Published online Mar 15, 2019. doi: 10.4251/wjgo.v11.i3.227
Peer-review started: October 23, 2018
First decision: November 29, 2018
Revised: December 12, 2018
Accepted: January 5, 2019
Article in press: January 6, 2019
Published online: March 15, 2019
Processing time: 143 Days and 15.8 Hours
It is usually difficult to adequately conduct percutaneous ultrasound-guided radiofrequency (RF) ablation for hepatocellular carcinomas (HCCs) abutting the diaphragm. Our hypothesis was that the subphrenic location of HCC could have an effect on the long-term therapeutic outcomes after hepatic resection and RF ablation.
To compare the long-term therapeutic outcomes of hepatic resection and percutaneous RF ablation for HCCs abutting the diaphragm.
A total of 143 Child-Pugh class A patients who had undergone hepatic resection (n = 80) or percutaneous ultrasound-guided RF ablation (n = 63) for an HCC (≤ 3 cm) abutting the right diaphragm were included. Cumulative local tumor progression (LTP), cumulative intrahepatic distant recurrence (IDR), disease-free survival (DFS), and overall survival (OS) rates were estimated. Prognostic factors for DFS and OS were analyzed. Complications were evaluated.
The cumulative IDR rate, DFS rate, and OS rate for the hepatic resection group and RF ablation group at 5 years were “35.9% vs 65.8%”, “64.1% vs 18.3%”, and “88.4% vs 68.7%”, respectively. Hepatic resection was an independent prognostic factor for DFS (P ≤ 0.001; hazard ratio, 0.352; 95%CI: 0.205, 0.605; with RF ablation as the reference category); however, treatment modality was not an independent prognostic factor for OS. The LTP rate was 46.6% at 5 years for the RF ablation group. The major complication rate was not significantly different between the groups (P = 0.630). The rate of occurrence of peritoneal seeding was higher in the RF ablation group (1.3% vs 9.5%, P = 0.044).
Although OS was not significantly different between patients who had gone hepatic resection or percutaneous RF ablation for HCCs abutting the diaphragm, DFS was better in the hepatic resection group.
Core tip: The aim of this study was to compare the long-term therapeutic outcomes of hepatic resection and percutaneous radiofrequency (RF) ablation for hepatocellular carcinomas abutting the diaphragm. The disease-free survival (DFS) rate was 64.1% and 18.3% for the hepatic resection group and the RF ablation group, and overall survival (OS) rate was 88.4% and 68.7% for the hepatic resection group and the RF ablation group at 5 years. The local tumor progression rate was as high as 46.6% for the RF ablation group. Although OS was not significantly different between two groups, DFS was better in the hepatic resection group.
- Citation: Song KD, Lim HK, Rhim H, Lee MW, Kang TW, Paik YH, Kim JM, Joh JW. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol 2019; 11(3): 227-237
- URL: https://www.wjgnet.com/1948-5204/full/v11/i3/227.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i3.227
Both hepatic resection and radiofrequency (RF) ablation are considered curative procedures for very early or early-stage hepatocellular carcinoma (HCC)[1]. Many studies have revealed that RF ablation is comparable to hepatic resection in terms of long-term survival for patients with early-stage HCC[2-4]. However, most studies have not taken into account the location of HCCs. Tumor location is an important factor affecting local tumor control especially for RF ablation due to its technical complexity[5].
When an HCC is located in the liver abutting the right diaphragm, an adequate accomplishment of percutaneous ultrasound (US)-guided RF ablation is difficult due to the poor sonic window resulting from lung shadowing and the potential risk of collateral thermal injury to the diaphragm. According to a preliminary study, local tumor progression (LTP) after percutaneous RF ablation was more frequent in patients with subphrenic HCCs (29%) than in nonsubphrenic HCCs (6%)[6]. To overcome this inherent limitation, many investigators have used the infusion of artificial ascites or pleural effusion. Several studies have reported that percutaneous RF ablation with infusion of artificial ascites or pleural effusion was safe and effective[7-10]. However, the LTP rate after RF ablation for subphrenic HCCs remained high even with the application of these special techniques[9]. The effect of the specific location of HCC on the long-term therapeutic outcomes after hepatic resection and RF ablation has not yet been investigated. Thus, the aim of this study was to compare the long-term therapeutic outcomes of hepatic resection vs percutaneous RF ablation for the curative treatment of HCCs abutting the diaphragm.
Our Institutional Review Board approved this retrospective study, and informed consent was waived.
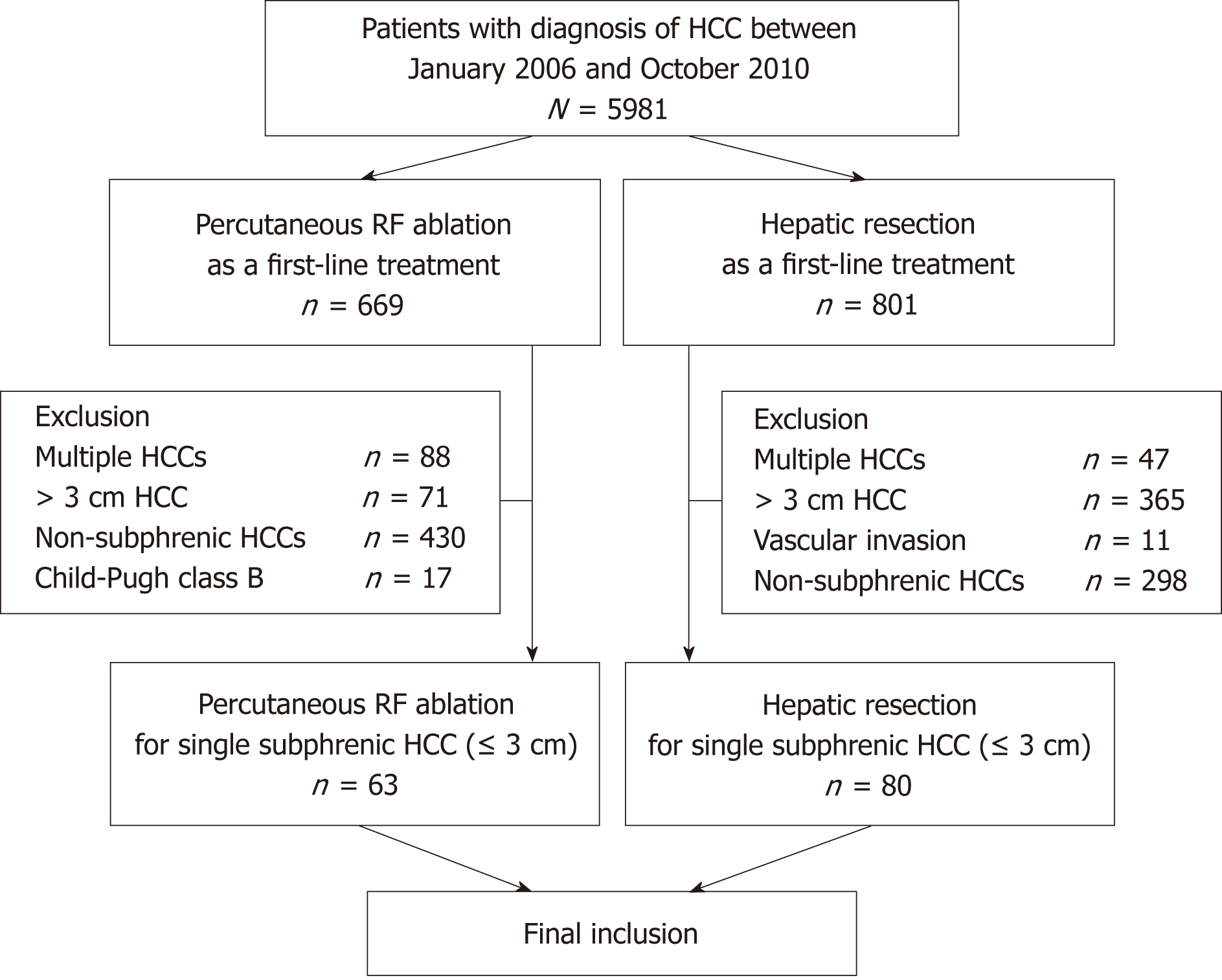
Between January 2006 and October 2010, 5981 patients were diagnosed with HCC at our institution. This study included patients from the same population as in a previous study that was conducted at our institution; however, the study design and result analysis methods are different[11]. Inclusion criteria for our study were as follows: (1) patients who had undergone percutaneous US-guided RF ablation or hepatic resection for HCC as a first-line treatment; (2) patients who had a single HCC ≤ 3 cm; (3) patients with HCC abutting the right diaphragm (subphrenic HCC); and (4) patients with Child-Pugh class A. A subphrenic HCC in our study was defined as a tumor that abutted the right diaphragm on axial or coronal images of computed tomography (CT) or magnetic resonance imaging. We excluded tumors that abutted the left diaphragm because they are different from the tumors abutting the right diaphragm in many ways, in terms of treatment. Most tumors abutting the left diaphragm are located under the heart and are, hence, considered more technically difficult to treat compared to those close to the right diaphragm. In addition, the use of artificial ascites or pleural effusion is usually ineffective for tumors abutting the left diaphragm. Instead, hepatic resection of tumors abutting the left diaphragm (especially in the left lateral segment) is easily performed either after laparotomy or with a laparoscopic approach. Finally, our study included 63 patients (49 men, 14 women; mean age, 60.3 years; range, 41–78 years) who had undergone percutaneous RF ablation and 80 patients (62 men, 18 women; mean age, 53.5 years; range, 30–78 years) who had been treated with hepatic resection. The patient inclusion flowchart is shown in Figure 1.
In 3 (4.7%) patients in the RF ablation group, HCC was confirmed histologically via percutaneous US-guided biopsy. In the remainder of the patients in the RF ablation group, HCC was diagnosed based on one of two clinical guidelines from the American Association for the Study of Liver Diseases at the time of RF ablation[1,12]. For all patients in the hepatic resection group, HCC was diagnosed histologically after hepatic resection.
The general inclusion criteria for hepatic resection at our institution were as follows: (1) a single tumor or oligonodular tumors within a monosegment of the liver; (2) an indocyanine green retention rate less than 20% at 15 min; (3) serum total bilirubin level less than 1.5 mg/dL; (4) no severe portal hypertension; and (5) no gross ascites. The inclusion criteria for percutaneous RF ablation at our institution were as follows: (1) a single tumor (≤ 5 cm in the greatest dimension) or multiple nodular tumors (three or fewer, each ≤ 3 cm in the greatest dimension); (2) Child-Pugh class A or B disease; (3) no evidence of portal vein thrombosis or extrahepatic metastasis; and (4) prothrombin time ratio > 50%, and platelet count > 50000/mm3 (50 × 109/L). Treatment modality was decided based on age, liver function reserve, tumor location, surgical risk, and patient preference by a multidisciplinary tumor board composed of hepatologists, radiologists, surgeons, and medical and radiation oncologists.
Hepatic resection was performed by one of two surgeons (JHK and JWJ) with more than 10 years of experience in hepatobiliary surgery by the end of the study. The types of hepatic resection were as follows: subsegmentectomy in 58 patients, bisegmentectomy in five patients, posterior sectionectomy in 12 patients, right hemihepatectomy in two patients, anterior sectionectomy in one patient, central hepatectomy in one patient, and extended left hemihepatectomy in one patient. As a result, anatomical resection was performed in 17 (21.3%) patients and non-anatomical resection was performed in 63 (78.8%) patients[13]. Hepatic resection was performed after laparotomy in 78 (97.5%) patients and with laparoscopy in two (2.5%) patients. RF ablation was performed by one of five interventional radiologists (MWL, DC, HR, HKL, and YK) with more than 6 years of experience in RF ablation by the end of the study. The process and method of RF ablation were the same as those described in a previous study[14]. In brief, RF ablation was performed percutaneously under the guidance of real-time US. We used internally cooled electrode systems with generators (Cool-tip RF System, Covidien, Mansfield, MA, United States; or VIVA RFA System, STARmed, Goyang, South Korea). Sedation was performed via an intravenous injection of pethidine hydrochloride (Samsung Pharmaceuticals, Seoul, South Korea) and fentanyl citrate (GUJU Pharma, Seoul, South Korea). To improve the sonic window and avoid thermal injury to the diaphragm, artificial ascites (5% dextrose in a water solution) was infused into the perihepatic space using a 5F angiosheath in 39 (61.9%) patients.
After RF ablation, immediate follow-up contrast agent-enhanced CT was performed to evaluate the therapeutic response and possible complications. Contrast agent-enhanced CT was performed at the 1 mo follow-up, every 3 mo during the first 2 years, followed by every 4-6 mo according to the risk of recurrence for both the hepatic resection group and RF ablation group.
Baseline characteristics of patients and HCCs were obtained through review of their electronic medical record from our institution. To compare the therapeutic outcomes between the two groups, intrahepatic distant recurrence (IDR), disease-free survival (DFS), and overall survival (OS) were calculated. IDR was defined as a new tumor appearing in the liver separate from the treated area. DFS was defined as the time interval from the date of treatment to one of the following events: intrahepatic recurrence, extrahepatic recurrence, or death. OS was defined as the time interval from the date of treatment to death. If the patients had undergone liver transplantation, they were considered to have been censored at the time of liver transplantation. Complications were stratified according to the Clavien classification of postoperative complications, and complications of grade II or higher were considered major complications[15]. Local tumor progression (LTP) was evaluated for the RF ablation group. LTP was defined as the appearance of new tumor foci at the margin of the ablation zone after at least one contrast-enhanced follow-up study had demonstrated an absence of viable tumors[16].
Continuous data were compared using two-sample t tests, and categorical variables were compared using chi-squared tests between the two groups. Cumulative LTP, cumulative IDR, DFS, and OS rates were estimated using the Kaplan-Meier method. Prognostic factors for DFS and OS were assessed using Cox regression models. Proportional hazard (PH) assumption for the Cox proportional hazard model was tested using Schoenfeld’s method. For the variables with violation of PH assumption, the time-dependent Cox regression was applied. When the time dependence was not significant, the Cox proportional hazard model was applied. Possible risk factors with P values of 0.1 or less at univariate analyses were entered into the multivariate Cox proportional hazard models. Subgroup analysis for patients with ≤ 2 cm HCCs was performed with Cox proportional hazard models. All statistical analyses were performed using a software (PASW statistical software, version 18.0; SPSS, Chicago, IL). For all tests, a P value < 0.05 was defined as a significant difference.
Baseline characteristics of patients and HCCs are shown in Table 1. The median follow-up period was 74.9 mo (range, 10.3-117.8 mo) in the hepatic resection group and 65.3 mo (range, 4.1-113.9 mo) in the RF ablation group. The RF ablation group was significantly older, and they exhibited a lower α-fetoprotein level, platelet count, and serum albumin level, and a higher prothrombin time. In the RF ablation group, the proportion of patients with liver cirrhosis and hepatitis C virus was higher and the proportion of patients with hepatitis B virus was lower compared to that in the hepatic resection group. The mean size of HCCs was not significantly different between the two groups.
| Variable | Hepatic resection (n = 80) | RF ablation (n = 63) | P value |
| Mean age (yr) | 53.5 ± 9.0 | 60.3 ± 8.7 | < 0.001 |
| Male sex | 62 (78) | 49 (78) | 0.968 |
| Etiology | 0.042 | ||
| HBV | 68 (85) | 43 (68) | |
| HCV | 6 (8) | 13 (20) | |
| NBNC | 6 (8) | 7 (11) | |
| Liver cirrhosis | 50 (63) | 50 (79) | 0.029 |
| Tumor size | 2.1 ± 0.6 | 2.1 ± 0.5 | 0.906 |
| α-fetoprotein (ng/mL) | 200 ± 489 | 72 ± 165 | 0.031 |
| Platelet count (× 103/mm3) | 148 ± 49 | 107 ± 49 | < 0.001 |
| Alanine aminotransferase (IU/L) | 41 ± 21 | 40 ± 30 | 0.849 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 | 0.7 ± 0.4 | 0.99 |
| Albumin (g/dL) | 4.2 ± 0.4 | 3.9 ± 0.4 | < 0.001 |
| Prothrombin time (INR) | 1.1 ± 0.1 | 1.2 ± 0.1 | < 0.001 |
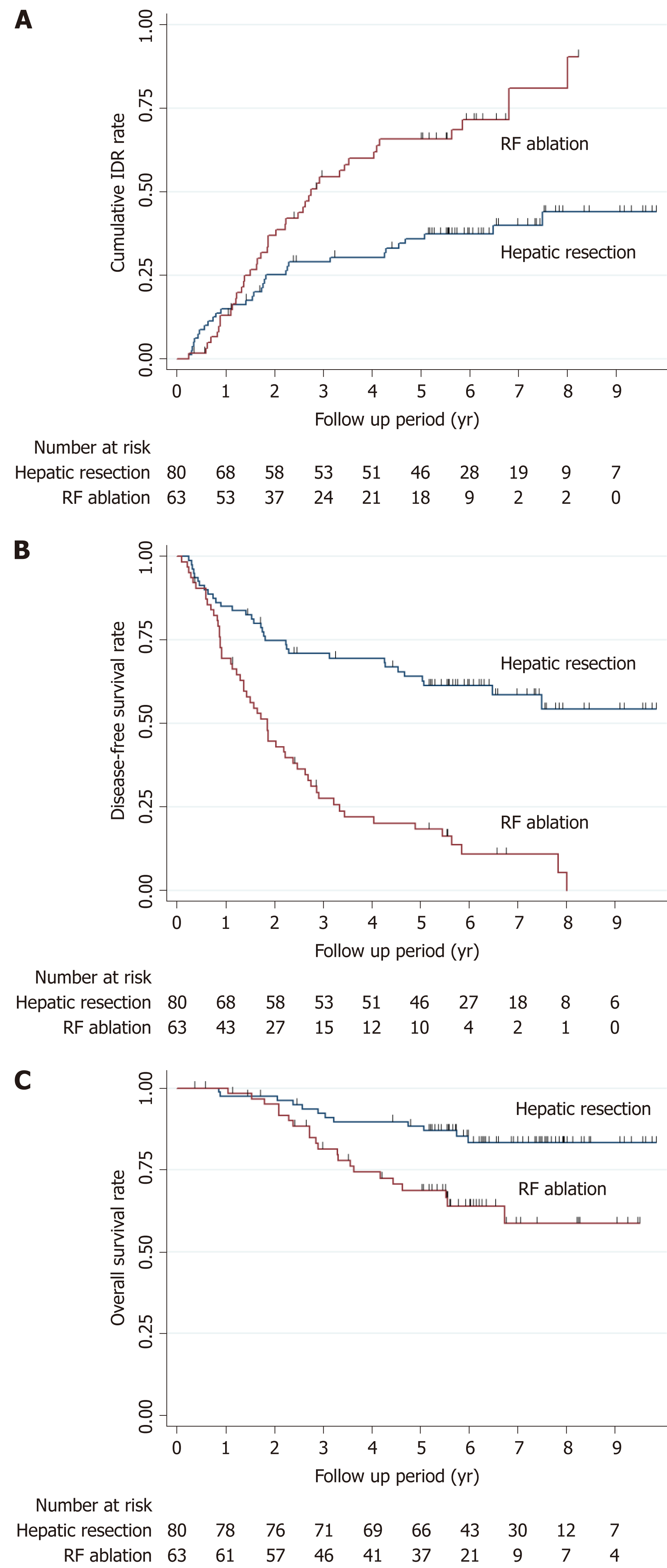
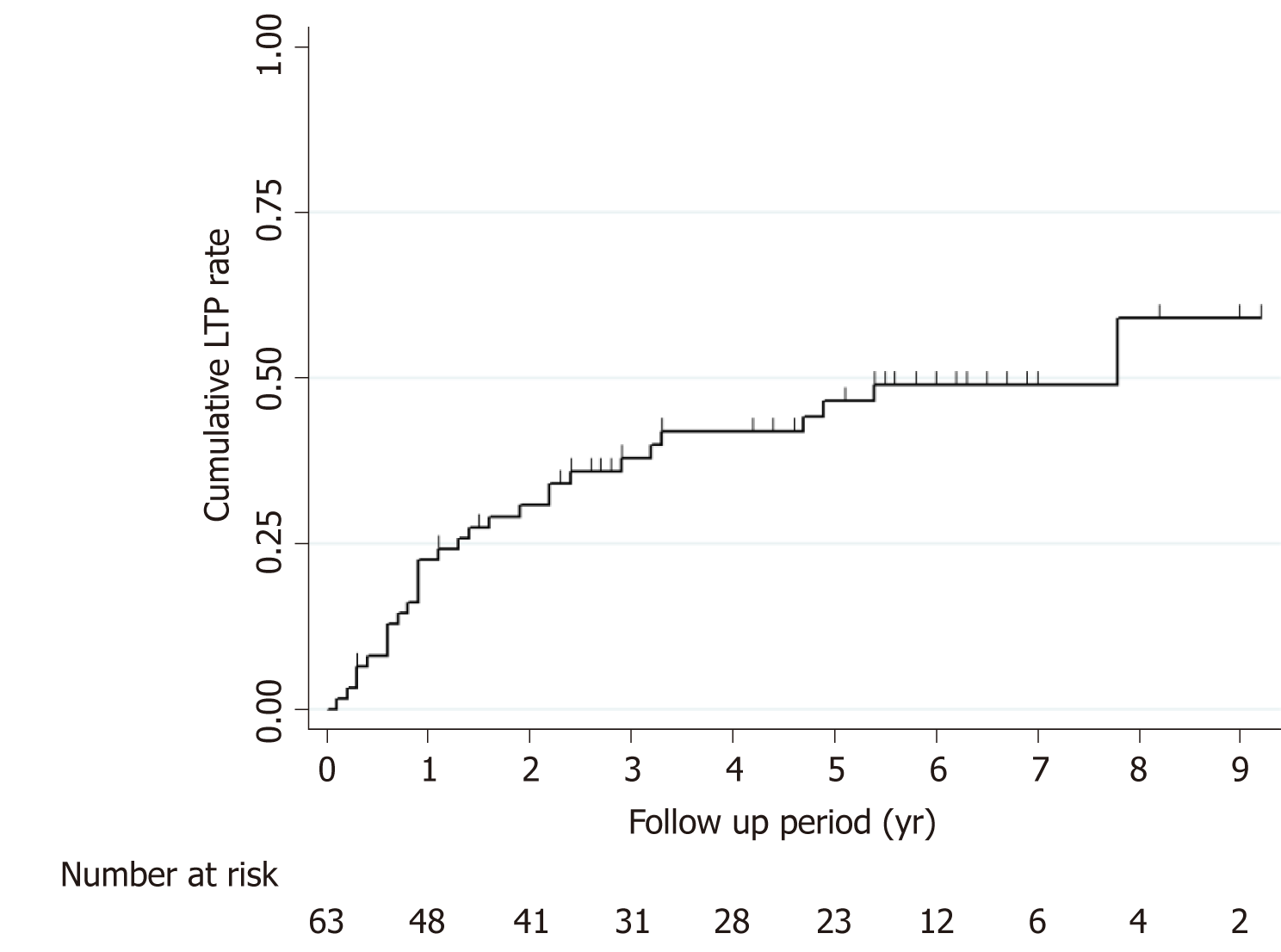
The cumulative IDR rates at 1-, 3-, and 5-years were 15.0%, 29.1%, and 35.9%, respectively, for the hepatic resection group and 13.1%, 54.5%, and 65.8%, respectively, for the RF ablation group (Figure 2A). The estimated DFS rates at 1-, 3-, and 5-years were 85.0%, 70.9%, and 64.1%, respectively, for the hepatic resection group and 69.5%, 27.5%, and 18.3%, respectively, for the RF ablation group (Figure 2B). The estimated OS rates at 1-, 3-, and 5-years were 97.5%, 92.3%, and 88.4%, respectively, for the hepatic resection group and 100%, 81.4%, and 68.7%, respectively, for the RF ablation group (Figure 2C). For the RF ablation group, the cumulative LTP rates were 22.5%, 37.8%, and 46.6% at 1-, 3-, and 5-years, respectively (Figure 3).
Based on multivariate analysis, there was no independent prognostic factor for OS. Hepatic resection [P ≤ 0.001; hazard ratio (HR), 0.352; 95% confidence interval (CI): 0.205, 0.605; with RFA as the reference category], alanine aminotransferase level (P = 0.006; HR, 1.011; 95%CI: 1.003, 1.020), and serum albumin level (P = 0.014; HR, 0.481; 95%CI: 0.269, 0.860) were independent prognostic factors for DFS (Tables 2 and 3).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR | P value | HR | P value | |
| Group (RF ablation) | 0.364 (0.179, 0.742) | 0.005 | 0.676 (0.309, 1.482) | 0.329 |
| Age | 1.025 (0.988, 1.063) | 0.187 | ||
| Sex (female) | 0.975 (0.439, 2.165) | 0.95 | ||
| Etiology (hepatitis B virus)1 | 0.028 | 0.176 | ||
| Hepatitis C virus | 3.053 (1.240, 7.516) | 0.01 | 2.180 (0.854, 5.566) | 0.124 |
| NBNC | 2.292 (0.671, 7.831) | 0.26 | 2.433 (0.686, 8.636) | 0.232 |
| Liver cirrhosis (absence) | 1.834 (0.756, 4.446) | 0.18 | ||
| Tumor size | 1.505 (0.782, 2.899) | 0.221 | ||
| α-fetoprotein | 1.000 (1.000, 1.001) | 0.345 | ||
| Platelet count | 0.989 (0.982, 0.996) | 0.003 | 0.991 (0.982, 1.000) | 0.051 |
| Alanine aminotransferase | 1.006 (0.993, 1.019) | 0.36 | ||
| Total bilirubin | 1.031 (0.393, 2.704) | 0.951 | ||
| Albumin | 0.327 (0.148, 0.727) | 0.006 | 0.485 (0.189, 1.244) | 0.132 |
| Prothrombin time (INR) | 19.351 (1.354, 1798.539) | 0.034 | 0.325 (0.002, 46.731) | 0.657 |
| Variable | Univariate analysis | Multivariate analysis | ||
| HR | P value | HR | P value | |
| Group (RF ablation) | 0.272 (0.174, 0.427) | < 0.001 | 0.352 (0.205, 0.605) | < 0.001 |
| Age | 1.036 (1.012, 1.060) | 0.003 | 1.015 (0.987, 1.043) | 0.306 |
| Sex (female) | 1.096 (0.650, 1.847) | 0.732 | ||
| Etiology (hepatitis B virus) 1 | 0.726 | |||
| Hepatitis C virus | 1.507 (0.789, 2.878) | 0.31 | ||
| NBNC | 1.121 (0.483, 2.601) | 1 | ||
| Liver cirrhosis (absence) | 1.526 (0.916, 2.544) | 0.105 | ||
| Tumor size | 0.976 (0.658, 1.448) | 0.904 | ||
| α-fetoprotein | 1.000 (0.999, 1.000) | 0.391 | ||
| Platelet count | 0.991 (0.987, 0.995) | < 0.001 | 0.998 (0.993, 1.004) | 0.542 |
| Alanine aminotransferase | 1.008 (1.000, 1.017) | 0.045 | 1.011 (1.003, 1.020) | 0.006 |
| Total bilirubin | 0.995 (0.540, 1.833) | 0.988 | ||
| Albumin | 0.281 (0.168, 0.470) | < 0.001 | 0.481 (0.269, 0.860) | 0.014 |
| Prothrombin time (INR) | 147.887 (13.992, 1563.115) | < 0.001 | 4.212 (0.195, 90.886) | 0.359 |
Thirty-seven patients in the hepatic resection group and 27 patients in the RF ablation group had ≤ 2 cm HCC. The cumulative IDR rates at 1-, 3-, and 5-years were 13.5%, 27.3%, and 33.1%, respectively, for the hepatic resection group and 15.3%, 60.3%, and 70.2%, respectively, for the RF ablation group. The estimated DFS rates at 1-, 3-, and 5-years were 86.5%, 72.7%, and 66.9%, respectively, for the hepatic resection group and 81.0%, 27.4%, and 18.3%, respectively, for the RF ablation group. The estimated OS rates at 1-, 3-, and 5-years were 100%, 94.5%, and 91.7%, respectively, for the hepatic resection group and 100%, 83.8%, and 65.4%, respectively, for the RF ablation group. In multivariate analysis, hepatic resection was an independent prognostic factor for DFS (P = 0.018; HR, 0.365; CI: 0.158-0.844), but was not an independent prognostic factor for OS.
There was no treatment-related mortality in either group. Major complications occurred in three patients (3.8%) in the hepatic resection group: Grade II, pneumonia (n = 1) and intraperitoneal hemorrhage (n = 1); and Grade III, wound infection requiring surgery (n = 1). In the RF ablation group, a major complication occurred in one patient (1.6%): Grade III, pleural effusion requiring drainage. The major complication rate was not significantly different between the two groups (P = 0.060). The posttreatment hospital stay was significantly longer in the hepatic resection group (median, 9 d; range, 5-23 d) than in the RF ablation group (median, 1.0 d; range, 1-4 d; P < 0.001).
During the follow-up period, peritoneal seeding occurred in one patient (1.3%) in the hepatic resection group and six patients (9.5%) in the RF ablation group, and the rate of peritoneal seeding was significantly different (P = 0.044).
During the follow-up period, LTP occurred in 29 (46.0%) of the 63 patients in the RF ablation group. The initial treatment modalities for LTP were as follows: transarterial chemoembolization (TACE) (n = 14), RF ablation (n = 12), hepatic resection (n = 1), combined TACE and RF ablation (n = 1), and combined TACE and radiation therapy (n = 1). In 26 of 29 patients, LTP was controlled with additional treatments, and the number of additional treatments was as follows: One (n = 17), two (n = 3), three (n = 4), and six (n = 2). For the remaining three patients, LTP was not controlled even though they received repeated treatments with TACE or RF ablation. In addition, multiple intra- and extrahepatic metastases occurred. Finally, sorafenib treatment was administered. IDR occurred in 31 (38.8%) of the 80 patients in the hepatic resection group, and treatment modalities were as follows: TACE (n = 18), RF ablation (n = 11), cryoablation (n = 1), and hepatic resection (n = 1). IDR occurred in 42 (66.7%) of the 63 patients in the RF ablation group, and treatment modalities were as follows: TACE (n = 16), RF ablation (n = 20), combined TACE and RF ablation (n = 3), hepatic resection (n = 1), liver transplantation (n = 1), and sorafenib treatment (n = 1).
In our study, we compared long-term therapeutic outcomes for treatments using hepatic resection and percutaneous RF ablation for HCCs (≤ 3 cm) abutting the right diaphragm; we found that the treatment modality was a significant prognostic factor for DFS, but was not an independent prognostic factor for OS. For the RF ablation group, the LTP rate was as high as 46.6% at 5 years. The location of tumors can affect the technical difficulty in local control of tumors, especially for RF ablation. Although there have been many studies that compared therapeutic outcomes between hepatic resection and RF ablation for HCC, most of them did not consider the location of tumors. In this way, the results of our study, which compares hepatic resection and percutaneous RF ablation for HCCs with consideration of the location of tumors, can provide important data for the proper management of HCCs abutting the diaphragm.
In our study, the LTP rate was 46.6% at 5 years for the RF ablation group. The LTP rate was much higher than rates reported in previous studies that included all HCCs located in the liver[11,14,17-19]. Percutaneous RF ablation for subphrenic HCCs is difficult to adequately perform for several reasons. First, the poor sonic window resulting from the lung shadow makes it difficult to accurately target tumors with the electrodes. Second, all tumors were subcapsular HCCs in our study. In general, subcapsular HCCs are considered to be more difficult to treat with percutaneous HCC than nonsubcapsular HCCs because of the difficulty of placing an electrode and not being able to obtain enough ablative margin along the hepatic capsule.
In this study, patients who had undergone hepatic resection exhibited longer DFS compared to those who had undergone RF ablation. This result is in line with previous studies that compared DFS outcomes for hepatic resection and RF ablation for HCC[20,21]. In our study, the estimated DFS rates at 1-, 3-, and 5-years were 85.0%, 70.9%, and 64.1%, respectively, for the hepatic resection group and 69.5%, 27.5%, and 18.3%, respectively, for the RF ablation group. In the previous study at our institution that compared RF ablation with hepatic resection for single HCC ≤ 3 cm located in the liver, the estimated DFS rate at 5 years was 61.1% for the hepatic resection group and 31.7% for the RF ablation group[11]. The DFS rate for the hepatic resection group of this study was similar to our previous result. However, the DFS rate for the RF ablation group of this study was lower than our previous result. This difference can most likely be explained by the high LTP rate for the RF ablation group in this study.
According to previous studies, RF ablation was comparable to hepatic resection for very early and early-stage HCCs in terms of OS[22-24]. In our study, estimated OS rates for the hepatic resection group (97.5%, 92.3%, and 88.4% at 1-, 3-, and 5-years, respectively) appeared to be better than those for the RF ablation group (100%, 81.4%, and 68.7% at 1-, 3-, and 5-years). However, similar to previous studies, treatment modality was not an independent prognostic factor for OS according to multivariate analyses in our study.
Previous studies have reported comparable outcomes between RF ablation and hepatic resection in terms of long-term survival for patients with early-stage HCC. Based on these results, both hepatic resection and RF ablation are considered as curative treatment options for early stage HCC. Although treatment modality was not an independent prognostic factor for OS in patients with subphrenic HCCs, there were some differences in treatment outcomes between patients with subphrenic HCCs and nonsubphrenic HCCs that need to be considered when treatment modality is determined. First, the LTP rate after RF ablation was much higher for patients with subphrenic HCCs. Second, recurrent LTP was common in patients with subphrenic HCCs. In 12 (41%) of 29 patients who had LTP, multiple treatments were performed to control the LTP. Third, the peritoneal seeding rate for subphrenic HCCs was as high as 9.5% in the RF ablation group. Considering these unfavorable outcomes of RF ablation for subphrenic HCCs, it may be reasonable to preferentially consider hepatic resection as the first-line treatment for subphrenic HCCs rather than percutaneous RF ablation. Otherwise, laparoscopic RF ablation or combined TACE and RF ablation should be considered because these modalities can be more effective than percutaneous RF ablation alone in terms of local tumor control[25-27]. However, this issue needs to be investigated further.
Our study has some limitations. First, because this is a retrospective study, the treatment groups were not randomized, and we could not exclude the possibility of selection bias. However, we analyzed the effect of treatment modality (hepatic resection vs percutaneous RF ablation) after controlling for potential compounding factors. Second, HCC was diagnosed based on clinical guidelines in most patients in the RF ablation group. Therefore, there was a possibility of false-positive diagnosis, which could affect the outcomes. Third, this is a single-center study. In general, the outcomes of both hepatic resection and RF ablation greatly depend on the expertise and experience of the operators. In addition, we only used the single straight type of RF electrode and US as a guiding modality. Using other types of RF electrodes or guiding modalities may result in different therapeutic outcomes. Therefore, care should be taken when generalizing our results to that from other institutions.
In conclusion, although OS was not significantly different between patients who had undergone hepatic resection or percutaneous RF ablation for HCCs abutting the diaphragm, DFS was better in the hepatic resection group, and LTP was as high as 46.6% at 5 years in the RF ablation group. Therefore, it may be reasonable that hepatic resection should be preferentially considered over percutaneous US-guided RF ablation as a first-line treatment for HCCs abutting the diaphragm.
Many studies have revealed that radiofrequency (RF) ablation is comparable to hepatic resection in terms of long-term survival for patients with early stage hepatocellular carcinoma (HCC). However, most studies have not taken into account the location of HCCs.
Our study attempted to analyze the effect of the subphrenic location of HCC on the long-term therapeutic outcomes after hepatic resection and RF ablation.
To compare the long-term therapeutic outcomes between hepatic resection vs percutaneous RF ablation for HCCs abutting the diaphragm.
A total of 143 Child-Pugh class A patients who had undergone hepatic resection (n = 80) or percutaneous RF ablation (n = 63) for an HCC (≤ 3 cm) abutting the right diaphragm were included. Therapeutic outcomes were compared.
Hepatic resection was an independent prognostic factor for disease-free survival (DFS) (P ≤ 0.001; hazard ratio, 0.352; 95%CI: 0.205, 0.605; with RF ablation as the reference category); however, treatment modality was not an independent prognostic factor for overall survival (OS). The local tumor progression rate was 46.6% at 5 years for the RF ablation group.
Although OS was not significantly different between patients who had undergone hepatic resection or percutaneous RF ablation for HCCs abutting the diaphragm, DFS was better in the hepatic resection group.
Further studies with large sample size and multicenter prospective studies are needed to confirm the conclusion of this study.
STROBE Statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aykan NF, He J, Huang C, Lambrecht NW S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6560] [Article Influence: 468.6] [Reference Citation Analysis (1)] |
| 2. | Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Molinari M, Helton S. Hepatic resection versus radiofrequency ablation for hepatocellular carcinoma in cirrhotic individuals not candidates for liver transplantation: a Markov model decision analysis. Am J Surg. 2009;198:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Chen J, Peng K, Hu D, Shen J, Zhou Z, Xu L, Chen J, Pan Y, Wang J, Zhang Y, Chen M. Tumor Location Influences Oncologic Outcomes of Hepatocellular Carcinoma Patients Undergoing Radiofrequency Ablation. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, Lim HK. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:34-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004;183:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Kang TW, Rhim H, Lee MW, Kim YS, Choi D, Lee WJ, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol. 2011;196:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Kondo Y, Yoshida H, Tateishi R, Shiina S, Kawabe T, Omata M. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg. 2008;95:996-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, Choi D, Song KD, Kwon CH, Joh JW, Paik SW, Paik YH, Ahn JH. Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection--Propensity Score Analyses of Long-term Outcomes. Radiology. 2015;275:908-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4500] [Article Influence: 225.0] [Reference Citation Analysis (0)] |
| 13. | Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, Shen F, Pinna AD. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Gwak GY, Yoo BC. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24667] [Article Influence: 1174.6] [Reference Citation Analysis (0)] |
| 16. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology,; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 894] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 17. | Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, Paik YH, Kim MJ, Ahn JH. Long-term Therapeutic Outcomes of Radiofrequency Ablation for Subcapsular versus Nonsubcapsular Hepatocellular Carcinoma: A Propensity Score Matched Study. Radiology. 2016;280:300-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-577; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 19. | Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, Han JK, Choi BI. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 20. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 639] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 21. | Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Ishikawa T, Saito S, Nasu A, Kita R, Kimura T, Arimoto A, Osaki Y. Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMC Gastroenterol. 2011;11:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC, Rhee JC, Choi D, Lim HK, Lee KW, Joh JW. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005;39:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Hasegawa K, Makuuchi M, Takayama T, Kokudo N, Arii S, Okazaki M, Okita K, Omata M, Kudo M, Kojiro M, Nakanuma Y, Takayasu K, Monden M, Matsuyama Y, Ikai I. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol. 2008;49:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 27. | Hirooka M, Kisaka Y, Uehara T, Ishida K, Kumagi T, Watanabe Y, Abe M, Matsuura B, Hiasa Y, Onji M. Efficacy of laparoscopic radiofrequency ablation for hepatocellular carcinoma compared to percutaneous radiofrequency ablation with artificial ascites. Dig Endosc. 2009;21:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |