Published online Sep 15, 2019. doi: 10.4251/wjgo.v11.i9.761
Peer-review started: May 21, 2019
First decision: July 31, 2019
Revised: August 6, 2019
Accepted: August 27, 2019
Article in press: August 28, 2019
Published online: September 15, 2019
Processing time: 119 Days and 23.3 Hours
Bile duct cancer constitutes gallbladder cancer (GBC), intrahepatic cholangiocarcinoma (ICA), and extrahepatic cholangiocarcinoma (ECA). These three entities show morphological and immunohistochemical resemblance so that it is difficult to differentiate between primary ICA and liver metastasis of GBC, which sometimes becomes a point of discussion in clinical practice. Although these cancers demonstrate significant differences in their mutational landscape, several reports demonstrated shared genomic alteration in paired primary and metastatic site aids in distinguishing metastatic recurrence from second primary cancers.
We present a 73-year-old female patient who underwent curative resection for GBC harboring epidermal growth factor receptor 2 (ERBB2) activating mutation on next-generation sequencing (NGS)-based genomic testing. One year later, a hepatic lesion was observed on follow-up imaging and she underwent surgical resection for a pathological diagnosis. The histological findings of the hepatic lesion were similar to those of the primary lesion. Additionally, using NGS panel testing, the hepatic lesion was found to have ERBB2 activating mutation, which is the identical mutation detected in the sequencing result of the primary site. ERBB2 activating mutation occurs more frequently in GBC than ICA and ECA. Therefore, in the present case, we think this molecular finding potentiated the diagnosis of the liver mass toward a metastatic recurrence. Additionally, this patient underwent HER2-targeted treatment with lapatinib in combination with capecitabin and obtained clinical benefit.
This case illustrated NGS panel usefulness in distinguishing GBC recurrence from second primary cancer and HER2-targeted agent efficacy on ERBB2 mutated GBC.
Core tip: We present a case report of a patient with gallbladder cancer (GBC) harboring epidermal growth factor receptor 2 (ERBB2) hotspot extracellular domain mutation (Ser310Phe) on both the primary site and metachronous liver metastasis. Given that pathological differentiation between hepatic metastasis and primary cancer of the liver is often difficult, next-generation sequencing panel could be a novel option for patients who need to distinguish a metastatic lesion from a second malignancy, which would affect staging and the treatment strategy. This case also illustrated the benefit of the HER2-targeted agent in the treatment of GBC harboring ERBB2 activating mutation.
- Citation: Inagaki C, Maeda D, Kimura A, Otsuru T, Iwagami Y, Nishida N, Sakai D, Shitotsuki R, Yachida S, Doki Y, Satoh T. Gallbladder cancer harboring ERBB2 mutation on the primary and metastatic site: A case report. World J Gastrointest Oncol 2019; 11(9): 761-767
- URL: https://www.wjgnet.com/1948-5204/full/v11/i9/761.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i9.761
Gallbladder cancer (GBC) is an uncommon malignancy with an aggressive clinical course. Its prevalence varies among geographical areas and is higher in Asia and the Andes region[1]. GBC constitutes bile duct cancer along with intrahepatic cholangiocarcinoma (ICA) and extrahepatic cholangiocarcinoma (ECA). However, these are distinct entities with significant differences in their mutational landscape[2]. For example, isocitrate dehydrogenase 1 or 2 mutations, breast cancer 1 associated protein-1 mutations, and fibroblast growth factor receptors fusions are frequently seen in ICA, whereas Kirsten rat sarcoma viral oncogene homolog and, mothers against decapentaplegic homolog 4 mutations are more likely to occur in ECA. However, GBC has a high frequency of avian erythroblastosis oncogene B2 (ERBB2), transformation-related protein 53 (TP53), and cyclin-dependent kinase inhibitor 2A mutations.
Generally, surgical resection is the only curative option for localized GBC, and chemotherapy is the primary treatment for unresectable or recurrent disease. Despite recent advances in the treatments, more than half of the patients have experienced a recurrence after radical resection and prognosis of metastatic disease is very poor with 5-year survival around 5%[3,4]. The liver is the most common site of recurrence in GBC[4]. The histological differentiation between primary ICA and liver metastasis of GBC is often difficult owing to morphological and immunohistochemical resemblance, whereas the distinction between the metastasis of the primary malignancy and newly developed second primary malignancy is clinically important for accurate staging and tailoring treatment strategies[5]. Reflecting recent technical advances in high-throughput next-generation sequencing, there are several reports describing the utility of genomic profiling in the differential diagnosis of a metastatic recurrence and distinguishing it from second primary malignancy[6].
Here, we present a case report of a patient with GBC harboring ERBB2 activating mutation on both the primary site and metachronous liver metastasis, which aids in the differentiation from secondary malignancy. Additionally, this patient was treated with human epidermal growth factor receptor-2 (HER2)-targeted agent, lapatinib, and achieved clinical benefits.
The patient was a previously healthy 73-year-old female who underwent curative resection for GBC (pT2N0M0 according to the eighth International union against cancer TNM classification). We performed next-generation sequencing (NGS)-based genomic profiling of the resected specimen using the NGS gene panel, Oncomine® Comprehensive Assay version 3 (OCA v.3, Thermo Fisher Scientific), which revealed ERBB2 Ser310Phe (c.929C>T; VAF, 18%) and TP53 Ser241Tyr (c.722C>A; VAF, 19%) mutations. One year later, a hepatic lesion was observed on follow-up imaging and she underwent surgical total biopsy for a pathological diagnosis.
A patient had no symptoms and was in good health at the time of total biopsy.
The patient had no previous medical history.
The patient’s physical examination was not remarkable and laboratory testing was within normal limits, including tumor markers, such as CA19-9 and CEA.
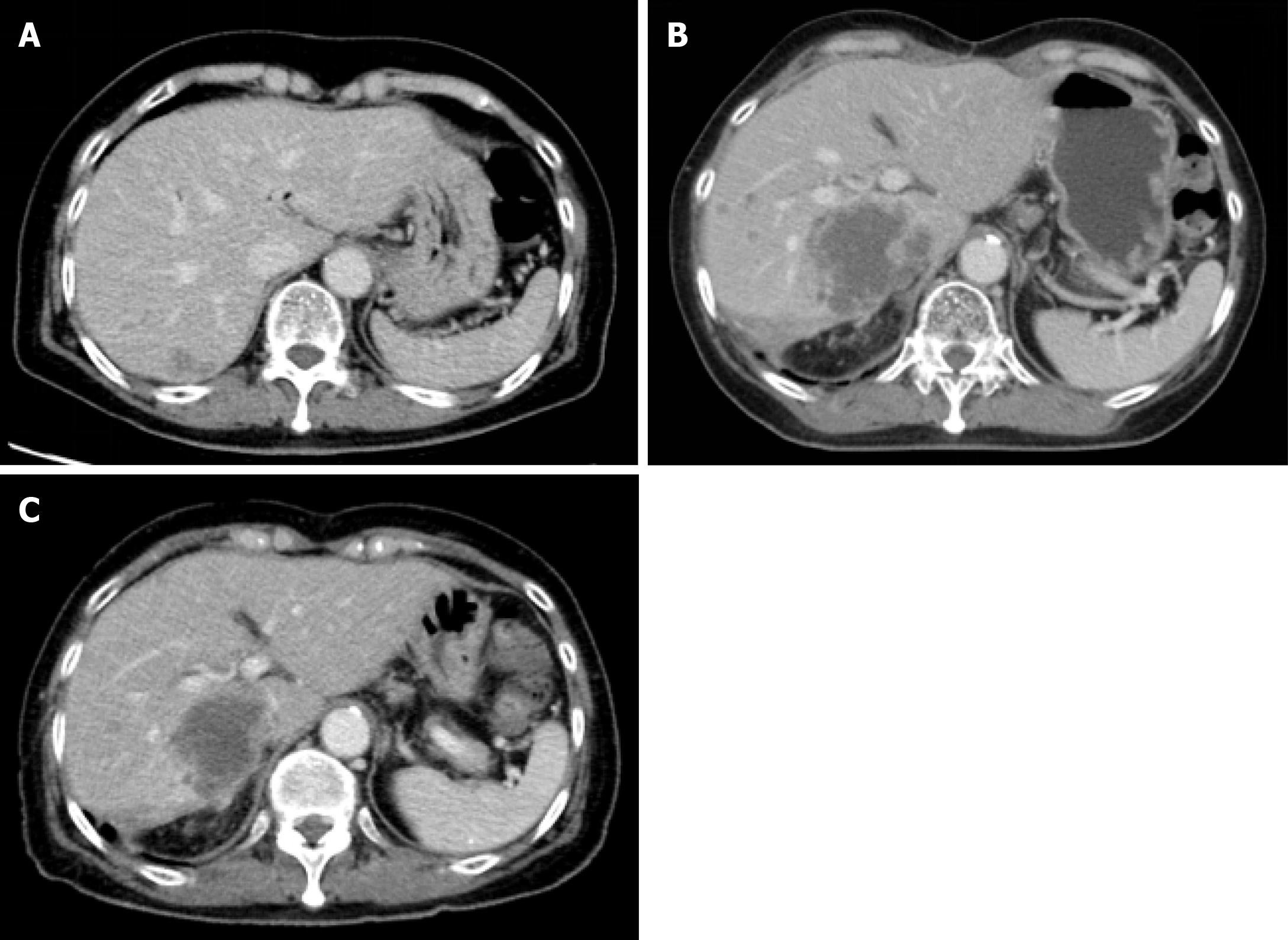
Contrasted computed tomography (CT) showed an ill-defined low attenuation lesion in the posterior lobe of the liver (Figure 1).
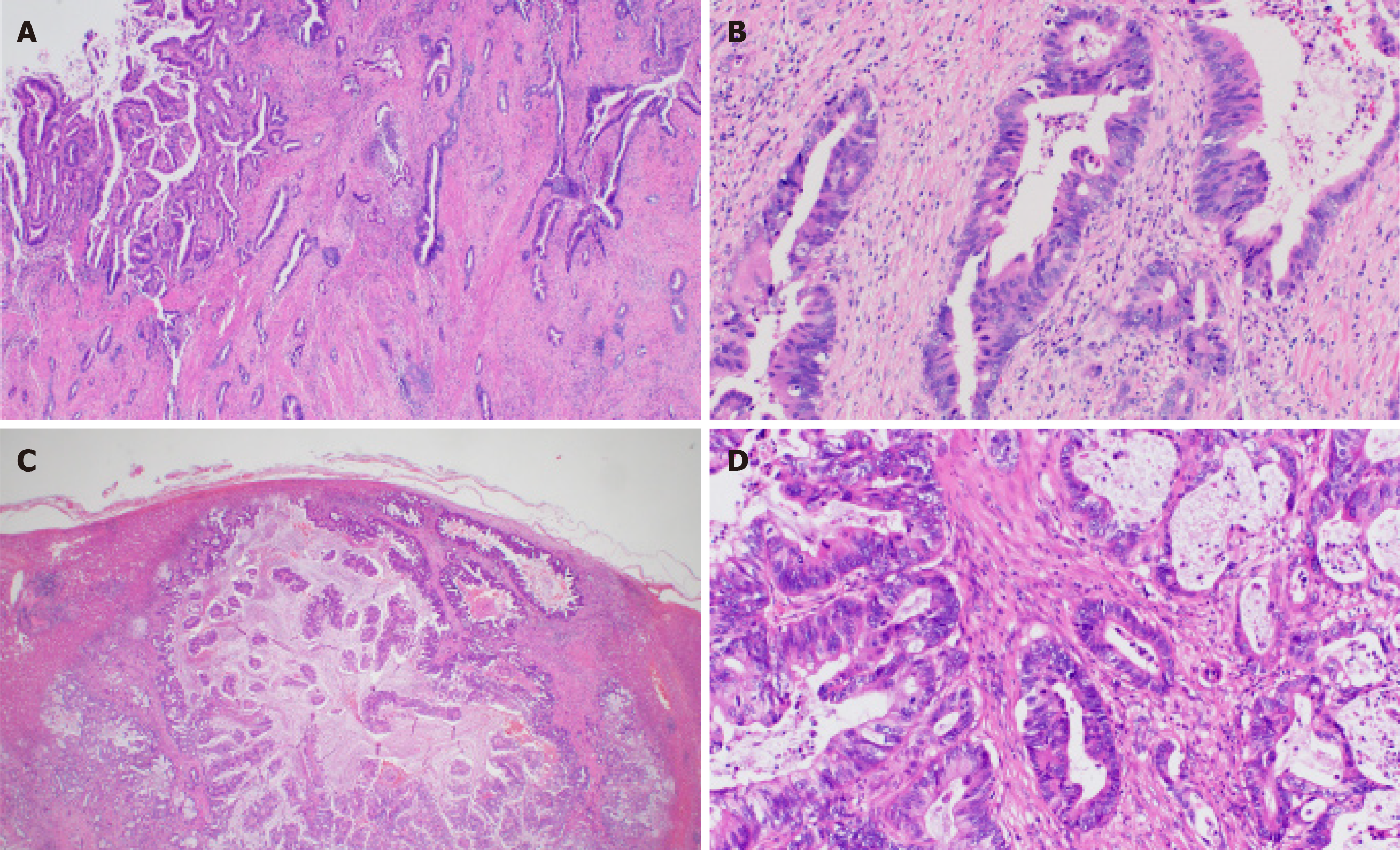
The hepatic lesion was histologically diagnosed as well-differentiated adenocarcinoma and the histological findings of the hepatic lesion were similar to those of GBC (Figure 2). Therefore, the lesion was considered a metastasis. Moreover, we performed genomic profiling from the liver tumor using the NGS panel, Oncomine® Target Test system (OTT, Thermo Fisher Scientific). This revealed ERBB2 Ser310Phe (c.929C>T; VAF, 26%), which was identical to the mutation detected in the sequencing result of the primary site; thus, the liver tumor was the most consistent with a metastasis of GBC rather than localized ICC. To evaluate HER2 overexpression in tumor cells, we performed immunohistochemistry of HER2, which was negative (HER2 score 0). Since TP53 was not included in the gene list of OTTs, TP53 mutation status at the metastatic site was not assessed.
The final diagnosis of the presented case is hepatic recurrence of GBC.
After the total biopsy of liver metastasis, she was treated with two standard chemotherapy regimens, namely gemcitabine and cisplatin, and TS-1; however, her disease did not obtain clinical benefit from these treatments. After six months from hepatic resection, she was confirmed to have a progressive disease during second-line chemotherapy. At that time, she had liver and pulmonary recurrence, as well as pulmonary and inferior vena cava tumor embolism, which caused tachycardia and peripheral edema.
Considering no standard treatment beyond second-line for GBC, we treated the patient with lapatinib with a combination of capecitabine (lapatinib at a dose of 1250 mg per day continuously plus capecitabine at a dose of 2000 mg per square meter of body-surface area on days 1 through 14 of a 21 d cycle) based on the accumulating preclinical and clinical evidence that tumors with ERBB2 mutation benefit from HER2-targeted treatment.
Within a week of treatment, she experienced major subjective clinical improvement, which included resolution of peripheral edema. After 2 cycles of treatment, contrasted CT imaging showed a decrease in the size of tumor emboli and hepatic lesions (Figure 1). However, after 4 cycles of treatment, the patient discontinued treatment due to grade 3 mucositis. Mucositis was gradually subsided over two weeks after discontinuation of the treatment. One month after discontinuation, her disease progressed, and she chose best supportive care.
We observed the same ERBB2 Ser310Phe mutation in the primary tumors, as well as the metachronous hepatic lesion of this patient using NGS panels. We believe this molecular finding potentiated the diagnosis of the liver mass toward a metastatic recurrence. In addition, this patient exhibited a favorable effect of the HER2-targeted agent on GBC with ERBB2 activating mutation.
Histologically, metastatic adenocarcinoma of the biliary tract cannot be distinguished from ICA or pancreatic origin owing to similarities in appearance and immunohistochemical staining patterns[5]. In addition, there is no particular method established to assess genetic relationships and clonality in primary and metastatic sites in malignancy. However, limited studies have shown the potential of genetic profiling to distinguish between a metastatic recurrence of the primary cancer and newly developed second primary cancer in several malignancies[6-8]. Previous reports demonstrated shared genomic alteration in paired primary and metastatic sites was useful in differentiating multifocal non-small cell lung cancer from intrapulmonary metastasis[7,8]. Moreover, Vignot et al[9] showed that genomic profiles of the first metastatic recurrent sites are highly concordant to the primary site in colorectal cancer. In the present case, the primary site and hepatic lesion shared the identical ERBB2 mutation. ERBB2 mutations are relatively frequent (9%-10%) in GBC, in contrast to ICA, as shown in previous studies[10-12]. Currently, there are no reports evaluating the concordance between primary and metastatic sites in CA; however, we considered this molecular finding supported the diagnosis of metastatic recurrence rather than the primary carcinoma of liver origin.
Additionally, ERBB2 Ser310Phe is a known activating hotspot mutation in the extracellular domain[13]. The growing body of preclinical evidence and early phase trials supports HER2-targeted therapy for cancers harboring ERBB2 activating mutations, whereas standardized molecular treatment has not been determined for this population[14-16]. Furthermore, the efficacy of HER2-targeted treatment on ERBB2-mutated tumors seems to vary between tumor types and mutation loci. Neratinib is an irreversible pan-HER tyrosine kinase inhibitor and clinical efficacy of neratinib for various ERBB2-mutated cancers was evaluated in the basket trial[17]. Neratinib exhibited the greatest activity in patients with breast cancer [Overall response rate; ORR 32% (8/25)], followed by biliary tract cancer [ORR 22.2% (2/9)). When stratified by a mutant allele, response was greatest in patients with kinase domain hotspot mutation [ORR 21.4% (9/42)], followed by Ser310 mutation [ORR 10% (3/30)] and exon 20 insertion mutation [ORR 7.1% (2/28)]. Among two biliary tract cancer patients with ERBB2 Ser310 mutation included in this trial, one patient responded to neratinib. Ado-trastuzumab emtansine, a HER2-targeted antibody-drug conjugate linking trastuzumab with emtansine, demonstrated ORR of 44% (8/18) for patients with lung cancer harboring ERBB2 mutation, including Ser310, in phase II basket trial; however, to the best of our knowledge, there are no reports evaluating its benefit for GBC with ERBB2 mutation[18]. Javle et al[19] reported a case series of biliary tract cancer harboring ERBB2 mutations. In this report, one cholangial cancer patient with ERBB2 Ser310 mutation treated with trastuzumab, a humanized monoclonal antibody directed to HER2, in combination with FOLFOX, was not effective. Our patient obtained clinical benefit from lapatinib and capecitabin combination treatment. Lapatinib is a dual tyrosine kinase inhibitor that targets epidermal growth factor receptor and HER2 and the combination treatment of lapatinib and capecitabin was evaluated initially in HER2 positive breast cancer patients and showed prolonged survival with tolerable toxicity[20]. A previous case report indicated substantial efficacy of this combination treatment in a patient with metastatic extramammary Paget’s disease harboring ERBB2 Ser310 mutation[21]. Given that TS-1 monotherapy, which is oral fluoropyrimidine as with capecitabin, was prescribed as second line treatment and was not effective to this patient, modest benefit from this combination treatment would be attributed to lapatinib. As both lapatinib and capecitabin are off-label use in Japan for patients with cholangiocarcinoma, we prescribed these agents following patients’ written consent.
This case highlighted the usefulness of NGS panels in distinguishing hepatic metastasis from primary cancer of the liver, which sometimes becomes a point of discussion in daily practice. Although we need a large cohort for verification, NGS panel may be a novel option for patients who need to distinguish a metastatic lesion from a second malignancy, which would affect staging and treatment strategies.
This case also illustrated the value of lapatinib in combination with capecitabine in the treatment of GBC harboring ERBB2 activating mutation. We recognized HER2-targeted agent as a potential treatment for ERBB2 mutated tumors. Further investigation of HER2-targeted agent in this population is warranted.
We thank SCRUM-Japan, nation-wide cancer genome screening and patient registry program, which provided genomic tests free of charge using the NSG panel OCA v.3 for the participants, including this patient.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tajiri K, Barreto SG S-Editor: Zhang L L-Editor: A E-Editor: Qi LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Fujimoto A, Furuta M, Totoki Y, Tsunoda T, Kato M, Shiraishi Y, Tanaka H, Taniguchi H, Kawakami Y, Ueno M, Gotoh K, Ariizumi S, Wardell CP, Hayami S, Nakamura T, Aikata H, Arihiro K, Boroevich KA, Abe T, Nakano K, Maejima K, Sasaki-Oku A, Ohsawa A, Shibuya T, Nakamura H, Hama N, Hosoda F, Arai Y, Ohashi S, Urushidate T, Nagae G, Yamamoto S, Ueda H, Tatsuno K, Ojima H, Hiraoka N, Okusaka T, Kubo M, Marubashi S, Yamada T, Hirano S, Yamamoto M, Ohdan H, Shimada K, Ishikawa O, Yamaue H, Chayama K, Miyano S, Aburatani H, Shibata T, Nakagawa H. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet. 2016;48:500-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 536] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 3. | Takahashi Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Nimura Y, Nagino M. Surgery for Recurrent Biliary Tract Cancer: A Single-center Experience With 74 Consecutive Resections. Ann Surg. 2015;262:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Boutros C, Gary M, Baldwin K, Somasundar P. Gallbladder cancer: past, present and an uncertain future. Surg Oncol. 2012;21:e183-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Shimonishi T, Miyazaki K, Nakanuma Y. Cytokeratin profile relates to histological subtypes and intrahepatic location of intrahepatic cholangiocarcinoma and primary sites of metastatic adenocarcinoma of liver. Histopathology. 2000;37:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Weinberg BA, Gowen K, Lee TK, Ou SI, Bristow R, Krill L, Almira-Suarez MI, Ali SM, Miller VA, Liu SV, Klempner SJ. Comprehensive Genomic Profiling Aids in Distinguishing Metastatic Recurrence from Second Primary Cancers. Oncologist. 2017;22:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Klempner SJ, Ou SH, Costa DB, VanderLaan PA, Sanford EM, Schrock A, Gay L, Ali SM, Miller VA. The Clinical Use of Genomic Profiling to Distinguish Intrapulmonary Metastases From Synchronous Primaries in Non-Small-Cell Lung Cancer: A Mini-Review. Clin Lung Cancer. 2015;16:334-339.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Patel SB, Kadi W, Walts AE, Marchevsky AM, Pao A, Aguiluz A, Mudalige T, Liu Z, Deng N, Lopategui J. Next-Generation Sequencing: A Novel Approach to Distinguish Multifocal Primary Lung Adenocarcinomas from Intrapulmonary Metastases. J Mol Diagn. 2017;19:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Vignot S, Lefebvre C, Frampton GM, Meurice G, Yelensky R, Palmer G, Capron F, Lazar V, Hannoun L, Miller VA, André F, Stephens PJ, Soria JC, Spano JP. Comparative analysis of primary tumour and matched metastases in colorectal cancer patients: evaluation of concordance between genomic and transcriptional profiles. Eur J Cancer. 2015;51:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, Offerhaus GJA, Roa JC, Roberts LR, Gores GJ, Popescu I, Alexandrescu ST, Dima S, Fassan M, Simbolo M, Mafficini A, Capelli P, Lawlor RT, Ruzzenente A, Guglielmi A, Tortora G, de Braud F, Scarpa A, Jarnagin W, Klimstra D, Karchin R, Velculescu VE, Hruban RH, Vogelstein B, Kinzler KW, Papadopoulos N, Wood LD. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 11. | Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, Liu C, Shen B, Wang XA, Wu W, Zhou D, Zhang D, Wang T, Liu B, Qu K, Ding Q, Weng H, Ding Q, Mu J, Shu Y, Bao R, Cao Y, Chen P, Liu T, Jiang L, Hu Y, Dong P, Gu J, Lu W, Shi W, Lu J, Gong W, Tang Z, Zhang Y, Wang X, Chin YE, Weng X, Zhang H, Tang W, Zheng Y, He L, Wang H, Liu Y, Liu Y. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014;46:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 12. | Ueno M, Morizane C, Kawamoto Y, Takahashi H, Naruge D, Shimizu S, Nakamura K, Nakajima TE, Kato T, Kudo T, Mizuno N, Ohtsubo K, Itoh S, Ishii H, Sudo T, Nomura S, Fujii S, Shitara K, Ohtsu A, Yoshino T. 716P The nationwide cancer genome screening project in Japan, SCRUM-Japan GI-screen: Efficient identification of cancer genome alterations in advanced biliary tract cancer 2017. Ann Oncol. 2017;28:v209-v268. |
| 13. | Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, Zhang X, Lee SH, Cho J, Ambrogio L, Liao R, Imielinski M, Banerji S, Berger AH, Lawrence MS, Zhang J, Pho NH, Walker SR, Winckler W, Getz G, Frank D, Hahn WC, Eck MJ, Mani DR, Jaffe JD, Carr SA, Wong KK, Meyerson M. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109:14476-14481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Connell CM, Doherty GJ. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open. 2017;2:e000279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Iyer P, Shrikhande SV, Ranjan M, Joshi A, Gardi N, Prasad R, Dharavath B, Thorat R, Salunkhe S, Sahoo B, Chandrani P, Kore H, Mohanty B, Chaudhari V, Choughule A, Kawle D, Chaudhari P, Ingle A, Banavali S, Gera P, Ramadwar MR, Prabhash K, Barreto SG, Dutt S, Dutt A. ERBB2 and KRAS alterations mediate response to EGFR inhibitors in early stage gallbladder cancer. Int J Cancer. 2019;144:2008-2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Barreto SG, Dutt A, Chaudhary A. A genetic model for gallbladder carcinogenesis and its dissemination. Ann Oncol. 2014;25:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, Juric D, Quinn DI, Moreno V, Doger B, Mayer IA, Boni V, Calvo E, Loi S, Lockhart AC, Erinjeri JP, Scaltriti M, Ulaner GA, Patel J, Tang J, Beer H, Selcuklu SD, Hanrahan AJ, Bouvier N, Melcer M, Murali R, Schram AM, Smyth LM, Jhaveri K, Li BT, Drilon A, Harding JJ, Iyer G, Taylor BS, Berger MF, Cutler RE, Xu F, Butturini A, Eli LD, Mann G, Farrell C, Lalani AS, Bryce RP, Arteaga CL, Meric-Bernstam F, Baselga J, Solit DB. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554:189-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 600] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 18. | Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, Ulaner GA, Offin M, Feldman D, Hembrough T, Cecchi F, Schwartz S, Pavlakis N, Clarke S, Won HH, Brzostowski EB, Riely GJ, Solit DB, Hyman DM, Drilon A, Rudin CM, Berger MF, Baselga J, Scaltriti M, Arcila ME, Kris MG. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol. 2018;36:2532-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 401] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 19. | Javle M, Churi C, Kang HC, Shroff R, Janku F, Surapaneni R, Zuo M, Barrera C, Alshamsi H, Krishnan S, Mishra L, Wolff RA, Kaseb AO, Thomas MB, Siegel AB. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol. 2015;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15:924-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Vornicova O, Hershkovitz D, Yablonski-Peretz T, Ben-Itzhak O, Keidar Z, Bar-Sela G. Treatment of metastatic extramammary Paget's disease associated with adnexal adenocarcinoma, with anti-HER2 drugs based on genomic alteration ERBB2 S310F. Oncologist. 2014;19:1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |