14 | Flórez et al[18] | 48 | M | Nodule | Buttock | Head | 2000 |
| 15 | Gawrieh et al[19] | 45 | F | Nodule | Temporal scalp | No details | 2002 |
| 16 | Takeuchi et al[3] | 77 | M | Nodule | Left axilla | Tail | 2003 |
| 17 | Otegbayo et al[20] | 59 | M | Nodule | Face, chest, abdomen, back | No details | 2005 |
| 18 | Jun et al[21] | 68 | M | Nodule | Right forearm, chest | Body, tail | 2005 |
| 19 | Ambro et al[22] | 63 | M | Nodule | Scalp | Ductal | 2006 |
| 20 | Takemura et al[23] | 85 | M | Nodule | Left temple | No details | 2007 |
| 21 | Hafez et al[24] | 55 | F | Nodule | Neck | Head | 2008 |
| 22 | Kimura et al | 50 | M | Nodule | Lateral abdomen | Body | 2008 |
| 23 | van Akkooi et al[25] | 59 | M | Nodule | Scalp | No details | 2010 |
| 24 | Bdeiri et al[1] | 59 | F | Nodule | Scalp | Tail | 2010 |
| 25 | Pontinen et al[26] | 67 | F | Nodule | Lower abdomen | Tail | 2010 |
| 26 | Saif et al[27] | 46 | F | Nodule | Chest, abdomen, right supraclavicular area | No details | 2011 |
| 27 | Horino et al[6] | 58 | F | Nodule | Lower abdomen | Body | 2012 |
| 28 | Horino et al[6] | 65 | F | Nodule | Lower abdomen | Tail | 2012 |
| 29 | Bdeiri et al[1] | 70 | F | Nodule | Scalp | Tail | 2013 |
| 30 | Kaoutzanis et al[28] | 43 | M | Nodule | Scalp | Head | 2013 |
| 31 | Zhou et al[2] | 76 | F | Nodule | Scalp, chest, abdomen | Tail | 2014 |
| 32 | Shin et al[29] | 60 | M | Mass | Left hip | Body | 2015 |
| 33 | Kotsantis et al[11] | 62 | M | Edema | Scrotum, trunk, chest wall | Head | 2017 |
| 34 | Ito et al[30] | 71 | F | Ulcer | Scalp | Tail | 2020 |
Our case is unique in that the cutaneous pancreatic cancer metastases were found on the labia majora. Our patient had papules on the labia majora, with diffuse swelling on the skin of the chest, abdomen, and vulva. In addition, there was oozing of chyle from the skin biopsy sites.
Chyle originates in the bowel lacteals (lymphatic capillaries that absorb dietary fats), is absorbed by the intestinal lymphatic vessels, passes through the collecting lymphatic vessels into the intestinal trunk (truncus intestinalis), and then passes through the cisterna chyli and thoracic duct to the bloodstream. If the reflux is obstructed, chylous reflux occurs; the chyle flows backward or leaks into the serosal cavity, external genitalia, lower limbs, etc. Chylous reflux is divided into primary and secondary types. Secondary chylous reflux is usually caused by neoplasia, trauma, inflammation, or surgery[7]. According to the PET-CT scan, the metastatic cancer cells may have traveled to the labia majora via the lymphatic pathways. The abnormal lymphatic system, caused by tumor metastasis, may have been the underlying cause for the backflow of chyle to the skin. The patient was advised to undergo lymphangiography to confirm this, but declined for financial reasons.
The serum levels of tumor biomarkers CA199, CA242, and CA125 were significantly elevated in our case. It has been shown that the sensitivity and specificity of CA199 for diagnosing pancreatic cancer are 77% and 88%, respectively. Ozkan et al[8] revealed that CA242 was significantly increased in 75% of pancreatic cancer patients. To sum up, Mao et al[9] suggested that assessment of the combination of CA199, CA242, CEA, and CA125 levels is a simple, noninvasive, and effective method for early diagnosis of pancreatic cancer.
We present a case of pancreatic adenocarcinoma metastasizing to the vulva, with a number of flat papules. This condition is extremely rare and easy to misdiagnose.
Cutaneous metastasis from pancreatic carcinoma is a rare finding and mostly occurs around the umbilicus. Subcutaneous nodules are the most common clinical manifestation. To our knowledge, we describe the first case of cutaneous pancreatic metastasis to the vulva, with a number of flat papules. Clinicians should be aware of the possibility of metastatic cutaneous lesions in pancreatic adenocarcinoma and screen for tumor markers as promptly as possible.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Buanes T S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bdeiri K, Kamar FG. Cutaneous metastasis of pancreatic adenocarcinoma as a first clinical manifestation: a case report and review of the literature. Gastrointest Cancer Res. 2013;6:61-63. [PubMed] |
| 2. | Zhou HY, Wang XB, Gao F, Bu B, Zhang S, Wang Z. Cutaneous metastasis from pancreatic cancer: A case report and systematic review of the literature. Oncol Lett. 2014;8:2654-2660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Takeuchi H, Kawano T, Toda T, Minamisono Y, Nagasaki S, Yao T, Sugimachi K. Cutaneous metastasis from pancreatic adenocarcinoma: a case report and a review of the literature. Hepatogastroenterology. 2003;50:275-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Taniguchi S, Hisa T, Hamada T. Cutaneous metastases of pancreatic carcinoma with unusual clinical features. J Am Acad Dermatol. 1994;31:877-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Gabriele R, Conte M, Egidi F, Borghese M. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Horino K, Takamori H, Ikuta Y, Nakahara O, Chikamoto A, Ishiko T, Beppu T, Baba H. Cutaneous metastases secondary to pancreatic cancer. World J Gastrointest Oncol. 2012;4:176-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Huang HY, Chiu WT. Primary chylous reflux presenting with vulvar vesicles. Arch Dermatol. 2010;146:683-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Ozkan H, Kaya M, Cengiz A. Comparison of tumor marker CA 242 with CA 19-9 and carcinoembryonic antigen (CEA) in pancreatic cancer. Hepatogastroenterology. 2003;50:1669-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 371] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 9. | Mao QQ, Yan WL, Sun X, Hu Y, Zhong L. The value of serum CAl99,CA242 and CAl25 in the diagnosis and prognosis of pancreatic cancer. Biaoji Mianyi Fenxi Yu Linchunag Zazhi. 2009;16:79-82. [DOI] [Full Text] |
| 10. | EDELSTEIN JM. Pancreatic carcinoma with unusual metastasis to the skin and subcutaneous tissue simulating cellulitis. N Engl J Med. 1950;242:779-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Kotsantis I, Economopoulou P, Dritsakos K, Oikonomopoulos N, Bakogeorgos M, Rapti C, Kentepozidis N. Extensive cutaneous metastases of pancreatic adenocarcinoma: a case report and review of the literature. Clin Case Rep. 2017;5:51-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Sironi M, Radice F, Taccagni GL, Braga M, Zerbi M. Fine needle aspiration of a pancreatic oxyphilic carcinoma with pulmonary and subcutaneous metastases. Cytopathology. 1991;2:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 583] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 14. | Puri AS, Saraswat VA, Krishnani N, Salunke PN. Cutaneous metastases in pancreatic adenocarcinoma. Indian J Pathol Microbiol. 1995;38:99-101. [PubMed] |
| 15. | Nakano S, Narita R, Yamamoto M, Ogami Y, Osuki M. Two cases of pancreatic cancer associated with skin metastases. Am J Gastroenterol. 1996;91:410-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Miyahara M, Hamanaka Y, Kawabata A, Sato Y, Tanaka A, Yamamoto A, Ueno T, Nishihara K, Suzuki T. Cutaneous metastases from pancreatic cancer. Int J Pancreatol. 1996;20:127-130. [PubMed] |
| 17. | Horino K, Hiraoka T, Kanemitsu K, Tsuji T, Inoue K, Tanabe D, Takamori H, Matsuoka M, Kitamura N. Subcutaneous metastases after curative resection for pancreatic carcinoma: a case report and review of the literature. Pancreas. 1999;19:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Flórez A, Rosón E, Sánchez-Aguilar D, Peteiro C, Toribio J. Solitary cutaneous metastasis on the buttock: a disclosing sign of pancreatic adenocarcinoma. Clin Exp Dermatol. 2000;25:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Gawrieh S, Massey BT, Komorowski RA. Scalp metastases as the first manifestation of pancreatic cancer. Dig Dis Sci. 2002;47:1469-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Otegbayo JA, Oluwasola OA, Akere A, Yakubu A, Daramola OO, Ogun GO. Pancreatic carcinoma presenting as cutaneous nodules in a diabetic Nigerian male. West Afr J Med. 2005;24:180. [PubMed] |
| 21. | Jun DW, Lee OY, Park CK, Choi HS, Yoon BC, Lee MH, Lee DH. Cutaneous metastases of pancreatic carcinoma as a first clinical manifestation. Korean J Intern Med. 2005;20:260-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Ambro CM, Humphreys TR, Lee JB. Epidermotropically metastatic pancreatic adenocarcinoma. Am J Dermatopathol. 2006;28:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Takemura N, Fujii N, Tanaka T. Cutaneous metastasis as the first clinical manifestation of pancreatic adenocarcinoma: a case treated with gemcitabine. J Dermatol. 2007;34:662-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Hafez HZ. Cutaneous pancreatic metastasis: a case report and review of literature. Indian J Dermatol. 2008;53:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | van Akkooi AC, Dokter J, Boxma H. Unusual first presentation of metastatic pancreatic cancer as skin metastases in a burn patient. Burns. 2010;36:e111-e114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Pontinen T, Melin A, Varadi G, Khanmoradi K, Chewaproug D, Kung SC, Zaki R, Ortiz J. Cutaneous metastasis of pancreatic adenocarcinoma after kidney transplant: a case report and review of the literature. Exp Clin Transplant. 2010;8:273-276. [PubMed] |
| 27. | Saif MW, Brennan M, Penney R, Hotchkiss S, Kaley K. Cutaneous metastasis in a patient with pancreatic cancer. JOP. 2011;12:306-308. [PubMed] |
| 28. | Kaoutzanis C, Chang MC, Abdul Khalek FJ, Kreske E. Non-umbilical cutaneous metastasis of a pancreatic adenocarcinoma. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Shin WY, Lee KY, Ahn SI, Park SY, Park KM. Cutaneous metastasis as an initial presentation of a non-functioning pancreatic neuroendocrine tumor. World J Gastroenterol. 2015;21:9822-9826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Ito H, Tajiri T, Hiraiwa SI, Sugiyama T, Ito A, Shinma Y, Kaneko M, Anzai K, Tsuda S, Ichikawa H, Nagata J, Kojima S, Watanabe N. A Case of Rare Cutaneous Metastasis from Advanced Pancreatic Cancer. Case Rep Oncol. 2020;13:49-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |