Published online Nov 15, 2020. doi: 10.4251/wjgo.v12.i11.1325
Peer-review started: July 26, 2020
First decision: August 9, 2020
Revised: August 20, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: November 15, 2020
Processing time: 108 Days and 17.1 Hours
Although Borrmann type IV (B-4) gastric cancer has a higher mortality rate and presents distant metastasis easily, especially peritoneal metastasis, when diagnosed, some B-4 patients were found to have no distant metastasis by preoperative detection and underwent curative surgery, which was defined as circumscribed B-4 in our study. In this study, we focused on the circumscribed B-4 patients without distant metastasis during surgery to identify factors related to prognosis and postoperative peritoneal cavity metastasis (PPCM), which is important for selecting an appropriate therapeutic strategy.
To identify factors related to the prognosis and PPCM of B-4 patients.
A total of 117 B-4 patients who underwent gastrectomy between January 2005 and December 2012 were included in this study. Survival analysis was performed using Kaplan–Meier analysis and Cox multivariate models. Pearson correlation analyses were performed to identify the factors related to PPCM. All statistical analyses were performed using SPSS 20.0.
Lymph node status, gastrectomy type, and postoperative chemotherapy were independent prognostic factors in 117 circumscribed B-4 patients. Subtotal gastrectomy combined with chemotherapy could significantly improve the long-term survival time. Six patients who were diagnosed with pN0 and received the combination therapy had a 3-year survival rate of 100% and a median survival of 77.7 mo. Even for patients with metastatic lymph nodes (n = 13), the combination therapy also increased the 3-year overall survival rate to 57.1%. In addition, positive lymph node status was the only factor (P = 0.005) correlated with PPCM in certain B-4 patients, and chemotherapy was useful for suppressing PPCM in patients with subtotal gastrectomy but not in those with total gastrectomy.
Lymph node status is an independent prognostic factor for circumscribed B-4 patients. In addition, subtotal gastrectomy and postoperative chemotherapy could effectively improve prognosis and even suppress PPCM.
Core Tip: This is a retrospective study to evaluate the factors related to prognosis and prognostic postoperative peritoneal cavity metastasis for circumscribed Borrmann type IV (B-4) patients. We reported that lymph node metastatic status, gastrectomy type, and postoperative chemotherapy were the independent prognostic factors. Subtotal gastrectomy combined with chemotherapy could significantly improve the long-term survival time of circumscribed B-4 patients. And chemotherapy was also useful for suppressing postoperative peritoneal cavity metastasis in patients with subtotal gastrectomy. We believe that our study makes a significant contribution to the literature because it recommended reasonable treatment schedules for the B-4 patients, which can increase survival time to a certain extent.
- Citation: Huang HB, Gao ZM, Sun AQ, Liang WT, Li K. Subtotal gastrectomy combined with chemotherapy: An effective therapy for patients with circumscribed Borrmann type IV gastric cancer. World J Gastrointest Oncol 2020; 12(11): 1325-1335
- URL: https://www.wjgnet.com/1948-5204/full/v12/i11/1325.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i11.1325
Gastric cancer (GC) is the fourth most common malignancy and the second most frequent cause of cancer-related death worldwide, with approximately 951600 new cases and 723100 deaths every year[1-3]. Borrmann type IV (B-4) GC, as an aggressive type of GC, accounts for approximately 10% of all GC cases in Asia[4, 5]. B-4 lesions are characterized as lesions that diffusely infiltrate the gastric wall without ulceration or elevation. Compared with other types of GC, the occurrence rates of serosal invasion, positive lymph nodes, and distant metastasis in B-4 patients are higher.
Currently, surgery with chemotherapy is the major treatment method for advanced GC, which significantly improves the rate of long-term overall survival (OS). However, many B-4 patients are not suitable for surgical treatment at the time of diagnosis, and the 3-year OS rate of B-4 patients is only approximately 15%-20%[6-8]. Studies on the clinicopathology and prognosis of B-4 patients have had controversial findings[4,8-12]. Some B-4 patients were found to have no distant metastasis by preoperative detection and underwent curative surgery, which was defined as circumscribed B-4 in our study. Circumscribed B-4 accounts for relatively fewer cases of B-4 patients, and the clinicopathological characteristics and prognostic analysis of these patients would help us to understand the recurrence and metastasis of GC. There is no related research, especially for circumscribed B-4 patients.
In this study, we aimed to identify the factors related to prognosis and postoperative peritoneal cavity metastasis (PPCM) for circumscribed B-4 patients and to further explore the appropriate therapeutic strategies.
A total of 1803 patients were diagnosed with GC and underwent gastrectomy at the First Affiliated Hospital of China Medical University from January 2005 to December 2012. Among them, 117 circumscribed B-4 patients were included in our analysis. The inclusion criteria for the patients were as follows: (1) B-4 gastric lesions and no distant metastasis were confirmed by preoperative detection and histopathology postoperatively; (2) Gastrectomy, including subtotal or total, was performed without neoadjuvant therapy; and (3) Detailed clinicopathological and follow-up data could be obtained for each patient. This study was approved by the Ethics Committee of China Medical University, and informed consent was obtained from the patients.
The following data were collected: Age, gender, tumor size, tumor location, radical degree (R0/R1), histological type (well/poor), tumor invasion depth (pT), lymph node status (pN), lymphatic vessel infiltration, subtotal/total gastrectomy, postoperative chemotherapy, PPCM, and overall survival time. Of these, highly or moderately differentiated adenocarcinoma was classified as the well differentiated histological type, while others were classified as the poorly differentiated histological type. Selection indication of chemotherapy for GC patients was based on the National Comprehensive Cancer Network (NCCN) Guidelines. The adjuvant chemotherapy regimen was FOLFOX6 in postoperative 6 mo, including 5-fluorouracil and platinum. Completion degree of chemotherapy was heterogeneous, with eight or less cycles. Follow-up was completed by December 2017, and stage was classified according to the 8th edition of the American Joint Committee on Cancer (AJCC) classification system.
Continuous variables are expressed as the mean ± standard deviation (SD), and categorical variables are expressed as frequencies. We performed multivariate analyses with the Cox proportional hazards model to identify independent prognostic factors. In addition, Kaplan-Meier analysis and the log-rank test were also used to evaluate the prognostic difference between groups. Pearson correlation analyses were performed for related factors of PPCM. The above statistical analyses were performed using SPSS 20.0, and P < 0.01 was considered statistically significant.
A total of 117 patients were diagnosed with circumscribed B-4 GC and finally included in our analyses. As shown in Table 1, the mean age of the patients was 57.55 years, and the mean diameter of tumors was 7.81 cm. According to TNM stage, there were 27 cases of stage II and 90 cases of stage III. Of these, 81 (69.2%) patients had stage pT4, and 65 (55.6%) had stage pN3. A total of 116 patients received D2 lymphadenectomy, and the other one underwent D2+ lymphadenectomy. None of the 117 patients had distant metastasis, and cytology result of peritoneal lavage fluid was negative in our study.
| Variable | B-4 patients (n = 117) |
| Age (yr) | 57.55 ± 10.69 |
| Gender | |
| Male | 75 |
| Female | 42 |
| Diameter (cm) | 7.81 ± 3.07 |
| Tumor location | |
| Upper 1/3–1/2 (U or MU) | 22 |
| Lower 1/3–1/2 (M or L or ML) | 59 |
| Total (LAU or LMU) | 36 |
| Radical degree | |
| R0 | 45 |
| R1+ | 72 |
| Histological type | |
| Well | 8 |
| Poor | 109 |
| pT stage | |
| pT3 | 36 |
| pT4 | 81 |
| pN stage | |
| pN0 | 22 |
| pN1 | 15 |
| pN2 | 15 |
| pN3 | 65 |
| Venous/lymphatic infiltration | |
| Yes | 74 |
| No | 43 |
| Gastrectomy type | |
| Subtotal gastrectomy | 26 |
| Total gastrectomy | 91 |
| Adjuvant chemotherapy | |
| Yes | 74 |
| No | 43 |
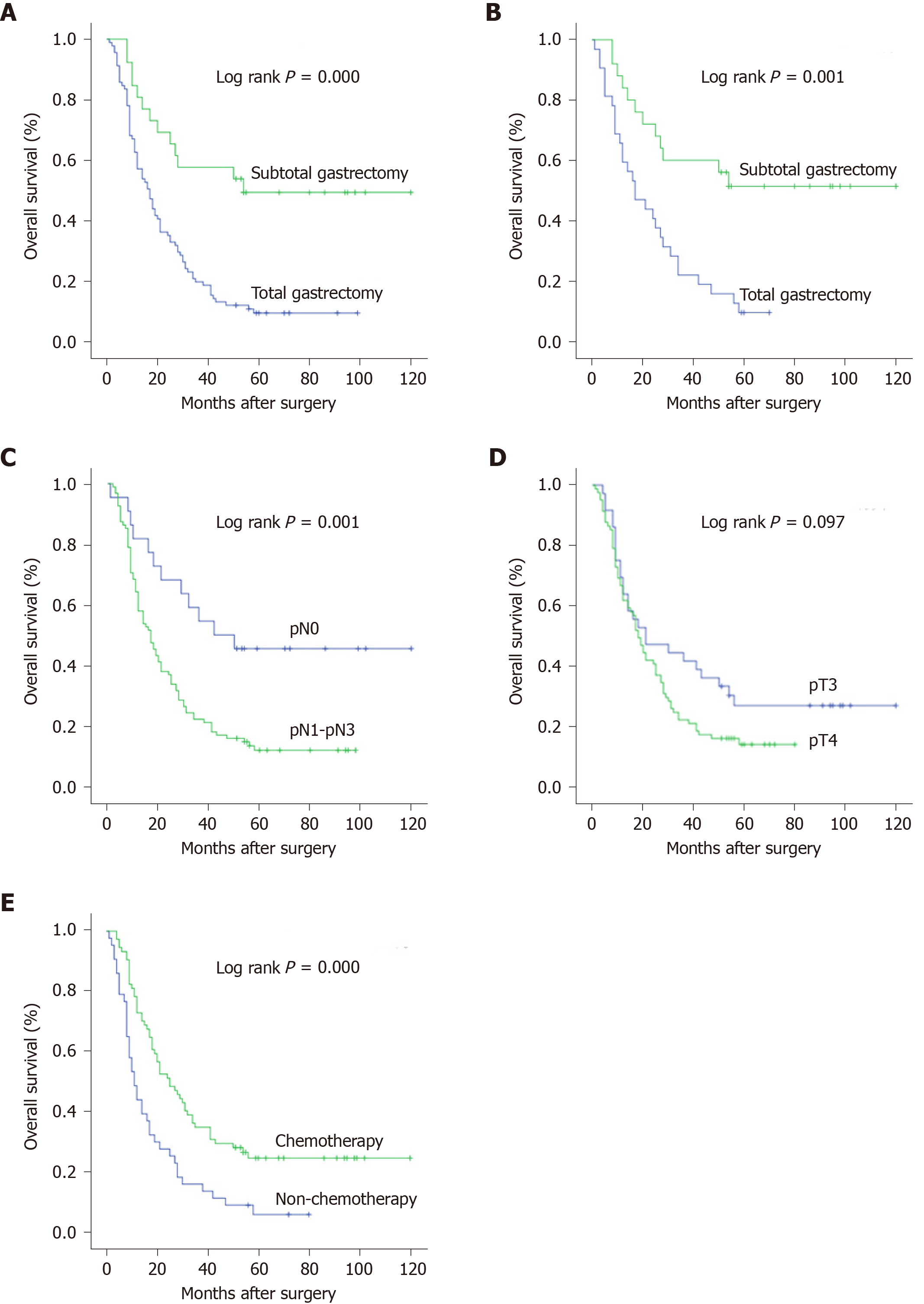
According to the Cox analysis, gastrectomy type (P = 0.003), pN stage (P = 0.000), and postoperative chemotherapy (P = 0.007) were crucial for predicting the prognosis of these circumscribed B-4 patients (Table 2). As shown in Figure 1A, the prognosis of patients who underwent subtotal gastrectomy was better than that of patients who underwent total gastrectomy (P = 0.000). After screening out patients with lower-middle lesions, the prognostic superiority of subtotal gastrectomy was consistent (Figure 1B). Notably, patients with a higher pN stage always had a worse prognosis (Figure 1C), but the prognostic value of pT stage was not significant (Figure 1D). In addition, as shown in Figure 1E, postoperative chemotherapy improved the long-term survival rate of circumscribed B-4 patients (P = 0.000).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 1.012 (0.992-1.033) | 0.226 | ||
| Gender | 0.969 (0.638-1.470) | 0.881 | ||
| Diameter (cm) | 1.086 (1.013-1.164) | 0.02 | ||
| Tumor location | 1.206 (0.879-1.655) | 0.246 | ||
| Radical degree | 1.575 (0.687-3.612) | 0.283 | ||
| Histological type | 0.708 (0.310-1.620) | 0.414 | ||
| pT stage | 1.422 (1.021-1.982) | 0.037 | ||
| pN stage | 1.482 (1.224-1.794) | 0.000 | 1.433 (1.179-1.742) | 0.000 |
| Venous/lymphatic infiltration | 0.934 (0.615-1.418) | 0.748 | ||
| Gastrectomy type | 0.330 (0.182-0.595) | 0.000 | 0.400 (0.219-0.728) | 0.003 |
| Postoperative chemotherapy | 0.482 (0.320-0.727) | 0.001 | 0.564 (0.373-0.853) | 0.007 |
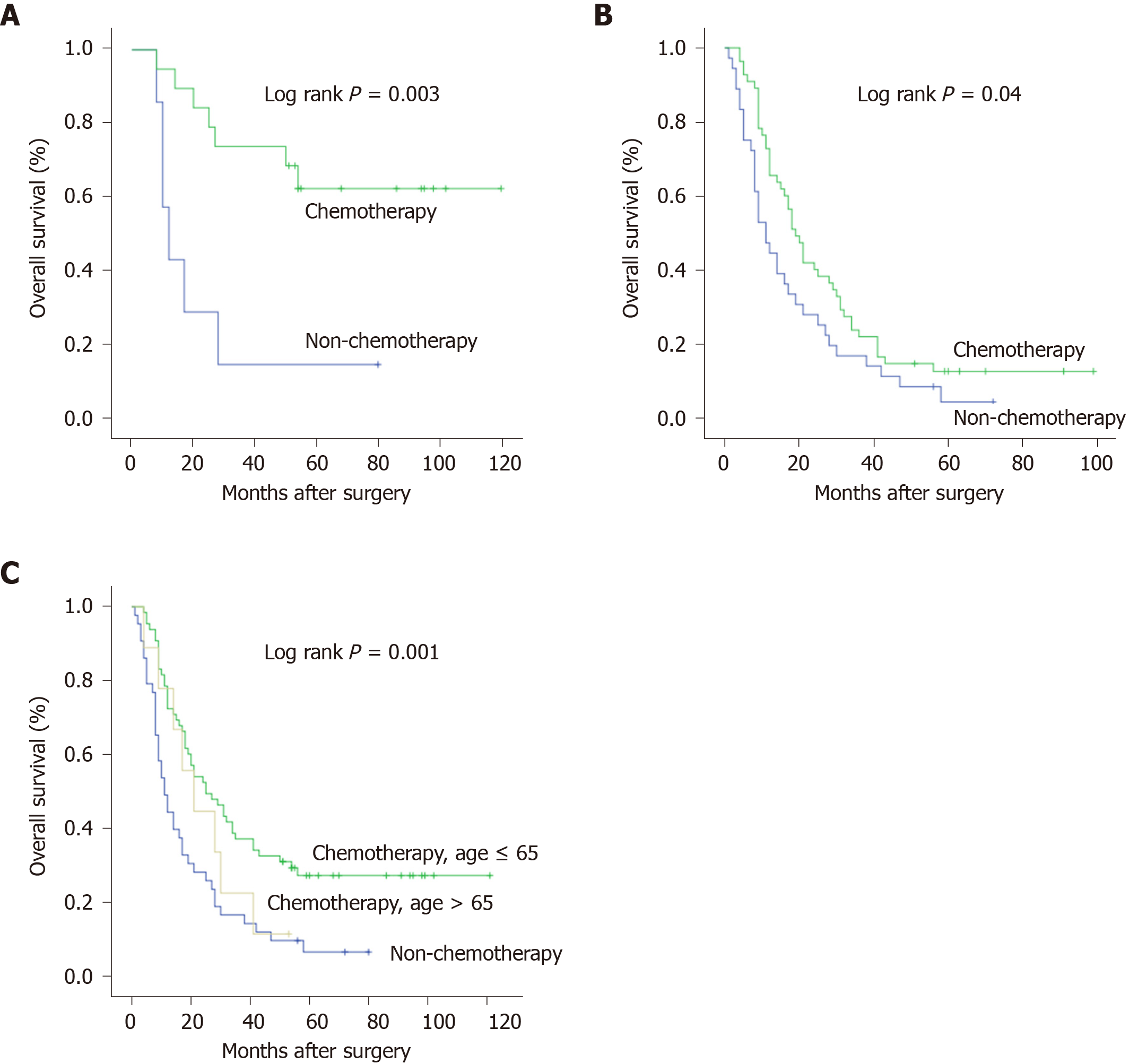
The survival curves of chemotherapy for subtotal or total gastrectomy are shown in Figure 2A-B. We revealed that circumscribed B-4 patients who underwent subtotal gastrectomy gained more benefit from postoperative chemotherapy (P = 0.003) than those who underwent total gastrectomy (P = 0.04). But therapeutic duration of chemotherapy was not the prognostic factor for circumscribed B-4. In addition, the efficacy of chemotherapy also seemed to be associated with patient age, and patients younger than 65 years had a better prognosis than the others (Figure 2C).
The prognostic results, including the median survival (MS) and 3-year OS rate, are shown in Figure 2. There was an obvious prognostic tendency of the different groups. For circumscribed B-4 patients with gastrectomy, postoperative chemotherapy could significantly increase the MS time, regardless of lymph node metastasis. Notably, we revealed that circumscribed B-4 patients (n = 6) who were diagnosed with pN0 and finally underwent subtotal gastrectomy and chemotherapy had a 3-year OS rate of 100% and a median survival of 77.7 mo. Even for patients with metastatic lymph nodes (n = 13), combination therapy also increased the 3-year OS rate to 57.1% and the MS to 51.0 mo.
Peritoneal metastasis is the most common type of GC metastasis. In our analysis, 79 (67.5%) patients were diagnosed with peritoneal cavity metastasis (PPCM) after gastrectomy, 11 (9.4%) had liver metastasis, and 5 (4.3%) had lung metastasis. We selected the other 22 patients without postoperative metastasis as a non-PPCM group. Positive pN stage was the only factor (P = 0.005) correlated with PPCM in circumscribed B-4 patients by Pearson correlation analyses (Table 3). Notably, there was no difference in the PPCM rate between different pT stages (pT3 vs pT4). In addition, although chemotherapy did not seem to be associated with PPCM according to the results shown in Table 3, we further explored the association of chemotherapy and PPCM in different gastrectomy types. As shown in Table 4, postoperative chemotherapy was useful for suppressing PPCM in patients with subtotal gastrectomy (P = 0.018) but not in those with total gastrectomy (P = 0.281).
| Variable | PPCM (79) | Non-PPCM (22) | Pearson P value |
| Tumor location | 0.497 | ||
| Upper 1/3–1/2 (U or MU) | 13 | 4 | |
| Lower 1/3–1/2 (M or L or ML) | 41 | 13 | |
| Total (LAU or LMU) | 25 | 5 | |
| Radical degree | 0.107 | ||
| R0 | 28 | 12 | |
| R1+ | 51 | 10 | |
| Gastrectomy type | 0.112 | ||
| Subtotal gastrectomy | 13 | 7 | |
| Total gastrectomy | 66 | 15 | |
| Venous/lymphatic infiltration | 0.416 | ||
| Yes | 50 | 16 | |
| No | 29 | 6 | |
| pT stage | 0.559 | ||
| pT3 | 23 | 5 | |
| pT4 | 56 | 17 | |
| pN stage | 0.005b | ||
| pN0 | 9 | 8 | |
| pN1-3 | 70 | 14 | |
| Chemotherapy | 0.105 | ||
| Yes | 46 | 17 | |
| No | 33 | 5 |
| Subtotal gastrectomy (20) | Total gastrectomy (81) | |||||
| PPCM (13) | Non-PPCM (7) | Pearson P value | PPCM (66) | Non-PPCM (15) | Pearson P value | |
| Chemotherapy | 4 | 6 | 0.018a | 45 | 8 | 0.281 |
| Non-chemotherapy | 9 | 1 | 21 | 7 |
In this study, we first defined B-4 patients without distant metastasis as circumscribed B-4 patients. By multivariate analysis, we revealed that subtotal/total gastrectomy, pN stage, and postoperative chemotherapy were independent prognostic factors for these patients. pN stage, but not pT stage, was crucial for predicting the prognosis of circumscribed B-4 patients. In a previous study, we suggested classifying B-4 patients into pT4b stage according to prognosis[4]. According to the statistical analysis, subtotal gastrectomy combined with chemotherapy can effectively improve the prognosis of circumscribed B-4. Even for the patients (n = 13) with positive pN, the combination therapy also improved the 3-year OS rate to 57.1%.
Surgical resection remains the main therapeutic method for GC, but the role of curative resection in B-4 is controversial[10,13,14]. Luo et al[8] and Kim et al[10] reported that the 3- to 5-year OS of curative (R0) patients was higher than that of noncurative patients, but surgical curability was not an independent predictor of prognosis, particularly for B-4 patients. In addition, studies found that total gastrectomy had no advantages of long-term prognosis and led to nutritional deficiency and worse quality of life for advanced GC compared with subtotal gastrectomy[15-20]. In our analysis, we also found that no survival benefit existed in the R0 patients compared with the R1 patients, and subtotal gastrectomy could significantly improve the prognosis of circumscribed B-4 patients compared with total gastrectomy. The pathological features of B-4 GC suggested that the lesion invaded the whole layers of the gastric wall, and even if there was no venous or lymphatic infiltration, some micrometastases had occurred before macroscopic distant metastasis. Thus, it is difficult to restrict metastasis and improve survival by surgical resection. The results from circumscribed B-4 patients provided more evidence for this conclusion.
Considering the limited effect of surgery, adjuvant chemotherapy was also found to be beneficial for survival in advanced GC. In a meta-analysis, 5-FU plus oxaliplatin (OXA) and 5-FU plus docetaxel (DOC) were recommended as postoperative chemotherapy regimens for advanced GC according to the efficacy[21]. In this study, chemotherapy, including 5-FU and platinum, significantly improved the prognosis of local B-4, serving as one of the independent factors, even for patients who were older than 65 years. Notably, as shown in Supplementary Table 1, significant differences in age and pT stage existed between the chemotherapy and nonchemotherapy groups (P < 0.01). Thus, we revealed that elderly patients with advanced GC stage seem to have a lower tendency to receive postoperative chemotherapy in the clinic, which should be rectified according to our conclusions. In addition, studies found that although S-1 or S-1/cisplatin might have positive effects against B-4, the prognostic role of neoadjuvant chemotherapy did not reach the expected survival rate, with a median survival of only 17.3 mo and a 3-year OS rate of 24.5%[22-24]. Therefore, neoadjuvant chemotherapy was not included and analyzed in our study.
Above all, as shown in Figure 2, the survival time and OS rates of the two groups were significantly different, and there was an obvious tendency. Subtotal gastrectomy with chemotherapy improved the median survival of negative and positive pN patients to 77.7 mo and 51.0 mo, respectively. Thus, for advanced GC patients, after confirmation as circumscribed B-4, we recommend striving for subtotal gastrectomy and performing postoperative chemotherapy, especially for the lower 1/3-1/2 lesions, regardless of whether the patient has lymph node metastases.
Additionally, our results showed that positive pN was correlated with the occurrence of PPCM in circumscribed B-4 patients, which indicated that lymph node metastasis was an important course for peritoneal metastasis. Some newly developed methods like endoscopic ultrasound guided fine needle aspiration may provide cytological confirmation for lymph node and other metastases[25-27]. Of course, serosal invasion and the shedding of cancer cells are also important routes for peritoneal metastasis.
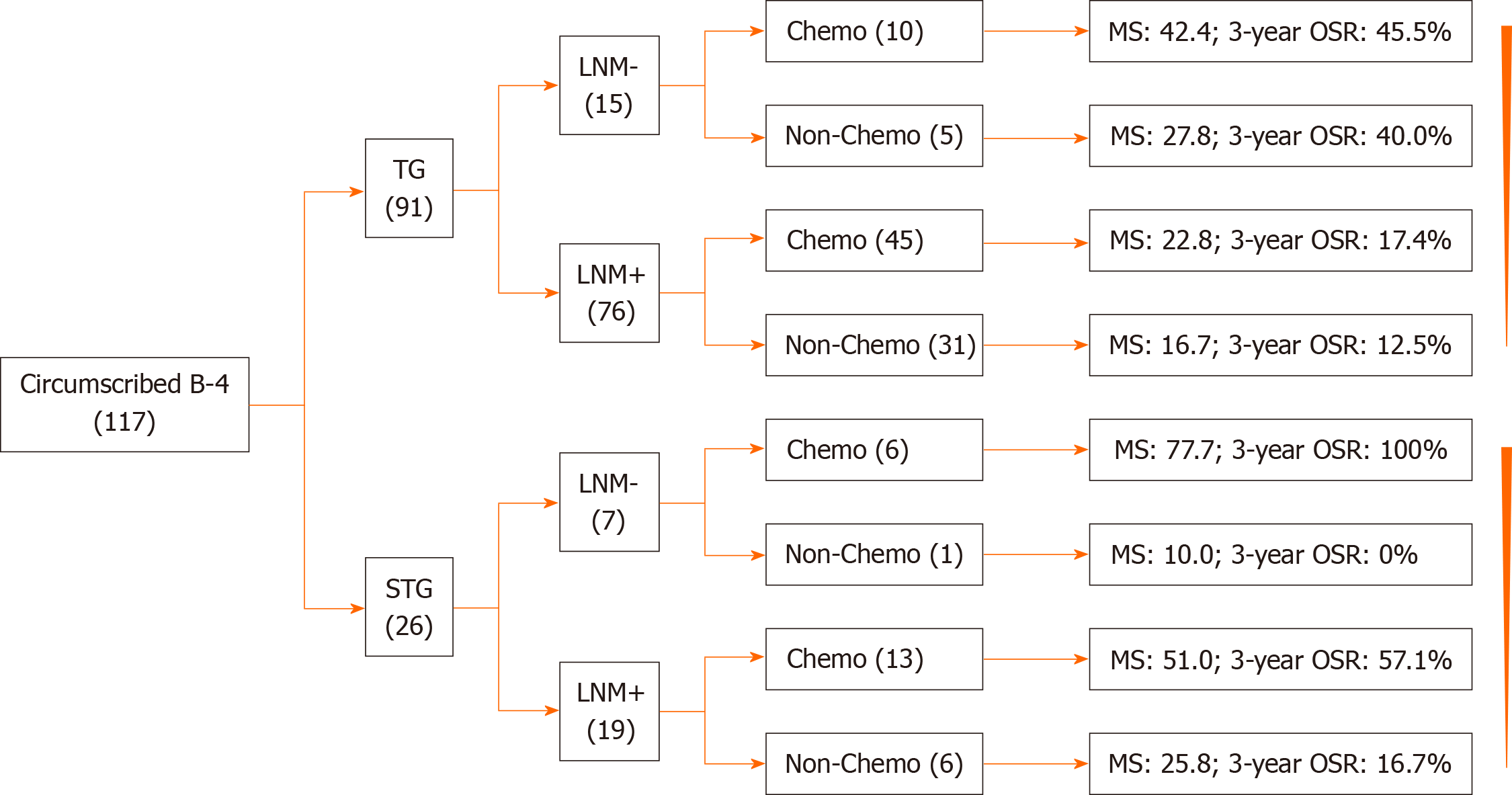
There were also some limitations in our study. First, this is a retrospective, single-center and small-sample study, which reduced the significance of our study. For example, only one patient was divided in a group, as shown in Figure 3. Second, no data of neoadjuvant or targeted therapy were referred to in our study. Thus, a prospective study on a larger scale is necessary in the future.
Lymph node metastasis is an independent risk factor for poor prognosis in circumscribed B-4 patients. Subtotal gastrectomy combined with postoperative chemotherapy could significantly improve the long-term OS rate and median survival time for circumscribed B-4 patients.
Borrmann type IV (B-4) gastric cancer (GC) accounts for about 10% of all GC cases in Asia. Some B-4 patients were found to have no distant metastasis by preoperative detection and underwent curative surgery, which was defined as circumscribed B-4 in our study.
Research of clinicopathological characteristics and prognosis in B-4 is rare, especially for the circumscribed B-4 patients.
In this study, we aimed to identify the factors related to prognosis and postoperative peritoneal cavity metastasis (PPCM) for circumscribed B-4 patients and to further explore the appropriate therapeutic strategies.
A total of 117 circumscribed B-4 patients were included in this study. Survival analysis and Pearson correlation analyses were performed to identify the factors related to prognosis.
Subtotal gastrectomy combined with chemotherapy could significantly improve the long-term survival time for circumscribed B-4. Positive lymph node staus was the only factor correlated with PPCM, and chemotherapy was useful for suppressing PPCM in patients with subtotal gastrectomy but not in those with total gastrectomy.
Lymph node status is an independent prognostic factor for circumscribed B-4 patients. Subtotal gastrectomy and chemotherapy could effectively improve prognosis and suppress PPCM.
This study recommended reasonable treatment schedules for circumscribed B-4 patients, but a multi-center study on a larger scale is necessary in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arigami T S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21362] [Article Influence: 2136.2] [Reference Citation Analysis (3)] |
| 2. | Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 3. | Sak K. A Hypothetical Approach on Gender Differences in Cancer Diagnosis. J Transl Int Med. 2019;7:90-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Huang JY, Wang ZN, Lu CY, Miao ZF, Zhu Z, Song YX, Xu HM, Xu YY. Borrmann type IV gastric cancer should be classified as pT4b disease. J Surg Res. 2016;203:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | An JY, Kang TH, Choi MG, Noh JH, Sohn TS, Kim S. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J Gastrointest Surg. 2008;12:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Dong RZ, Guo JM, Zhang ZW, Zhou YM, Su Y. Prognostic impact and implications of extracapsular lymph node spread in Borrmann type IV gastric cancer. Oncotarget. 2017;8:97593-97601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu ZG, Noh SH. Macroscopic Borrmann type as a simple prognostic indicator in patients with advanced gastric cancer. Oncology. 2009;77:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Luo Y, Gao P, Song Y, Sun J, Huang X, Zhao J, Ma B, Li Y, Wang Z. Clinicopathologic characteristics and prognosis of Borrmann type IV gastric cancer: a meta-analysis. World J Surg Oncol. 2016;14:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Zhu YL, Yang L, Sui ZQ, Liu L, Du JF. Clinicopathological features and prognosis of Borrmann type IV gastric cancer. J BUON. 2016;21:1471-1475. [PubMed] |
| 10. | Kim EY, Yoo HM, Song KY, Park CH. Limited significance of curative surgery in Borrmann type IV gastric cancer. Med Oncol. 2016;33:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Yook JH, Oh ST, Kim BS. Clinicopathological analysis of Borrmann type IV gastric cancer. Cancer Res Treat. 2005;37:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Kim DY, Kim HR, Kim YJ, Kim S. Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg. 2002;72:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Should scirrhous gastric carcinoma be treated surgically? Hepatogastroenterology. 2001;48:1509-1512. [PubMed] |
| 14. | Pedrazzani C, Marrelli D, Pacelli F, Di Cosmo M, Mura G, Bettarini F, Rosa F, de Manzoni G, Roviello F. Gastric linitis plastica: which role for surgical resection? Gastric Cancer. 2012;15:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Jang YJ, Park MS, Kim JH, Park SS, Park SH, Kim SJ, Kim CS, Mok YJ. Advanced gastric cancer in the middle one-third of the stomach: Should surgeons perform total gastrectomy? J Surg Oncol. 2010;101:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Sugoor P, Shah S, Dusane R, Desouza A, Goel M, Shrikhande SV. Proximal gastrectomy versus total gastrectomy for proximal third gastric cancer: total gastrectomy is not always necessary. Langenbecks Arch Surg. 2016;401:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Bozzetti F. Total versus subtotal gastrectomy in cancer of the distal stomach: facts and fantasy. Eur J Surg Oncol. 1992;18:572-579. [PubMed] |
| 18. | Liu Z, Feng F, Guo M, Liu S, Zheng G, Xu G, Lian X, Fan D, Zhang H. Distal gastrectomy versus total gastrectomy for distal gastric cancer. Medicine (Baltimore). 2017;96:e6003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Jentschura D, Winkler M, Strohmeier N, Rumstadt B, Hagmüller E. Quality-of-life after curative surgery for gastric cancer: a comparison between total gastrectomy and subtotal gastric resection. Hepatogastroenterology. 1997;44:1137-1142. [PubMed] |
| 20. | Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, Miller G, Martin I. Total or subtotal gastrectomy for gastric carcinoma? World J Surg. 1998;22:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Sun J, Ren Z, Sun X, Hou H, Li K, Ge Q. Efficacy and safety comparison of chemotherapies for advanced gastric cancer: A network meta-analysis. Oncotarget. 2017;8:39673-39682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kinoshita T, Sasako M, Sano T, Katai H, Furukawa H, Tsuburaya A, Miyashiro I, Kaji M, Ninomiya M. Phase II trial of S-1 for neoadjuvant chemotherapy against scirrhous gastric cancer (JCOG 0002). Gastric Cancer. 2009;12:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A; Gastric Cancer Surgical Study Group of Japan Clinical Oncology Group. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Mohri J, Katada C, Ueda M, Sugawara M, Yamashita K, Moriya H, Komori S, Hayakawa K, Koizumi W, Atsuda K. Predisposing Factors for Chemotherapy-induced Nephrotoxicity in Patients with Advanced Esophageal Cancer Who Received Combination Chemotherapy with Docetaxel, Cisplatin, and 5-fluorouracil. J Transl Int Med. 2018;6:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Uberoi AS, Bhutani MS. Has the role of EUS in rectal cancer staging changed in the last decade? Endosc Ultrasound. 2018;7:366-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Mizuno S, Nakai Y, Isayama H, Suzuki T, Saito K, Uchino R, Takahara N, Kogure H, Tada M, Koike K. EUS-FNA of gastric cancer metastatic to the head of pancreas using a forward oblique viewing echoendoscope in a case with Roux-en-Y anatomy. Endosc Ultrasound. 2018;7:420-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Yang F, Wang H, Liu X, Ge N, Guo J, Wang S, Song X, Cao L, Sun S. EUS-guided fine-needle technique-derived cancer organoids: A tailored "Shennong deity" for every patient with cancer. Endosc Ultrasound. 2019;8:73-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |