Published online Jun 15, 2019. doi: 10.4251/wjgo.v11.i6.489
Peer-review started: March 8, 2019
First decision: May 13, 2019
Revised: May 15, 2019
Accepted: May 28, 2019
Article in press: May 29, 2019
Published online: June 15, 2019
Processing time: 100 Days and 0.1 Hours
Radical surgical resection is regarded as the best treatment for hepatic hilar cholangiocarcinoma. However, 60%-70% of patients have lost the chance of surgery at the time of diagnosis. Simple biliary stent or drainage tube placement may fail in a short time due to tumor invasion or overgrowth, bile accumulation, or biofilm formation. Effective palliative treatments to extend the effective drainage time are of great significance for improving the quality of life of patients and changing the prognosis of patients.
To investigate the clinical efficacy of gemcitabine and cisplatin-based transcatheter arterial chemoembolization (TACE) combined with radiotherapy in hilar cholangiocarcinoma.
A retrospective analysis was conducted on patients clinically diagnosed with hilar cholangiocarcinoma from June 2014 to January 2017 at the Liaoning Provincial Cancer Hospital. Patients were evaluated by specialists, and those who were not suitable for surgery or unwilling to undergo surgery and met the inclusion criteria were included in the study. There were a total of 72 patients (34 males and 38 females) with an average age of 59.9 years (range, 40-72 years). According to percutaneous transhepatic biliary angiography and the patients’ wishes, stent implantation or biliary drainage tube implantation was used to relieve biliary obstruction. The patients were divided into either a control group or a combined treatment group according to their follow-up treatment. The control group consisted of a total of 35 patients who received simple biliary drainage tube placement and biliary stent implantation (7 patients with bilateral stents and 6 with a unilateral stent) and 22 patients receiving biliary drainage tube placement alone. The combined treatment group received TACE and extracorporeal radiotherapy after biliary drainage or biliary stent implantation and consisted of a total of 37 patients, including 21 patients receiving combined treatment after biliary stent placement (14 patients with bilateral stents and 7 with a unilateral stent) and 16 undergoing combined therapy after implanting the biliary drainage tube. In the combination treatment group, the TACE chemotherapy regimen employed gemcitabine and cisplatin, and the embolic agent was iodized oil. A particular dose was determined according to the patient's body surface area and the tumor staining indicated by DSA. In vitro radiotherapy was performed with intensity-modulated radiotherapy or three-dimensional conformal radiotherapy at an average dose of 48.3 Gy. Both groups were followed from stent implantation or drainage tube implantation until the patient quitted or died. The median length of follow-up observation was 13 mo. The differences in overall survival time and the effect of different jaundice reducing methods (single stent, double stent, or biliary drainage) on the patency time and survival time of biliary stents were compared between the two groups; the related factors affecting overall survival time were analyzed.
The median survival time of the control group was 10.5 mo; the median survival time of patients with biliary stent implantation and those with percutaneous biliary drainage was 9.6 mo and 11.4 mo, respectively, and there was no statistically significant difference between them. The median survival time of the combined treatment group was 20.0 mo, which was significantly higher than that of the control group (P < 0.05). Among patients in the combined treatment group, the median survival time of patients who underwent biliary stent implantation and those who accepted percutaneous biliary drainage before the combination therapy was 19.5 mo and 20.1 mo, respectively, and there was no significant difference between them. In the combination treatment group, the mean time of median stent patency was 15.6 mo, which was significantly higher than that of the control group (7.0 mo; P < 0.05). The independent factors affecting survival time included age, whether to receive combination therapy, percutaneous biliary drainage tube implantation, and Bismuth-Corlette classification as type IV.
Gemcitabine and cisplatin-based TACE combined with radiotherapy can prolong the survival of patients with hilar cholangiocarcinoma. Independent predictors of survival include selection of combination therapy, Bismuth-Corlette classification as type IV, selection of percutaneous biliary drainage tube implantation, and age.
Core tip: In this study, hilar cholangiocarcinoma patients with obstructive jaundice were observed by different methods of reducing jaundice. The effectiveness of transcatheter arterial chemoembolization combined with radiotherapy was observed in extending the effective drainage time of the stent or drainage tube, improving the quality of life, and changing the prognosis of patients. The independent factors affecting survival were analyzed. The results may be helpful to improve the systematic palliative treatment of hilar cholangiocarcinoma.
- Citation: Zheng WH, Yu T, Luo YH, Wang Y, Liu YF, Hua XD, Lin J, Ma ZH, Ai FL, Wang TL. Clinical efficacy of gemcitabine and cisplatin-based transcatheter arterial chemoembolization combined with radiotherapy in hilar cholangiocarcinoma. World J Gastrointest Oncol 2019; 11(6): 489-498
- URL: https://www.wjgnet.com/1948-5204/full/v11/i6/489.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i6.489
Hepatic hilar cholangiocarcinoma originates from the biliary mucosal epithelium and affects the left and right hepatic ducts at or near the junction of the biliary tract. Hilar cholangiocarcinoma is one of the most common malignant tumors of the biliary system, accounting for more than 70% of biliary tract tumors[1]. Radical surgical resection is the best treatment for long-term survival or cure in patients with hilar cholangiocarcinoma[2-5]. However, the incidence of hilar cholangiocarcinoma is a concealed, invasive growth, lacking specific symptoms in the early stage of the disease, and approximately 60%-70% of patients have lost the chance of surgery at the time of diagnosis.
The purpose of palliative biliary drainage[8-11] is to relieve bilirubinemia and cholangitis, provide conditions for surgery or other adjuvant treatments and has many advantages, such as reducing jaundice, being a relatively simple operation, and having a low cost. However, if the external drainage bile duct is carried for a long time, the quality of life may be seriously degraded due to inflammation of the puncture site, intercostal pain, and inconvenience in managing the tube[12-14]. Drainage may be reduced due to tube detachment or tumor bile duct growth. Percutaneous biliary stent implantation is considered a preferred solution for relieving high malignant biliary obstruction and achieving intrahepatic drainage. However, simple biliary stent placement may result in blockage due to tumor invasion or overgrowth, bile accumulation, or biofilm formation[15-18]. Extending the effective drainage time of the stent or drainage tube is of great significance for improving the quality of life of patients and the prognosis of patients.
A large number of studies have confirmed that gemcitabine combined with cisplatin chemotherapy[19,20], arterial chemoembolization[21-24], and radiation therapy[25,26] are safe and effective in the palliative treatment of cholangiocarcinoma. Therefore, this study retrospectively studied 72 patients with hilar cholangiocarcinoma with obstructive jaundice and explored the efficacy and prognosis of transcatheter arterial chemoembolization (TACE) combined with radiotherapy after percutaneous biliary drainage or stent implantation.
A total of 72 patients with hilar cholangiocarcinoma complicated with obstructive jaundice were enrolled in this study from June 2014 to January 2017 at the Liaoning Provincial Cancer Hospital (Table 1), including 34 males and 38 females, with an average age of 59.9 years (range, 40-72 years old). All patients underwent contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MR) scanning and magnetic resonance cholangiopancreatography (MRCP). The inclusion criteria were: (1) Informed consent obtained from the patient; (2) Clinical or pathological diagnosis of hilar cholangiocarcinoma with obstructive jaundice and undergoing biliary stenting; (3) Imaging evaluations of tumors confined to the liver before initial treatment; and (4) Estimated survival periods > 3 mo. The exclusion criteria were: (1) Severe liver function disorder, kidney function disorder, or severe coagulopathy; (2) Extrahepatic metastases or multiple intrahepatic lesions; (3) General condition failure; and (4) History of hepatitis and cirrhosis.
| Group | n | Age (yr) | Sex | Bismuth classification (cases) | Implantation method | ||||
| M | F | II | III | IV | Unilateral | Bilateral | |||
| Control (35 cases) | 61.6 ± 7.1 | 15 | 20 | 2 | 18 | 15 | 4 | 31 | |
| Stent | 13 | 5 | 8 | 2 | 7 | 4 | 4 | 9 | |
| Drainage tube | 22 | 10 | 12 | 0 | 11 | 11 | 0 | 22 | |
| Combined (37 cases) | 58.2 ± 7.7 | 19 | 18 | 4 | 18 | 15 | 7 | 30 | |
| Stent | 21 | 14 | 7 | 4 | 12 | 5 | 7 | 14 | |
| Drainage tube | 16 | 5 | 11 | 0 | 6 | 10 | 0 | 16 | |
| P-value | 0.91 | 0.49 | 0.74 | 0.88 | |||||
The basic information of the two groups of patients (Table 1), including age, sex, Bismuth-Corlette classification, and jaundice reduction, were not statistically significant (P > 0.05).
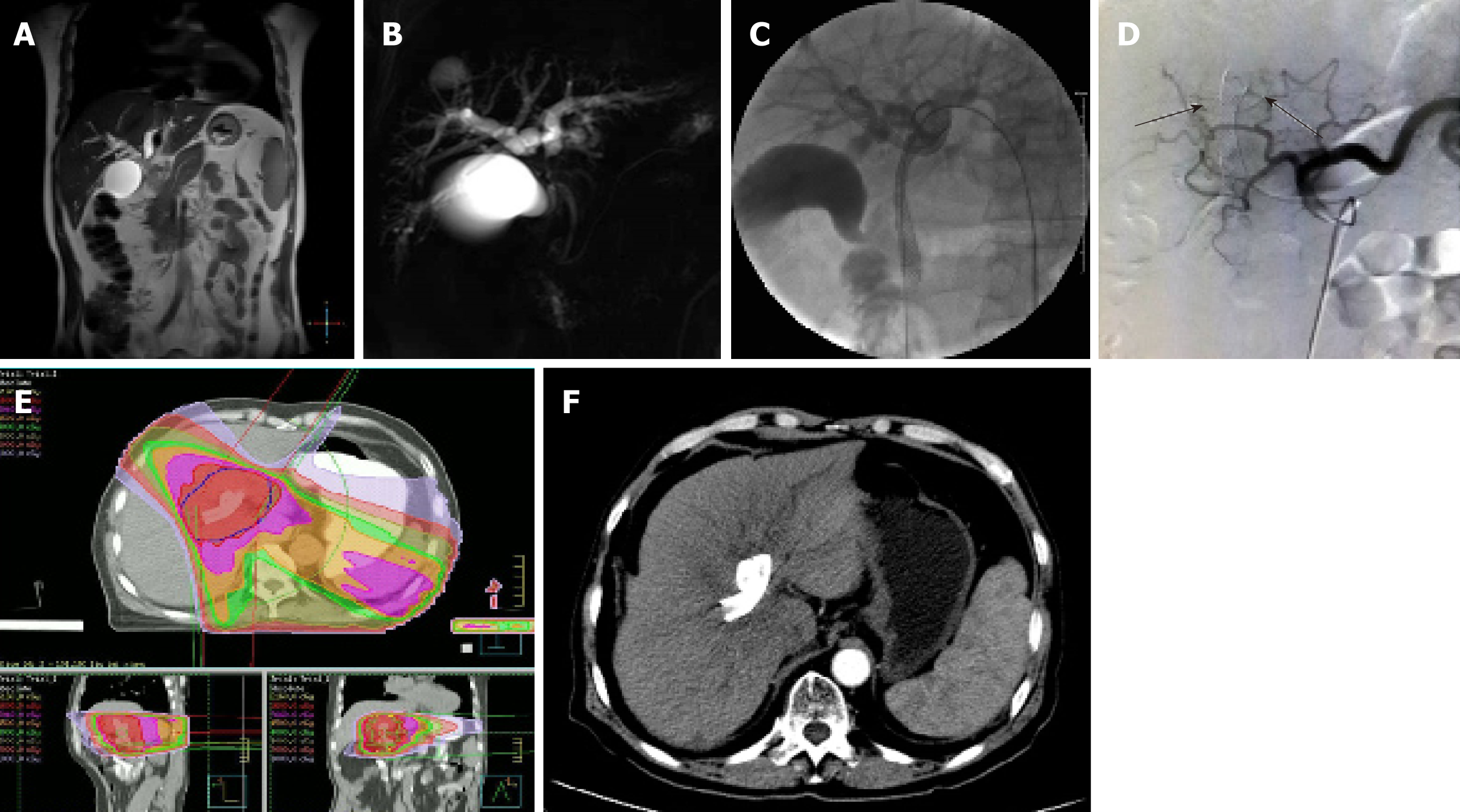
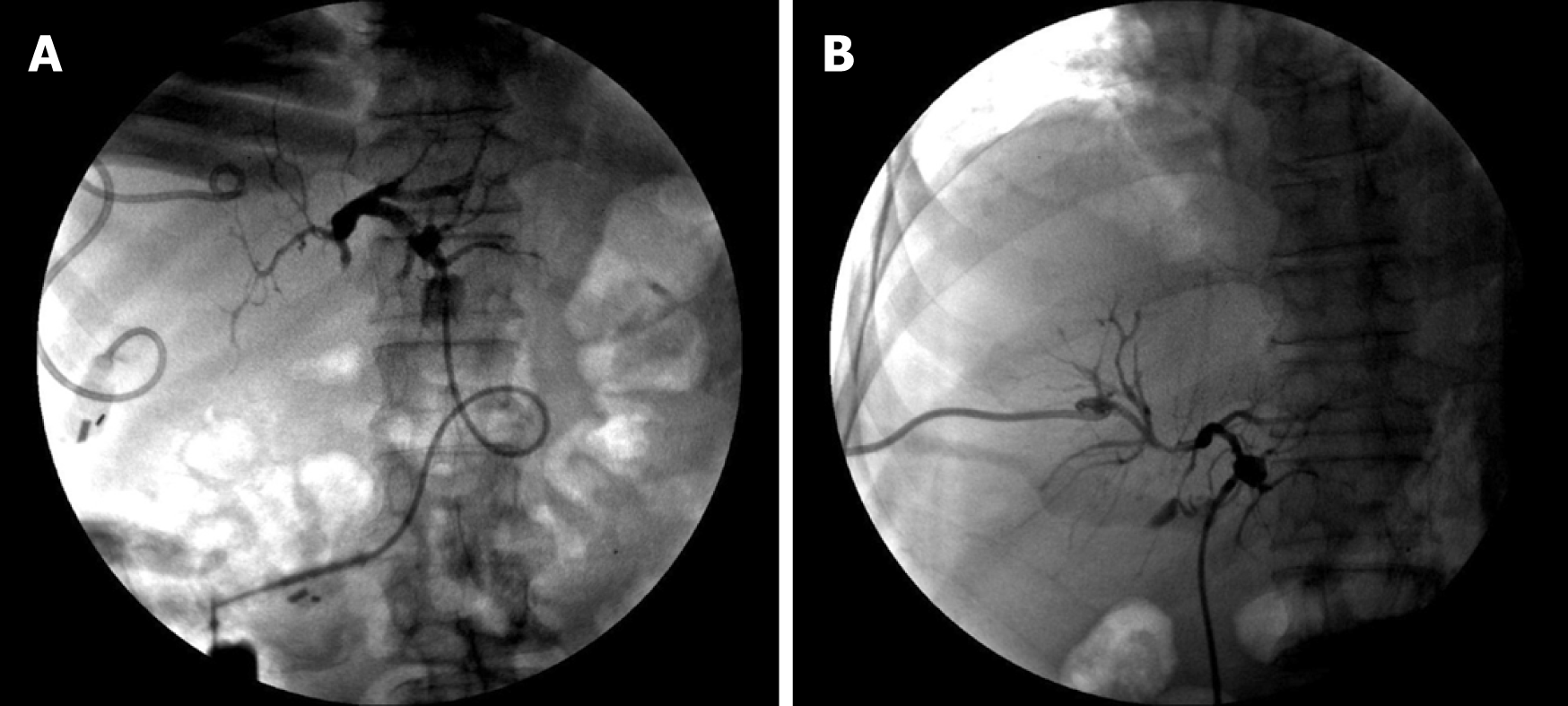
Percutaneous biliary puncture drainage tube placement and stent implantation were performed under X-ray and ultrasound guidance. The drainage tubes (Cook, USA) with a diameter of 7F or 8.5F were used. The stent was a self-expanding bare metal stent (Wall stent, Boston Scientific Corp., USA), model 8 mm (diameter) x 60 mm (length) and 8 mm (diameter) x 40 mm (length). First, ultrasound-guided percutaneous transhepatic cholangiography was performed. The puncture device was a 22G micropuncture needle kit (NPAS-100-RH-NT, Cook). According to the angiographic results and preoperative imaging analysis, bile duct involvement was determined, and whether to use bilateral or unilateral drainage, as well as the diameter, length, and implanting methods of the biliary stent were also determined, as shown in Figures 1 and 2.
Patients needed to have tubes changed every 3-6 mo. In patients where the tube could not be recanalized or detached, a drainage tube was percutaneously implanted.
The chemotherapy drugs for TACE were gemcitabine and cisplatin, and the doses used were 1/2 of the systemic doses, i.e., gemcitabine 500 mg/m2 and cisplatin 35 mg/m2, and the embolization agent was made with iodized oil. After treatment of the definite blood supply artery, selective embolization was performed (Figure 1). If the blood supply to the artery was not clear, arterial infusion was used. Rehydration and hydration were performed before and after treatment.
The radiation therapy plan was developed on the ADAC Pinnacle3 Treatment Planning System workstation (Philips, Best, The Netherlands). The gross tumor volume (GTV) included tumor and regional metastatic lymph nodes in the imaging findings. GTV was expanded outward by 0.5 cm as the clinical tumor volume (CTV), and CTV was expanded outward by 1 cm (for the cephalo-caudal direction) and 0.5 cm (for other directions), together with the tumor movement range, to form the planning tumor volume (PTV). The treatments were delivered with a VARIAN 6-MV X-ray linear accelerator (Varian Medical Systems, California, USA) using the following parameters: 3 to 7 coplanar or noncoplanar illumination fields, 90% isodose curve wrapped around PTV, 2.0 Gy each time, 5 times/wk, and total 50 Gy/25F.
The follow-up period began after stent or drainage tube implantation until the patient quitted study or died. Laboratory tests (routine blood and urine tests, indexes of hepatic and renal function, and tumor markers) and abdominal ultrasound were reviewed once a month; liver contrast-enhanced CT or MRI scans were reviewed every 3 mo. According to the imaging results and blood bilirubin levels, it was determined whether there was clogging in the biliary stent. For cases of obstructive jaundice, infection and other complications caused by biliary stent occlusion, biliary puncture drainage, anti-infection, and supportive treatments were given.
Statistical analyses were performed using SPSS version 19.0 statistical software. Data that were normally distributed are expressed as the mean ± SD, while those with a non-normal distribution are expressed as the median, and independent sample t-test was performed. The Kaplan-Meier method was used to compare the stent patency time and survival time. Cox proportional hazard regression analysis was used to analyze the risk factors affecting the prognosis of patients. P < 0.05 was considered statistically significant.
The total bilirubin values of the control group and the combined treatment group were both less than 80 μmol/L 5-6 wk after receiving biliary drainage or stent implantation. Postoperative complications included biliary hemorrhage and cholangitis. No operation-related deaths were observed within 30 d of the operation.
Twelve patients received intensity-modulated radiotherapy, and 25 patients received three-dimensional conformal radiotherapy. There were 10 (27.0%) patients with grade II-III adverse reactions 2 wk after radiotherapy, and 3 (8.1%) of these patients could not complete the treatment course due to upper abdominal pain, nausea, and vomiting. A total of 102 TACE treatments were given in 37 cases. Twenty-one (29.2%) patients developed grade II adverse events of neutropenia after the first or subsequent TACE treatments.
The main causes of death in the control group and the combination treatment group included multiple organ failure caused by extensive metastasis in 12 patients, biliary infection and upper gastrointestinal bleeding after biliary recanalization in 21, liver failure after biliary recanalization in 35, unexplained death in 2, and loss to follow-up in 2.
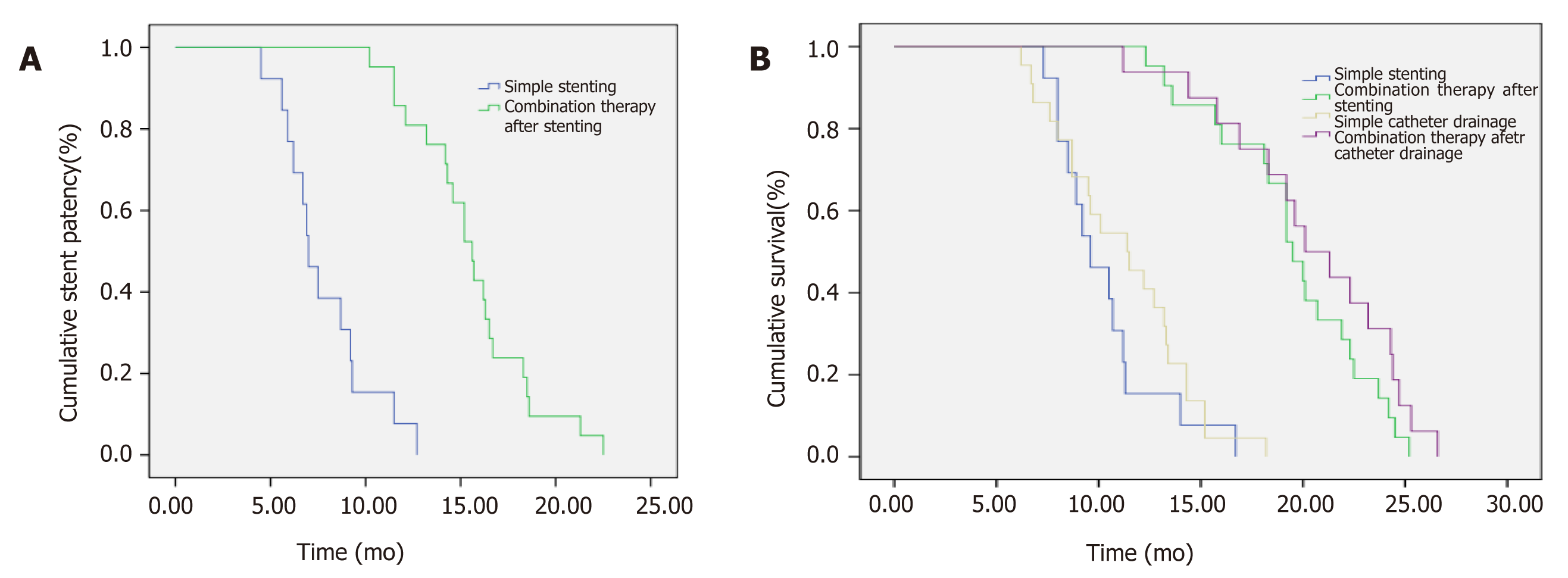
The median patency time of the biliary stent was 15.6 mo in the combined treatment group and 7.0 mo in the control group, and there was a significant difference between them (P < 0.05) (Figure 3).
The median survival time of the control group was 10.5 mo; the median survival time of patients with biliary stent implantation and those with percutaneous biliary drainage was 9.6 mo and 11.4 mo, respectively, and there was no statistically significant difference between them. The median survival time of the combined treatment group was 20.0 mo and was significantly higher than that of the control group (10.5 mo; P < 0.05). In the combined treatment group, the median survival time of the patients who underwent biliary stent implantation those who underwent percutaneous biliary drainage before combined therapy was 19.5 mo and 20.1 mo, respectively, and there was no significant difference between them (Figure 4).
Multivariate analysis was performed on the sex, age, Bismuth-Corlette classification, jaundice reduction, combination therapy or not, and baseline value of CA19-9 (reviewed 3 wk after jaundice) in the study cases, and the results are shown in Table 2.
| Influencing factor | Proportional risk factor | 95%CI | P-value | |
| Gender | M | 1 | ||
| F | 1.312 | 0.771-2.223 | 0.316 | |
| Age | ≤60 yr | 1 | ||
| >60 yr | 1.819 | 1.032-3.207 | 0.038 | |
| Bismuth-Corlett classification | II | 1 | ||
| III | 2.633 | 0.992-6.984 | 0.052 | |
| IV | 6.102 | 2.040-18.251 | 0.001 | |
| Ways to reduce jaundice | Stenting | 1 | ||
| Drainage | 0.263 | 0.134-0.516 | 0.000 | |
| Comprehensive treatment | No | 1 | ||
| Yes | 0.039 | 0.016-0.091 | 0.000 | |
| CA19-9 | - | 1.002 | 0.999-1.004 | 0.147 |
Obstructive jaundice is a common clinical symptom of hilar cholangiocarcinoma. Persistent biliary obstruction can lead to liver failure, secondary renal failure[27,28], severe hypoproteinemia, electrolyte imbalance, septic shock, etc., affecting the quality of life and survival of patients[16,29,30]. Clinically, percutaneous drainage tube or biliary stent implantation in various ways is an effective treatment for obstructive jaun-dice[17,31-39].
Most of hilar cholangiocarcinoma cases progress along the bile duct and invade the surrounding tissue, but distant metastasis rate is relatively low[40]. After the metal stent is placed, tumor invasion or overgrowth, bile accumulation, or biofilm formation may cause clogging in the stent. In this study, the median patency time of a simple metal stent was 7 mo in patients with high biliary obstruction, which is similar to that of other previous studies[16,17,29,30](7-9 mo).
The Asia-Pacific Consensus for Hepatic Portal Cholangiocarcinoma[31] indicates that percutaneous stent drainage is superior to endoscopic palliative care in advanced cases of type III or IV, and the purpose of palliative stent implantation is to ensure adequate liver drainage (>50%). Studies have shown that[17] effective liver drainage is important for patient survival, and the study by Vienne et al[33] showed that effective liver drainage (HR = 4.158, P = 0.040) was an independent predictor of survival. The most common classification of hilar cholangiocarcinoma is the Bismuth-Corlette classification, which evaluates local tumor spread but does not provide information on vascular invasion or metastatic disease and may be limited in assessment of prognostic value[34,40,41]. However, the Bismuth-Corlette classification determines the involvement of the biliary tract branch to a certain extent, which indicates the feasibility of achieving the maximum effective drainage of the liver and the stent or drainage tube implantation method; low classification (I or II) is easier to achieve the maximum effective drainage of the liver than high classification (III or IV). Type III or IV often requires multiple stents or drainage tubes. Our study showed that Bismuth-Corlette type IV was an independent predictor of survival and was inversely related to survival, and these results are similar to a previous study[33]. The control group showed a median survival time that was not statistically different from that in patients who underwent stent implantation or drainage tube implantation.
Drainage tubes were routinely required to undergo cholangiography and replaced every 3-6 mo; however, there were still 10 (26.3%) patients with 13 tube shedding events. In addition, 2 (5.3%) patients with implanted metastases at puncture sites of the right intercostal space were observed in the study. Studies have shown that compared with biliary stent implantation, the choice of biliary drainage tube implantation is an independent predictor of survival that is positively correlated with survival and the reimplantation and replacement of the drainage tube may lead to prolonged effective drainage time.
This study showed that CA19-9 level cannot be used as a predictor of survival. In addition, serum CA19-9 concentration was determined by the Lewis blood group antigen phenotype of red blood cells[42], and approximately 5%-14% of the population had the Lewis α-β-type, which cannot produce CA19-9. In the CA19-9 test of this study, we did not take these factors into consideration, which may also result in biased results.
Studies have shown that gemcitabine plus cisplatin chemotherapy can prolong the survival of patients with cholangiocarcinoma in palliative care. A meta-analysis of 1368 patients with locally advanced cholangiocarcinoma by Eckel et al[19] showed that gemcitabine and platinum combination therapy resulted in higher response rates and tumor control rates. The ABC-02 trial by Valle et al[20] explored the addition of cisplatin plus gemcitabine to unresectable and metastatic cholangiocarcinoma and suggested that cisplatin plus gemcitabine resulted in a significant increase in progression-free survival (PFS) (8.0 mo vs 5.0 mo, P < 0.001) and overall survival (OS) (11.7 mo vs 8.1 mo, P < 0.001) compared to gemcitabine alone. Some research on the treatment of cholangiocarcinoma with arterial therapy has also achieved some results. The results of the study by Gusani et al[23] indicate that gemcitabine-based TACE was well-tolerated in patients with unresectable cholangiocarcinoma and that combination therapy (cisplatin or oxaliplatin) prolonged patient survival (OS 11.7 mo) compared to controls. Park et al[24] reported that conventional lipiodol-based chemoembolization (C-TACE) increased survival from 3.3 mo to 12.2 mo. Mahadevan et al[25] recently reported the results of 32 patients with major unresectable hepatocellular carcinoma treated with stereotactic body radiotherapy (SBRT), with local control and OS rates of 88% and 58%, respectively, and a median survival of 17 mo. Adverse events included duodenal ulcer in 2 patients, liver abscess in 1, and cholangitis in 1. Kopek et al[26] reported 27 patients with hepatic hilar cholangiocarcinoma treated with SBRT, with median PFS and OS of 6.7 and 10.6 mo, respectively, of which 6 patients developed gastroduodenal ulcers.
In patients with type III or IV cholangiocarcinoma in the combination treatment group, complete drainage was not achieved even by bilateral stent placement, and liver damage caused by obstructive jaundice may exist throughout the course of the disease. Therefore, combined treatment requires more attention to the patient's liver function status. In our institution, patients with hilar cholangiocarcinoma were strictly evaluated for liver function before receiving chemotherapy, TACE, and radiotherapy. Patients with a total bilirubin index less than 51.3 μmol/L (3 mg/dL) and Child-Pugh grade B or better might be more tolerant to treatment-induced liver damage. A liver protectant was given during the treatment, and the biochemical indicators of liver function were closely monitored during TACE and radiotherapy in our study. In radiation therapy, the total dose was controlled at approximately 48.3 Gy. There were no patients with peptic ulcer, hemorrhage, or hepatic abscess in this study; 10 (27.0%) patients had grade II-III adverse reactions 2 wk after radiotherapy, 3 (8.1%) of whom were unable to complete the treatment course due to upper abdominal pain, nausea, and vomiting. The indications for TACE treatment during follow-up after radiotherapy were as follows: (1) The CA 19-9 index continued to increase for 3 mo during follow-up; (2) Imaging evaluation could measure lesion enlargement; (3) The total bilirubin index less than 3 mg/dL and Child-Pugh grade B or better; and (4) From the last treatment interval of more than 6 mo. During the operation, selective arterial administration was strictly required, and lesions with insignificant arterial blood supply were carefully embolized. In most patients, decreased appetite and fatigue occurred after treatment. Twenty-one (29.2%) patients developed grade II adverse reactions and neutropenia after the first or subsequent TACE treatment. No other serious complications were found to result in the inability to accept TACE. However, it was observed that 5 patients in the combination treatment group had irreversible cirrhosis and portal hypertension within 7-12 mo after treatment, which may be related to liver damage caused by TACE and radiotherapy.
This study showed that gemcitabine plus cisplatin-based TACE combined with radiotherapy extended the median survival time of patients with hilar cho-langiocarcinoma after receiving stent or drainage tube implantation (20.0 mo vs 10.5 mo, P < 0.05). Accepting combination therapy, Bismuth-Corlette type IV, and accepting biliary drainage tube implantation were the independent predictors of survival of patients with hilar cholangiocarcinoma. Limitations of the study include that this study was a retrospective study conducted in only one center, and cases of hepatitis, cirrhosis, and extrahepatic metastasis were excluded from the case selection. These factors may affect the patient's survival and the observation of other research indicators.
Because of the occultation of hepatic hilar cholangiocarcinoma, most patients have lost the opportunity for surgical radical treatment at the time of diagnosis. Palliative treatment is important for patients with hepatic hilar cholangiocarcinoma.
Simple reduction of jaundice may fail due to tumor progression, and effective and combined palliative treatment may prolong the effective drainage time of the biliary stent or drainage tube, thereby prolonging patient survival. However, relevant research is rare at present.
This study mainly investigated the effects of transcatheter arterial chemoembolization (TACE) combined with radiotherapy on the survival of patients with hilar cholangiocarcinoma after biliary stent or drainage tube implantation, and analyzed the influencing factors.
This study used a retrospective cohort analysis to determine the significance of TACE combined with radiotherapy by comparing the differences between the two groups and comparing the different methods of reducing jaundice (stent or drainage tube placement) within the group. Regression analysis of the overall data helps to identify the independent factors influencing survival.
There was no significant difference in survival between the control group and the combination treatment group, which were treated by stent or tube implantation for the treatment of reducing jaundice, while the survival of patients receiving TACE combined with radiotherapy was significantly longer than that of patients receiving simple reduction of jaundice.
The results showed that TACE combined with radiotherapy can significantly extend the effective drainage time of stent or tube and prolong the survival of patients. Co-treatment, Bismuth-Corlett type IV, percutaneous biliary drainage, and age were independent predictors of survival.
Accepting a reasonable and standardized palliative treatment will prolong the survival of patients with unresectable hilar cholangiocarcinoma and improve their living conditions. With the development of immunotherapy and targeted therapy, relevant research may be carried out in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Takamatsu S, Tsegmed U S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Weiss MJ, Cosgrove D, Herman JM, Rastegar N, Kamel I, Pawlik TM. Multimodal treatment strategies for advanced hilar cholangiocarcinoma. Langenbecks Arch Surg. 2014;399:679-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Launois B, Reding R, Lebeau G, Buard JL. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000;7:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (1)] |
| 6. | Squires MH, Cloyd JM, Dillhoff M, Schmidt C, Pawlik TM. Challenges of surgical management of intrahepatic cholangiocarcinoma. Expert Rev Gastroenterol Hepatol. 2018;12:671-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Poultsides GA, Zhu AX, Choti MA, Pawlik TM. Intrahepatic cholangiocarcinoma. Surg Clin North Am. 2010;90:817-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Covey AM, Brown KT. Palliative percutaneous drainage in malignant biliary obstruction. Part 2: Mechanisms and postprocedure management. J Support Oncol. 2006;4:329-335. [PubMed] |
| 9. | Deipolyi AR, Covey AM. Palliative Percutaneous Biliary Interventions in Malignant High Bile Duct Obstruction. Semin Intervent Radiol. 2017;34:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Tuqan W, Innabi A, Alawneh A, Farsakh FA, Al-Khatib M. Prediction of Survival Following Percutaneous Biliary Drainage for Malignant Biliary Obstruction. J Transl Int Med. 2017;5:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Robson PC, Heffernan N, Gonen M, Thornton R, Brody LA, Holmes R, Brown KT, Covey AM, Fleischer D, Getrajdman GI, Jarnagin W, Sofocleous C, Blumgart L, D'Angelica M. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol. 2010;17:2303-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Saluja SS, Gulati M, Garg PK, Pal H, Pal S, Sahni P, Chattopadhyay TK. Endoscopic or percutaneous biliary drainage for gallbladder cancer: a randomized trial and quality of life assessment. Clin Gastroenterol Hepatol. 2008;6:944-950.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Sharaiha RZ, Natov N, Glockenberg KS, Widmer J, Gaidhane M, Kahaleh M. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci. 2014;59:3099-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Shim DJ, Gwon DI, Han K, Kim Y, Ko GY, Shin JH, Ko HK, Kim JH, Kim JW, Yoon HK, Sung KB. Percutaneous Metallic Stent Placement for Palliative Management of Malignant Biliary Hilar Obstruction. Korean J Radiol. 2018;19:597-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Maybody M, Brown KT, Brody LA, Covey AM, Sofocleous CT, Thornton RH, Getrajdman GI. Primary patency of Wallstents in malignant bile duct obstruction: single vs. two or more noncoaxial stents. Cardiovasc Intervent Radiol. 2009;32:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Brountzos EN, Ptochis N, Panagiotou I, Malagari K, Tzavara C, Kelekis D. A survival analysis of patients with malignant biliary strictures treated by percutaneous metallic stenting. Cardiovasc Intervent Radiol. 2007;30:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553. [PubMed] |
| 19. | Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy. 2014;60:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3169] [Article Influence: 211.3] [Reference Citation Analysis (1)] |
| 21. | Boehm LM, Jayakrishnan TT, Miura JT, Zacharias AJ, Johnston FM, Turaga KK, Gamblin TC. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Kim JH, Yoon HK, Sung KB, Ko GY, Gwon DI, Shin JH, Song HY. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer. 2008;113:1614-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Gusani NJ, Balaa FK, Steel JL, Geller DA, Marsh JW, Zajko AB, Carr BI, Gamblin TC. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg. 2008;12:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Mahadevan A, Dagoglu N, Mancias J, Raven K, Khwaja K, Tseng JF, Ng K, Enzinger P, Miksad R, Bullock A, Evenson A. Stereotactic Body Radiotherapy (SBRT) for Intrahepatic and Hilar Cholangiocarcinoma. J Cancer. 2015;6:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Kopek N, Holt MI, Hansen AT, Høyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Bonnel D, André T, Mader B, Lefebvre JF, Bensoussan E, Liguory C. [Malignant biliary obstruction, general review and clinical practice]. Bull Cancer. 2013;100:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Kogure H, Isayama H, Nakai Y, Tsujino T, Matsubara S, Yashima Y, Ito Y, Hamada T, Takahara N, Miyabayashi K, Mizuno S, Mohri D, Kawakubo K, Sasaki T, Yamamoto N, Hirano K, Sasahira N, Tada M, Koike K. High single-session success rate of endoscopic bilateral stent-in-stent placement with modified large cell Niti-S stents for malignant hilar biliary obstruction. Dig Endosc. 2014;26:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Song T, Jia Z, Guo X, Zhao H, Bao W, Han D, Zhou X, Qi X. Does Hepatic Impairment Influence Renal Function Parameters in Liver Cirrhosis? J Transl Int Med. 2018;6:90-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Djiambou-Nganjeu H. Hepatic Encephalopathy in Liver Cirrhosis. J Transl Int Med. 2017;5:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Hwang JC, Kim JH, Lim SG, Kim SS, Yoo BM, Cho SW. Y-shaped endoscopic bilateral metal stent placement for malignant hilar biliary obstruction: prospective long-term study. Scand J Gastroenterol. 2011;46:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, Khor CJ, Ponnudurai R, Moon JH, Seo DW, Pantongrag-Brown L, Sangchan A, Pisespongsa P, Akaraviputh T, Reddy ND, Maydeo A, Itoi T, Pausawasdi N, Punamiya S, Attasaranya S, Devereaux B, Ramchandani M, Goh KL; Asia-Pacific Working Group on Hepatobiliary Cancers. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:593-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 33. | Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, Chryssostalis A, Gaudric M, Pelletier G, Buffet C, Chaussade S, Prat F. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Pranculis A, Kievišas M, Kievišienė L, Vaičius A, Vanagas T, Kaupas RS, Dambrauskas Ž. Percutaneous Transhepatic Biliary Stenting with Uncovered Self-Expandable Metallic Stents in Patients with Malignant Biliary Obstruction - Efficacy and Survival Analysis. Pol J Radiol. 2017;82:431-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Paul A, Kaiser GM, Molmenti EP, Schroeder T, Vernadakis S, Oezcelik A, Baba HA, Cicinnati VR, Sotiropoulos GC. Klatskin tumors and the accuracy of the Bismuth-Corlette classification. Am Surg. 2011;77:1695-1699. [PubMed] |
| 36. | Adler DG. EUS-guided gallbladder drainage: Current status and future prospects. Endosc Ultrasound. 2018;7:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Baars JE, Kaffes AJ, Saxena P. EUS-guided biliary drainage: A comprehensive review of the literature. Endosc Ultrasound. 2018;7:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Coro O, Caillol F, Poincloux L, Bories E, Pesenti C, Ratone JP, Giovannini M. Hepaticogastrostomy under EUS guidance for a patient with a history of bypass surgery with a new stent design (with video). Endosc Ultrasound. 2019;8:66-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Nam K, Kim DU, Lee TH, Iwashita T, Nakai Y, Bolkhir A, Castro LA, Vazquez-Sequeiros E, de la Serna C, Perez-Miranda M, Lee JG, Lee SS, Seo DW, Lee SK, Kim MH, Park DH. Patient perception and preference of EUS-guided drainage over percutaneous drainage when endoscopic transpapillary biliary drainage fails: An international multicenter survey. Endosc Ultrasound. 2018;7:48-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Ogura T, Okuda A, Miyano A, Nishioka N, Higuchi K. Stent release within scope channel technique to prevent stent migration during EUS-guided hepaticogastrostomy (with video). Endosc Ultrasound. 2018;7:67-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Suarez-Munoz MA, Fernandez-Aguilar JL, Sanchez-Perez B, Perez-Daga JA, Garcia-Albiach B, Pulido-Roa Y, Marin-Camero N, Santoyo-Santoyo J. Risk factors and classifications of hilar cholangiocarcinoma. World J Gastrointest Oncol. 2013;5:132-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 42. | Juntermanns B, Sotiropoulos GC, Radunz S, Reis H, Heuer M, Baba HA, Canbay A, Schuler M, Gerken G, Paul A, Kaiser GM. Comparison of the sixth and the seventh editions of the UICC classification for perihilar cholangiocarcinoma. Ann Surg Oncol. 2013;20:277-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |