Published online Apr 15, 2019. doi: 10.4251/wjgo.v11.i4.295
Peer-review started: October 2, 2018
First decision: October 25, 2018
Revised: November 27, 2018
Accepted: December 31, 2018
Article in press: January 1, 2019
Published online: April 15, 2019
Processing time: 196 Days and 12.9 Hours
Colorectal cancer is the third most common cancer in men and the second most common in women worldwide. Almost a third of the patients has or will develop liver metastases. Neoadjuvant chemotherapy (NAC) has recently become nearly systematic prior to surgery of colorectal livers metastases (CRLMs). The response to NAC is evaluated by radiological imaging according to morphological criteria. More recently, the response to NAC has been evaluated based on histological criteria of the resected specimen. The most often used score is the tumor regression grade (TRG), which considers the necrosis, fibrosis, and number of viable tumor cells.
To analyze the predictive factors of the histological response, according to the TRG, on CRLM surgery performed after NAC.
From January 2006 to December 2013, 150 patients who had underwent surgery for CRLMs after NAC were included. The patients were separated into two groups based on their histological response, according to Rubbia-Brandt TRG. Based on their TRG, each patient was either assigned to the responder (R) group (TRG 1, 2, and 3) or to the non-responder (NR) group (TRG 4 and 5). All of the histology slides were re-evaluated in a blind manner by the same specialized pathologist. Univariate and multivariate analyses were performed.
Seventy-four patients were classified as responders and 76 as non-responders. The postoperative mortality rate was 0.7%, with a complication rate of 38%. Multivariate analysis identified five predictive factors of histological response. Three were predictive of non-response: More than seven NAC sessions, the absence of a radiological response after NAC, and a repeat hepatectomy (P < 0.005). Two were predictive of a good response: A rectal origin of the primary tumor and a liver-first strategy (P < 0.005). The overall survival was 57% at 3 yr and 36% at 5 yr. The disease-free survival rates were 14% at 3 yr and 11% at 5 yr. The factors contributing to a poor prognosis for disease-free survival were: No histological response after NAC, largest metastasis > 3 cm, more than three preoperative metastases, R1 resection, and the use of a targeted therapy with NAC (P < 0.005).
A non-radiological response and a number of NAC sessions > 7 are the two most pertinent predictive factors of non-histological response (TRG 4 or 5).
Core tip: In this study, we analyzed the histological responses of colorectal liver metastasis from 74 responders and 76 non-responders after neoadjuvant chemotherapy. We identified that the absence of a radiological response and extended neoadjuvant chemotherapy, comprising more than seven treatment sessions, are the two most pertinent predictive factors of non-histological response. This study also confirmed that the histological response of colorectal liver metastases after neoadjuvant chemotherapy has an influence on survival and, hence, warrants consideration. However, this influence on overall survival was lacking in cases of particularly aggressive disease that revealed microscopic vascular invasion in histological analyses.
- Citation: Serayssol C, Maulat C, Breibach F, Mokrane FZ, Selves J, Guimbaud R, Otal P, Suc B, Berard E, Muscari F. Predictive factors of histological response of colorectal liver metastases after neoadjuvant chemotherapy. World J Gastrointest Oncol 2019; 11(4): 295-309
- URL: https://www.wjgnet.com/1948-5204/full/v11/i4/295.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i4.295
Colorectal cancer is the third most common cancer in men and the second most common in women worldwide[1-6]. Almost a third of the patients have or will develop colorectal liver metastases (CRLMs)[2,7,8]. Only 20% of these patients are amenable to curative treatment by liver resection and/or thermo-ablation[7,9,10]. According to the most recent series, when combined with chemotherapy, this can result in a 5-year survival rate of up to 60%[6,11-13].
Neoadjuvant chemotherapy (NAC) has recently become nearly systematic prior to surgical management of CRLMs[7,14-16]. It can be administered to patients who are initially considered to be non-resectable, and to patients with liver disease that is at the limit of resectability[14,17-19], while it is also used in cases of CRLMs that are initially resectable[20,21]. The response to NAC is evaluated by radiological imaging, according to morphological criteria such as RECIST, mRECIST, and CHOI[22-28]. Surgery is mostly indicated in the case of liver lesions that are responsive to NAC, or for lesions that remain stable. Indeed, progression of the disease is a poor prognostic factor, and sometimes results in a temporary postponement of liver ablation surgery[15,28,29]. More recently, the response to NAC has also been evaluated based on histological criteria of the resected specimen. Rubbia-Brandt and Blazer scores indicate that histological regression correlates with the overall and disease-free survival (DFS) of patients after resection[13,30,31]. The most commonly used score in the world is the one established by Rubbia-Brandt and his team[31]. It reflects the tumor regression grade (TRG), which takes into account the level of necrosis and fibrosis, as well as the number of viable tumor cells. Few studies to date have documented the influence of TRG on patient survival, and these were mostly published by the authors of the scoring systems. This explains why the histological regression score of CRLM specimens is rarely used in current practice[15,32]. Therefore, at present, analysis of the histological response (HR) by the TRG has no influence on whether or not adjuvant chemotherapy is administered. To our knowledge, no study has attempted to identify the predictive factors of HR after NAC.
This study aimed to analyze the HR, according to the Rubbia-Brandt TRG, on CRLM surgery performed after NAC. It also sought to identify independent predictive factors of a good response, and to analyze the influence of this response on DFS and overall.
From January 2006 to December 2013, patients who underwent surgery for CRLMs after NAC in our department were included. They were retrospectively analyzed with regard to their pre-treatment characteristics. Patients for whom the primary tumor was resected or who had chemotherapy at another center were also included.
The patients were separated into two groups based on their HR, according to Rubbia-Brandt TRG. Based on their TRG, each patient was either assigned to the responder (R) group (TRG 1, 2, and 3) or to the non-responder (NR) group (TRG 4 and 5).
For each patient, the following preoperative parameters were included prospectively: Age, body mass index, American Society of Anesthesiologists’ score, history of liver surgery or a liver procedure (e.g., portal vein embolization or drainage), date when the metastases were discovered, the existence of extra-hepatic metastases, NAC (e.g., the number of treatment sessions and the types of chemotherapy), associated targeted therapy and type, radiological evaluation (e.g., the morphological criteria of the response to NAC according to RECIST, mRECIST or CHOI criteria[22-28]), two-stage hepatectomy, the surgical strategy proposed by the multidisciplinary team meeting, the location of the primary, the treatment date, and the lymphatic status of the primary tumor. Perioperative parameters included: The types of procedures and number of segments resected. The postoperative parameters included: The length of hospitalization, occurrence of a severe or mild (according to the Clavien-Dindo classification) medical or surgical complication, mortality at 30 d, repeat procedures, initiation of postoperative chemotherapy, and the type of chemotherapy. The histological data comprised: The TRG, number of lesions on the resected specimens, resection margins (a resection was considered to be R0 when the smallest microscopic margin was more than or equal to 1 mm), presence of emboli, tumor differentiation grade, and size of the largest metastasis. The follow-up data comprised: The most recent update and status, date of death (if applicable), date of recurrence (if any) and its location.
The patients’ records were assessed at a multidisciplinary team meeting that included at least one radiologist, one liver surgeon, one digestive oncologist and one pa-thologist.
The minimal time period between the end of NAC and the surgery was 4 to 6 wk. Surgical resections were anatomical or non-anatomical (atypical resections), and combined or not combined with perioperative radiofrequency ablation (RFA) according to the rules regarding size and localization for this method. Surgical resections were performed in one or two steps irrespective of the strategy chosen (i.e., conventional, liver-first, or combined). Surgical resections were most often performed by laparotomy, and occasionally by laparoscopy if the anatomical and oncological conditions suited this approach. A perioperative ultrasound scan was systematically carried out to explore the liver disease, and it was used to guide the identification of the hepatectomy cuts in order to obtain an adequate margin. In the case of RFA, this was done perioperatively by two of our hospital's experienced radiologists. Ultrasound with a contrast agent (SonoVue) was sometimes used in case of difficulty viewing the anatomical area.
All of the histology slides were re-evaluated in a blind manner by the same specialized pathologist. The slides had already been fixed, embedded in paraffin, cut, spread, and colored according to standard pathology methods. These slides were microscopically analyzed according to the Rubbia-Brandt TRG score. For the resected specimens that had several tumors, and in case of a dissociated response, the worst TRG was taken into account. The following criteria were also analyzed: The existence of a “dangerous halo”, thickness of the invasive front, ablation margin, degree of tumor differentiation, and presence of vascular emboli.
Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
The search for factors predictive of the response rate was based on percentage comparison tests (chi-squared or Fisher’s exact tests, according to the theoretical sizes) for the qualitative variables, and on comparison of means (Student’s t-test, in case of normalcy and equality of the variances) or distribution tests (the Mann-Whitney non-parametric test) for the quantitative variables. The predictive factors associated with the response at a threshold of 20% in the univariate analysis were introduced in this logistic regression model. The final model including the variables that were significantly and independently associated with the response rate was obtained by stepwise regression. The intermediate nested models were compared using the likelihood ratio test. The interactions between the independent variables of the final model were identified (they were all non-significant). The adequacy of the model for the data was tested. The degree of significance was set as P < 0.05. Firstly, the analysis of TRG impact on overall survival (OS) was based on a bivariate comparison of Kaplan-Meier survival curves using the log-rank test. The survival curves were described with the use of the survival median (and the interquartile range) and hazard ratio (confidence interval at 95%) based on a Cox model. Secondly, the analysis of the independent influence of TRG on OS was based on a Cox model adjusted according to other predictive factors. The predictive factors (excluding recurrence) associated with OS at the threshold of 20% in the univariate analysis were jointly introduced with the TRG in this Cox model. The final model, including the variables that were significantly and independently associated with OS, was obtained by the stepwise regression method. The intermediate nested models were compared using the likelihood ratio test. The interactions between the TRG and the independent variables of the final model were identified (in particular, the interaction between the TRG and postoperative chemotherapy was identified and, if necessary, this was done during the stepwise regression procedure). The level of significance was set at 5% (P < 0.05). The conditions for application (log-linearity and proportional risks) of the Cox model were verified. The quantitative variables were dichotomized by the median.
Between January 2006 and December 2013, 521 liver resections for CLRM were carried out in our department. During this period, 150 patients underwent a liver resection for synchronous colon or rectal cancer liver metastases after NAC. Of these 150 patients, 74 (49%) were responders (R) and 76 (51%) were non-responders (NR) based on the resected specimen.
As shown in Table 1, 75% of the patients had synchronous liver metastases. Sixty-three percent of the patients had a bilobar distribution of the lesions, with an average of 4 ± 3 lesions. The location of the primary tumor had a significant influence on the HR, which was better for the tumors of rectal origin: 47.3% vs 25%. The presence of a radiological response to NAC was significantly associated with a good HR. In 94% of the cases, NAC included 5-fluorouracil, in 72% it included irinotecan, and for 43% of the patients it included oxaliplatin. In the majority of cases (69%), chemotherapy was combined with a targeted therapy, without this having any statistically significant effect on the HR. However, when the type of targeted therapy was examined, it was noted that the use of bevacizumab led to a greater HR: 58% vs 43%. The number of NAC treatment sessions was significantly related to the HR, and the patients who were classified as R had a median number of six treatment sessions, which is fewer than for the NR patients. The patients for whom the treatment strategy comprised a liver-first procedure (i.e., the liver resected first in case of synchronous liver metastases with the primary tumor left in place) had a significantly better HR rate. However, the patients who underwent repeat liver resections were more often histologically non-responsive.
| NR | R | P-value | Total | |
| N = 76, n (%) | N = 74, n (%) | N = 150, n (%) | ||
| Gender | ||||
| F | 33 (43.4) | 38 (51.4) | 0.33 | 71 (47.3) |
| M | 43 (56.6) | 36 (48.6) | 79 (52.7) | |
| Age, in yr | ||||
| Median | 631 | 641 | 0.91 | 63.31 |
| ASA | ||||
| 1 | 3 (3.9) | 4 (5.4) | 0.33 | 7 (4.7) |
| 2 | 70 (92.1) | 70 (94.6) | 140 (93.3) | |
| 3 | 3 (3.9) | 0 (0) | 3 (2) | |
| Number of lesions before treatment | ||||
| Median | 31 | 31 | 0.35 | 31 |
| Bilobar lesions | ||||
| No | 26 (34.2) | 30 (40.5) | 0.42 | 56 (37.3) |
| Yes | 50 (65.8) | 44 (59.5) | 94 (62.7) | |
| Synchronous liver metastases | ||||
| No | 21 (27.6) | 17 (23) | 0.51 | 38 (25.3) |
| Yes | 55 (72.4) | 57 (77) | 112 (74.7) | |
| Metachronous livers metastases | ||||
| No | 55 (72.4) | 57 (77) | 0.51 | 112 (74.7) |
| Yes | 21 (27.6) | 17 (23) | 38 (25.3) | |
| Time period if metachronous, in mo | ||||
| Median | 141 | 151 | 0.96 | 141 |
| Extra-hepatic metastases | ||||
| N | 56 (73.7) | 57 (77) | 0.63 | 113 (75.3) |
| Yes | 20 (26.3) | 17 (23) | 37 (24.7) | |
| Primary cancer | ||||
| Colon | 57 (75.0) | 39 (52.7) | 0.004 | 96 (64) |
| Rectum | 19 (25.0) | 35 (47.3) | 54 (36) | |
| N+ status primary tumor | ||||
| No | 20 (28.2) | 27 (37) | 0.26 | 47 (32.6) |
| Yes | 51 (71.8) | 46 (63) | 97 (67.4) | |
| Cytotoxic chemotherapy | ||||
| 5-FU | 72 (94.7) | 69 (93.2) | 0.74 | 141 (94) |
| Oxaliplatin | 33 (43.4) | 31 (41.9) | 0.85 | 64 (42.7) |
| Irinotecan | 54 (71.1) | 54 (73) | 0.79 | 108 (72) |
| Capecitabine | 1 (1.3) | 1 (1.4) | 1 | 2 (1.3) |
| Raltitrexed | 0 (0.0) | 1 (1.4) | 0.49 | 1 (0.7) |
| Targeted preoperative therapy | ||||
| No | 24 (31.6) | 23 (31.1) | 0.95 | 47 (31.3) |
| Yes | 52 (68.4) | 51 (68.9) | 103 (68.7) | |
| Type of targeted therapy | ||||
| None | 24 (31.6) | 23 (31.1) | 0.056 | 47 (31.3) |
| Bevacizumab | 33 (43.4) | 43 (58.1) | 76 (50.7) | |
| Cetuximab | 17 (22.4) | 6 (8.1) | 23 (15.3) | |
| Panitumumab | 1 (1.3) | 2 (2.7) | 3 (2) | |
| Aflibercept | 1 (1.3) | 0 (0) | 1 (0.7) | |
| Number of preoperative treatment sessions | ||||
| Median | 8* | 6* | 0.038 | 7.5* |
| Response to preoperative chemotherapy | ||||
| No | 25 (32.9) | 13 (17.6) | 38 (25.3) | |
| Yes | 51 (67.1) | 61 (82.4) | 0.031 | 112 (74.7) |
| Liver-first strategy | ||||
| No | 74 (97.4) | 61 (82.4) | 0.002 | 135 (90) |
| Yes | 2 (2.6) | 13 (17.6) | 15 (10) | |
| Combined surgery | ||||
| No | 72 (94.7) | 68 (91.9) | 0.53 | 140 (93.3) |
| Yes | 4 (5.3) | 6 (8.1) | 10 (6.7) | |
| Two-stage procedure | ||||
| No | 54 (71.1) | 60 (81.1) | 0.15 | 114 (76) |
| Yes | 22 (28.9) | 14 (18.9) | 36 (24) | |
| Repeat hepatectomy | ||||
| No | 63 (82.9) | 69 (93.2) | 0.051 | 132 (88) |
| Yes | 13 (17.1) | 5 (6.8) | 18 (12) | |
| Hepatectomy | ||||
| Minor | 39 (51.3) | 33 (44.6) | 0.41 | 72 (48) |
| Major | 37 (48.7) | 41 (55.4) | 78 (52) | |
The median number of lesions found on the resected specimens was three (1.0–4.0). The rate of resection with healthy margins (R0) was 78%. There were multiple lesions in 72% of the cases. In the group of patients classified as R, there were significantly more homogeneous HR, fewer R1 resections, and fewer vascular neoplastic microemboli (VNME) (Table 2).
| NR | R | P-value | Total | |
| N = 76,n (%) | N = 74,n (%) | N = 150,n (%) | ||
| Dissociated histological response | ||||
| No | 33 (43.4) | 55 (74.3) | 0.0001 | 88 (58.7) |
| Yes | 43 (56.6) | 19 (25.7) | 62 (41.3) | |
| Number of lesions on specimen | ||||
| Median | 31 | 21 | 0.07 | 31 |
| Lesions on specimen | ||||
| Single | 18 (23.7) | 24 (32.4) | 0.23 | 42 (28) |
| Multiple | 58 (76.3) | 50 (67.6) | 108 (72) | |
| Lesions on specimen | ||||
| ≤ 3 | 46 (60.5) | 53 (71.6) | 0.15 | 99 (66) |
| > 3 | 30 (39.5) | 21 (28.4) | 51 (34) | |
| Resection | ||||
| R0 | 52 (68.4) | 65 (87.8) | 0.004 | 117 (78) |
| R1 | 24 (31.6) | 9 (12.2) | 33 (22) | |
| Emboli | ||||
| No | 60 (78.9) | 68 (91.9) | 0.025 | 128 (85.3) |
| Yes | 16 (21.1) | 6 (8.1) | 22 (14.7) | |
| Size of the largest metastasis | ||||
| Median | 2.91 | 31 | 0.43 | 31 |
| Differentiation | ||||
| Unclassifiable | 2 (2.6) | 5 (6.8) | 0.45 | 7 (4.7) |
| Undifferentiated/barely differentiated | 5 (6.6) | 3 (4.1) | 8 (5.3) | |
| Moderately differentiated | 30 (39.5) | 34 (45.9) | 64 (42.7) | |
| Well-differentiated | 39 (51.3) | 32 (43.2) | 71 (47.3) | |
The postoperative mortality was 0.7% (one patient died of hepatocellular insufficiency), with a complication rate of 38% (Table 3). The average length of the hospital stay was 12.5 ± 7 d. Postoperative chemotherapy was administered to 55% of the patients, and in 83% of these cases, the chemotherapy protocol was similar to the one administered preoperatively. There was no significant difference between the R group and the NR group in terms of the choice of chemotherapy type. Regardless of the location, the tumor recurred in 89% of the cases. NR patients had significantly more recurrences in the liver.
| NR | R | P-value | Total | |
| N = 76, n (%) | N = 74, n (%) | N = 150, n (%) | ||
| Complication | ||||
| No | 45 (59.2) | 48 (64.9) | 0.48 | 93 (62) |
| Yes | 31 (40.8) | 26 (35.1) | 57 (38) | |
| Medical complication | ||||
| No | 53 (69.7) | 54 (73) | 0.66 | 107 (71.3) |
| Yes | 23 (30.3) | 20 (27) | 43 (28.7) | |
| Surgical complication | ||||
| No | 57 (75) | 61 (82.4) | 0.27 | 118 (78.7) |
| Yes | 19 (25) | 13 (17.6) | 32 (21.3) | |
| Length of the hospitalization | ||||
| Median | 111 | 111 | 0.36 | 111 |
| Postoperative chemotherapy | ||||
| Absent | 36 (47.4) | 31 (41.9) | 0.33 | 67 (44.7) |
| Similar | 31 (40.8) | 38 (51.4) | 69 (46) | |
| Different | 9 (11.8) | 5 (6.8) | 14 (9.3) | |
| Recurrence | ||||
| No | 5 (6.6) | 12 (16.2) | 0.06 | 17 (11.3) |
| Yes | 71 (93.4) | 62 (83.8) | 133 (88.7) | |
| Recurrence | ||||
| Hepatic | 59 (77.6) | 39 (52.7) | 0.001 | 98 (65.3) |
| Extra-hepatic | 51 (67.1) | 46 (62.2) | 0.53 | 97 (64.7) |
| Multiple sites | 43 (56.6) | 33 (44.6) | 0.14 | 76 (50.7) |
The multivariate analysis identified five predictive factors of HR. Three were predictive of non-response (NR): More than seven NAC sessions, the absence of a radiological response after NAC, and a repeat hepatectomy. Two were predictive of a good response (R): Rectal origin of the primary tumor and liver-first strategy (Table 4).
| Odds ratio | 95%CI | P-value | |
| Number of preoperative treatment sessions | |||
| ≤ 7 | 1 | - | - |
| > 7 | 0.47 | [0.23; 0.98] | 0.044 |
| Radiological response to preoperative chemotherapy | |||
| Yes | 1 | - | - |
| No | 0.33 | [0.14; 0.79] | 0.013 |
| Liver-first strategy | |||
| No | 1 | - | - |
| Yes | 5.11 | [1.06; 24.73] | 0.042 |
| Repeat hepatectomy | |||
| No | 1 | - | - |
| Yes | 0.30 | [0.09; 0.97] | 0.045 |
| Primary cancer | |||
| Colon | 1 | - | - |
| Rectal | 2.81 | [1.28; 6.14] | 0.010 |
The median survival of the patients was 6 yr (ranging from 4–7.5 yr).
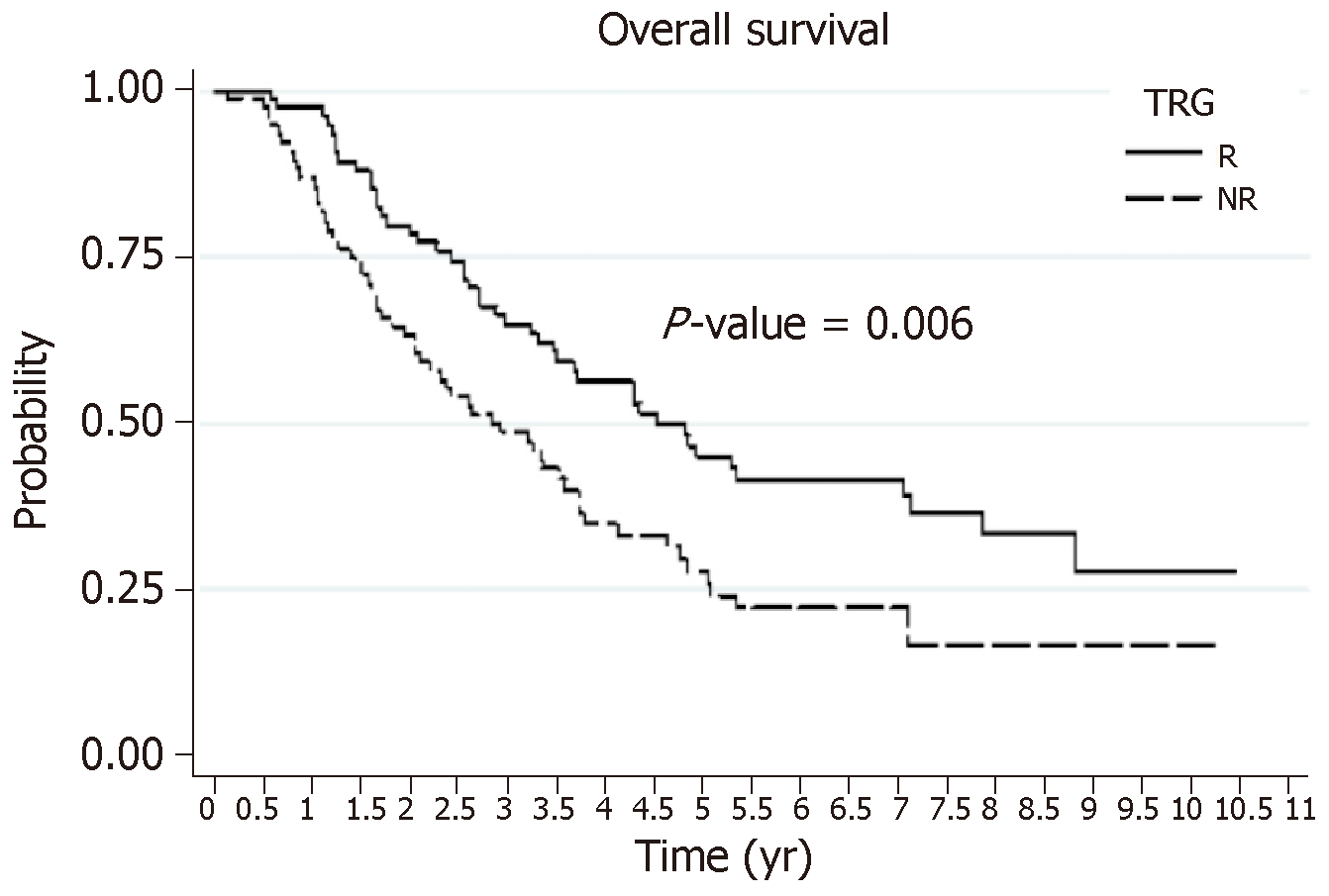
Overall survival: The OS rates at 3 yr and 5 yr were 57% and 36%, respectively. These OS rates were significantly better for patients with an HR (the R group): 65% and 45%, respectively, vs 47% and 26%, respectively, for the NR group (Figure 1). The identification of independent prognostic factors of OS (Table 5) revealed that the effect of HR was dependent on the presence or absence of VNME. In patients without VNME, HR was an independent prognostic factor of OS, while this was no longer the case in the presence of VNME upon analysis of the ablated specimen. Other factors that were prognostic of poor OS were: Male gender, preoperative targeted therapy, a two-stage hepatectomy protocol, and a N+ status of the primary tumor.
| No vascular neoplastic microemboli on histology | |||
| Hazard ratio | 95%CI | P-value | |
| Responder | |||
| NR | 1 | [0.35; 0.86] | 0.010 |
| R | 0.55 | ||
| Gender | |||
| F | 1 | [1.11; 2.58] | 0.014 |
| M | 1.70 | ||
| Targeted preoperative therapy | |||
| No | 1 | [1.40; 3.62] | 0.001 |
| Yes | 2.25 | ||
| Two-stage procedure | |||
| No | 1 | [1.18; 3.06] | 0.008 |
| Yes | 1.90 | ||
| N+ status of the primary tumor | |||
| No | 1 | [1.10; 2.84] | 0.018 |
| Yes | 1.77 | ||
| Presence of vascular neoplastic microemboli on histology | |||
| Hazard ratio | 95%CI | P-value | |
| Gender | |||
| F | 1 | [1.11; 2.58] | 0.014 |
| M | 1.70 | ||
| Targeted preoperative therapy | |||
| No | 1 | [1.40; 3.62] | 0.001 |
| Yes | 2.25 | ||
| Two-stage procedure | |||
| No | 1 | [1.18; 3.06] | 0.008 |
| Yes | 1.90 | ||
| N+ status of the primary tumor | |||
| No | 1 | [1.10; 2.84] | 0.018 |
| Yes | 1.77 | ||
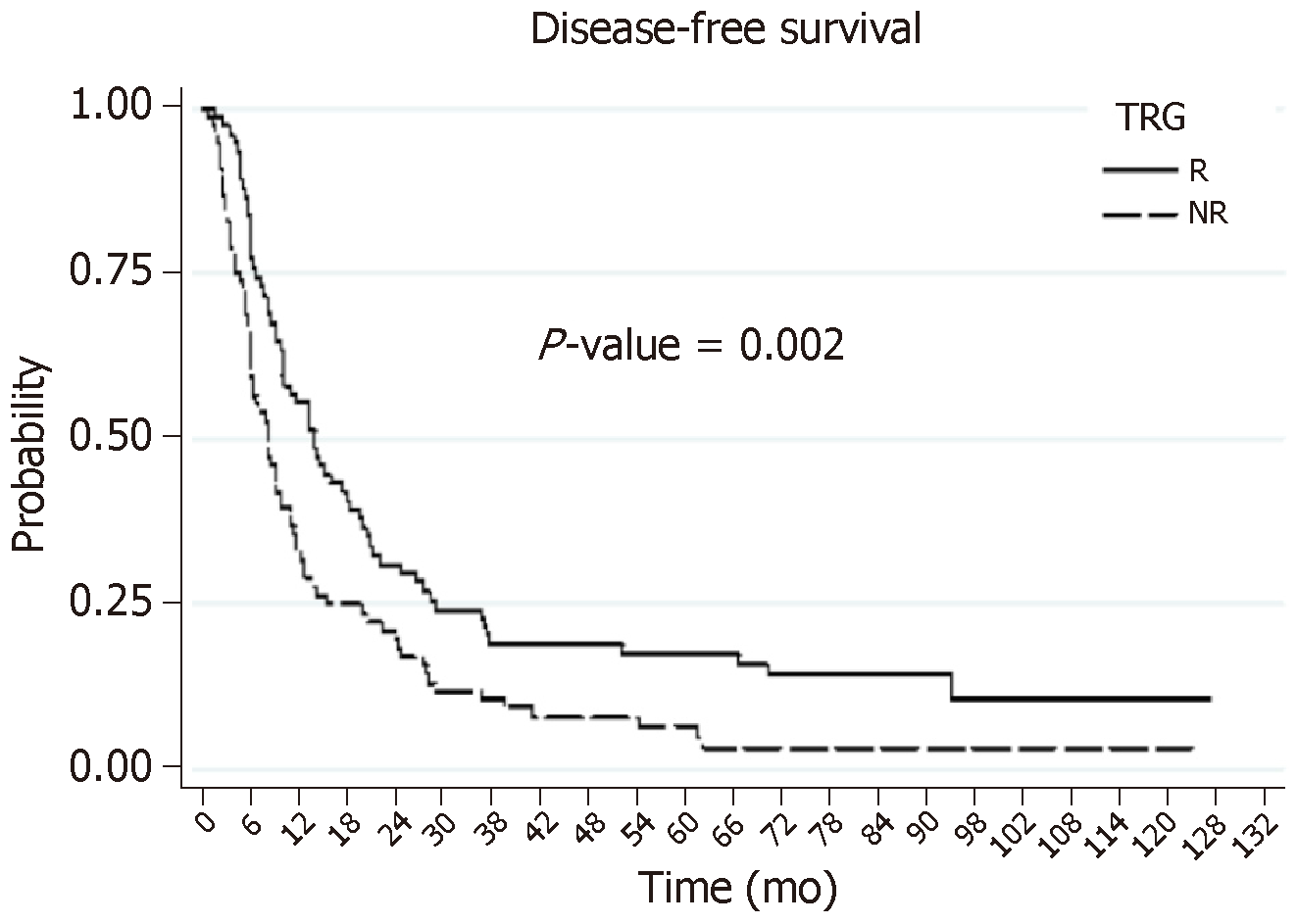
DFS: The DFS rates at 3 yr and 5 yr were 14% and 11%, respectively. These DFS survival rates were significantly better for patients with HR (the R group): 19% and 11%, respectively, vs 9% and 5%, respectively, for the NR patients (Figure 2). The search for independent prognostic factors of DFS identified one good prognostic factor, which was the presence of HR with NAC (the R group, P = 0.013), and four poor prognostic factors: NAC with targeted therapy (P = 0.004), more than three preoperative metastases (P = 0.001), R1 resection (P = 0.022), and a size greater than 3 cm for the largest metastasis (P = 0.011).
Our work demonstrates that the HR of CRLMs operated on after NAC has a significant influence on OS and DFS, as evaluated by the Rubbia-Brandt score. In this population, the OS rate at 5 yr was equivalent to that stated in the literature, which varies from 18%-58%[8,10-13,33-40]. However, the DFS in this population was at the lower limit of that reported in the literature, which varies from 11%-37%[10,35-39]. This can likely be explained by the particularly severe liver disease in our population of selected patients. Indeed, 75% of our patients had synchronous metastases, while in the literature this rate varies from 15%-50%[2,7,8,38]. We noted HR in 49% of the patients (TRG 1 to 3), including five patients (7%) with complete tumor regression (TRG1). In research published in 2007, the Rubbia-Brandt team found a good HR rate of 27%-82%, which varied according to the chemotherapy protocol[31]. Viganò et al[13] had figures similar to ours, with a good HR (TRG 1 to 3) for 44% of the patients and complete tumor regression (TRG1) for 8% of the patients, as was the case with the series reported by Loupakis et al[41], with HR for 48% and complete tumor regression (TRG 1) for 14% of the patients. We used the Rubbia-Brandt score, as it was the first to define standardized histological criteria of response to NAC in cases of CRLMs, and also because of its use in the management of rectal cancers[42].
We identified five independent predictive factors of HR after NAC. Three factors were predictive of the absence of HR (more than seven NAC treatment sessions, the absence of a radiological response after NAC, and repeat hepatectomy). The high number of NAC treatment sessions is probably a reflection of tumor chemotherapy resistance. This factor likely indicates that the earlier the response (evaluated radiologically) (i.e., before the first seven treatment sessions), the greater the chances of a good associated HR. In the study by Viganò et al[13], the number of treatment sessions also appeared to be a predictive factor of HR based on multivariate analysis, with a better HR when there were fewer than six chemotherapy treatment sessions. The absence of a radiological response (i.e., stability or progression) after NAC is recognized as a major factor for cancelling or delaying surgery after NAC[20,43-45]. The influence of a repeat hepatectomy on HR has not been reported in the literature to date. One of the explanations, aside from the actual severity of a liver recurrence after the first hepatectomy, could be a change in the chemotherapy sensitivity of the recurring tumor. In our series, rectal origin of the primary tumor was associated with a three-fold greater probability of having a good HR. We found two studies in the literature for which this location of the primary tumor was a poor prognostic factor of OS[46,47]. It has also been reported that a primary tumor that originated in the ascending colon is a poor prognostic factor[48-53]. A liver-first strategy in case of synchronous CRLMs was a factor predictive of a good HR. This can probably be explained by the fact that only the patients who were radiological responders after NAC have access to this strategy. To our knowledge, only two studies in the literature, namely Viganò et al[13] and Pietrantonio et al[54], have examined the factors predictive of a HR of CRLMs, based on multivariate analysis involving 323 and 93 patients, respectively. Viganò et al[13] were able to identify four predictive factors of a good response, which were the combination of oxaliplatin and irinotecan, the radiological response, the largest lesion ≤ 5 cm, and a carcinoembryonic antigen level ≤ 5 ng/mL.
OS was influenced by the HR according to whether or not there were VNME on the resected specimen. In fact, in the absence of VNME, the HR is an independent factor of a good prognosis for OS. Although the impact of the HR on OS has already been reported in the literature[13,41,54] , this is the first time that it has been shown that its influence is abrogated by the presence of VNME on the resected specimen, which is a known factor of poor prognosis. Therefore, tumor aggressiveness as reflected by VNME appears to be a more significant determining factor than the HR for the OS prognosis of patients. Four other independent factors of poor prognosis for OS were identified: Male gender, use of targeted therapy, two-stage hepatectomy, and N+ status of the primary tumor. Male gender[40,46,47,55-58] and a positive lymphatic status of the primary tumor[34,58-61] are prognostic factors that have been reported in the literature. The administration of NAC with a targeted therapy, which was identified in our analysis, can be explained by the fact that bevacizumab was typically used (74% of the targeted therapies). This is the treatment of choice for mutant RAS tumors, which carry worse prognoses[59,61-67]. In terms of the two-stage hepatectomies, this can be explained by the fact that 13% of our patients did not undergo the second stage of surgery due to progression of the tumor after the first procedure. These figures are comparable to those presented in the literature, which vary from 15%-31%[68-73].
DFS was significantly influenced by the HR, with 17% survival at 5 yr for the responders compared to 5% for the non-responders. A similar difference in DFS was found in the study by Rubbia-Brandt et al[31], with a three-fold better survival for the responders compared to the non-responders. Four independent factors of poor prognosis were identified: Use of neoadjuvant targeted therapy, more than three preoperative lesions, metastasis with a size greater than 3 cm, and R1 resection. The number and size of metastases[13,35,36,38,58,74], and R1 resection[35,36,38,75-77] are the typical factors of poor prognosis found in the literature.
In conclusion, this study confirmed that the HR of CRLMs after NAC has an influence on survival and, hence, warrants attention. We found, however, that this influence on OS was lacking in cases of particularly aggressive disease, with microscopic vascular invasion in the histological analysis. Two simple criteria enable the prediction of HR after NAC: More than seven treatment sessions and the absence of a radiological response.
Colorectal cancer is the third most common cancer in men and the second most common in women worldwide. Almost a third of the patients has or will develop liver metastases. Neoadjuvant chemotherapy (NAC) has recently become nearly systematic prior to surgery of colorectal livers metastases (CRLMs). The response to NAC is evaluated by radiological imaging according to morphological criteria. More recently, the response to NAC has been evaluated based on histological criteria of the resected specimen. The most often used score is the tumor regression grade (TRG), which considers the necrosis, fibrosis, and number of viable tumor cells.
Few studies to date have documented the influence of TRG on patient survival, and they were mostly published by the authors of the scoring systems. This explains why the histological regression score of CRLM specimens is rarely used in current practice[15,32]. Therefore, at present, analysis of the histological response by the TRG has no influence on whether or not adjuvant chemotherapy is administered. To our knowledge, no study has attempted to identify the predictive factors of histological response after NAC.
Our research aimed to analyze the histological response, according to the Rubbia-Brandt TRG, on CRLM surgery performed after NAC. It also sought to identify independent predictive factors of a good response, and to analyze the influence of this response on overall and disease-free survival.
From January 2006 to December 2013, 150 patients who had undergone surgery for CRLMs after NAC were included. The patients were separated into two groups based on their histological response, according to Rubbia-Brandt TRG. Based on their TRG, each patient was either assigned to the responder (R) group (TRG 1, 2, and 3) or to the non-responder (NR) group (TRG 4 and 5). All of the histology slides were re-evaluated in a blind manner by the same specialized pathologist. Univariate and multivariate analyses were performed.
Seventy-four patients were classified as responders and 76 as non-responders. The postoperative mortality rate was 0.7%, with a complication rate of 38%. Multivariate analysis identified five predictive factors of histological response. Three were predictive of non-response (NR): More than seven NAC sessions, absence of a radiological response after NAC, and repeat hepatectomy (P < 0.005). Two were predictive of a good response (R): Rectal origin of the primary tumor and a liver-first strategy (P < 0.005). The overall survival was 57% at 3 yr and 36% at 5 yr. The disease-free survival rates were 14% at 3 yr and 11% at 5 yr. Factors contributing to a poor prognosis for the DFS were: No histological response after NAC, largest metastasis > 3 cm, more than three preoperative metastases, R1 resection, and the use of a targeted therapy with NAC (P < 0.005).
The histological response of CRLMs after NAC has an influence on survival, hence warranting consideration. We found, however, that this influence on overall survival was lacking in cases of particularly aggressive disease, with microscopic vascular invasion upon histological analysis.
Two simple criteria enable the prediction of a histological response after NAC: More than seven treatment sessions and absence of a radiological response.
STROBE Statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hori T, Marin JJG, Muhammad JS, Shibata T, Wang YF S-Editor: Yan JP L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3652] [Article Influence: 304.3] [Reference Citation Analysis (2)] |
| 2. | Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 1005] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 3. | Binder-Foucard F, Bossard N, Delafosse P, Belot A, Woronoff AS, Remontet L; French network of cancer registries (Francim). Cancer incidence and mortality in France over the 1980-2012 period: solid tumors. Rev Epidemiol Sante Publique. 2014;62:95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2905] [Article Influence: 363.1] [Reference Citation Analysis (3)] |
| 5. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3274] [Article Influence: 409.3] [Reference Citation Analysis (3)] |
| 6. | Cowppli-Bony A, Uhry Z, Remontet L, Voirin N, Guizard AV, Trétarre B, Bouvier AM, Colonna M, Bossard N, Woronoff AS, Grosclaude P; French Network of Cancer Registries (FRANCIM). Survival of solid cancer patients in France, 1989-2013: a population-based study. Eur J Cancer Prev. 2017;26:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol. 2011;9:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 9. | Kelly ME, Spolverato G, Lê GN, Mavros MN, Doyle F, Pawlik TM, Winter DC. Synchronous colorectal liver metastasis: a network meta-analysis review comparing classical, combined, and liver-first surgical strategies. J Surg Oncol. 2015;111:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O'Callaghan C, Langer B, Martignoni G, Bouché O, Lazorthes F, Van Cutsem E, Bedenne L, Moore MJ, Rougier P. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906-4911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 11. | Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1105] [Cited by in RCA: 1117] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 12. | van Amerongen MJ, van der Stok EP, Fütterer JJ, Jenniskens SF, Moelker A, Grünhagen DJ, Verhoef C, de Wilt JH. Short term and long term results of patients with colorectal liver metastases undergoing surgery with or without radiofrequency ablation. Eur J Surg Oncol. 2016;42:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Viganò L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013;258:731-40; discussion 741-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Gruenberger B, Scheithauer W, Punzengruber R, Zielinski C, Tamandl D, Gruenberger T. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2414] [Article Influence: 268.2] [Reference Citation Analysis (31)] |
| 16. | Hebbar M, Chibaudel B, André T, Mineur L, Smith D, Louvet C, Dutel JL, Ychou M, Legoux JL, Mabro M, Faroux R, Auby D, Brusquant D, Khalil A, Truant S, Hadengue A, Dalban C, Gayet B, Paye F, Pruvot FR, Bonnetain F, Landi B, Flesch M, Carola E, Martin P, Vaillant E, de Gramont A; Group Coopérateur Multidisciplinaire en Oncologie (GERCOR) Group. FOLFOX4 versus sequential dose-dense FOLFOX7 followed by FOLFIRI in patients with resectable metastatic colorectal cancer (MIROX): a pragmatic approach to chemotherapy timing with perioperative or postoperative chemotherapy from an open-label, randomized phase III trial. Ann Oncol. 2015;26:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | de Haas RJ, Wicherts DA, Andreani P, Pascal G, Saliba F, Ichai P, Adam R, Castaing D, Azoulay D. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg. 2011;253:1069-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Torzilli G, Adam R, Viganò L, Imai K, Goransky J, Fontana A, Toso C, Majno P, de Santibañes E. Surgery of Colorectal Liver Metastases: Pushing the Limits. Liver Cancer. 2016;6:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Wang CC, Li J. An update on chemotherapy of colorectal liver metastases. World J Gastroenterol. 2012;18:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1434] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 21. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 915] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 22. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13071] [Article Influence: 522.8] [Reference Citation Analysis (0)] |
| 23. | Jang HJ, Kim BC, Kim HS, Kim JH, Song HH, Kim JB, Park JJ, Yoon SN, Woo JY, Zang DY. Comparison of RECIST 1.0 and RECIST 1.1 on computed tomography in patients with metastatic colorectal cancer. Oncology. 2014;86:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Boonsirikamchai P, Asran MA, Maru DM, Vauthey JN, Kaur H, Kopetz S, Loyer EM. CT findings of response and recurrence, independent of change in tumor size, in colorectal liver metastasis treated with bevacizumab. AJR Am J Roentgenol. 2011;197:W1060-W1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Chung WS, Park MS, Shin SJ, Baek SE, Kim YE, Choi JY, Kim MJ. Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria. AJR Am J Roentgenol. 2012;199:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21477] [Article Influence: 1342.3] [Reference Citation Analysis (1)] |
| 27. | Shindoh J, Loyer EM, Kopetz S, Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA, Charnsangavej C, Aloia TA, Vauthey JN. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566-4572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Suzuki K, Muto Y, Ichida K, Fukui T, Takayama Y, Kakizawa N, Kato T, Hasegawa F, Watanabe F, Kaneda Y, Kikukawa R, Saito M, Tsujinaka S, Futsuhara K, Takata O, Noda H, Miyakura Y, Kiyozaki H, Konishi F, Rikiyama T. Morphological response contributes to patient selection for rescue liver resection in chemotherapy patients with initially un-resectable colorectal liver metastasis. Oncol Lett. 2017;14:1491-1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Adam R, Aloia T, Lévi F, Wicherts DA, de Haas RJ, Paule B, Bralet MP, Bouchahda M, Machover D, Ducreux M, Castagne V, Azoulay D, Castaing D. Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy. J Clin Oncol. 2007;25:4593-4602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Blazer DG, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D, Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK, Vauthey JN. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344-5351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 475] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 31. | Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, Chaussade S, Mentha G, Terris B. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 391] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 32. | Knijn N, de Ridder JA, Punt CJ, de Wilt JH, Nagtegaal ID. Histopathological evaluation of resected colorectal cancer liver metastases: what should be done? Histopathology. 2013;63:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1029] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 34. | Matias M, Casa-Nova M, Faria M, Pires R, Tato-Costa J, Ribeiro L, Costa L. Prognostic Factors after Liver Resection for Colorectal Liver Metastasis. Acta Med Port. 2015;28:357-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Imai K, Allard MA, Benitez CC, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Baba H, Adam R. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist. 2016;21:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Hallet J, Sa Cunha A, Adam R, Goéré D, Bachellier P, Azoulay D, Ayav A, Grégoire E, Navarro F, Pessaux P; French Colorectal Liver Metastases Working Group, Association Française de Chirurgie (AFC). Factors influencing recurrence following initial hepatectomy for colorectal liver metastases. Br J Surg. 2016;103:1366-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay D, Bismuth H, Castaing D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26:3672-3680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Angelsen JH, Viste A, Løes IM, Eide GE, Hoem D, Sorbye H, Horn A. Predictive factors for time to recurrence, treatment and post-recurrence survival in patients with initially resected colorectal liver metastases. World J Surg Oncol. 2015;13:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | de Haas RJ, Wicherts DA, Salloum C, Andreani P, Sotirov D, Adam R, Castaing D, Azoulay D. Long-term outcomes after hepatic resection for colorectal metastases in young patients. Cancer. 2010;116:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 41. | Loupakis F, Schirripa M, Caparello C, Funel N, Pollina L, Vasile E, Cremolini C, Salvatore L, Morvillo M, Antoniotti C, Marmorino F, Masi G, Falcone A. Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer. 2013;108:2549-2556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Miccichè F, Ricci R, Morganti AG, Gambacorta MA, Maurizi F, Coco C. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 328] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 43. | Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052-61; discussion 1061-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 758] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 44. | Nordlinger B, Vauthey JN, Poston G, Benoist S, Rougier P, Van Cutsem E. The timing of chemotherapy and surgery for the treatment of colorectal liver metastases. Clin Colorectal Cancer. 2010;9:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Tabernero J, Teh C, Van Cutsem E; Jean-Nicolas Vauthey of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 411] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 46. | Kattan MW, Gönen M, Jarnagin WR, DeMatteo R, D’Angelica M, Weiser M, Blumgart LH, Fong Y. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 623] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 49. | Adam R, de Haas RJ, Wicherts DA, Vibert E, Salloum C, Azoulay D, Castaing D. Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg. 2011;253:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 50. | Modest DP, Schulz C, von Weikersthal LF, Quietzsch D, von Einem JC, Schalhorn A, Vehling-Kaiser U, Laubender RP, Giessen C, Stintzing S, Heinemann V. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment). Anticancer Drugs. 2014;25:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, Passalacqua R, Sgroi G, Barni S. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 534] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 52. | O'Dwyer PJ, Manola J, Valone FH, Ryan LM, Hines JD, Wadler S, Haller DG, Arbuck SG, Weiner LM, Mayer RJ, Benson AB. Fluorouracil modulation in colorectal cancer: lack of improvement with N -phosphonoacetyl- l -aspartic acid or oral leucovorin or interferon, but enhanced therapeutic index with weekly 24-hour infusion schedule--an Eastern Cooperative Oncology Group/Cancer and Leukemia Group B Study. J Clin Oncol. 2001;19:2413-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ, Heinemann V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 527] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 54. | Pietrantonio F, Mazzaferro V, Miceli R, Cotsoglou C, Melotti F, Fanetti G, Perrone F, Biondani P, Muscarà C, Di Bartolomeo M, Coppa J, Maggi C, Milione M, Tamborini E, de Braud F. Pathological response after neoadjuvant bevacizumab- or cetuximab-based chemotherapy in resected colorectal cancer liver metastases. Med Oncol. 2015;32:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Sakamoto M, Fukuda H. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br J Surg. 1999;86:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 56. | Hohenberger P, Schlag PM, Gerneth T, Herfarth C. Pre- and postoperative carcinoembryonic antigen determinations in hepatic resection for colorectal metastases. Predictive value and implications for adjuvant treatment based on multivariate analysis. Ann Surg. 1994;219:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | John SK, Robinson SM, Rehman S, Harrison B, Vallance A, French JJ, Jaques BC, Charnley RM, Manas DM, White SA. Prognostic factors and survival after resection of colorectal liver metastasis in the era of preoperative chemotherapy: an 11-year single-centre study. Dig Surg. 2013;30:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Viganò L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, Ijzermans JN, Mirza DF, Elias D, Adam R. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21:1276-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 59. | Ribero D, Viganò L, Amisano M, Capussotti L. Prognostic factors after resection of colorectal liver metastases: from morphology to biology. Future Oncol. 2013;9:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Zhou L, Zhag G, Tang T, Lian R, Wang W. Retrospective analysis of clinical and pathologic risk factors in liver resection for hepatic colorectal metastases. J Cancer Res Ther. 2013;9 Suppl:S178-S182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Brudvik KW, Jones RP, Giuliante F, Shindoh J, Passot G, Chung MH, Song J, Li L, Dagenborg VJ, Fretland ÅA, Røsok B, De Rose AM, Ardito F, Edwin B, Panettieri E, Larocca LM, Yamashita S, Conrad C, Aloia TA, Poston GJ, Bjørnbeth BA, Vauthey JN. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann Surg. 2019;269:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 62. | Denbo JW, Yamashita S, Passot G, Egger M, Chun YS, Kopetz SE, Maru D, Brudvik KW, Wei SH, Conrad C, Vauthey JN, Aloia TA. RAS Mutation Is Associated with Decreased Survival in Patients Undergoing Repeat Hepatectomy for Colorectal Liver Metastases. J Gastrointest Surg. 2017;21:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA, Donehower RC, Hirose K, Ahuja N, Pawlik TM, Choti MA. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119:4137-4144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 64. | Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, Sobrero A, Ychou M; European Colorectal Metastases Treatment Group; Sixth International Colorectal Liver Metastases Workshop. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 65. | Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, Curley SA, Aloia TA, Maru DM. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619-26; discussion 626-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 66. | Zimmitti G, Shindoh J, Mise Y, Kopetz S, Loyer EM, Andreou A, Cooper AB, Kaur H, Aloia TA, Maru DM, Vauthey JN. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 67. | Brudvik KW, Mise Y, Chung MH, Chun YS, Kopetz SE, Passot G, Conrad C, Maru DM, Aloia TA, Vauthey JN. RAS Mutation Predicts Positive Resection Margins and Narrower Resection Margins in Patients Undergoing Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2016;23:2635-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 68. | Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 536] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 69. | Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-49; discussion 1049-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 364] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 70. | Adam R, Miller R, Pitombo M, Wicherts DA, de Haas RJ, Bitsakou G, Aloia T. Two-stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am. 2007;16:525-536, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Regimbeau JM, Cosse C, Kaiser G, Hubert C, Laurent C, Lapointe R, Isoniemi H, Adam R. Feasibility, safety and efficacy of two-stage hepatectomy for bilobar liver metastases of colorectal cancer: a LiverMetSurvey analysis. HPB (Oxford). 2017;19:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | de Haas RJ, Adam R, Wicherts DA, Azoulay D, Bismuth H, Vibert E, Salloum C, Perdigao F, Benkabbou A, Castaing D. Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. Br J Surg. 2010;97:1279-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 73. | Turrini O, Viret F, Guiramand J, Lelong B, Bège T, Delpero JR. Strategies for the treatment of synchronous liver metastasis. Eur J Surg Oncol. 2007;33:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Park MS, Yi NJ, Son SY, You T, Suh SW, Choi YR, Kim H, Hong G, Lee KB, Lee KW, Jeong SY, Park KJ, Suh KS, Park JG. Histopathologic factors affecting tumor recurrence after hepatic resection in colorectal liver metastases. Ann Surg Treat Res. 2014;87:14-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Angelsen JH, Horn A, Eide GE, Viste A. Surgery for colorectal liver metastases: the impact of resection margins on recurrence and overall survival. World J Surg Oncol. 2014;12:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318; discussion 318-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2789] [Article Influence: 107.3] [Reference Citation Analysis (1)] |
| 77. | Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715-722, discussion 722-discussion 724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 811] [Article Influence: 40.6] [Reference Citation Analysis (0)] |