Published online Jun 15, 2019. doi: 10.4251/wjgo.v11.i6.499
Peer-review started: January 9, 2019
First decision: January 18, 2019
Revised: February 27, 2019
Accepted: March 12, 2019
Article in press: March 13, 2019
Published online: June 15, 2019
Processing time: 157 Days and 1.5 Hours
Radical D2 lymphadenectomy for advanced gastric cancer as a standard procedure has gained global consensus. Mounting studies have shown that the number of lymph nodes dissection directly affects the prognosis and recurrence of gastric cancer. Our previous study showed that there was no obvious lymph node around the abnormal hepatic artery derived from the superior mesenteric artery.
To investigate the relationship between celiac artery variation and the number of lymph nodes dissection in gastric cancer surgery.
The clinicopathological data of 421 patients treated with radical D2 lymphadenectomy were analyzed retrospectively. The difference of the number of lymph nodes dissection between the celiac artery variation group and the normal vessels group and the relationship with prognosis were analyzed.
Celiac artery variation was found in 110 patients, with a variation rate of 26.13%. Celiac artery variation, tumor staging, and Borrmann typing were factors that affected lymph node clearance in gastric cancer, and the number of lymph nodes dissection in patients with celiac artery variation was significantly less than that of non-variant groups (P < 0.05). Univariate analysis showed that there was no significant difference in survival time between the two groups (P > 0.05). Univariate and multiple Cox regression analysis showed that celiac artery variation was not a prognostic factor for gastric cancer (P > 0.05). Tumor staging, intraoperative bleeding, and positive lymph node ratio were prognostic factors for gastric cancer patients (all P < 0.05).
The number of lymph nodes dissection in patients with celiac artery variation was reduced, but there was no obvious effect on prognosis. Therefore, lymph nodes around the abnormal hepatic artery may not need to be dissected in radical D2 lymphadenectomy.
Core tip:Celiac artery variation has been given great importance by surgeons. However, the distribution of the lymph nodes around the variant celiac artery and its effect on prognosis has rarely been examined. This study shows that variation of the celiac artery is an important factor affecting the lymph node clearance of gastric cancer, and the decrease in the number of lymph nodes dissection does not affect the prognosis. Therefore, lymph nodes dissection around abnormal hepatic artery, especial for the abnormal hepatic artery derived from the superior mesenteric artery, is not recommended.
- Citation: Mu GC, Huang Y, Liu ZM, Chen ZB, Wu XH, Qin XG, Zeng YJ. Relationship between celiac artery variation and number of lymph nodes dissection in gastric cancer surgery. World J Gastrointest Oncol 2019; 11(6): 499-508
- URL: https://www.wjgnet.com/1948-5204/full/v11/i6/499.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i6.499
Gastric cancer is the fifth most common malignant tumor, and its mortality rate ranks second in the world. In 2015 alone, 679,000 new cases were estimated in China, and about 498,000 patients died of gastric cancer[1]. Radical D2 lymphadenectomy as a standard procedure has gained global consensus. The current seventh edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/ AJCC) tumor, node, and metastases (TNM) staging for gastric cancer recommended that at least 16 or more lymph nodes should be dissected for satisfactory histological examination[2]. A German multicenter study showed that clearance of more than 25 lymph nodes was an independent prognostic factor for all types of pathological staging[3]. Studies showed that the number of lymph nodes dissection directly affected the prognosis and recurrence of gastric cancer[4].
According to our previous results[5], there was no obvious lymph node around the abnormal hepatic artery derived from the superior mesenteric artery, and the relationship was unclear between the total number of lymph nodes dissection and the impact on the prognosis in celiac artery variation patients. In addition, Arifuzzamanet al[6] found that the celiac artery variation rate was 30.9%, suggesting that it may be important clinically to investigate the difference in the number of lymph nodes dissection in patients with celiac artery variation and those with normal vessels. These findings could provide clinical basis for precise individualized lymph nodes dissection of gastric cancer.
Four hundred and fifty-two gastric cancer patients who underwent D2 lymphade-nectomy that was performed by the same surgical team at the Department of Gastrointestinal Surgery of the First Affiliated Hospital of Guangxi Medical University from January 2009 to March 2017 were included in this study and screened according to the following criteria: (1) Preoperative gastroscopic pathology was gastric carcinoma; (2) Patient underwent upper abdominal multi-slice spiral computed tomography angiography(MSCTA) examination; (3) Preoperative comprehensive evaluations indicated D2 lymphadenectomy; (4) Patient was adopted for D2 lymphadenectomy. For the patients with vascular variations, we carefully dissected and cleaned the surrounding lymph nodes during the operation; (5) Complete clinical and pathological data were recorded; (6) No neoadjuvant therapy was accepted; and (7) Excluding other malignant tumors. Finally, 421 cases satisfied the conditions mentioned above. Three hundred and six cases (72.7%) were men, and the median age was 56.1 years old (19-86 years old). After the surgery, all the patients were staged according to the 7th AJCC TNM staging standard: IA stage-49 cases, IB stage-41 cases, IIA stage-47 cases, IIB stage-62 cases, IIIA stage-52 cases, IIIB stage-56 cases, and 114 in stage IIIC. This study was approved by ethical review committee at 27 August 2018 [Approval number: 2018(KY-E-056)].
Inspection equipment (LightspeedVCT) was provided by the American GE company. The preparation, scanning parameters, and data and image processing of the CT scan have been detailed in the literature[7]. After image reconstruction, two senior radiologists analyzed the reconstructed three-dimensional vascular images and observed whether celiac artery variation was present.
The 421 gastric cancer patients underwent standard radical gastrectomy by the same experienced surgical team. Lymph node clearance was carried out according to the requirements of the Japanese Gastric Cancer Association protocol. The range of lymph nodes dissection and gastrectomy were performed in accordance with the requirements of the Japanese Gastric Cancer Association guidelines[8]. The lymph nodes in each group were selected by the senior resident who participated in the operation, in accordance with the regulations of the Japanese gastric cancer protocol at the end of the operation. After the operation, additional sorting was carried out with touch method, and the lymph nodes extracted in the operation were sent for pathological examination.
The standard follow-up protocol for patients with gastric cancer was every 3 mo for at least 2 years, every 6 mo for the next 3 years, and every 12 mo after five years for life.The follow-up items included physical examination, tumor markers, computed tomographic scan, and gastroscopy. Follow-up deadline was to 31 May 2018, and the survival time was calculated from operation time to death or follow-up deadline. Sixteen cases were lost midway during follow-up, and the loss rate was 3.8%. The patients were followed up for 12.0-112.0 mo, and the median follow-up period was 42.6 mo.
SPSS 16 statistical software (Chicago, IL, United States) was used to analyze the data. The count data was compared by χ2 test. Two independent samples t test or one-way analysis of variance were used to analyze normal distribution data. Survival analysis was performed by Kaplan-Meier method, and survival rate was compared by Log-rank. Univariate and multivariate Cox regression analysis was used to analyze the survival of gastric cancer. The difference was statistically significant if P < 0.05.
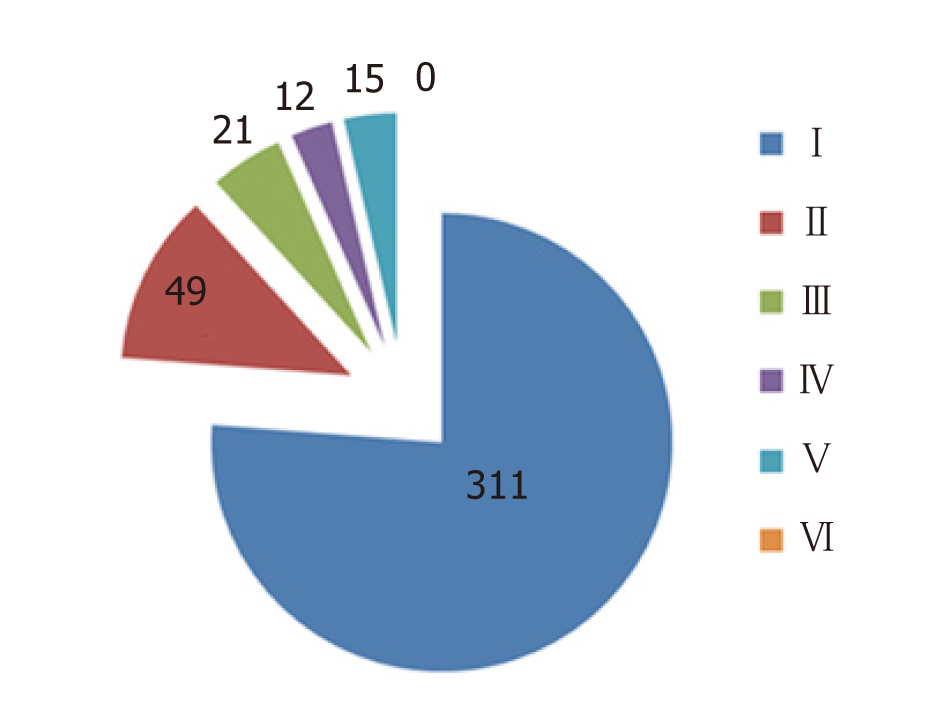
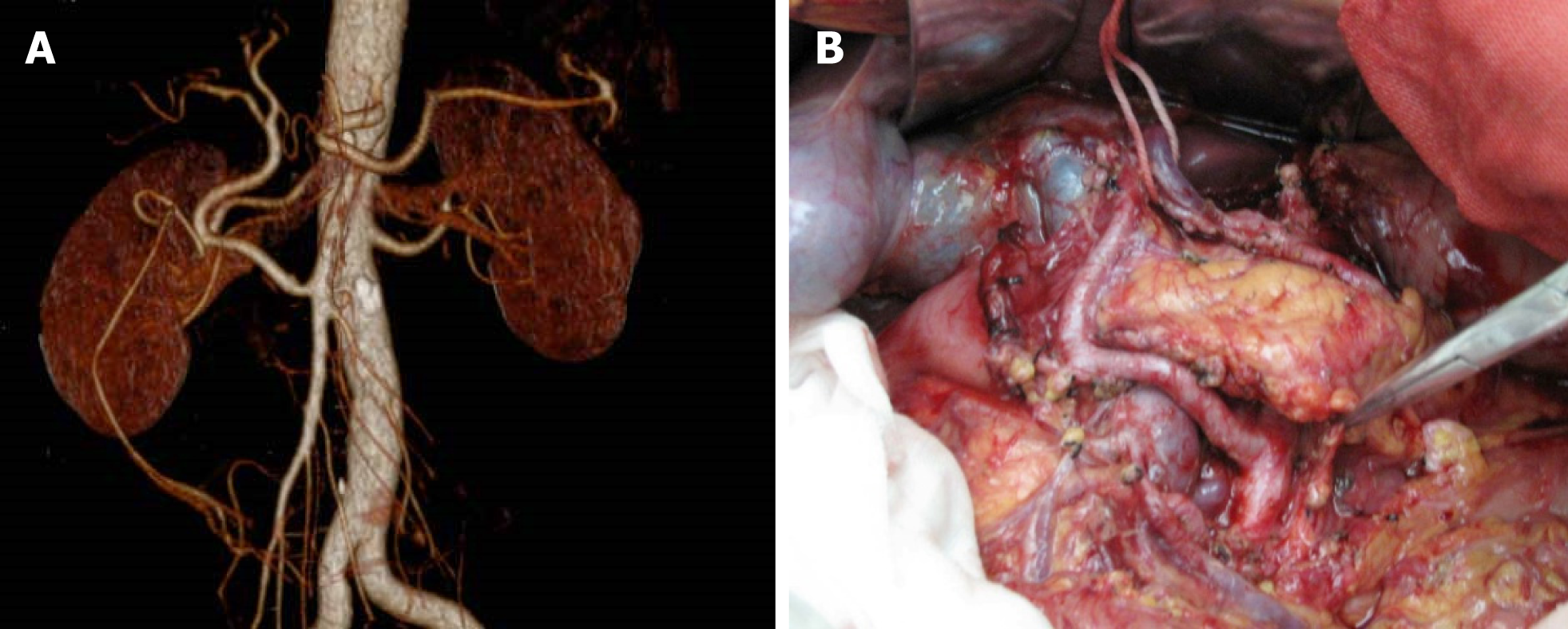
The preoperative MSCTA images showed 311 cases of normal celiac artery, 110 cases of variant celiac artery, and the variation rate was 26.13%. Celiac artery types in all 421 cases detected by preoperative MSCTA were conformed intraoperatively. Ninety-seven cases had an abnormal hepatic artery and were classified according to Hiatt’s standard[9] (Figure 1). Among them, abnormal hepatic artery derived from superior mesenteric artery was seen in 48 cases, the hepatic artery ran in front of the pancreas in two cases(Figure 2) and behind the pancreas in 46 cases. In the post-pancreas type, the hepatic artery arising from the superior mesenteric artery ran behind the pancreatic neck and the initial segment of the portal vein. Then, it ran behind the right hepatic duct and entered the liver ligament.
The left gastric artery derived from the abdominal aorta in eight cases, the splenic artery derived from the superior mesenteric artery in two cases, and in three cases, the celiac trunk and the superior mesenteric artery with a common trunk derived from the abdominal aorta directly.
In total, 2243 lymph nodes were dissected in 110 cases of celiac artery variation, with an average of 20.4 (4-50)/case, 671 positive lymph nodes, and 6.1 average lymph node metastases. The total number of lymph nodes detection in 311 cases without vascular variation was 7373, with an average of 23.7 (3-70/case), 1663 positive lymph nodes, and 5.3 average lymph node metastases. In general, the number of lymph nodes dissection in patients with celiac artery variation was significantly less than that in patients without celiac artery variation (P = 0.000). In stage I and II, there was significant difference between the two groups of lymph node clearance (P = 0.000),but there was no significant difference in stage III (P = 0.229). There was no significant difference in age, sex, tumor location, tumor stage, pathological type, Borrmann typing, or adjuvant chemotherapy between the two groups (P > 0.05) (Table 1).
| Clinicopathological features | Abnormal vessel group, n = 110 | Normal vessel group, n = 311 | χ2 | P value |
| Gender | ||||
| male | 74 | 232 | 2.196 | 0.138 |
| female | 36 | 79 | ||
| Agein yr | ||||
| ≤45 | 23 | 54 | 0.881 | 0.644 |
| 46-60 | 46 | 143 | ||
| >60 | 41 | 114 | ||
| Tumor stage | ||||
| I | 24 | 66 | 2.848 | 0.241 |
| II | 22 | 87 | ||
| III | 64 | 158 | ||
| Tumor location | ||||
| Proximal stomach | 13 | 38 | 0.977 | 0.802 |
| Gastric body | 14 | 45 | ||
| Gastric antrum | 73 | 208 | ||
| Whole stomach | 10 | 20 | ||
| Pathology classification | ||||
| Adenocarcinoma | 99 | 270 | 2.489 | 0.477 |
| Mucous carcinoma | 5 | 10 | ||
| Signet ring cell carcinoma | 2 | 13 | ||
| Undifferentiated carcinoma | 4 | 18 | ||
| Borrmann classification | ||||
| I | 8 | 11 | 3.830 | 0.280 |
| II | 31 | 75 | ||
| III | 53 | 164 | ||
| IV | 18 | 61 | ||
| Adjuvant chemotherapy | ||||
| Yes | 79 | 217 | 0.163 | 0.687 |
| No | 31 | 94 | ||
| Complication | ||||
| Yes | 18 | 29 | 4.059 | 0.044 |
| No | 92 | 282 | ||
| Lymph node clearance | 20.391 ± 0.693 | 23.707 ± 0.587 | 3.651 | 0.000 |
| Lymph node clearance in different stages | ||||
| I | 13.917 ± 0.558 | 20.470 ± 1.010 | 3.822 | 0.000 |
| II | 17.863 ± 0.728 | 23.402 ± 1.095 | 4.231 | 0.000 |
| III | 23.688 ± 0.932 | 25.228 ± 0.871 | 1.207 | 0.229 |
The number of lymph nodes dissection in radical D2 lymphadenectomy for gastric cancer was not only related to the variation of celiac artery but also affected by late tumor stage and high Borrmann typing (P < 0.05)and was not affected by sex, age, tumor location, and pathological type (all P > 0.05) (Table 2).
| Influence factors | n | mean ± S | SS | MS | t or F value | P value |
| Gender | ||||||
| Male | 306 | 22.869 ± 0.573 | - | - | 0.097 | 0.922 |
| Female | 115 | 22.765 ± 0.839 | ||||
| Agein yr | ||||||
| ≤ 45 | 77 | 23.091 ± 1.313 | 15.536 | 7.768 | 0.081 | 0.922 |
| 46-60 | 189 | 22.937 ± 0.625 | ||||
| > 60 | 155 | 22.600 ± 0.817 | ||||
| Variation of celiac artery | ||||||
| Yes | 110 | 20.391 ± 0.693 | - | - | 3.651 | 0.000 |
| No | 311 | 23.707 ± 0.587 | ||||
| Tumor stage | ||||||
| I | 90 | 18.722 ± 0.814 | - | - | 13.365 | 0.000 |
| II | 109 | 22.284 ± 0.910 | ||||
| III | 222 | 24.784 ± 0.676 | ||||
| Tumor location | ||||||
| Proximal stomach | 51 | 21.588 ± 1.273 | 510.539 | 170.180 | 1.801 | 0.146 |
| Gastric body | 59 | 21.509 ± 1.199 | ||||
| Gastric antrum | 281 | 23.000 ± 0.576 | ||||
| Whole stomach | 30 | 26.100 ± 2.207 | ||||
| Pathology classification | ||||||
| Adenocarcinoma | 369 | 22.846 ± 0.511 | 54.376 | 18.125 | 0.190 | 0.903 |
| Mucous carcinoma | 15 | 24.333 ± 2.656 | ||||
| Signet ring cell carcinoma | 15 | 21.733 ± 1.963 | ||||
| Undifferentiated carcinoma | 22 | 22.500 ± 2.123 | ||||
| Borrmann classification | ||||||
| I | 19 | 17.053 ± 1.733 | 2492.710 | 830.903 | 9.261 | 0.000 |
| II | 105 | 20.171 ± 0.842 | ||||
| III | 218 | 23.303 ± 0.655 | ||||
| IV | 79 | 26.506 ± 1.165 |
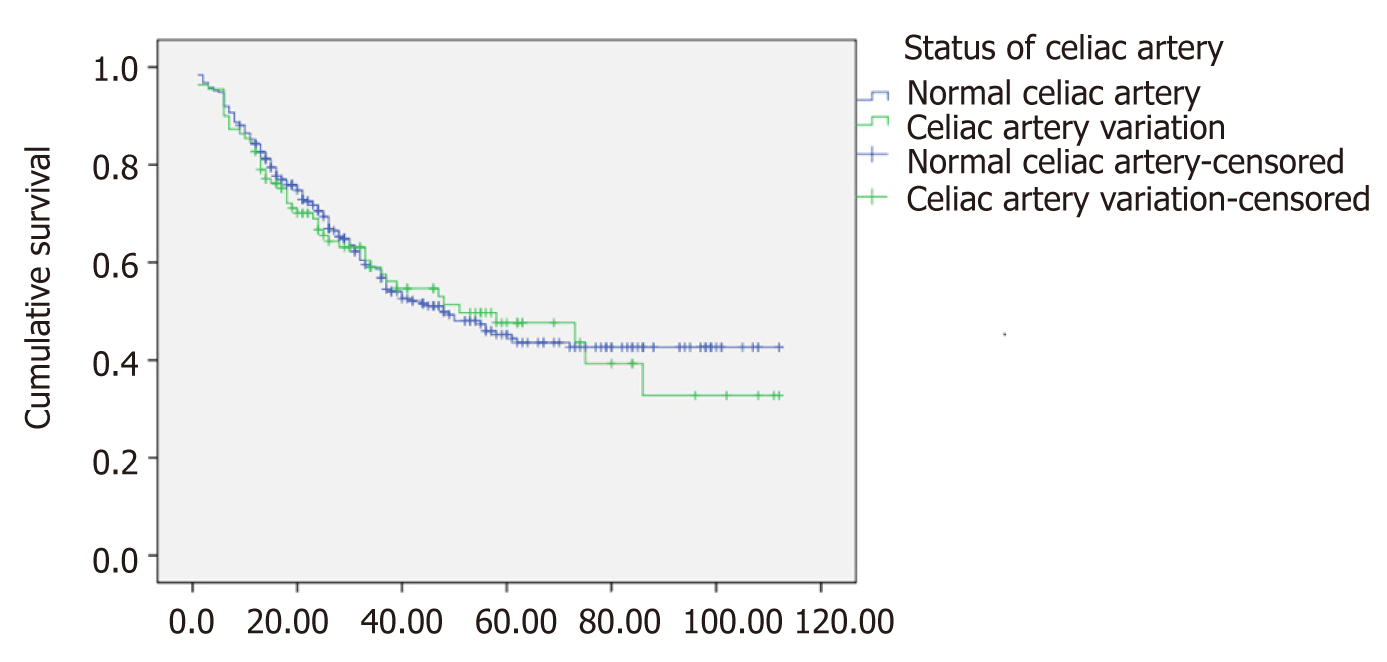
The survival rate at 1, 3, and 5 years in the celiac artery variation group was 84.5%, 57.6%, and 47.6%, respectively. The survival rate at 1, 3, and 5 years in the non-variation group was 85.2%, 56.8%, and 45.2%, respectively. There was no statistical difference in the survival time between the two groups (χ2 = 0.056, P = 0.813) (Figure 3).
The age, sex, tumor staging, tumor location, pathological type, Borrmann typing, number of lymph nodes, lymph node metastasis, positive lymph node ratio, celiac artery variation, operation time, and intraoperative bleeding amount and postoperative survival of gastric cancer patients were analyzed by univariate Cox regression analysis. The results showed that tumor staging, tumor location, Borrmann typing, operation time, intraoperative bleeding amount, number of lymph nodes dissection, number of lymph node metastases, and positive lymph nodes ratio were prognostic factors of gastric cancer(Table 3). Variables of statistical significance in univariate Cox regression analysis were introduced into the multivariate Cox regression analysis equation (Forward: LR method), and the selected standard was 0.05. The results showed that tumor stage, intraoperative bleeding amount, and positive lymph node ratio were independent risk factors for prognosis of gastric cancer, as shown in Table 4.
| Clinicopathological features | B | SE | Wald | OR (95%CI) | P value |
| Age | 0.204 | 0.104 | 3.820 | 1.227(0.999-1.505) | 0.051 |
| Gender | 0.104 | 0.159 | 0.429 | 1.110(0.812-1.517) | 0.512 |
| Tumor stage | 1.004 | 0.124 | 65.481 | 2.728(2.140-3.479) | 0.000 |
| Tumor location | -0.265 | 0.088 | 8.958 | 0.767 (0.645-0.913) | 0.003 |
| Pathology classification | 0.020 | 0.089 | 0.050 | 1.020 (0.857-1.214) | 0.823 |
| Borrmann classification | 0.605 | 0.098 | 37.822 | 1.832 (1.511-2.222) | 0.000 |
| Celiac artery variation | 0.038 | 0.163 | 0.055 | 1.039 (0.755-1.430) | 0.815 |
| Operation time | 0.002 | 0.001 | 8.270 | 1.002 (1.001-1.003) | 0.004 |
| Intraoperative bleeding | 0.001 | 0.000 | 21.216 | 1.001 (1.000-1.001) | 0.000 |
| No. of lymph nodes metastases | 0.071 | 0.008 | 90.075 | 1.074 (1.058-1.090) | 0.000 |
| No. of lymph nodes | 0.018 | 0.007 | 6.083 | 1.018 (1.004-1.032) | 0.014 |
| Positive lymph node ratio | 2.491 | 0.242 | 106.057 | 12.072 (7.514-19.393) | 0.000 |
| Variables | B | SE | Wald | OR (95%CI) | P value |
| Comprehensive staging | 0.626 | 0.145 | 18.494 | 1.870 (1.406-2.486) | 0.000 |
| Intraoperative bleeding | 0.001 | 0.000 | 10.575 | 1.001 (1.000-1.001) | 0.001 |
| Positive lymph node ratio | 1.466 | 0.318 | 21.206 | 4.330 (2.320-8.079) | 0.000 |
At present, D2 lymphadenectomy is widely accepted as the standard of surgery for advanced gastric cancer all around the world. The current 7th UICC/AJCC TNM staging of gastric cancer requires that lymph nodes dissection should include at least 16 or more lymph nodes for histological examination[2]. In recent years, many studies have shown that there is a correlation between the overall survival time and the number of lymph nodes dissection after gastric cancer surgery[10,11]. However, the scope of precise lymph nodes dissection for different stages and tumor locations remains controversial. Lu et al[12] found that radical distal gastrectomy with more than 16 lymph nodes dissection and radical total gastrectomy with more than 21 lymph nodes dissection was the standard and will be more conducive to the analysis and evaluation of the prognosis of the patients. A multicenter study in the United States has shown that clearance of more than 16 lymph nodes in patients with IA-III-A can significantly improve long-term survival[13]. According to the results of a multicenter study including 1654 cases in Germany, more than 25 lymph nodes should be removed for gastric cancer patients with stage II[3]. In a word, the number of lymph nodes dissection in gastric cancer is still controversial. In the era of precision surgery, many scholars suggest that a reasonable range of lymph nodes dissection should be selected according to the individual factors, such as tumor location, tumor staging, and human anatomy, in order to reduce postoperative complications and improve the long-term survival rate and postoperative living quality. The variation of the celiac artery is an important anatomical factor for gastric cancer patients. An earlier study found that the abnormal hepatic artery derived from the superior mesenteric artery was not obviously linked to lymph node distribution[5]. We speculate that the anatomic variation of the celiac artery may affect the number of lymph nodes dissection in gastric cancer.
The results of this study showed that the variation rate of the celiac artery was as high as 26.13%, which was similar to that reported in Ugurel et al[14]. Among them, hepatic artery system variation was the most common, and the abnormal hepatic artery derived from superior mesenteric artery accounted for 43.6% of the hepatic artery system variation, which was a little higher than our previous report(37.0%)[15]. The existence of celiac artery variation increases the difficulty of operation, prolongs the operation time, increases the amount of bleeding during the operation, and may increase the incidences of intraoperative and postoperative complications. Therefore, the surgeon attaches great importance to celiac artery variation[16-18]. However, the distribution of the lymph nodes around the variant celiac artery has rarely been concerned. The results of this study show that celiac artery variation, tumor stage, and Borrmann typing are factors that affect the lymph nodes dissection of gastric cancer. The higher tumor stage and Borrmann typing, the more lymph nodes could be observed. At present, lymph nodes were sorted with touch method after surgery, which may be associated with overlooking some hidden tiny lymph nodes. High tumor stage and Borrmann typing may increase the rate of lymph node enlargement around the stomach, thus potentially increasing the number of lymph nodes dissection.
The number of lymph nodes dissection in patients with celiac artery variation is significantly less than those without variation, which further validates the prediction of the results in our earlier study. We speculate that the reasons for the reduction of the number of lymph nodes may be as follows. Firstly, the lymphatic reflux system of the stomach is special and complex. Kajitani in Japan suggests that the lymph around the stomach flows retrograde along the artery, and the artery is more fixed. Finally, it was determined to use the trip of the arterial system and the branch of the artery as a fixed anatomical sign to describe the lymph circumfluence of the stomach[19]. Variation of the celiac artery may be accompanied by a change in the lymphatic reflux, leading to a reduction in the distribution of the perivascular lymph nodes. Secondly, the 14th version of the Japanese gastric cancer treatment protocol lists No.12a as a routine cleaning object and No.12p and No.12b as unconventional cleaning objects. The No.12a lymph node is distributed along the hepatic artery from the confluence part of the left and right hepatic duct to the superior border of the pancreas. Normally, the proper hepatic artery goes ahead of the left anterior of the hepatoduodenal ligament and anterior of the portal vein, but the abnormal right hepatic artery and common hepatic artery derived from the superior mesenteric artery are common in the rear of the portal vein and medial of the common bile duct[20].The lymph nodes around this part of the abnormal hepatic artery are easily ignored without dissection during the operation because they are mistaken for No.12b or No.12p, eventually leading to a reduction in the number of lymph nodes dissection. Thirdly, the abnormal arteries, especiallythe abnormal hepatic artery derived from the superior mesenteric artery, are mostly of the post-pancreas type, and it is difficult to dissect the peripheral lymph nodes and adipose tissue around the root of the abnormal hepatic artery and the posterior part of the pancreas.
The number of lymph nodes dissection in the patients with celiac artery variation was not more than those of normal blood vessels, but there was no difference in the prognosis of the two groups. The univariate and multivariate Cox regression analysis showed that the variation of celiac artery was not an independent risk factor for the prognosis of gastric cancer. In view of this, we do not recommend routine cleaning the lymph nodes around the variant celiac artery, especially the abnormal hepatic artery derived from the superior mesenteric artery. The reasons are three points: (1) No lymph nodes are found around the abnormal hepatic artery of the post-pancreas and pre-pancreas type arising from the superior mesenteric artery during the D2 radical lymphadenectomy. Also, the tissues around the abnormal vessels were dissected for routine HE staining and CK20, CEA immunization, and no metastasis was found[5]; (2) The majority of abnormal hepatic arteries derived from the superior mesenteric artery belonged to the post-pancreas type, greatly increasing the difficulty of lymph nodes dissection and the risk of damaging the abnormal hepatic artery and pancreas, which may lead to increased risk of intraoperative bleeding, postoperative liver function damage, and pancreatic fistula and an increase in operation time; and (3) The results of this study showed that the number of lymph nodes dissection was reduced in celiac artery variation patients. However, prognosis was not affected, and the variation of the celiac artery was not an independent risk factor for the prognosis of gastric cancer.
In summary, variation of the celiac artery is an important factor affecting the lymph node clearance of gastric cancer, and the decrease in the number of lymph nodes dissection does not affect the prognosis. We do not recommend routine cleaning for the abnormal hepatic artery, especially the abnormal hepatic artery derived from the superior mesenteric artery. As this study was retrospective, the next step is to perform a prospective control study on the distribution difference of the peripheral lymph nodes based on the detailed vascular variation types, which would yield a more reliable basis for the development of a precise and individualized treatment plan for patients with gastric cancer.
The number of lymph nodes dissection directly affects the prognosis and recurrence of gastric cancer. In addition, celiac artery variation is quite common clinically. However, there are few studies that discuss the relationship between celiac artery variation and the number of lymph nodes dissection in gastric cancer surgery.
According to our previous study, the number of lymph nodes dissection in gastric cancer surgery might be different between variant celiac artery patients and normal celiac artery patients. Therefore, we conducted this study to investigate the relationship between celiac artery variation and the number of lymph nodes dissection in gastric cancer surgery.
To investigate the relationship between celiac artery variation and the number of lymph nodes dissection in radical D2 lymphadenectomy of gastric cancer and the effect on prognosis.
The clinicopathological data of 421 patients treated with radical D2 lymphadenectomy were analyzed retrospectively. The difference in the number of lymph nodes dissection between celiac artery variation group and normal vessels group and the relationship with prognosis were analyzed.
The number of lymph nodes dissection in patients with celiac artery variation was significantly less than that of non-variant groups, but there was no significant difference in survival time between the two groups. Univariate and multiple Cox regression analysis showed that celiac artery variation was not a prognostic factor for gastric cancer.
Celiac artery variation is an important factor affecting lymph node clearance in patients with gastric cancer. The number of lymph nodes dissection in patients with celiac artery variation is reduced, but there is no obvious effect on the prognosis. Therefore, lymph nodes around the abnormal artery, especially for the abnormal hepatic artery derived from superior mesenteric artery, may not need to be dissected in radical D2 lymphadenectomy.
As this was a small-scale study, we propose future studies with a larger sample sizes. At the same time, the relationship between celiac artery variation and the number of lymph nodes dissection in different celiac artery variation types should be evaluated. We propose that lymph nodes around the abnormal artery, especially for the abnormal hepatic artery derived from superior mesenteric artery, do not need to dissected in radical D2 lymphadenectomy. However, further prospective and controlled studies are required to verify this theory.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Avital S, Kimura A, Yarema RR S-Editor: Ji FF L-Editor: Filipodia E-Editor: Xing YX
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 2. | Sobin LH, Wittekind C. TNM Classification of Malignant Tumors (UICC). 7th ed. New York: Willry-Less 2010; 305. |
| 3. | Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 799] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 4. | Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114-7124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 475] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 5. | Huang Y, Liu C, Lin JL, Mu GC, Zeng Y. Is it necessary to dissect the lymph nodes around an abnormal hepatic artery in D2 lymphadenectomy for gastric cancer? Clin Transl Oncol. 2013;15:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Arifuzzaman M, Nasim Naqvi SS, Adel H, Adil SO, Rasool M, Hussain M. Anatomical Variants Of Celiac Trunk, Hepatic And Renal Arteries In A Population Of Developing Country Using Multidetector Computed Tomography Angiography. J Ayub Med Coll Abbottabad. 2017;29:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Li XH, Sun CH, Feng ST, Yan CG, He YL, Han FH, Li ZP, Meng QF. Assessment of 64-slice spiral computed tomography angiography with image fusion for perigastric arteries anatomy. Zhonghua Weichang Waike Zazhi. 2012;15:594-598. [PubMed] |
| 8. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 9. | Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 501] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Morgan JW, Ji L, Friedman G, Senthil M, Dyke C, Lum SS. The role of the cancer center when using lymph node count as a quality measure for gastric cancer surgery. JAMA Surg. 2015;150:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Deutsch GB, O'Connor V, Sim MS, Lee JH, Bilchik AJ. Incorporating surgical quality into the AJCC 7th edition improves staging accuracy in gastric cancer. Ann Surg Oncol. 2015;22:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Huang CM, Zhou ZW. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol. 2017;24:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Gholami S, Janson L, Worhunsky DJ, Tran TB, Squires MH, Jin LX, Spolverato G, Votanopoulos KI, Schmidt C, Weber SM, Bloomston M, Cho CS, Levine EA, Fields RC, Pawlik TM, Maithel SK, Efron B, Norton JA, Poultsides GA. Number of Lymph Nodes Removed and Survival after Gastric Cancer Resection: An Analysis from the US Gastric Cancer Collaborative. J Am Coll Surg. 2015;221:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Ugurel MS, Battal B, Bozlar U, Nural MS, Tasar M, Ors F, Saglam M, Karademir I. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol. 2010;83:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Mu GC, Huang Y, Liu ZM, Lin JL, Zhang LL, Zeng YJ. Clinical research in individual information of celiac artery CT imaging and gastric cancer surgery. Clin Transl Oncol. 2013;15:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Tu RH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Huang CM, Zheng CH. Development of lymph node dissection in laparoscopic gastrectomy: safety and technical tips. Transl Gastroenterol Hepatol. 2017;2:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Randjelovic DT, Filipovic RB, Bilanovic LD, Stanisavljevic SN. Perigastric vascular abnormalities and the impact on esophagogastrectomy. Dis Esophagus. 2007;20:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Chen W, Gao J, Chen D. Guiding values of multislice spiral computed tomography angiography in laparoscopic D2 radical gastrectomy of local advanced gastric carcinoma. J Cancer Res Ther. 2018;14:S197-S201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Li GL, Ji JF. The formulation of Japan stomach cancer treatment statute and skip metastasis of lymph node in gastric cancer. Zhonghua Weichang Waike Zazhi. 2009;12:197-198. |
| 20. | Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, Cho BH, So YH, Park JH. Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology. 2010;255:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |