Published online Jan 15, 2020. doi: 10.4251/wjgo.v12.i1.21
Peer-review started: March 19, 2019
First decision: July 31, 2019
Revised: October 9, 2019
Accepted: November 4, 2019
Article in press: November 4, 2019
Published online: January 15, 2020
Processing time: 287 Days and 15.2 Hours
In addition to the popularity of laparoscopic gastrectomy (LG), many reconstructive procedures after LG have been reported. Surgical resection and lymphatic dissection determine long-term survival; however, the election of a reconstruction procedure determines the postoperative quality of life for patients with gastric cancer (GC). Presently, no consensus exists regarding the optimal reconstructive procedure. In this review, the current state of digestive tract reconstruction after LG is reviewed. According to the determining influence of the tumor site on the procedures of surgical resection and reconstruction, we divide these reconstruction procedures into three categories consistent with the resection procedures. We focus on the technical tips of every reconstruction procedure and examine the surgical outcomes (length of surgery and blood loss) and postoperative complications (anastomotic leakage and stricture) to facilitate gastrointestinal surgeons to understand the merits and demerits of every reconstruction procedure.
Core tip: This article systematically reviews almost all the reconstruction methods currently used and divides them into three categories according to the method of resection (laparoscopic distal gastrectomy, laparoscopic total gastrectomy, and laparoscopic proximal gastrectomy). This review clearly demonstrates the key steps, merits, and demerits of every reconstruction method via drawing schematics based on the authors’ personal experience.
- Citation: Shen J, Ma X, Yang J, Zhang JP. Digestive tract reconstruction options after laparoscopic gastrectomy for gastric cancer. World J Gastrointest Oncol 2020; 12(1): 21-36
- URL: https://www.wjgnet.com/1948-5204/full/v12/i1/21.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i1.21
Gastric cancer (GC) remains a disease with high incidence and mortality worldwide[1,2]. GC patients demonstrate reliable survival results due to the implementation of D2 lymphadenectomy, which has become the cornerstone of GC treatment in the past decades[3-5]. Kitano et al[6] first reported a case of laparoscopic-assisted distal gastrectomy in 1994. GC surgery has gradually changed from open to laparoscopic-assisted and ultimately to total laparoscopic during the past 20 years. Presently, the main indication for laparoscopic gastrectomy (LG) is early GC because recent studies have shown that the oncologic outcomes of LG were comparable to those of open surgery[7-9]. Three multicenter trials, the JLSSG 0901[10], CLASS-01[11], and KLASS-02[12,13] trials, are current large-scale randomized controlled trials (RCTs) to obtain evidence-based oncological outcomes of LG for advanced GC (AGC)[14]. The results of these RCTs were expected to establish concrete evidence of the widely carried out LG in the treatment of AGC. Various new laparoscopic lymph node dissection procedures were reported and have been shown to achieve pathologically reliable lymphadenectomy during this development process. These technical summaries based on the surgeon’s clinical experience made lymph node dissection standardized with reliable quality[15-17].
In addition to the improved survival, quality of life (QoL) attracted more attention, and total laparoscopic surgery has gained widespread global popularity owing to its well-known benefits, such as reduced surgical trauma, decreased pain, low rates of morbidities, and a shorter length of hospital stay[14,18]. Digestive tract reconstruction is a key technique in laparoscopic surgery. However, no definitive consensus is currently available regarding how to choose among the various methods. This review focuses on describing technical tips and discussing the merits and demerits of commonly used laparoscopic reconstruction procedures at present.
To eliminate the influence of the learning curve on complications, a literature search was performed using the terms “laparoscopic gastrectomy”, “digestive tract reconstruction”, and “gastric cancer” along with their synonyms or abbreviations after 2015. Studies on different reconstructive procedures including less than 10 patients were excluded. The length of surgery, intraoperative blood loss, anastomotic leakage and stricture, esophagitis reflux, and gastric stasis were examined. Data extraction was confirmed manually.
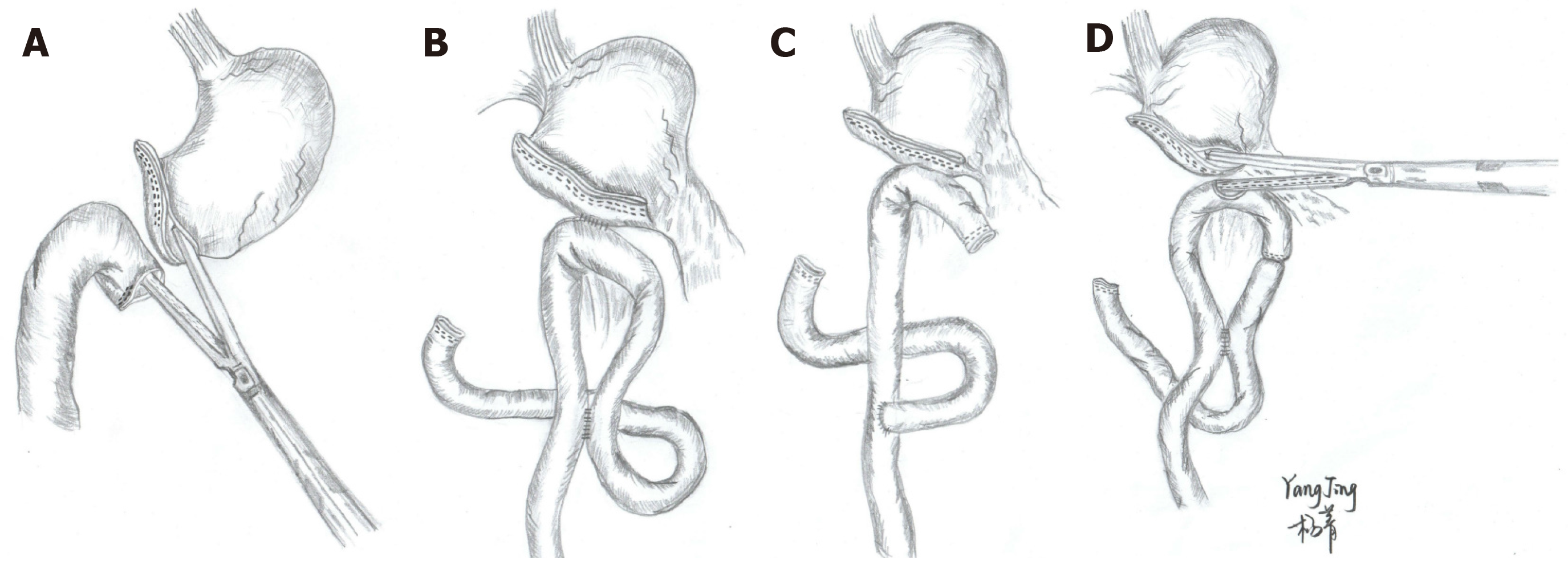
Billroth-I (B-I), Billroth-II (B-II), Roux-en-Y, and uncut Roux-en-Y reconstruction are the most popular methods of reconstruction after LDG for GC.
B-I reconstruction, one of the most popular procedures of reconstruction after distal gastrectomy, is associated with the physiological anatomy and involves only one anastomosis site without stump nor input loop. It is often performed using an extracorporeal procedure with a mini-laparotomy scar in laparoscopy-assisted distal gastrectomy or an intracorporeal procedure in total LDG. The delta-shaped anastomosis (DA) is the most common intracorporeal B-I anastomosis for LDG that was first reported by Kanaya et al[19] in 2002. DA is completed by side-to-side gastroduodenostomy with laparoscopic linear staplers (LS) intracorporeally (Figure 1A). This procedure is becoming widely used due to its simplicity and safety[20], and it can be performed safely even by an inexperienced surgeon[21]. Several studies have demonstrated that DA resulted in less blood loss and faster recovery than B-I, especially in obese patients. However, no difference was found in the surgical outcomes (operative time, number of harvested lymph nodes, and proximal margin) and postoperative complications (anastomotic leakage, stricture, hemorrhage, and wound complications)[22-24] (Table 1). However, some researchers have indicated that DA may affect the blood supply during cutting and result in an increased risk of anastomosis-related complications[25]. Another limitation of DA is that it is difficult to locate the tumor to obtain a pathologically safe margin; sometimes the tumor location requires being marked preoperatively or intraoperatively[20], a step that is likely the main shortcoming and limitation of the operation. Additionally, the cost of DA procedure is higher as it requires more endoscopic liner stapler cartridges[26].
| Ref. | Publication year | Reconstruc-tion procedure | n | Length of surgery, min (mean ± SD or range) | Blood loss, mL (mean ± SD or range) | Anastomotic leakage (n) | Anastomotic stricture (n) | Stasis (n) |
| Fukunaga et al[51] | 2018 | B-I (augmented rectangle technique) | 160 | 227 ± 75 | 47.3 ± 50 | 0 | 0 | 0 |
| Lin et al[52] | 2016 | LTDG BI | 158 | 154.4 ± 30.1 | 51.1 ± 30.9 | 5 | 0 | NA |
| LADG BI | 484 | 155.6 ± 46.2 | 61.6 ± 78.3 | 1 | 0 | NA | ||
| Jeong et al[20] | 2015 | Intracorporeal B-I | 42 | 116 ± 23 | 105 ± 69 | 0 | NA | 1 |
| Extracorporeal B-I | 179 | 142 ± 19 | 50 ± 39 | 2 | NA | 5 | ||
| Jian-Cheng et al[53] | 2015 | DA | 24 | 175.3 ± 64.7 | 50.8 ± 25.3 | NA | NA | NA |
| Lee et al[24] | 2015 | DA | 138 | 220.4 ± 70.5 | 99.8 ± 99.0 | 2 | 2 | NA |
| B-I | 100 | 220.5 ± 64.7 | 133.3 ± 152.1 | 0 | 4 | NA | ||
| Jang et al[27] | 2015 | Overlap | 42 | 228.3 ± 42.5 | NA | 0 | 0 | 5 |
| Watanabe et al[28] | 2019 | B-I | 247 | 203 (107–418) | 10 (0–380) | 4 | 0 | 3 |
| R-Y | 286 | 257 (134–495) | 27.5 (1–915) | 5 | 3 | 11 | ||
| Toyomasu et al[54] | 2018 | B-I | 123 | 191.2 ± 51.6 | 58.2 ± 45.3 | 1 | 0 | 0 |
| R-Y | 24 | 244.5 ± 40.2 | 84.8 ± 60.9 | 0 | 0 | 2 | ||
| Okuno et al[55] | 2018 | R-Y | 159 | 320 ± 65 | 61 ± 109 | 4 | 1 | NA |
| B-I (β) | 78 | 250 ± 61 | 70 ± 100 | 3 | 3 | NA | ||
| Kim et al[43] | 2015 | B-I | 165 | 173.4 ± 44.7 | 92.1 ± 92.1 | 3 | 4 | NA |
| B-II | 371 | 198.7 ± 48.5 | 172.2 ± 130.8 | 2 | 2 | NA | ||
| R-Y | 161 | 185.7 ± 55.5 | 87.1 ± 65.9 | 1 | 3 | NA | ||
| Kim et al[56] | 2017 | B-II LADG | 60 | 205.0 ± 22.4 | 117.2 ± 81.6 | NA | NA | NA |
| B-II LTDG | 60 | 197.3 ± 40.1 | 100.5 ± 36.8 | NA | NA | NA | ||
| Cui et al[57] | 2017 | R-Y | 30 | 157.3 ± 33.9 | 89.2 ± 85.5 | 1 | NA | NA |
| B-II + Braun | 26 | 134.6 ± 28.8 | 96.0 ± 89.8 | 0 | NA | NA | ||
| In Choi et al[58] | 2016 | B-II + Braun | 26 | 198.1 ± 33.0 | 161.7 ± 146.6 | NA | 1 | NA |
| R-Y | 40 | 242.3 ± 58.1 | 245.0 ± 207.0 | NA | 1 | NA | ||
| Du et al[59] | 2019 | R-Y | 24 | 203.6 ± 26.2 | 168.3 ± 83.1 | 0 | 0 | 2 |
| Seo et al[60] | 2018 | Uncut R-Y | 30 | 170.0 ± 26.0 | 122.8 ± 109.0 | 0 | 0 | 4 |
| Ma et al[61] | 2017 | Uncut R-Y | 51 | 170 (135-210) | 60 (30-110) | 0 | 0 | 0 |
| Zang et al[62] | 2018 | Uncut R-Y (ERAS) | 20 | 217.9 ± 52.5 | 166.1 ± 12.5 | NA | 0 | 0 |
| Uncut R-Y (control) | 22 | 225.4 ± 61.7 | 150.9 ± 31.7 | NA | 0 | 0 | ||
| Park et al[63] | 2018 | Uncut R-Y | 230 | 185.0 [150.0; 230.0] | 100.0 [50.0; 150.0] | NA | 6 | 2 |
| R-Y | 46 | 200.0 [180.0; 230.0] | 100.0 [50.0; 100.0] | NA | 0 | 3 | ||
| Yang et al[50] | 2017 | Uncut Roux-en-Y | 79 | 154.8 ± 17.8 | 74.1 ± 26.7 | NA | NA | NA |
| B-II | 79 | 145.5 ± 15.1 | 74.0 ± 36.6 | NA | NA | NA |
To improve the disadvantages mentioned above, a modified reconstructive procedure using an overlap method for B-I is developed. In general, the anastomosis is performed by overlapping the remnant stomach and duodenal stump via LS[27]. Watanabe et al[28] believed that this method is safer and easier because the posterior wall of the remnant stomach and anterior wall of the duodenum are anastomosed, and it is not necessary to create a space around the posterior wall of the duodenum. Accordingly, this procedure reduces the possibility of damage to the surrounding structures and duodenum when compared with the formation of anastomosis on the posterior wall in DA[28]. High technical requirements, sufficiently long duodenal stump dissociation, high anastomotic tension, bile reflux, and gastric stump cancer surgery are the inherent shortcomings of B-I reconstruction, and surgeons should consider these issues when choosing this procedure.
Due to a combination of increased screening and improved diagnostic techniques, the diagnostic rate of early GC has increased in recent years. As a result of the satisfactory survival outcomes achieved in the treatment of early GC, surgeons pay more attention to the postoperative QoL[29,30]. Pylorus-preserving gastrectomy (PPG) is a function-preserving surgery for the treatment of patients with cT1N0 middle third GC, aiming to decrease the complication rate and improve the postoperative QoL. The infrapyloric artery and antral cuff (2 cm length) were preserved, D2 lymph node dissection was performed, and the gastrogastrostomy was similar to B-I anastomosis with LS[31-33].
It was reported that PPG has the benefits in postoperative nutrition and can reduce the incidence of bile reflux, dumping syndrome, and cholelithiasis meanwhile[34]. However, some surgeons worry that the intracorporeal reconstruction may lead to micro-dissemination of free cancer cells left over in the remnant gastric lumen[35].
B-II reconstruction is another frequently used method in total LDG. During the procedure, an LS is used to anastomose the greater curvature side of the remnant gastric stomach with the jejunum approximately 10-15 cm from the Treitz ligament.
The main advantages of this method are that the tension of the anastomotic stoma is small, there is no need to dissociate much duodenum, and there is no specific requirement for the location of the tumors. Therefore, B-II is usually used in cases in which B-I is inappropriate. Nevertheless, this method is limited because of a higher risk of complications such as reflux gastritis[36,37]. Considering these reasons, B-II with Braun anastomosis (side-to-side jejunojejunostomy away from the gastrojejunal anastomosis) was applied in total LDG (Figure 1B). Additionally, it can reduce the aï¬erent loop syndrome compared with B-II without Braun anastomosis[38]. Some researchers have revealed that B-II Braun anastomosis cannot reduce the high incidence of bile reflux[39], and Park et al[40] reported a high incidence of bile reflux in B-II Braun anastomosis patients (~43.3%). Therefore, some researchers have proposed that Roux-en-Y or uncut Roux-en-Y reconstruction may be an alternative to B-II reconstruction.
Roux-en-Y reconstruction (Figure 1C) is a very common procedure in the West, has gained popularity in Asia, and is often preferred if the remnant stomach is relatively small or the tumor is near the pylorus[41]. Previous studies have reported that Roux-en-Y reconstruction can reduce the incidence of food residues, esophagitis, gastritis, and bile reflux in follow-up endoscopic findings than the B-I and B-II groups[39,42,43] (Table 1). However, Roux-en-Y reconstruction in total LDG for GC is a more complicated procedure than B-I or B-II because it involves two anastomoses. Therefore, the operation time and anastomosis time were significantly longer for RY than for B-I[44], and multiple anastomotic lines could increase the probability of anastomotic leakage. Additionally, Roux-en-Y reconstruction has a specific problem named Roux stasis syndrome, which is characterized by vomiting, swelling, nausea, and postprandial pain. Its incidence rate is approximately 10%-30%[45,46]. To solve this problem, uncut Roux-en-Y reconstruction was first carried out in 1988 by Van Stiegmann et al[47]. Uncut Roux-en-Y is a simple modification of the B-II with the Braun anastomosis method, in which the jejunogastric pathway is occluded with a nonbladed LS (Figure 1D). It is believed that the mechanism of uncut Roux-en-Y reconstruction can reduce Roux stagnation syndrome by preserving intestinal integrity to facilitate the conduction of myenteric impulse[48]. Park et al[40] reported that there was no difference in the incidence of gastritis and bile reflux between the uncut RY and RY group, while the uncut RY group significantly improved stasis compared with the RY group (5.8% vs 35.3%). Accordingly, uncut RY reconstruction could technically overcome the gastric stasis, which is a major drawback of RY reconstruction. However, some studies have reported that the recanalization of the uncut stapled line was relatively high, with a rate of 2.9%-13%[49,50]. Future large-scale prospective randomized clinical trials are needed to evaluate the advantages or disadvantages of uncut RY reconstruction.
Proximal gastrectomy (PG) was mainly performed in patients with early GC in the upper-third of the stomach to preserve the physiological function of the remnant stomach[64,65]. Many reconstructive procedures have been reported, including esophagogastrostomy (EGS), jejunal interposition (JI)[66], jejunal pouch interposition (JPI)[67], and double tract reconstruction (DTR)[68,69]. The clinical applications of laparoscopic JI and JPI have not been popularized due to the complexity of the operations, and this review mainly focuses on the methods of EGS and DTR.
EGS is the most popular and a classical reconstruction method because it includes only one anastomosis site and is widely used worldwide. The EGS technique is similar to the esophagojejunostomy (EJS) mentioned above. It was widely recognized that the EGS procedure often leads to severe reflux esophagitis due to resection of the cardiac sphincter and some surgeons choose to perform total gastrectomy (TG)[70,71]. However, patients with early-stage GC usually have good survival outcomes and require higher QoL. Accordingly, there were some improved methods of EGS, such as gastric tube reconstruction after PG. This procedure showed advantages in the operating time and blood loss and could lead to a similar prognosis in patients compared with TG–Roux-en-Y reconstruction. More importantly, preservation of the duodenal passage could contribute to better iron uptake and may ameliorate body weight loss and nutritional status postoperatively[72]. Yamashita et al[73] developed a new method of side overlap with fundoplication (SOFY) for EGS that could be easily performed laparoscopically. Reflux esophagitis was rarely observed in the SOFY group (1/14) but was common in the non-SOFY group (5/16). Anastomotic stenosis was also more frequent in the non-SOFY group. One shortcoming was that the number of clinical cases using this method was too small, and a larger sample with higher levels of evidence is needed in the future to observe actual effects[74]. Double-flap (DF), also named Kamikawa’s method, is another novel technical development. Briefly, a DF window with a dimension of 2.5 cm × 3.0-3.5 cm (width × height) is created at the anterior wall of gastric remnant. Next, the posterior wall of the esophagus and superior opening of the mucosa on the gastric remnant are manually anastomosed laparoscopically. The anastomotic site is finally covered by the flaps to create the anastomotic valve[74]. Obviously, DF can significantly reduce the symptoms of esophageal reflux. However, a longer operative time is needed and the anastomotic stricture rate remained in a high range from 4.7% to 17.5% in different centers. Otherwise, DF requires complicated intracorporeal suturing and leads to a longer learning curve[75-77]. Additionally, gastric retention was also common in EGS due to vagotomy, and the simple EGS was gradually replaced by other reconstruction procedures.
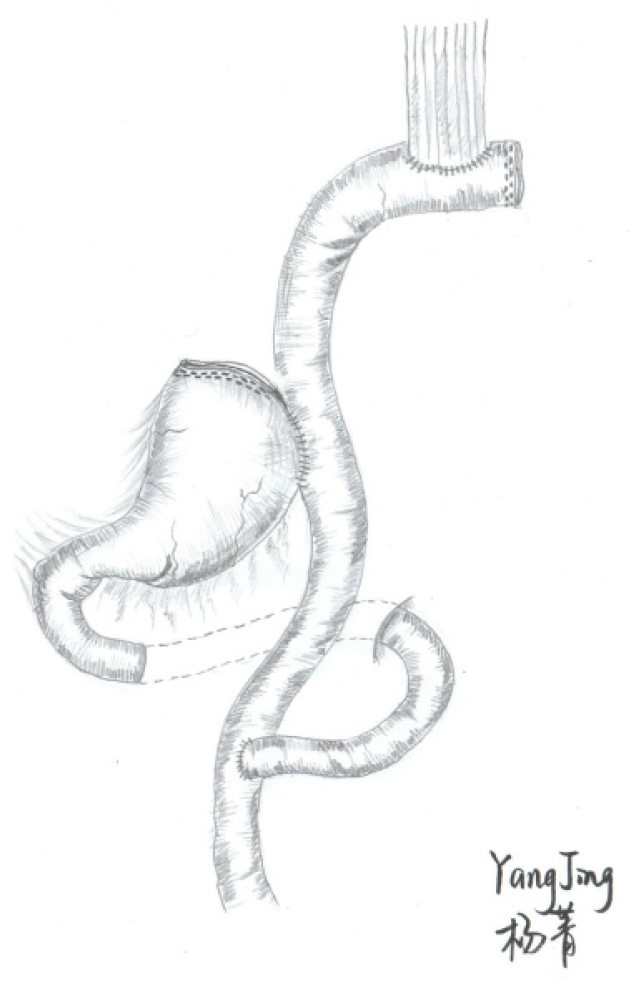
DTR is thought to be the best reconstruction procedure with respect to postoperative reflux esophagitis and is commonly used presently. Technically, a conventional Roux-en-Y EJS similar to TG is performed first. A side-to-side gastrojejunostomy is subsequently performed 10-15 cm below the EJS by LS (Figure 2). Reflux esophagitis can be theoretically reduced due to the interposition of the 10- to 15-cm jejunum between the remnant stomach and esophagus[58]. As reported by Aburatani et al[78], the DTR group (10.5%) had a lower incidence of reflux symptoms in the first year postoperatively than the EGS group (54.5%). Both EJS and EGS were completed with circular staplers (CS), and the frequency of anastomotic stenosis was also higher in the EG group (27.3% vs 0%) in that study[78]. The possible causes of benign anastomotic stenosis were different vascularization and erosive effects of the refluxed duodenal and gastric contents. The distance of anastomosis between gastrojejunostomy and EJS was also considered a risk factor for anastomosis-related late complications[79]. Similar to JI, the DTR procedure was also aimed to maintain gradual intestinal absorption and helped to improve QoL compared with TG. As reported by Nomura et al[80], the intestinal absorption and hormonal secretion in the DTR group were largely unaffected by the posture of the meal intake than JI. In the present literature, the DTR did not show a longer operation time and more blood loss. Anastomotic leakage was rarely or even not evident, the incidence of anastomotic stricture ranged from 0% to 6.67%, and the incidence of esophagitis reflux was reported from 0% to 20% (Table 2). These results indicate that DTR is a safe and feasible surgical procedure. In terms of the long-term effects, Cho et al[81]’s results showed similar hematologic and nutritional outcomes between the two procedures. However, other studies reported that DTR has advantages in hemoglobin change and vitamin B12 deficiency compared with laparoscopic total gastrectomy (LTG)[79,82,83]. Long-term results in a multicenter study with a larger number of patients should be evaluated in the future to fully elucidate the controversy.
| Ref. | Publication year | Reconstruc-tion procedure | n | Length of surgery, min (mean ± SD or range) | Blood loss, mL (mean ± SD or range) | Esophagitis reflux (n) | Anastomotic leakage (n) | Anastomotic stricture (n) |
| Nomura et al[80] | 2019 | DTR | 15 | 352.5 ± 67.3 | 90.5 ± 105.5 | 1 | 0 | 1 |
| JI | 15 | 322.5 ± 24.2 | 46.8 ± 69.8 | 1 | 0 | 1 | ||
| Aburatani et al[78] | 2017 | DTR | 19 | 325.7 ± 66.9 | 131.4 ± 118.7 | 2 | 0 | 0 |
| EGS | 22 | 290.3 ± 55.1 | 132.0 ± 129.7 | 12 | 0 | 6 | ||
| Tanaka et al[84] | 2017 | DTR | 10 | 285 (146–440) | 0 (0–25) | 20 | 0 | 0 |
| Yang et al[85] | 2015 | DTR | 16 | 219.6 ± 48.6 | 101.5 ± 71.6 | 0 | 0 | 0 |
| Hong et al[86] | 2015 | DTR | 21 | 173.8 ± 21.8 | 109.2 ± 96.3 | 1 | 0 | 0 |
| Cho et al[81] | 2018 | DTR | 38 | 217.7 ± 53.0 | 100.2 ± 92.0 | 0 | 1 | 0 |
| TG | 42 | 226.9 ± 66.2 | 118.8 ± 157.2 | 3 | 4 | 2 | ||
| Park et al[82] | 2018 | DTR | 34 | 212.9 ± 32.6 | 30 (6-600) | NA | NA | NA |
| TG | 46 | 240.7 ± 43.9 | 59 (20-85) | NA | NA | NA | ||
| Jung et al[79] | 2017 | DTR | 92 | 198.3 ± 38.8 | 84.7 ± 81.7 | 1 | 2 | 3 |
| TG | 156 | 225.4 ± 51.6 | 128.3 ± 112.5 | 3 | 3 | 2 | ||
| Kim et al[83] | 2016 | DTR | 17 | 268.2±40.9 | NA | 2 | 0 | 0 |
| TG | 17 | 270.2±43.4 | NA | 1 | 0 | 1 |
As reviewed, the DTR, improved EGS, and JI methods were used to prevent reflux esophagitis. LPG with DTR maintains comparable oncological safety and anastomosis-related late complications compared with LTG and is preferred as a reasonable alternative to LTG if oncological safety is assured. However, its advantage in nutrition postoperatively remains controversial compared with LTG. Accordingly, surgeons should be aware that LPG should be strictly limited to performance under the premise of radical resection.
The Roux-en-Y procedure is most commonly method for reconstruction between the esophagus and jejunum after LTG. EJS is difficult, and multiple intracorporeal techniques for EJS have been developed that can be divided into two categories: Those using a CS and those using an LS. Only a few reports exist concerning the hand-sewn technique for EJS, which is currently only safely performed in few high-volume centers because it is too difficult to be popularized and is not discussed in this review[87,88].
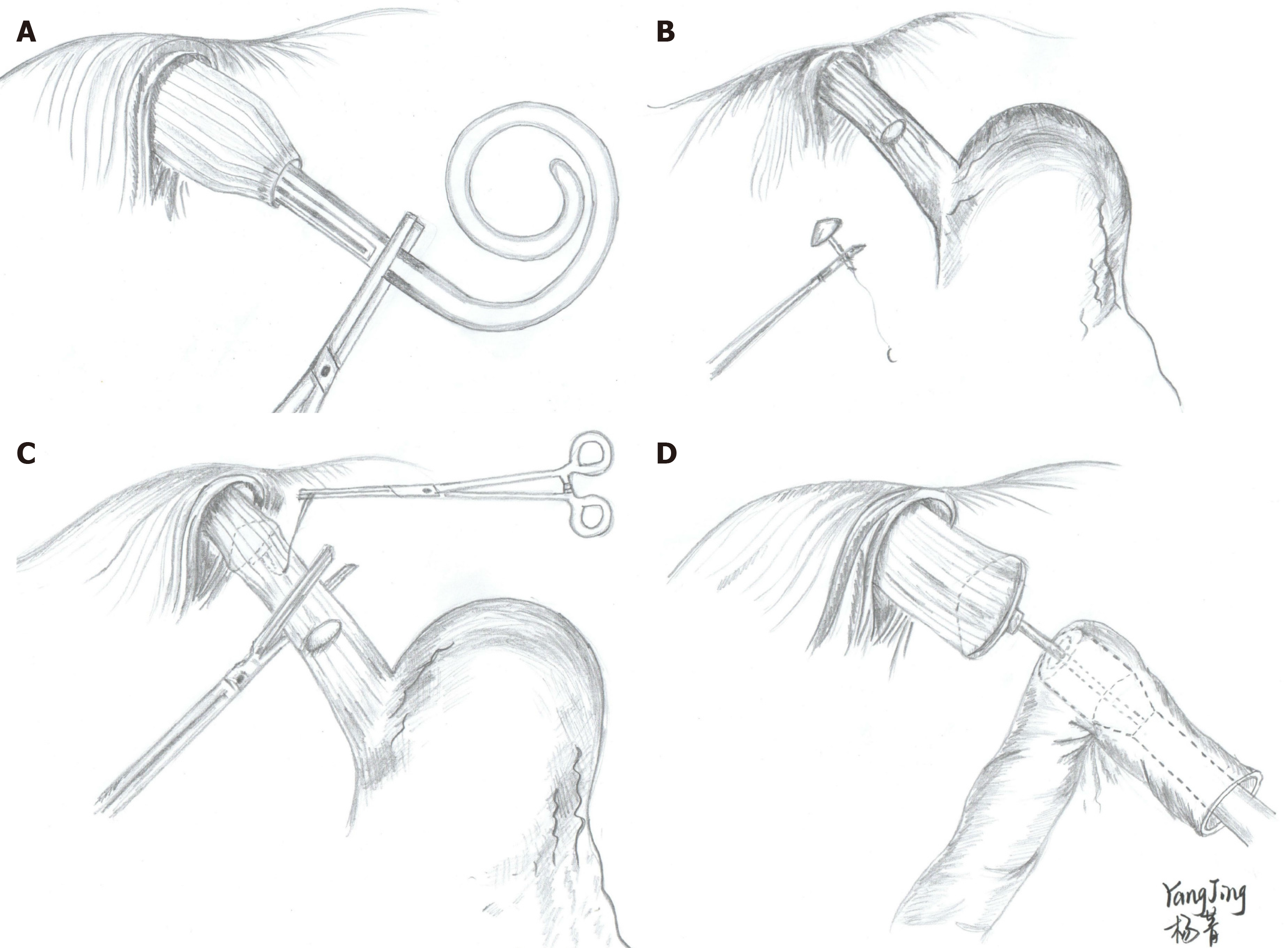
Similar to conventional open TG, the CS method of EJS is completed in an end-to-side manner using a CS. In the early LTG surgeries, the anvil was inserted into the esophagus stump using the purse-string instrument[89,90] or hand-sewn method[91,92]. In addition to the improvement in the laparoscopic devices and accumulation of experience, the application of these two methods has decreased and has been gradually replaced by other methods. Presently, the maneuver of inserting the anvil is commonly performed transorally or transabdominally, referred to as the OrVil™ or reverse puncture method (RPM), respectively. The OrVilTM was first reported by Jeong et al[93] in 2009. Briefly, the anvil connected to the OrVilTM tube was transorally introduced into the esophagus (Figure 3A) and intracorporeal EJS was consecutively performed with a CS through a minilaparotomy incision that was used to remove the stomach (Figure 3D). The RPM is another common method that was first reported by Omori et al[94] in 2009. The main steps of this procedure are as follows: Semicircumferential esophagotomy is performed at the anterior esophageal wall and the anvil secured with a prolene suture that is then inserted into the esophagus. Thereafter, the needle is reversely sutured out and the center rod of the anvil penetrates the esophageal wall by drawing the suture (Figure 3B and C). Finally, the esophagus is transected using a linear cutter, and EJS is achieved with a CS under laparoscopic monitoring.
The CS method has been widespread, especially in the introductory period, because it is similar to the conventional open surgeries. CS also has other merits compared with LS, such as no requirement for intracorporeal suturing and excessive esophageal dissociation[95]. Comparing the two CS methods, the device of OrVilTM is easier and very convenient to perform intracorporeal EJS. Otherwise, the RPM needs laparoscopic suturing and more esophagus may be sacrificed[96]. In terms of the surgical outcomes and postoperative course, no significant difference was found in the surgical time and blood loss between OrVilTM and RPM, and the incidence of anastomotic leakage was also similar. However, the incidence of anastomotic stricture was higher when performing OrVilTM, ranging from 0% to 8.3% (Table 2)[97-100]. As reviewed by Inokuchi et al[101], the incidence was decreased compared with the results from early surgeries. This progress might be attributed to the standardization of the procedures and accumulation of skills to use the OrVilTM device. Additionally, the anastomotic complications might be related to the insertion site in the abdominal wall for CS[102]. However, higher cost, possibility of bacterial contamination, and injury of the esophageal mucosa are important factors limiting the popularity of the OrVilTM method[96,103]. Many surgeons, including the authors, would choose the RPM after mastering laparoscopic techniques. The determination of the CS method should be selected by the preference and experience of the surgeons.
As described, all CS require a mini incision to finally complete the EJS, and these CS methods are actually laparoscopic-assisted surgeries. Furthermore, it is sometimes difficult to complete anastomosis through this mini incision, especially in obese patients. Additionally, in patients with a small esophageal diameter, the CS method is extremely difficult and would increase the risk of anastomotic complications that could be overcome by LS methods.
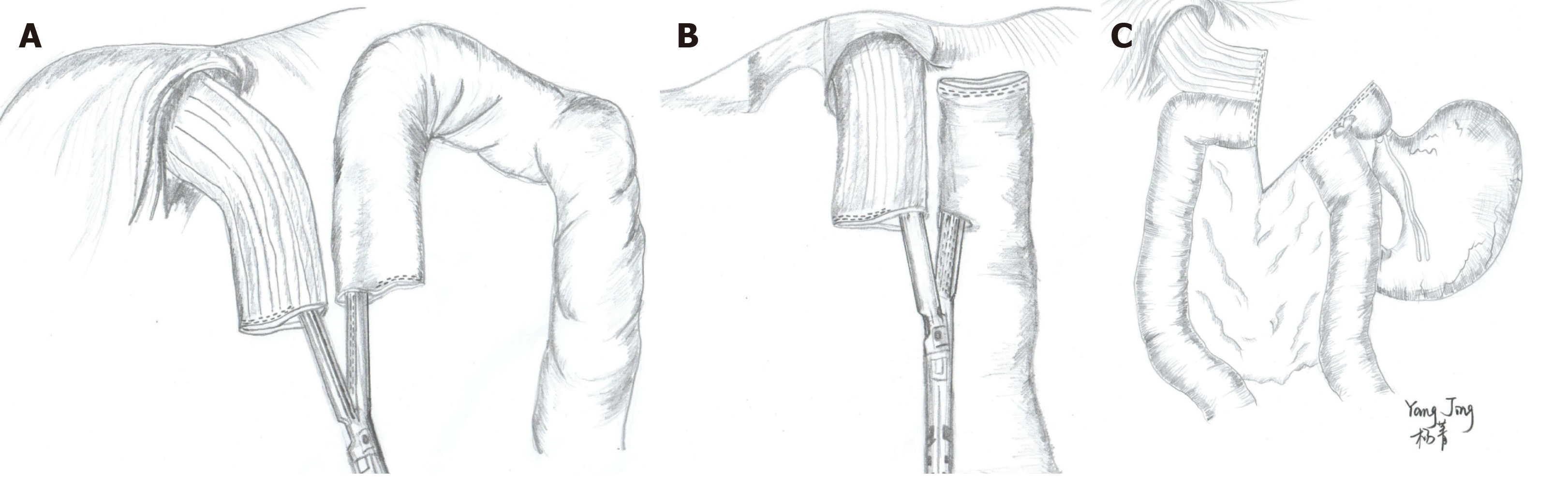
The LS methods are total laparoscopic surgeries because EJS is completed in a side-to-side manner using a LS without any assisted incisions. The main procedures include functional end-to-end anastomosis (FEEA), overlap method, and π-type anastomosis. The FEEA method, also called “inverse-peristaltic anastomosis”, was first reported by Uyama et al[104] in 1999. First, the distal jejunum loop is pulled to the left side of the esophageal stump after lymphadenectomy. A functional side-to-side anastomosis is subsequently completed with an LS via the stump of the esophagus and jejunum (Figure 4A). The common entry hole is finally closed by LS. The overlap method proposed by Inaba et al[105] in 2010 is similar to FEEA. The hole used for overlap anastomosis is not opened at the jejunal stump, and side-to-side anastomosis is completed along the peristaltic direction of the esophagus (Figure 4B). Another difference from FEEA is the closure of the common entry hole that is performed via an intracorporeal suture. The π-type anastomosis is an improvement of FEEA. Technically, neither the esophagus nor the jejunum is transected, and a side-to-side EJS is performed with an LS. The esophageal division, common entry hole closure, and jejunal division are subsequently performed using a single 60-mm LS (3-in-1 technique) (Figure 4C)[106].
Comparing these three LS methods, FEEA is time-saving because the common entry hole can be closed with an LS. However, the jejunal limb needs to be lifted further up when performing FEEA, and this step might lead to the tension of the jejunal limb of the mesentery, which might increase the risk of anastomotic leakage[107]. Second, the procedure of FEEA needs more working space than overlap as the jejunum is folded up when performing anastomosis (Figure 4A). No significant difference was found between the two methods in terms of actual anastomotic complications (Table 2). However, the anastomotic oral end tended to have greater tension, which was the high incidence site of anastomotic leakage. Moreover, this site is located in the mediastinum, and it is difficult to strengthen by laparoscopic suture. Accordingly, surgeons should pay more attention to this point, especially for beginners. The surgical procedure is simplified, and the surgical time is reduced by performing π-type anastomosis. However, the largest deficiency of this method is that the margin could not be checked until the reconstruction is completed, limiting its popularity.
Compared with the CS method, LS method shows some merits in surgical outcomes (Table 3): Being time-saving and less blood loss[108-110], with fewer intraoperative events and intraoperative anastomosis events[109]. Regarding anastomotic complications, LS methods seem to have less anastomotic stricture[97,111,112]. Additionally, LS can be performed in the narrow mediastinum because the tips are thinner and can achieve a suitable anastomotic size regardless of the esophageal diameter. Furthermore, the rotary connecter of LS enables the LS method to be performed in real time to reduce the jejunal tension by changing the anastomotic position and direction to improve the quality of anastomosis. Therefore, the LS methods have been favored by more surgeons in the past few years.
| Ref. | Publication year | LS or CS | EJS procedure | N | Length of surgery, min (mean ± SD or range) | Blood loss, mL (mean ± SD or range) | Anastomotic leakage (n) | Anastomotic stricture (n) |
| Tokuhara et al[99] | 2018 | CS | OrVilTM | 24 | NA | NA | 1 | 2 |
| Brenkman et al[114] | 2016 | CS | OrVilTM | 47 | 301 (148–454) | 300 (30–900) | 7 | NA |
| Ali et al[115] | 2016 | CS | RPM | 58 | 199.8 ± 57.0 | 81.6 ± 40.3 | 3 | 5 |
| Wang et al[96] | 2015 | CS | OrVilTM | 42 | 287.8 ± 38.4 | 96.4 ± 32.7 | 0 | 2 |
| RPM | 42 | 271.8 ± 46.1 | 88.2 ± 36.9 | 1 | 2 | |||
| Li et al[103] | 2017 | CS | OrVilTM | 19 | NA | NA | 0 | 1 |
| RPM | 24 | NA | NA | 1 | 0 | |||
| Lu et al[98] | 2016 | CS | OrVilTM | 25 | 216.5 ± 24.9 | 141.2 ± 121.1 | 0 | 0 |
| LATG-PSI | 25 | 224.0 ± 30.5 | 138.8 ± 79.9 | 0 | 0 | |||
| Duan et al[116] | 2017 | CS | End-to-side EJS | 176 | 250.0 ± 54.1 | 114.1 ± 74.0 | 7 | 11 |
| Semi-end-to-end EJS | 92 | 238.0 ± 50.4 0.079 | 110.5 ± 82.8 | 1 | 0 | |||
| Kyogoku et al[108] | 2018 | CS | OrVilTM / RPM | 83 | 330 (123–627) | 100 (0–1108) | 3 | 6 |
| LS | FEEA/overlap | 208 | 297 (171–553) | 23 (0–1070) | 4 | 7 | ||
| Lee et al[117] | 2017 | LS | Overlap | 50 | 144.6 ± 29.9 | NA | 0 | 0 |
| Son et al[118] | 2017 | LS | Overlap | 27 | 171.1 ± 50.9 | 119.4 ± 107.1 | 0 | 0 |
| Kitagami et al[119] | 2016 | LS | Overlap | 100 | 379 (248–649) | 65 (5–750) | 0 | 0 |
| Miura et al[107] | 2017 | LS | FEEA | 120 | 350.8 | 0 | 2 | 1 |
| Overlap | 48 | 402.5 | 6.5 | 3 | 0 | |||
| Yoshikawa et al[112] | 2018 | CS | OrVilTM | 36 | 345 ± 9.9 | 45 ± 15 | 0 | 3 |
| LS | Overlap | 47 | 398 ± 8 | 126 ± 13 | 2 | 0 | ||
| Kawamura et al[97] | 2017 | CS | OrVilTM | 49 | 259.5 ± 51.4 | 53.3 ± 70.0 | 2 | 2 |
| LS | Overlap | 139 | 276.5 ± 53.0 | 69.7 ± 116.6 | 1 | 0 | ||
| Yasukawa et al[100] | 2017 | CS | OrVilTM | 51 | 346.1 ± 52.7 | 34 (10-556) | 2 | 0 |
| LS | FEEA | 18 | 348.4 ± 53.5 | 35 (10-750) | 0 | 1 | ||
| Gong et al[109] | 2017 | CS | NA | 266 | 170 (65-453) | NA | 15 | 3 |
| LS | NA | 421 | 149 (75-342) | NA | 15 | 2 | ||
| Huang et al[110] | 2017 | CS | NA | 456 | 203.6 ± 49.3 | 98.4 ± 149.1 | 22 | 4 |
| LS | IJOM (overlap) | 51 | 209.3 ± 41.0 | 48.3 ± 38.5 | 1 | 0 | ||
| Chen et al[111] | 2016 | CS | RPM | 18 | 305.6 ± 45.9 (250-380) | 80.6 ± 29.4 (50-160) | 1 | 1 |
| LS | FEEA | 22 | 266.8 ± 38.7 (230-360) | 86.4 ± 39.7 (50-200) | 0 | 0 | ||
| Kim et al[120] | 2016 | CS | PSI | 29 | 230.3 ± 56.5 | 106.3 ± 70.3 | 0 | 1 |
| LS | Overlap | 27 | 228.9 ± 33.6 | 90.9 ± 46.0 | 1 | 0 | ||
| Chen et al[88] | 2016 | CS + LS | CS + LS | 40 | 284.3 ± 45.6 (230–380) | 83.8 ± 35.2 (30–200) | 1 | 3 |
| Hand-sewn | 59 | 257.4 ± 47.2 (170–350) | 87.6 ± 42.4 (30–200) | 0 | 0 |
Almost all the literature included in this review originated from the East and was mainly reported from Japan and Korea. This highlights the prominent position of these two countries in the field of GC treatment, while Western surgeons have less experience in treating GC laparoscopically due to the low incidence and respectability[113]. The results of the RCTs[9,11,12] mentioned above were expected to establish concrete evidence of widely carried out LG in the treatment of AGC. Therefore, LG will encounter a period of rapid development, and controversy concerning reconstruction is expected to be resolved by large-scale and multicenter RCTs in the near future.
In conclusion, the choice of specific reconstruction method remains unclear presently, and surgeons must understand the merits and demerits of every anastomotic device and procedure. Under the premise of radical gastrectomy and lymphadenectomy, a reasonable reconstruction procedure should be selected to improve the QoL postoperatively by considering the following factors: Safety (anastomosis with sufficient blood supply and free tension), efficiency (simple and time-saving), minimal invasion (less blood loss), stability (surgeon’s preference and experience), and QoL (function preservation, if possible, reflux prevention, and nutrition).
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Lazăr DC, Trkulja V S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13158] [Article Influence: 1879.7] [Reference Citation Analysis (4)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13210] [Article Influence: 1467.8] [Reference Citation Analysis (3)] |
| 3. | Agolli L, Nicosia L. Between evidence and new perspectives on the current state of the multimodal approach to gastric cancer: Is there still a role for radiation therapy? World J Gastrointest Oncol. 2018;10:271-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1914] [Article Influence: 239.3] [Reference Citation Analysis (1)] |
| 5. | Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH, Kim HI, Jung HY, Kim YS, Zang DY, Cho JY, Park JO, Lim DH, Jung ES, Ahn HS, Kim HJ. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 7. | Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH; Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J Korean Surg Soc. 2013;84:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Nakamura K, Katai H, Mizusawa J, Yoshikawa T, Ando M, Terashima M, Ito S, Takagi M, Takagane A, Ninomiya M, Fukushima N, Sasako M. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer (JCOG0912). Jpn J Clin Oncol. 2013;43:324-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 10. | Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, Shiraishi N, Kitano S. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg. 2015;39:2734-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 11. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 530] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 12. | Kim HI, Hur H, Kim YN, Lee HJ, Kim MC, Han SU, Hyung WJ. Standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC): a prospective, observational, multicenter study [NCT01283893]. BMC Cancer. 2014;14:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Kim MC; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 14. | Huh YJ, Lee JH. The Advances of Laparoscopic Gastrectomy for Gastric Cancer. Gastroenterol Res Pract. 2017;2017:9278469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Shinohara H, Haruta S, Ohkura Y, Udagawa H, Sakai Y. Tracing Dissectable Layers of Mesenteries Overcomes Embryologic Restrictions when Performing Infrapyloric Lymphadenectomy in Laparoscopic Gastric Cancer Surgery. J Am Coll Surg. 2015;220:e81-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Yang XT. Laparoscopic spleen-preserving no. 10 lymph node dissection for advanced proximal gastric cancer using a left approach. Ann Surg Oncol. 2014;21:2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Shen J, Dong X, Liu Z, Wang G, Yang J, Zhou F, Lu M, Ma X, Li Y, Tang C, Luo X, Zhao Q, Zhang J. Modularized laparoscopic regional en bloc mesogastrium excision (rEME) based on membrane anatomy for distal gastric cancer. Surg Endosc. 2018;32:4698-4705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Best LM, Mughal M, Gurusamy KS. Laparoscopic versus open gastrectomy for gastric cancer. Cochrane Database Syst Rev. 2016;3:CD011389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284-287. [PubMed] |
| 20. | Jeong O, Jung MR, Park YK, Ryu SY. Safety and feasibility during the initial learning process of intracorporeal Billroth I (delta-shaped) anastomosis for laparoscopic distal gastrectomy. Surg Endosc. 2015;29:1522-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Kanaya S, Kawamura Y, Kawada H, Iwasaki H, Gomi T, Satoh S, Uyama I. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer. 2011;14:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Ding W, Tan Y, Xue W, Wang Y, Xu XZ. Comparison of the short-term outcomes between delta-shaped anastomosis and conventional Billroth I anastomosis after laparoscopic distal gastrectomy: A meta-analysis. Medicine (Baltimore). 2018;97:e0063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Wang SY, Hong J, Hao HK. A comparative study of delta-shaped and conventional Billroth I anastomosis after laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. 2017;31:3191-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Lee HH, Song KY, Lee JS, Park SM, Kim JJ. Delta-shaped anastomosis, a good substitute for conventional Billroth I technique with comparable long-term functional outcome in totally laparoscopic distal gastrectomy. Surg Endosc. 2015;29:2545-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Noshiro H, Iwasaki H, Miyasaka Y, Kobayashi K, Masatsugu T, Akashi M, Ikeda O. An additional suture secures against pitfalls in delta-shaped gastroduodenostomy after laparoscopic distal gastrectomy. Gastric Cancer. 2011;14:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Matsuhashi N, Osada S, Yamaguchi K, Saito S, Okumura N, Tanaka Y, Nonaka K, Takahashi T, Yoshida K. Oncologic outcomes of laparoscopic gastrectomy: a single-center safety and feasibility study. Surg Endosc. 2013;27:1973-1979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Jang CE, Lee SI. Modified intracorporeal gastroduodenostomy in totally laparoscopic distal gastrectomy for gastric cancer: early experience. Ann Surg Treat Res. 2015;89:306-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Watanabe Y, Watanabe M, Suehara N, Saimura M, Mizuuchi Y, Nishihara K, Iwashita T, Nakano T. Billroth-I reconstruction using an overlap method in totally laparoscopic distal gastrectomy: propensity score matched cohort study of short- and long-term outcomes compared with Roux-en-Y reconstruction. Surg Endosc. 2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Wang Z, Ma L, Zhang XM, Zhou ZX. Long-term outcomes after D2 gastrectomy for early gastric cancer: survival analysis of a single-center experience in China. Asian Pac J Cancer Prev. 2014;15:7219-7222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, Ryu KW, Nam BH, Kook MC, Kim YW. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. 2015;81:333-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Lee SW, Bouras G, Nomura E, Yoshinaka R, Tokuhara T, Nitta T, Tsunemi S, Tanigawa N. Intracorporeal stapled anastomosis following laparoscopic segmental gastrectomy for gastric cancer: technical report and surgical outcomes. Surg Endosc. 2010;24:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Kim JJ, Song KY, Chin HM, Kim W, Jeon HM, Park CH, Park SM. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc. 2008;22:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Koeda K, Chiba T, Noda H, Nishinari Y, Segawa T, Akiyama Y, Iwaya T, Nishizuka S, Nitta H, Otsuka K, Sasaki A. Intracorporeal reconstruction after laparoscopic pylorus-preserving gastrectomy for middle-third early gastric cancer: a hybrid technique using linear stapler and manual suturing. Langenbecks Arch Surg. 2016;401:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Hosoda K, Yamashita K, Sakuramoto S, Katada N, Moriya H, Mieno H, Watanabe M. Postoperative quality of life after laparoscopy-assisted pylorus-preserving gastrectomy compared With laparoscopy-assisted distal gastrectomy: A cross-sectional postal questionnaire survey. Am J Surg. 2017;213:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Han TS, Kong SH, Lee HJ, Ahn HS, Hur K, Yu J, Kim WH, Yang HK. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol. 2011;18:2818-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Fukuhara K, Osugi H, Takada N, Takemura M, Higashino M, Kinoshita H. Reconstructive procedure after distal gastrectomy for gastric cancer that best prevents duodenogastroesophageal reflux. World J Surg. 2002;26:1452-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Kumagai K, Shimizu K, Yokoyama N, Aida S, Arima S, Aikou T; Japanese Society for the Study of Postoperative Morbidity after Gastrectomy. Questionnaire survey regarding the current status and controversial issues concerning reconstruction after gastrectomy in Japan. Surg Today. 2012;42:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Vogel SB, Drane WE, Woodward ER. Clinical and radionuclide evaluation of bile diversion by Braun enteroenterostomy: prevention and treatment of alkaline reflux gastritis. An alternative to Roux-en-Y diversion. Ann Surg. 1994;219:458-65; discussion 465-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 40. | Park JY, Kim YJ. Uncut Roux-en-Y Reconstruction after Laparoscopic Distal Gastrectomy Can Be a Favorable Method in Terms of Gastritis, Bile Reflux, and Gastric Residue. J Gastric Cancer. 2014;14:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | He Z, Zang L. Reconstruction after laparoscopic assisted distal gastrectomy: technical tips and pitfalls. Transl Gastroenterol Hepatol. 2017;2:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Inokuchi M, Kojima K, Yamada H, Kato K, Hayashi M, Motoyama K, Sugihara K. Long-term outcomes of Roux-en-Y and Billroth-I reconstruction after laparoscopic distal gastrectomy. Gastric Cancer. 2013;16:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Kim CH, Song KY, Park CH, Seo YJ, Park SM, Kim JJ. A comparison of outcomes of three reconstruction methods after laparoscopic distal gastrectomy. J Gastric Cancer. 2015;15:46-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | An JY, Cho I, Choi YY, Kim YM, Noh SH. Totally laparoscopic Roux-en-Y gastrojejunostomy after laparoscopic distal gastrectomy: analysis of initial 50 consecutive cases of single surgeon in comparison with totally laparoscopic Billroth I reconstruction. Yonsei Med J. 2014;55:162-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today. 2009;39:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Pan Y, Li Q, Wang DC, Wang JC, Liang H, Liu JZ, Cui QH, Sun T, Zhang RP, Kong DL, Hao XS. Beneficial effects of jejunal continuity and duodenal food passage after total gastrectomy: a retrospective study of 704 patients. Eur J Surg Oncol. 2008;34:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Van Stiegmann G, Goff JS. An alternative to Roux-en-Y for treatment of bile reflux gastritis. Surg Gynecol Obstet. 1988;166:69-70. [PubMed] |
| 48. | Morrison P, Miedema BW, Kohler L, Kelly KA. Electrical dysrhythmias in the Roux jejunal limb: cause and treatment. Am J Surg. 1990;160:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Huang Y, Wang S, Shi Y, Tang D, Wang W, Chong Y, Zhou H, Xiong Q, Wang J, Wang D. Uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Expert Rev Gastroenterol Hepatol. 2016;10:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 50. | Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: Which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol. 2017;23:6350-6356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 51. | Fukunaga T, Ishibashi Y, Oka S, Kanda S, Yube Y, Kohira Y, Matsuo Y, Mori O, Mikami S, Enomoto T, Otsubo T. Augmented rectangle technique for Billroth I anastomosis in totally laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. 2018;32:4011-4016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Lin M, Zheng CH, Huang CM, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Tu RH. Totally laparoscopic versus laparoscopy-assisted Billroth-I anastomosis for gastric cancer: a case-control and case-matched study. Surg Endosc. 2016;30:5245-5254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Jian-Cheng T, Bo Z, Jian F, Liang Z. Delta-Shaped Gastroduodenostomy in Fully Laparoscopic Distal Gastrectomy: A Retrospective Study. Medicine (Baltimore). 2015;94:e1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Toyomasu Y, Ogata K, Suzuki M, Yanoma T, Kimura A, Kogure N, Ohno T, Kamiyama Y, Mochiki E, Kuwano H. Comparison of the Physiological Effect of Billroth-I and Roux-en-Y Reconstruction Following Laparoscopic Distal Gastrectomy. Surg Laparosc Endosc Percutan Tech. 2018;28:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Okuno K, Nakagawa M, Kojima K, Kanemoto E, Gokita K, Tanioka T, Inokuchi M. Long-term functional outcomes of Roux-en-Y versus Billroth I reconstructions after laparoscopic distal gastrectomy for gastric cancer: a propensity-score matching analysis. Surg Endosc. 2018;32:4465-4471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Kim JH, Jun KH, Chin HM. Short-term surgical outcomes of laparoscopy-assisted versus totally laparoscopic Billroth-II gastrectomy for gastric cancer: a matched-cohort study. BMC Surg. 2017;17:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Cui LH, Son SY, Shin HJ, Byun C, Hur H, Han SU, Cho YK. Billroth II with Braun Enteroenterostomy Is a Good Alternative Reconstruction to Roux-en-Y Gastrojejunostomy in Laparoscopic Distal Gastrectomy. Gastroenterol Res Pract. 2017;2017:1803851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | In Choi C, Baek DH, Lee SH, Hwang SH, Kim DH, Kim KH, Jeon TY, Kim DH. Comparison Between Billroth-II with Braun and Roux-en-Y Reconstruction After Laparoscopic Distal Gastrectomy. J Gastrointest Surg. 2016;20:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Du J, Xue H, Hua J, Zhao L, Zhang Z. Intracorporeal classic circular-stapled gastrojejunostomy and jejunojejunostomy during laparoscopic distal gastrectomy: A simple, safe "intraluminal poke technique" for anvil placement. J Surg Oncol. 2019;119:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Seo HS, Jung YJ, Kim JH, Park CH, Lee HH. Three-Port Right-Side Approach-Duet Totally Laparoscopic Distal Gastrectomy for Uncut Roux-en-Y Reconstruction. J Laparoendosc Adv Surg Tech A. 2018;28:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Ma JJ, Zang L, Yang A, Hu WG, Feng B, Dong F, Wang ML, Lu AG, Li JW, Zheng MH. A modified uncut Roux-en-Y anastomosis in totally laparoscopic distal gastrectomy: preliminary results and initial experience. Surg Endosc. 2017;31:4749-4755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Zang YF, Li FZ, Ji ZP, Ding YL. Application value of enhanced recovery after surgery for total laparoscopic uncut Roux-en-Y gastrojejunostomy after distal gastrectomy. World J Gastroenterol. 2018;24:504-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Park YS, Shin DJ, Son SY, Kim KH, Park DJ, Ahn SH, Park DJ, Kim HH. Roux Stasis Syndrome and Gastric Food Stasis After Laparoscopic Distal Gastrectomy with Uncut Roux-en-Y Reconstruction in Gastric Cancer Patients: A Propensity Score Matching Analysis. World J Surg. 2018;42:4022-4032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Shiraishi N, Adachi Y, Kitano S, Kakisako K, Inomata M, Yasuda K. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. 2002;26:1150-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg Today. 2016;46:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 66. | Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Completely laparoscopic proximal gastrectomy with jejunal interposition and lymphadenectomy. J Am Coll Surg. 2000;191:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Namikawa T, Oki T, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K. Impact of jejunal pouch interposition reconstruction after proximal gastrectomy for early gastric cancer on quality of life: short- and long-term consequences. Am J Surg. 2012;204:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 68. | Ahn SH, Jung DH, Son SY, Lee CM, Park DJ, Kim HH. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer. 2014;17:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 69. | Aikou T, Natsugoe S, Shimazu H, Nishi M. Antrum preserving double tract method for reconstruction following proximal gastrectomy. Jpn J Surg. 1988;18:114-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 70. | Hosogi H, Yoshimura F, Yamaura T, Satoh S, Uyama I, Kanaya S. Esophagogastric tube reconstruction with stapled pseudo-fornix in laparoscopic proximal gastrectomy: a novel technique proposed for Siewert type II tumors. Langenbecks Arch Surg. 2014;399:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Chen S, Li J, Liu H, Zeng J, Yang G, Wang J, Lu W, Yu N, Huang Z, Xu H, Zeng X. Esophagogastrostomy plus gastrojejunostomy: a novel reconstruction procedure after curative resection for proximal gastric cancer. J Gastrointest Surg. 2014;18:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Toyomasu Y, Ogata K, Suzuki M, Yanoma T, Kimura A, Kogure N, Yanai M, Ohno T, Mochiki E, Kuwano H. Restoration of gastrointestinal motility ameliorates nutritional deficiencies and body weight loss of patients who undergo laparoscopy-assisted proximal gastrectomy. Surg Endosc. 2017;31:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Yamashita Y, Yamamoto A, Tamamori Y, Yoshii M, Nishiguchi Y. Side overlap esophagogastrostomy to prevent reflux after proximal gastrectomy. Gastric Cancer. 2017;20:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 74. | Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S, Kagawa S, Shirakawa Y, Fujiwara T. Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy. J Am Coll Surg. 2016;223:e7-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 75. | Muraoka A, Kobayashi M, Kokudo Y. Laparoscopy-Assisted Proximal Gastrectomy with the Hinged Double Flap Method. World J Surg. 2016;40:2419-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 76. | Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, Ida S, Watanabe M, Sano T, Yamaguchi T. Clinical Outcomes and Evaluation of Laparoscopic Proximal Gastrectomy with Double-Flap Technique for Early Gastric Cancer in the Upper Third of the Stomach. Ann Surg Oncol. 2017;24:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 77. | Hosoda K, Washio M, Mieno H, Moriya H, Ema A, Ushiku H, Watanabe M, Yamashita K. Comparison of double-flap and OrVil techniques of laparoscopy-assisted proximal gastrectomy in preventing gastroesophageal reflux: a retrospective cohort study. Langenbecks Arch Surg. 2019;404:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 78. | Aburatani T, Kojima K, Otsuki S, Murase H, Okuno K, Gokita K, Tomii C, Tanioka T, Inokuchi M. Double-tract reconstruction after laparoscopic proximal gastrectomy using detachable ENDO-PSD. Surg Endosc. 2017;31:4848-4856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Jung DH, Lee Y, Kim DW, Park YS, Ahn SH, Park DJ, Kim HH. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg Endosc. 2017;31:3961-3969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 80. | Nomura E, Kayano H, Lee SW, Kawai M, Machida T, Yamamoto S, Nabeshima K, Nakamura K, Mukai M, Uchiyama K. Functional evaluations comparing the double-tract method and the jejunal interposition method following laparoscopic proximal gastrectomy for gastric cancer: an investigation including laparoscopic total gastrectomy. Surg Today. 2019;49:38-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Cho M, Son T, Kim HI, Noh SH, Choi S, Seo WJ, Roh CK, Hyung WJ. Similar hematologic and nutritional outcomes after proximal gastrectomy with double-tract reconstruction in comparison to total gastrectomy for early upper gastric cancer. Surg Endosc. 2019;33:1757-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 82. | Park JY, Park KB, Kwon OK, Yu W. Comparison of laparoscopic proximal gastrectomy with double-tract reconstruction and laparoscopic total gastrectomy in terms of nutritional status or quality of life in early gastric cancer patients. Eur J Surg Oncol. 2018;44:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 83. | Kim DJ, Kim W. Laparoscopy-assisted Proximal Gastrectomy with Double Tract Anastomosis Is Beneficial for Vitamin B12 and Iron Absorption. Anticancer Res. 2016;36:4753-4758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Tanaka K, Ebihara Y, Kurashima Y, Nakanishi Y, Asano T, Noji T, Murakami S, Nakamura T, Tsuchikawa T, Okamura K, Shichinohe T, Hirano S. Laparoscopic proximal gastrectomy with oblique jejunogastrostomy. Langenbecks Arch Surg. 2017;402:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Yang K, Bang HJ, Almadani ME, Dy-Abalajon DM, Kim YN, Roh KH, Lim SH, Son T, Kim HI, Noh SH, Hyung WJ. Laparoscopic Proximal Gastrectomy with Double-Tract Reconstruction by Intracorporeal Anastomosis with Linear Staplers. J Am Coll Surg. 2016;222:e39-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Hong J, Qian L, Wang YP, Wang J, Hua LC, Hao HK. A novel method of delta-shaped intracorporeal double-tract reconstruction in totally laparoscopic proximal gastrectomy. Surg Endosc. 2016;30:2396-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Xu X, Huang C, Mou Y, Zhang R, Pan Y, Chen K, Lu C. Intra-corporeal hand-sewn esophagojejunostomy is a safe and feasible procedure for totally laparoscopic total gastrectomy: short-term outcomes in 100 consecutive patients. Surg Endosc. 2018;32:2689-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Chen K, Wu D, Pan Y, Cai JQ, Yan JF, Chen DW, Maher H, Mou YP. Totally laparoscopic gastrectomy using intracorporeally stapler or hand-sewn anastomosis for gastric cancer: a single-center experience of 478 consecutive cases and outcomes. World J Surg Oncol. 2016;14:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Liu W, Guo Y, Qiu Z, Niu D, Zhang J. Intracorporeal Circular Stapled Esophagojejunostomy Using Conventional Purse-String Suture Instrument After Laparoscopic Total Gastrectomy. J Laparoendosc Adv Surg Tech A. 2017;27:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Usui S, Nagai K, Hiranuma S, Takiguchi N, Matsumoto A, Sanada K. Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument "Endo-PSI (II)" and circular stapler. Gastric Cancer. 2008;11:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Kinoshita T, Oshiro T, Ito K, Shibasaki H, Okazumi S, Katoh R. Intracorporeal circular-stapled esophagojejunostomy using hand-sewn purse-string suture after laparoscopic total gastrectomy. Surg Endosc. 2010;24:2908-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 92. | Du J, Shuang J, Li J, Li J, Hua J. Intracorporeal circular-stapled esophagojejunostomy after laparoscopic total gastrectomy: a novel self-pulling and holding purse-string suture technique. J Am Coll Surg. 2014;218:e67-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc. 2009;23:2624-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 94. | Omori T, Oyama T, Mizutani S, Tori M, Nakajima K, Akamatsu H, Nakahara M, Nishida T. A simple and safe technique for esophagojejunostomy using the hemidouble stapling technique in laparoscopy-assisted total gastrectomy. Am J Surg. 2009;197:e13-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Kosuga T, Hiki N, Nunobe S, Ohashi M, Kubota T, Kamiya S, Sano T, Yamaguchi T. Does the Single-Stapling Technique for Circular-Stapled Esophagojejunostomy Reduce Anastomotic Complications After Laparoscopic Total Gastrectomy? Ann Surg Oncol. 2015;22:3606-3612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Wang H, Hao Q, Wang M, Feng M, Wang F, Kang X, Guan WX. Esophagojejunostomy after laparoscopic total gastrectomy by OrVil™ or hemi-double stapling technique. World J Gastroenterol. 2015;21:8943-8951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Kawamura H, Ohno Y, Ichikawa N, Yoshida T, Homma S, Takahashi M, Taketomi A. Anastomotic complications after laparoscopic total gastrectomy with esophagojejunostomy constructed by circular stapler (OrVil<sup>™</sup>) versus linear stapler (overlap method). Surg Endosc. 2017;31:5175-5182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 98. | Lu X, Hu Y, Liu H, Mou T, Deng Z, Wang D, Yu J, Li G. Short-term outcomes of intracorporeal esophagojejunostomy using the transorally inserted anvil versus extracorporeal circular anastomosis during laparoscopic total gastrectomy for gastric cancer: a propensity score matching analysis. J Surg Res. 2016;200:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Tokuhara T, Nakata E, Tenjo T, Kawai I, Kondo K, Ueda H, Tomioka A. Stenosis after esophagojejunostomy with the hemi-double-stapling technique using the transorally inserted anvil (OrVil™) in Roux-en-Y reconstruction with its efferent loop located on the patient's left side following laparoscopic total gastrectomy. Surg Endosc. 2019;33:2128-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Yasukawa D, Hori T, Kadokawa Y, Kato S, Machimoto T, Hata T, Aisu Y, Sasaki M, Kimura Y, Takamatsu Y, Ito T, Yoshimura T. Impact of stepwise introduction of esophagojejunostomy during laparoscopic total gastrectomy: a single-center experience in Japan. Ann Gastroenterol. 2017;30:564-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol. 2015;21:9656-9665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 102. | Kawaguchi Y, Shiraishi K, Akaike H, Ichikawa D. Current status of laparoscopic total gastrectomy. Ann Gastroenterol Surg. 2018;3:14-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 103. | Li X, Hong L, Ding D, Liu Y, Niu G, Li L, Wang X, Li X, Ke C. Comparison of OrVil™ and RPD in laparoscopic total gastrectomy for gastric cancer. Surg Endosc. 2017;31:4773-4779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 104. | Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 105. | Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010;211:e25-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 106. | Kwon IG, Son YG, Ryu SW. Novel Intracorporeal Esophagojejunostomy Using Linear Staplers During Laparoscopic Total Gastrectomy: π-Shaped Esophagojejunostomy, 3-in-1 Technique. J Am Coll Surg. 2016;223:e25-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Miura S, Kanaya S, Hosogi H, Kawada H, Akagawa S, Shimoike N, Okumura S, Okada T, Ito T, Arimoto A. Esophagojejunostomy With Linear Staplers in Laparoscopic Total Gastrectomy: Experience With 168 Cases in 5 Consecutive Years. Surg Laparosc Endosc Percutan Tech. 2017;27:e101-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Kyogoku N, Ebihara Y, Shichinohe T, Nakamura F, Murakawa K, Morita T, Okushiba S, Hirano S. Circular versus linear stapling in esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer: a propensity score-matched study. Langenbecks Arch Surg. 2018;403:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Gong CS, Kim BS, Kim HS. Comparison of totally laparoscopic total gastrectomy using an endoscopic linear stapler with laparoscopic-assisted total gastrectomy using a circular stapler in patients with gastric cancer: A single-center experience. World J Gastroenterol. 2017;23:8553-8561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 110. | Huang ZN, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Lin JL. Digestive tract reconstruction using isoperistaltic jejunum-later-cut overlap method after totally laparoscopic total gastrectomy for gastric cancer: Short-term outcomes and impact on quality of life. World J Gastroenterol. 2017;23:7129-7138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 111. | Chen K, Pan Y, Cai JQ, Xu XW, Wu D, Yan JF, Chen RG, He Y, Mou YP. Intracorporeal esophagojejunostomy after totally laparoscopic total gastrectomy: A single-center 7-year experience. World J Gastroenterol. 2016;22:3432-3440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Yoshikawa K, Shimada M, Higashijima J, Tokunaga T, Nishi M, Takasu C, Kashihara H, Ishikawa D. Usefulness of the Transoral Anvil Delivery System for Esophagojejunostomy After Laparoscopic Total Gastrectomy: A Single-institution Comparative Study of Transoral Anvil Delivery System and the Overlap Method. Surg Laparosc Endosc Percutan Tech. 2018;28:e40-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Lianos GD, Hasemaki N, Glantzounis GK, Mitsis M, Rausei S. Assessing safety and feasibility of 'pure' laparoscopic total gastrectomy for advanced gastric cancer in the West. Review article. Int J Surg. 2018;53:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Brenkman HJ, Correa-Cote J, Ruurda JP, van Hillegersberg R. A Step-Wise Approach to Total Laparoscopic Gastrectomy with Jejunal Pouch Reconstruction: How and Why We Do It. J Gastrointest Surg. 2016;20:1908-1915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Ali B, Park CH, Song KY. Intracorporeal esophagojejunostomy using hemi-double-stapling technique after laparoscopic total gastrectomy in gastric cancer patients. Ann Surg Treat Res. 2017;92:30-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 116. | Duan W, Liu K, Fu X, Shen X, Chen J, Su C, Yu P, Zhao Y. Semi-end-to-end esophagojejunostomy after laparoscopy-assisted total gastrectomy better reduces stricture and leakage than the conventional end-to-side procedure: A retrospective study. J Surg Oncol. 2017;116:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 117. | Lee TG, Lee IS, Yook JH, Kim BS. Totally laparoscopic total gastrectomy using the overlap method; early outcomes of 50 consecutive cases. Surg Endosc. 2017;31:3186-3190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Son SY, Cui LH, Shin HJ, Byun C, Hur H, Han SU, Cho YK. Modified overlap method using knotless barbed sutures (MOBS) for intracorporeal esophagojejunostomy after totally laparoscopic gastrectomy. Surg Endosc. 2017;31:2697-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Kitagami H, Morimoto M, Nakamura K, Watanabe T, Kurashima Y, Nonoyama K, Watanabe K, Fujihata S, Yasuda A, Yamamoto M, Shimizu Y, Tanaka M. Technique of Roux-en-Y reconstruction using overlap method after laparoscopic total gastrectomy for gastric cancer: 100 consecutively successful cases. Surg Endosc. 2016;30:4086-4091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 120. | Kim EY, Choi HJ, Cho JB, Lee J. Totally Laparoscopic Total Gastrectomy Versus Laparoscopically Assisted Total Gastrectomy for Gastric Cancer. Anticancer Res. 2016;36:1999-2003. [PubMed] |