Published online Nov 15, 2020. doi: 10.4251/wjgo.v12.i11.1296
Peer-review started: June 8, 2020
First decision: September 11, 2020
Revised: September 25, 2020
Accepted: October 28, 2020
Article in press: October 28, 2020
Published online: November 15, 2020
Processing time: 156 Days and 20 Hours
Patients with right sided colorectal cancer are known to have a poorer prognosis than patients with left sided colorectal cancer, whatever the cancer stage. To this day, primary tumor resection (PTR) is still controversial in a metastatic, non resectable setting.
To explore the survival impact of PTR in patients with metastatic colorectal cancer (mCRC) depending on PTL.
We retrospectively collected data from all consecutive patients treated for mCRC at the Centre Georges Francois Leclerc Hospital. Univariate and multivariate Cox proportional hazard regression models were used to assess the influence of PTR on survival. We then evaluated the association between PTL and overall survival among patients who previously underwent or did not undergo PTR. A propensity score was performed to match cohorts.
Four hundred and sixty-six patients were included. A total of 153 (32.8%) patients had unresected synchronous mCRC and 313 (67.2%) patients had resected synchronous mCRC. The number of patients with right colic cancer, left colic cancer and rectal cancer was respectively 174 (37.3%), 203 (43.6%) and 89 (19.1%). In the multivariate analysis only PTL, PTR, resection of hepatic and or pulmonary metastases and the use of oxaliplatin, EGFR inhibitors or bevacizumab throughout treatment were associated to higher overall survival rates. Survival evaluation depending on PTR and PTL found that PTR improved the prognosis of both left and right sided mCRC. Results were confirmed by using a weighted propensity score.
In mCRC, PTR seems to confer a higher survival rate to patients whatever the PTL.
Core Tip: This article presents a large, real life, cohort of patients treated for a metastatic colorectal cancer. Primary tumor resection in this setting is not a validated systematic treatment. However, in our hospital, primary tumor resection is performed widely. In our study, primary tumor resection was associated to higher overall survival rates even in patients with a poor prognosis. We also looked at the impact of primary tumor resection depending on primary tumor location. Primary tumor location had no impact on the benefit provided by primary tumor resection.
- Citation: Tharin Z, Blanc J, Charifi Alaoui I, Bertaut A, Ghiringhelli F. Influence of primary tumor location and resection on survival in metastatic colorectal cancer. World J Gastrointest Oncol 2020; 12(11): 1296-1310
- URL: https://www.wjgnet.com/1948-5204/full/v12/i11/1296.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i11.1296
Colorectal cancer (CRC) is a major public health issue and stands as the third most frequent cancer throughout the world with 1.8 million new cases diagnosed every year. It is the second cause of cancer related death with, according to GLOBOCAN estimates, 880000 deaths per year[1]. Between 20% and 30% of patients have metastases at the time of diagnosis[2,3] which classifies them as stage IV CRC. It has been established that primary tumor resection (PTR) and surgery of the metastases is a necessity for patients presenting resectable metastases as it allows to cure 20% to 25% of patients[4]. All resectable metastatic patients will undergo chemotherapy, in most cases they will receive peri-operative chemotherapy relying on the association of 5 fluorouracil (5 FU) and oxaliplatin[5,6].
For 75% to 90% of patients, the cancer is unresectable, and they will receive palliative chemotherapy[7] . Recent data underlines that primary tumor location (PTL) is one of the most important prognostic factors in our study population. Indeed, the CALGB/SWOG 80405 found that survival was doubled for patients with left-sided primary tumors vs those with right-sided primary tumors[8,9]. This data was corroborated by a meta-analysis that pooled all clinical trials, available up to October 2016, which assessed the impact of PTL in metastatic CRC (mCRC)[10].
For patients with unresectable metastases, PTR has not been validated as a systematic treatment in randomized clinical trials. At present, whether the patient will undergo PTR or not is decided in multidisciplinary reunions. This decision is based on quality of life improvement and prevention of complications related to the primary tumor for patients with unresectable metastases. In a recent analysis of the ARCAD patient data base, patients with synchronous metastases and no PTR had a significantly worse median overall survival (OS) (16.4 m) than patients with synchronous metastases who underwent PTR [22.2 m; hazard ratio (HR): 1.60, 95% carcinoembryonic antigen (CI): 1.43-1.78][11]. Moreover, a meta-analysis of 21 studies, including 44226 patients, found that patients who had PTR had a better OS than the patients who received chemotherapy alone[12].
However, to our knowledge, the outcome of PTR in terms of OS depending on PTL has never been evaluated.
The aim of this retrospective study, carried out at the Centre George François Leclerc Hospital in Dijon, was to evaluate if PTR allowed to improve the prognosis of either left or right mCRC.
Data was collected retrospectively from all consecutive patients treated at the Centre George François Leclerc Hospital for synchronous mCRC between January 31st, 2000 and the December 20th, 2018. Patients were included regardless of their tumor burden, of their resectability, of the treatments they had received (chemotherapy, molecular targeted agents, PTR, lung and/or liver metastases resection, hyperthermic chemotherapy). Patients were excluded if the PTL wasn’t specified in the medical file, if the CRC wasn’t the first or the only malignancy diagnosed or if it was appendicle cancer.
The following parameters were retrospectively collected in the patients’ medical file: Gender, age, performance status (PS), liver surgery or liver radiofrequency, lung surgery or lung radiofrequency, PTR, PTL: Right colon/Left colon/rectum (right colon cancers included right sided and transverse colon cancers ; Left colon cancers included left sided and sigmoid cancers), number of metastatic sites, KRAS and BRAF mutations, type of medical treatment, levels of lactate dehydrogenase (LDH), carcinoembryonic antigen, leucocytes and alkaline phosphatase (ALP).
The primary endpoint was to evaluate whether the use of PTR allowed to improve the prognosis of patients and if the benefit was correlated to PTL. All patients were followed until either their death or the date of last follow-up prior to the March 31st, 2020. The primary end point was OS, which was defined as the interval between the time of diagnosis of metastatic disease and the date of death as reported on medical record. Survivors were censored at last follow-up.
The characteristics of the whole population are presented according to whether PTR was performed and in function of PTL. Continuous variables were compared using ANOVA or Kruskal-Wallis tests depending on the distribution of the data. Qualitative variables were compared using either the χ2 test or the Fisher exact test.
The median follow-up was estimated using the reverse Kaplan Meier method. OS was estimated using the Kaplan-Meier method, described using medians with its 95% confidence interval (95%CI), and compared using a log-rank test. Univariate Cox regressions were performed to estimate HR with its 95%CI. The multivariate Cox regression model was generated with all the variables with a P < 0.20 and with less than 20% of missing data. The risks proportionality and log-linearity were verified for each variable. Correlations between all variables were tested and, in case of correlated variables, only one variable was included in the multivariate model. The interaction between PTL and PTR was tested.
A propensity score was generated using a multivariate logistic regression and stood as the likelihood of undergoing PTR. The inverse probability treatment weight was used to balance clinical variables associated with PTR and to eliminate potential selection biases. The weight allocated to patients who had undergone PTR was 1/PS while the patients without PTR received a weight of 1/(1-PS). Then a weighted Cox regression model was built using the same variables introduced in the raw Cox model. Statistical analyses were performed using SAS® software version 9.4.
The data from 466 patients with synchronous mCRC was collected from the Centre Georges Francois Leclerc Hospital database between January 31st 2000 and December 20th 2018.
The male gender was slightly predominant (54.7%) but not statistically significant. The mean age was 64 years. PS was good for most patients with 83.6% of patients with a 0 or 1 PS. The number of patients with right colic cancer, left colic cancer and rectal cancer was respectively 174 (37.3%), 203 (43.6%) and 89 (19.1%). Three hundred and thirteen (67.2%) patients had undergone PTR, 153 (32.8) patients had not been operated on. One hundred and thirty seven (29.6%) patients were treated for their metastatic disease with curative intent with either liver and/or pulmonary surgery or radiofrequency. RAS status was available for 356 patients (76.4%), 154 patients had a RAS mutation. BRAF status was available for 294 patients (63.1%), 29 patients had a BRAF mutation.
Patients’ characteristics according to PTR status are summarized in Table 1. Age, PTL, PS, lung metastases, ACE, LDH, leucocyte and ALP and surgery or radiofrequency of lung and/or liver metastases were the significantly different variables between the groups.
| Total | PTR | No PTR | P value | |
| Age | 0.0065 | |||
| n | 466 | 313 | 153 | |
| Mean (std) | 64.0 (11.6) | 63.0 (11.1) | 66.1 (12.4) | |
| Median [min-max] | 65.0 (24.0-92.0) | 63.0 (29.0-90.0) | 67.0 (24.0-92.0) | |
| Gender | 0.9563 | |||
| Male | 255 (54.7%) | 171 (54.6%) | 84 (54.9%) | |
| Female | 211 (45.3%) | 142 (45.4%) | 69 (45.1%) | |
| Primary tumor location | 0.0010 | |||
| Left colon + sigmoid | 203 (43.6%) | 153 (48.9%) | 50 (32.7%) | |
| Right colon + transverse colon | 174 (37.3%) | 112 (35.8%) | 62 (40.5%) | |
| Rectum | 89 (19.1%) | 48 (15.3%) | 41 (26.8%) | |
| PS | 0.0296 | |||
| 0-1 | 300 (83.6%) | 202 (86.7%) | 98 (77.8%) | |
| 2-4 | 59 (16.4%) | 31 (13.3%) | 28 (22.2%) | |
| Missing values | 107 | 80 | 27 | |
| Number of metastatic sites | 0.1080 | |||
| 1 | 283 (60.7%) | 200 (63.9%) | 83 (54.2%) | |
| 2 | 133 (28.5%) | 84 (26.8%) | 49 (32.0%) | |
| > 2 | 50 (10.7%) | 29 (9.3%) | 21 (13.7%) | |
| Metastatic sites | ||||
| Liver | 0.9689 | |||
| No | 89 (19.2%) | 60 (19.2%) | 29 (19.1%) | |
| Yes | 375 (80.8%) | 252 (80.8%) | 123 (80.9%) | |
| Missing values | 2 | 1 | 1 | |
| Lung | 0.0206 | |||
| No | 342 (73.9%) | 240 (77.2%) | 102 (67.1%) | |
| Yes | 121 (26.1%) | 71 (22.8%) | 50 (32.9%) | |
| Missing values | 3 | 2 | 1 | |
| Peritoneum | 0.8377 | |||
| No | 356 (76.9%) | 240 (77.2%) | 116 (76.3%) | |
| Yes | 107 (23.1%) | 71 (22.8%) | 36 (23.7%) | |
| Missing values | 3 | 2 | 1 | |
| Other | 0.2531 | |||
| No | 361 (78.1%) | 247 (79.7%) | 114 (75.0%) | |
| Yes | 101 (21.9%) | 63 (20.3%) | 38 (25.0%) | |
| Missing values | 4 | 3 | 1 | |
| KRAS mutation | 0.5956 | |||
| No | 202 (56.7%) | 143 (57.7%) | 59 (54.6%) | |
| Yes | 154 (43.3%) | 105 (42.3%) | 49 (45.4%) | |
| Missing values | 110 | 65 | 45 | |
| BRAF mutation | 0.3218 | |||
| No | 265 (90.1%) | 188 (91.3%) | 77 (87.5%) | |
| Yes | 29 (9.9%) | 18 (8.7%) | 11 (12.5%) | |
| Missing values | 172 | 107 | 65 | |
| CEA | 0.0002 | |||
| ≤ 200 | 251 (68.6%) | 177 (75.3%) | 74 (56.5%) | |
| > 200 | 115 (31.4%) | 58 (24.7%) | 57 (43.5%) | |
| Missing values | 100 | 78 | 22 | |
| LDH | < 0.0001 | |||
| ≤ 254 | 175 (53.2%) | 130 (62.2%) | 45 (37.5%) | |
| > 254 | 154 (46.8%) | 79 (37.8%) | 75 (62.5%) | |
| Missing values | 137 | 104 | 33 | |
| Leukocytes > 10000 | 0.0014 | |||
| No | 256 (70.9%) | 175 (76.8%) | 81 (60.9%) | |
| Yes | 105 (29.1%) | 53 (23.2%) | 52 (39.1%) | |
| Missing values | 105 | 85 | 20 | |
| Alkaline phosphatase > 300 | 0.0008 | |||
| No | 261 (77.9%) | 179 (83.6%) | 82 (67.8%) | |
| Yes | 74 (22.1%) | 35 (16.4%) | 39 (32.2%) | |
| Missing values | 131 | 99 | 32 | |
| Oxaliplatin | 0.3478 | |||
| No | 35 (7.5%) | 21 (6.7%) | 14 (9.2%) | |
| Yes | 431 (92.5%) | 292 (93.3%) | 139 (90.8%) | |
| 5 FU or capecitabine | 1.0000 | |||
| No | 3 (0.6%) | 2 (0.6%) | 1 (0.7%) | |
| Yes | 463 (99.4%) | 311 (99.4%) | 152 (99.3%) | |
| Irinotecan | 0.1278 | |||
| No | 105 (22.6%) | 64 (20.5%) | 41 (26.8%) | |
| Yes | 360 (77.4%) | 248 (79.5%) | 112 (73.2%) | |
| Missing values | 1 | 1 | 0 | |
| Bevacizumab | 0.1978 | |||
| No | 150 (32.4%) | 95 (30.4%) | 55 (36.4%) | |
| Yes | 313 (67.6%) | 217 (69.6%) | 96 (63.6%) | |
| Missing values | 3 | 1 | 2 | |
| EGFR inhibitors | 0.2681 | |||
| No | 267 (57.5%) | 174 (55.8%) | 93 (61.2%) | |
| Yes | 197 (42.5%) | 138 (44.2%) | 59 (38.8%) | |
| Missing values | 2 | 1 | 1 | |
| 1st line chemotherapy regimen | 0.7568 | |||
| Mono-chemotherapy | 48 (10.4%) | 32 (10.3%) | 16 (10.5%) | |
| Bi-chemotherapy | 326 (70.6%) | 216 (69.7%) | 110 (72.4%) | |
| Tri- chemotherapy | 88 (19.0%) | 62 (20.0%) | 26 (17.1%) | |
| Missing values | 4 | 3 | 1 | |
| Lung or Liver surgery or radio-frequency | < 0.0001 | |||
| No | 326 (70.4%) | 178 (57.4%) | 148 (96.7%) | |
| Yes | 137 (29.6%) | 132 (42.6%) | 5 (3.3%) | |
| Missing values | 3 | 3 | 0 | |
Patients’ characteristics depending on PTL are summarized in the supplementary data (Supplementary Table 1). PS, number of metastatic sites, lung and peritoneum metastases, KRAS and BRAF mutations, PTR, ALP levels, the use of EGFR inhibitors, chemotherapy regimen and surgery or radiofrequency of lung and/or liver metastases were the significantly different variables between the groups.
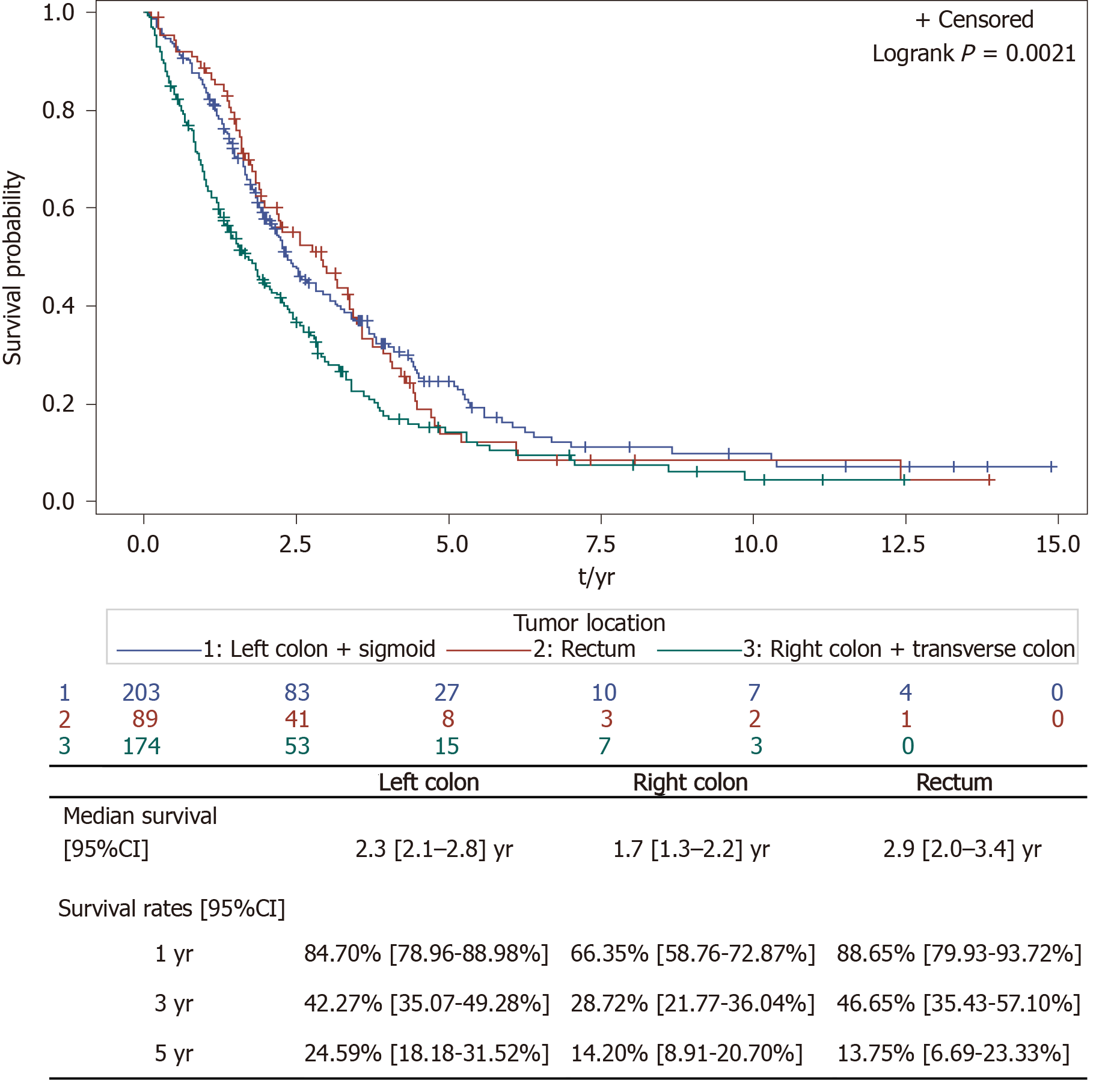
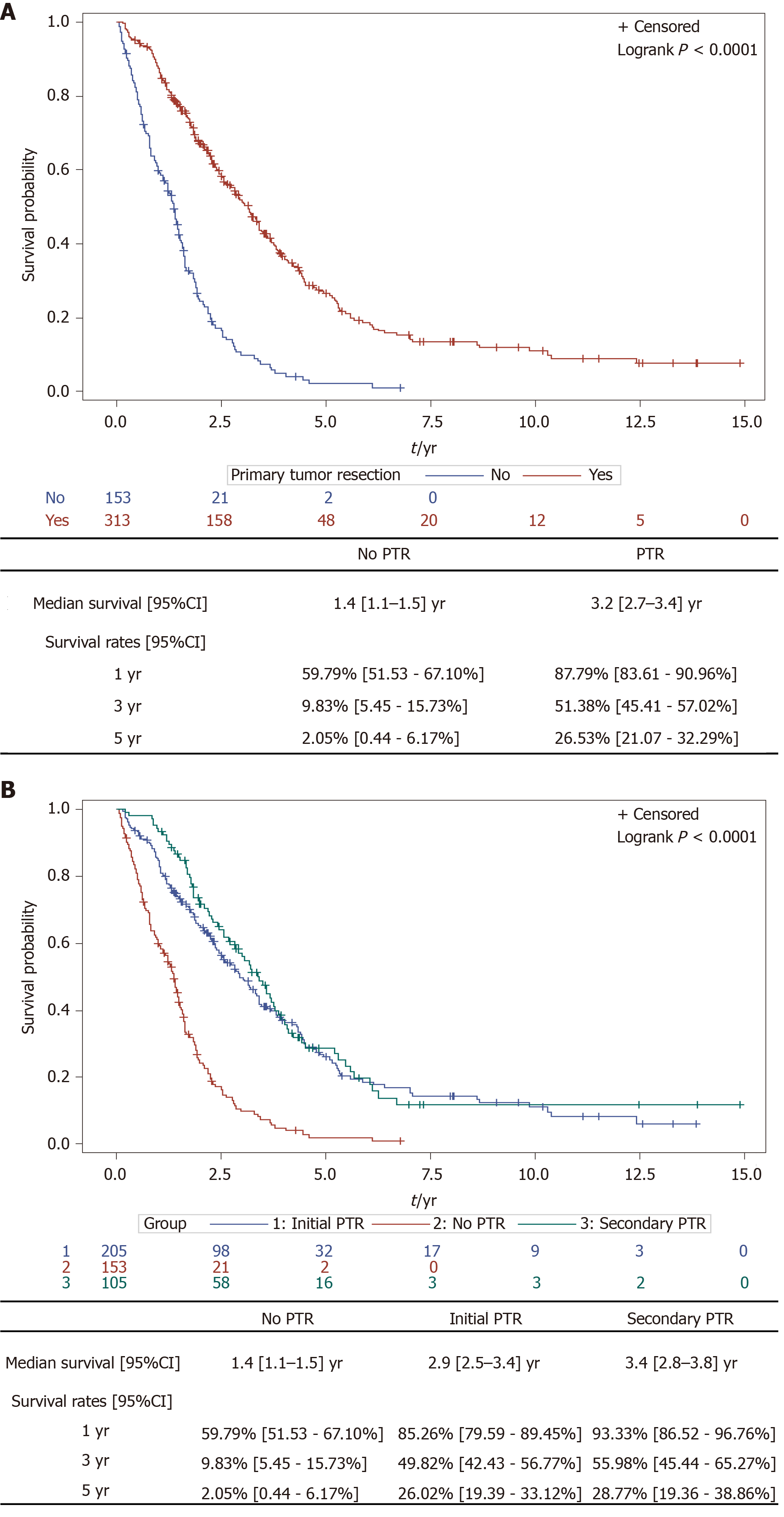
Median follow up was 8 years. As expected, we found that left sided colon cancers were associated to a better OS than right sided colon cancers. Results shown in Figure 1. As Kaplan Meier curves and Log Rank tests show that left colon cancers and rectal cancers share similar prognosis, we decided to pool them for further analyses. We found that patients who underwent PTR had higher OS rates than patients who didn’t. Results shown in Figure 2A. The benefit of PTR in terms of OS was observed regardless of PTR taking place before, primary PTR, or after the initiation of chemotherapy, secondary PTR. Results shown in Figure 2B. As Kaplan Meier curves and Log Rank tests show that synchronous patients with primary PTR and synchronous patients with secondary PTR share similar prognosis, we decided to pool them for further analyses.
Using a univariate Cox model, we tested the impact of each clinical variable on OS. We found that left sided mCRC, a unique metastatic site, low levels of ACE, LDH, leucocytes and ALP, PTR, resection of hepatic and/or pulmonary metastases in curative intent, the use of intensive first line chemotherapy and the use of oxaliplatin, EGFR inhibitors or bevacizumab throughout treatment were associated to a better OS (Table 2).
| HR | 95%CI | P value | ||
| Age | n = 466 | 0.0714 | ||
| > 65 yr vs ≤ 65 yr | 1.209 | [0.984-1.485] | ||
| Gender | n = 466 | 0.3701 | ||
| Female vs male | 1.099 | [0.894-1.350] | ||
| Tumor location | n = 466 | 0.0022 | ||
| Rectum vs left colon + sigmoid | 1.019 | [0.767-1.355] | ||
| Right colon + transverse colon vs Left colon + sigmoid | 1.464 | [1.16-1.841] | ||
| Number of metastatic sites | n = 466 | 0.0035 | ||
| 2 vs 1 | 1.219 | [0.965-1.539] | ||
| > 2 vs 1 | 1.749 | [1.245-2.455] | ||
| Number of metastatic sites | n = 466 | 0.0084 | ||
| ≥ 2 vs 1 | 1.328 | [1.076-1.641] | ||
| Liver metastases | n = 464 | 0.8836 | ||
| Yes vs No | 1.020 | [0.782-1.330] | ||
| Lung metastases | n = 463 | 0.6435 | ||
| Yes vs No | 1.057 | [0.835-1.338] | ||
| Peritoneum metastases | n = 463 | 0.0619 | ||
| Yes vs No | 1.260 | [0.989-1.606] | ||
| Other metastases | n = 462 | 0.0114 | ||
| Yes vs No | 1.377 | [1.075-1.765] | ||
| KRAS mutation | n = 356 | 0.0696 | ||
| Yes vs No | 1.256 | [0.982-1.606] | ||
| BRAF mutation | n = 294 | < 0.0001 | ||
| Yes vs No | 2.276 | [1.511-3.429] | ||
| CEA | n = 366 | < 0.0001 | ||
| > 200 vs ≤ 200 | 1.654 | [1.290-2.120] | ||
| LDH | n = 329 | < 0.0001 | ||
| > 254 vs ≤ 254 | 2.022 | [1.574-2.596] | ||
| Leukocytes > 10000 | n = 361 | 0.0016 | ||
| Yes vs No | 1.506 | [1.168-1.941] | ||
| Alkaline phosphatase > 300 | n = 335 | < 0.0001 | ||
| Yes vs No | 2.272 | [1.713-3.014] | ||
| PTR | n = 466 | < 0.0001 | ||
| Yes vs No | 0.313 | [0.250-0.392] | ||
| Oxaliplatin | n = 466 | < 0.0001 | ||
| Yes vs No | 0.361 | [0.248-0.524] | ||
| Irinotecan | n = 465 | 0.112 | ||
| Yes vs No | 0.807 | [0.619-1.051] | ||
| 5 FU or Capecitabine | n = 466 | 0.2869 | ||
| Yes vs No | 0.539 | [0.173-1.682] | ||
| Bevacizumab | n = 463 | 0.0117 | ||
| Yes vs No | 0.750 | [0.599-0.938] | ||
| EGFR inhibitors | n = 464 | 0.0129 | ||
| Yes vs No | 0.769 | [0.625-0.946] | ||
| 1rst line chemotherapy regimen | n = 462 | 0.0080 | ||
| Bi-chemotherapy vs Mono-chemotherapy | 0.682 | [0.496-0.937] | ||
| Tri-chemotherapy vs Mono-chemotherapy | 0.543 | [0.368-0.799] | ||
| Lung or liver surgery or radio-frequency | n = 463 | < 0.0001 | ||
| Yes vs No | 0.302 | [0.235-0.388] | ||
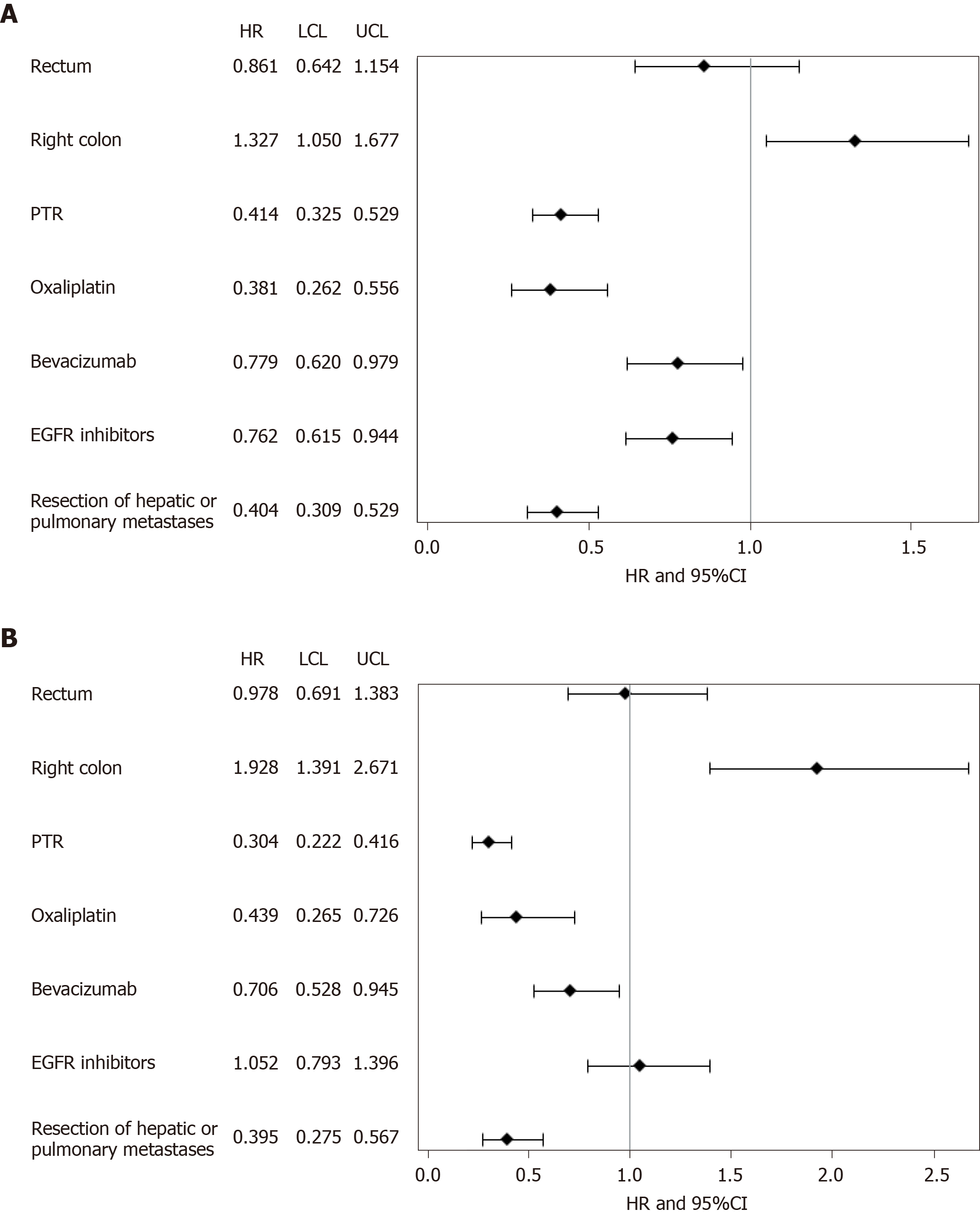
Using a multivariate COX model which only included variables with P < 0.2 on univariate analysis and with less than 20% of missing data, we observed that only left sided tumors, PTR, the use of oxaliplatin, EGFR inhibitors or bevacizumab throughout treatment and resection of hepatic and/or pulmonary metastases in curative intent were associated to higher OS rates. Harrell’s C statistic of the multivariate model is 0.75 which indicates a good discrimination quality of the model. To allow for potential biases between patients who do and do not undergo PTR, we performed a weighted propensity score to match both cohorts: 304 patients were retained. The weighted multivariate COX model obtained similar results to the raw multivariate COX model. Results shown in Forrest Plot shown in Figure 3A and B.
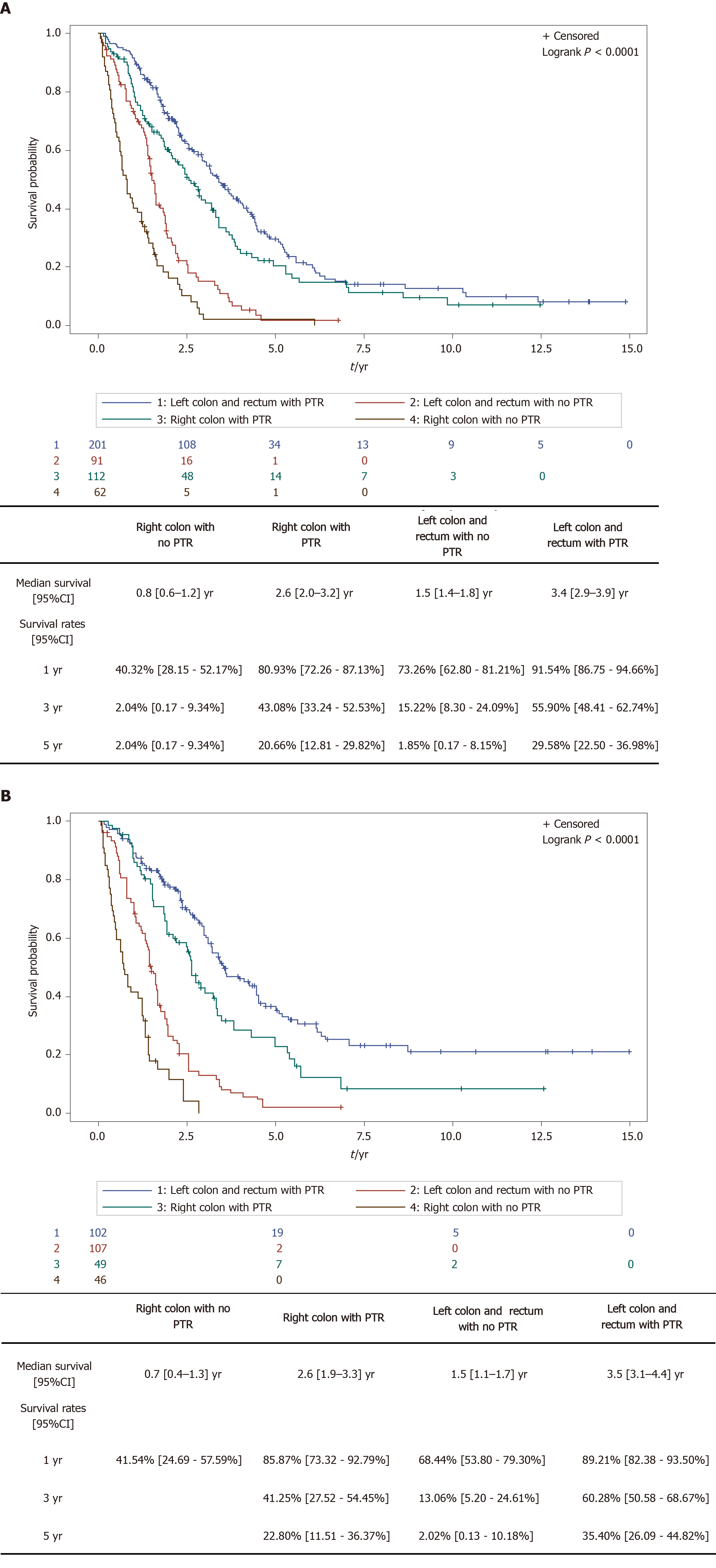
The interaction test carried out between PTR and PTL was not statistically significant (P = 0.2426), thus suggesting that the positive effect of PTR is independent of PTL. The improvement of the HR of OS when PTR was performed was observed regardless of PTL. In the raw cohort, the one year survival rate for right sided tumors went from 40.32% (28.15-52.17) without PTR to 80.93% (72.26-87.13) with PTR; for the left sided tumors, it went from 73.26% (62.80-81.21) without PTR to 91.54% (86.75-94.66) with PTR. Similar results were observed in the weighted cohorts. Results shown in Figure 4A and B.
This retrospective study is in line with previous studies and supports the idea that PTR improves OS in patients with mCRC and suggests that PTR could be important in disease control of both left and right sided mCRC.
Many articles on PTR in mCRC have been published in the last 20 years. In the early 2000’s, Cook et al[7] published the first article based on data extracted from a national prospective database in the United States, The Surveillance, Epidemiology and End Results where 26754 patients were included. Patients who underwent PTR had an improved survival[7]. In 2014, Ahmed et al[13] Published the first prospective observational study, designed to compare OS depending on PTR in patients with mCRC. All the results are in favor of PTR regardless of other important prognostic factors such as: Age, PS, comorbid illness and chemotherapy. However, many confounding factors could have influenced and biased these results.
More recently, using an Instrumental Variable analysis based on the annual hospital-levels of PTR rates, Alawadi et al[14] underlined in a large united states cohort that survival benefit linked to PTR is mainly related to inclusion biases and therefore do not recommend it in a non-resectable metastatic setting. A Japanese group reported, at the ASCO GI this year, the first clinical trial designed to evaluate the impact of primary PTR, in patients with unresectable mCRC. The study cohort only included 160 patients out of the 758 patients initially planned: 78 patients were assigned to the PTR + chemotherapy group, 83 were assigned to the chemotherapy alone group. They found that PTR followed by chemotherapy had no survival benefit over chemotherapy alone with respectively a 25.9 mo and 26.7 mo OS [hazard ratio: 1.10 (0.76-1.59), one-sided P = 0.69][15].
In contrast, in our study, the multivariate Cox proportional analysis and weighted Cox proportional analyses were operated on the known bad prognostic risk factors in order to evaluate, independently, the association between survival and PTR. In these models, we continued to observe a strong association between PTR and prognosis.
Nevertheless, PTR in patients with unresectable mCRC cancer stays controversial. Indeed, the results of the studies encouraging PTR are thought to be biased by confounding factors such as age, PS, metastases resectability. One of the main arguments against PTR is the risk of post-operative complications and therefore delayed chemotherapy[16]. However, in our study, regardless of its limitations, PTR is associated to an increased OS independently of the known bad prognostic risk factors and chemotherapy treatment. Our study also shows that PTR is associated to better outcomes in both left and right sided mCRC.
The limits to our study are of course its retrospective design and the mono centric recruitment. However, we studied a large cohort of unselected patients and our results in terms of outcome and population are very similar to the results observed in clinical trials testing new strategies in mCRC[6,17] or in studies evaluating survival in mCRC[18]. Other limitations to our study are that it compares a heterogeneous population of patients in terms of tumor burden and that there is a statically significant difference when it comes to PTR between our groups. However, the aim of the propensity score was to balance the disparities observed within our population. The last limitation to our study is that the reason behind the decision of PTR was not recorded and we therefore cannot exclude a confounding factor.
Our results support that PTR could be associated to OS improvement in patients with mCRC regardless of PTL. Our findings indicate a possible argument to promote surgical treatment for this category of patients. Such data needs to be validated in prospective clinical trials.
Patients with right sided colorectal cancer (CRC) are known to have a poorer prognosis than patients with left sided tumors and primary tumor resection (PTR) is controversial whatever the primary tumor location (PTL).
Results concerning PTR in unresectable metastatic colorectal cancer (mCRC) are non-consensual and PTR is not a standard practice. To our knowledge, the outcome of PTR in terms of overall survival (OS) depending on tumor sidedness has never been evaluated.
This study aimed to explore the survival impact of PTR in patients with mCRC depending on PTL.
We retrospectively collected data from all consecutive patients treated for mCRC at the Centre Georges Francois Leclerc Hospital. Univariate and multivariate Cox proportional hazard regression models were used to assess the influence of PTR on survival. We then evaluated associations between PTL and OS among patients who previously underwent or did not undergo PTR. A propensity score was performed to match cohorts.
Four hundred and sixty-six patients were included. A total of 153 (32.8%) patients had unresected synchronous mCRC and 313 (67.2%) patients had resected synchronous mCRC. The number of patients with right colic cancer, left colic cancer and rectal cancer was respectively 174 (37.3%), 203 (43.6%) and 89 (19.1%). In the multivariate analysis only PTL, PTR, resection of hepatic and or pulmonary metastases and the use of oxaliplatin, EGFR inhibitors or bevacizumab throughout treatment were associated to higher OS rates. Survival evaluation depending on PTR and PTL found that PTR improved the prognosis of both left and right sided unresectable mCRC. Results were confirmed by using a weighted propensity score.
In mCRC, PTR seems to confer higher survival rates whatever the PTL.
These results are in favor of PTR for patients treated for a mCRC but would need to be supported by a large scale, prospective trial.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aoki T, Boteon YL, Huang LY, MD JS, Yong T S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55773] [Article Influence: 7967.6] [Reference Citation Analysis (132)] |
| 2. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2911] [Article Influence: 363.9] [Reference Citation Analysis (3)] |
| 3. | Leufkens AM, van den Bosch MA, van Leeuwen MS, Siersema PD. Diagnostic accuracy of computed tomography for colon cancer staging: a systematic review. Scand J Gastroenterol. 2011;46:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Brown RE, Bower MR, Martin RC. Hepatic resection for colorectal liver metastases. Surg Clin North Am. 2010;90:839-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery vs surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1441] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 6. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery vs surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 918] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 7. | Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol. 2005;12:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Venook AP. Right-sided vs left-sided colorectal cancer. Clin Adv Hematol Oncol. 2017;15:22-24. [PubMed] |
| 9. | Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH, Atkins JN, Berry S, Polite BN, O'Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB 3rd, Mulkerin DL, Mayer RJ, Blanke C. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017;317:2392-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 679] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 10. | Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 400] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 11. | van Rooijen KL, Shi Q, Goey KKH, Meyers J, Heinemann V, Diaz-Rubio E, Aranda E, Falcone A, Green E, de Gramont A, Sargent DJ, Punt CJA, Koopman M. Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: Individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer. 2018;91:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Clancy C, Burke JP, Barry M, Kalady MF, Calvin Coffey J. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol. 2014;21:3900-3908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Ahmed S, Fields A, Pahwa P, Chandra-Kanthan S, Zaidi A, Le D, Haider K, Reeder B, Leis A. Surgical Resection of Primary Tumor in Asymptomatic or Minimally Symptomatic Patients With Stage IV Colorectal Cancer: A Canadian Province Experience. Clin Colorectal Cancer. 2015;14:e41-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Alawadi Z, Phatak UR, Hu CY, Bailey CE, You YN, Kao LS, Massarweh NN, Feig BW, Rodriguez-Bigas MA, Skibber JM, Chang GJ. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer. 2017;123:1124-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Moritani K, Kanemitsu Y, Shida D, Shitara K, Mizusawa J, Katayama H, Hamaguchi T, Shimada Y; Colorectal Cancer Study Group (CCSG) of Japan Clinical Oncology Group (JCOG). A randomized controlled trial comparing primary tumour resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 (iPACS study). Jpn J Clin Oncol. 2020;50:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Damjanov N, Weiss J, Haller DG. Resection of the primary colorectal cancer is not necessary in nonobstructed patients with metastatic disease. Oncologist. 2009;14:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 795] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 18. | Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1032] [Article Influence: 64.5] [Reference Citation Analysis (0)] |