Published online Feb 15, 2019. doi: 10.4251/wjgo.v11.i2.161
Peer-review started: October 16, 2018
First decision: December 7, 2018
Revised: December 17, 2018
Accepted: December 23, 2018
Article in press: December 23, 2018
Published online: February 15, 2019
Processing time: 123 Days and 5.2 Hours
There are several surgical options for treating early gastric cancers (EGCs), such as endoscopic resection, laparoscopic or open gastrectomy with D1 or D2 lymphadenectomy. Endoscopic resection for EGC with low risk of lymph node metastasis has been widely accepted as a therapeutic alternative. The role of endoscopic submucosal dissection (ESD) in treating EGC is not well established, especially when compared with resection surgery cases in a long-term follow-up scope.
To compare the safety and efficacy of the short- and long-term outcomes between ESD and resection surgery.
We searched the databases of PubMed, EMBASE, Web of Science, and the Cochrane Library from January 1990 to June 2018, enrolling studies reporting short- or long-term outcomes of ESD in comparison with resection surgery for EGC. The quality of the studies was assessed by the Newcastle-Ottawa Quality Assessment Scale. Stata software (version 12.0) was used for the analysis. Pooling analysis was conducted using either fixed- or random-effects models depending on heterogeneity across studies.
Fourteen studies comprising 5112 patients were eligible for analysis (2402 for EGC and 2710 for radical surgery). Our meta-analysis demonstrated that the ESD approach showed advantages through decreased operation time [weighted mean difference (WMD): -140.02 min, 95%CI: -254.23 to -34.82 min, P = 0.009], shorter hospital stay (WMD: -5.41 d, 95% CI: -5.93 to -4.89 d, P < 0.001), and lower postoperative complication rate [Odds ratio (OR) = 0.39, 95%CI: 0.28-0.55, P < 0.001). Meanwhile, EGC patients who underwent ESD had higher recurrence rate (OR = 9.24, 95%CI: 5.94-14.36, P < 0.001) than resection surgery patients. However, the long-term survival including overall survival [Hazard ratio (HR) = 0.51, 95%CI: 0.26-1.00, P = 0.05] and event-free survival (HR = 1.59, 95%CI: 0.66-9.81, P = 0.300) showed no significant differences between these two groups.
In the treatment of EGC, ESD was safe and feasible in comparison with resection surgery, with advantages in several surgical and post-operative recovery parameters. Although the recurrence rate was higher in ESD group, the long-term survival was still comparable in these two groups, suggesting ESD could be recommended as standard treatment for EGC with indications.
Core tip: The role of endoscopic submucosal dissection (ESD) in treating early gastric cancer (EGC) is not well established, especially when compared with resection surgery cases in a long-term follow-up scope. This study collected and analyzed up-to-date clinical data of comparison between ESD and surgical gastrectomy in EGC patients. The results turned out a comparable short- and long-term result between these two groups with more favorable short-term recovery in ESD patients.
- Citation: Li H, Feng LQ, Bian YY, Yang LL, Liu DX, Huo ZB, Zeng L. Comparison of endoscopic submucosal dissection with surgical gastrectomy for early gastric cancer: An updated meta-analysis. World J Gastrointest Oncol 2019; 11(2): 161-171
- URL: https://www.wjgnet.com/1948-5204/full/v11/i2/161.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i2.161
Gastric cancer remains the fifth common malignancy and the third leading cause of cancer mortality worldwide[1]. The prevalence of gastric cancer (GC) is relatively high in East Asia, especially in China, Korea, and Japan, accounting for nearly half of all new cases across the world annually[2]. Early GC (EGC) patients constitute a considerable proportion of all GC patients in these countries, therefore, Japan and Korea have implemented a national screening program for GC to detect EGC[2].
Early gastric cancer is defined as a gastric cancer with tumor invasion that is confined to the mucosa or submucosa, irrespective of the presence of lymph node metastasis[3].There are several surgical options for treating EGCs, such as endoscopic resection, laparoscopic or open gastrectomy with D1 or D2 lymphadenectomy. Endoscopic resection for EGC with low risk of lymph node metastasis has been widely accepted as a therapeutic alternative[4,5]. Endoscopic resection instead of surgery is currently recommended in certain selected EGC patients who have met these following criteria: (1) differentiated-type mucosal cancer without ulceration, regardless of tumor size; (2) differentiated-type mucosal cancer with ulceration no more than 3 cm in diameter; (3) superficial submucosal cancer no more than 3 cm in diameter; or (4) undifferentiated-type mucosal cancer no more than 2 cm in diameter without ulceration[6,7].
The common endoscopic approaches include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). The ESD technique is now a more acceptable endoscopic approach for the treatment of EGC without lymph node metastasis compared to EMR. ESD involves the resection of both benign neoplastic (premalignant) and malignant noninvasive lesions, and enjoys the advantages of higher curative resection and histologically complete resection rate, and lower local recurrence rate for EGCs, which have been confirmed by several large-scale clinical studies and meta-analyses[8,9].
Surgical treatment has been performed in a considerable proportion of EGC patients because of its predictable oncological outcomes. Compared with surgical resection, the endoscopic procedure tends to show advantages in perioperative recovery. And comparisons of clinical parameters or outcomes between ESD and resection surgery for EGC are still lacking and controversial. Several previous meta-analysis either enrolled limited studies nor didn’t include the survival comparison[10,11]. Moreover, the long-term outcomes in patients with sufficient follow-up who have undergone ESD or surgery remain unclear. Therefore, in this meta-analysis we aimed to compare clinical outcomes in EGC patients who underwent ESD and those who underwent surgery.
We searched the databases of PubMed, EMBASE, Web of Science, and the Cochrane Library dating from January 1990 to June 2018. The following search terms were applied: “ESD” or “endoscopic submucosal dissection,” “surgery,” “resection surgery,” “gastrectomy,” and “early gastric cancer” or “EGC.” Both free terms and MeSH words were included. Citations and references of identified studies were also reviewed for potential literature and trials. There was no restriction of language in the literature search.
The following studies were included: (1) studies involving patients diagnosed with EGC based on histology; (2) studies conducted to compare ESD and surgery for EGC; and (3) studies reporting clinical outcomes after ESD or surgery, long-term outcomes included overall survival (OS) or event-free survival (EFS) (disease-free survival or recurrence-free survival).
Exclusion criteria were: (1) case reports or reviews; (2) ESD or surgery performed in pathological types other than gastric adenocarcinoma; (3) involvement of EMR or other hybrid endoscopic resection techniques; and (4) studies including fewer than 20 patients in each group.
Two reviewers independently screened the titles and abstracts of the articles. The following information was extracted from the articles: authors; year of publication; country or region; study design; numbers of patients; and clinical or oncological data consisting of operation time, complete resection rate, estimated blood loss, postoperative complications, total cost, duration of hospital stay, local recurrence rate, and recurrence-free survival.
The quality of the enrolled studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS). The scale measured up to a maximum 9 points, and studies scoring more than 6 points were considered methodologically sound[12].
Weighted mean differences (WMDs) were chosen for continuous variables in this meta-analysis. And odds ratios (ORs) were chosen for measuring dichotomous variables while hazard ratios (HRs) for time-to-event variables. Statistical heterogeneity was assessed by performing χ2 tests and calculating the Higgins I2 statistic, and a value of P < 0.10 or I2 > 50% indicated statistical significance. A random-effect model was generally employed. If the heterogeneity was statistically insignificant, then a fixed-effect model was adopted. Publication bias was evaluated by Begg’s test. A P value of < 0.05 was considered significant. Statistical analyses were performed using Stata software (version 12.0; StataCorp, College Station, TX, USA).
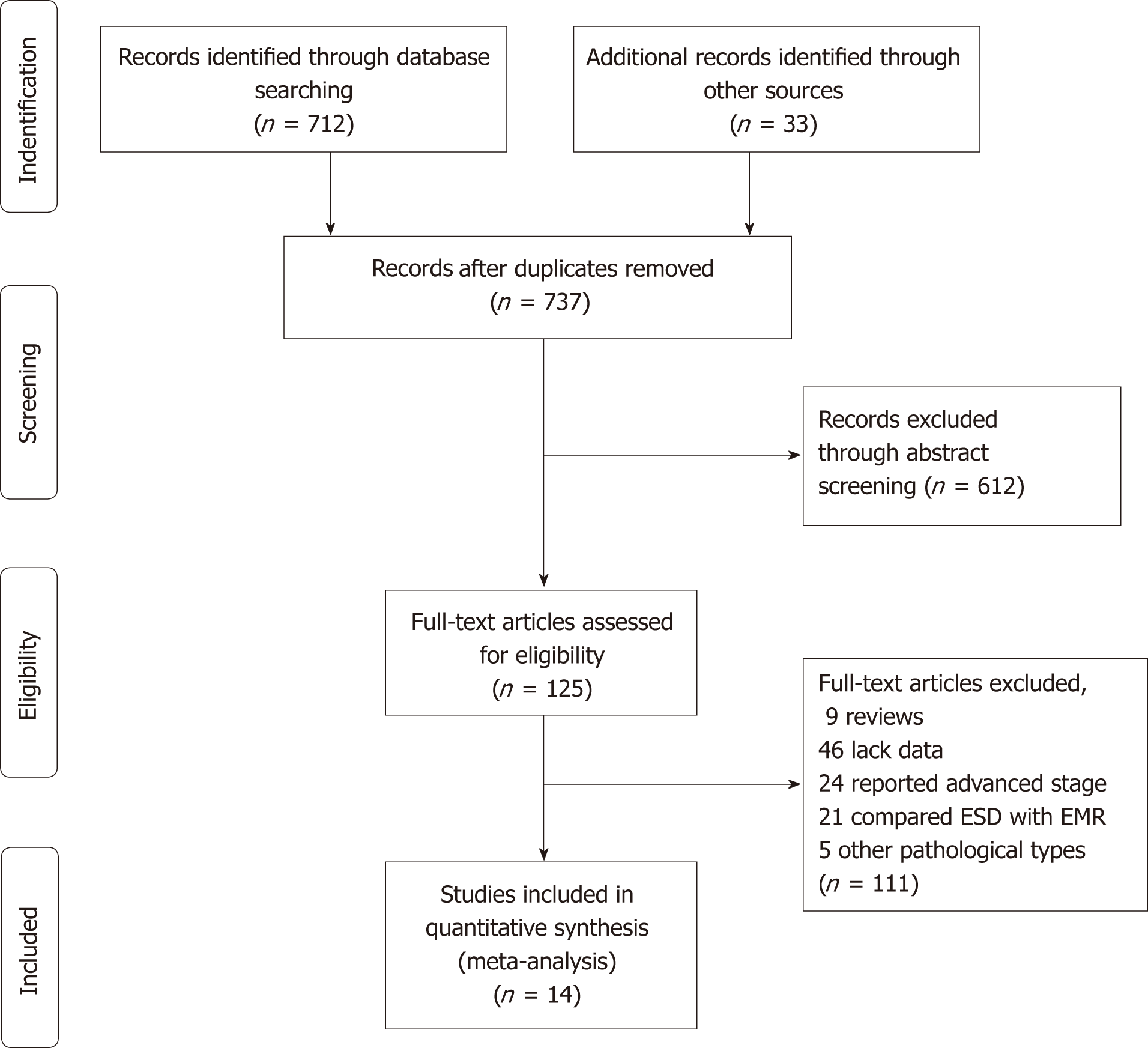
The flow chart of the search strategy is presented in Figure 1. A total of 745 studies were retrieved from a search of the aforementioned databases. After checking, 8 duplicate publications were excluded. Through abstract screening, 612 studies were excluded owing to irrelevance to our topics. In the remaining 125 articles with full-text review, 6 case reports and 9 reviews were excluded; 46 were excluded for lack of sufficient data; 24 were excluded because they reported advanced stages other than early stage; 21 were excluded because they reported comparison between EMR and ESD/surgery; and 5 were excluded because they reported pathological types other than adenocarcinoma. Ultimately, our meta-analysis comprised fourteen studies including a total of 5112 EGC patients.
The main characteristics of the eligible studies and quality assessment results are displayed in Table 1[13-26]. Among these studies, two were performed in China, one in Japan, one in Canada, and the remaining 11 were in Korea. Regarding quality assessment, two studies scored 6 in the NOS and all others achieved 7 or higher (Table 1).
| Ref. | Year/region | Number | Mean age | Sex (M/F) | Tumor size (mm) | Follow up (mo) | NOS | |||||
| ESD | Surgery | ESD | Surgery | ESD | Surgery | ESD | Surgery | ESD | Surgery | |||
| Chiu et al[13] | 2012/China | 74 | 40 | 66.3 | 67.0 | 49/25 | 23/17 | 18.5 (8-40) | 24.7 (10-40) | / | 6 | |
| Park et al[14] | 2014/Korea | 132 | 132 | 73.9 | 74.4 | 97/35 | 88/44 | 7 | ||||
| Kim et al[15] | 2014/Korea | 142 | 71 | 62.0 | 56.7 | 94/48 | 58/13 | / | / | 76.7 ± 16.5 | 65.5 ± 16.5 | 6 |
| Cho et al[16] | 2015/Korea | 88 | 88 | 61.8 | 61.3 | 63/25 | 62/26 | 21.8 ± 12.1 | 21.4 ± 10.1 | 77 (18-107) | 78 (11-113) | 7 |
| Song et al[17] | 2015/China | 29 | 59 | 65.3 | 45.8 | 15/14 | 38/21 | 26.9 ± 8.5 | 22.3 ± 9.4 | 7 | ||
| Fukunaga et al[18] | 2016/Japan | 74 | 74 | 67.3 | 67.1 | 57/17 | 58/16 | 23.1 ± 10.1 | 24.7 ± 11.4 | 43.5 (26.3-76) | 62.9 (36.5-91.7) | 8 |
| Gong et al[19] | 2016/Korea | 40 | 39 | 65 | 60 | 35/5 | 35/4 | 17.5 (13.0-24.8) | 24 (16-35) | 63.2 (53.6-83.5) | 60.3 (58.4-68.7) | 7 |
| Ryu et al[20] | 2016/Korea | 81 | 144 | 63.65 | 61.37 | 59/22 | 118/26 | 19.32 ± 11.31 | 20.55 ± 10.68 | 78.12 ± 9.72 | 80.56 ± 8.92 | 7 |
| Najmeh et al[21] | 2016/Canada | 30 | 37 | 74 | 75 | 23/7 | 24/13 | / | / | 20 (5-56) | 26.5 (11-94) | 7 |
| Shin et al[22] | 2017/Korea | 175 | 100 | 61.7 | 60.5 | 129/46 | 73/27 | / | / | 56 (45-58) | 53 (44-60) | 8 |
| Jeon et al[23] | 2017/Korea | 117 | 117 | 59.9 | 59.5 | 82/35 | 81/36 | 18 ± 11.0 | 18 ± 10 | 57.0 (35.5-65.5) | 58 (49.0-61.0) | 8 |
| Park et al[24] | 2017/Korea | 81 | 81 | 55.0 | 54.2 | 33/48 | 42/39 | 10.6 ± 5.2 | 10.3 ± 5.2 | 48.1 (33.6-71.4) | 60 (34.0-70.1) | 8 |
| Hahn et al[25] | 2017/Korea | 817 | 1206 | 61.9 | 57.0 | 605/212 | 752/454 | 13.0 ± 9.7 | 16.8 ± 11.0 | 37.5 (26.2-59.4) | 57.34 (37.63-60.47) | 8 |
| Lee et al[26] | 2017/Korea | 522 | 522 | 61 | 61 | 366/156 | 370/152 | 25 (20-35) | 25 (21-35) | 52.7 (37.7-67.9) | 59.2 (47.9-63.4) | 8 |
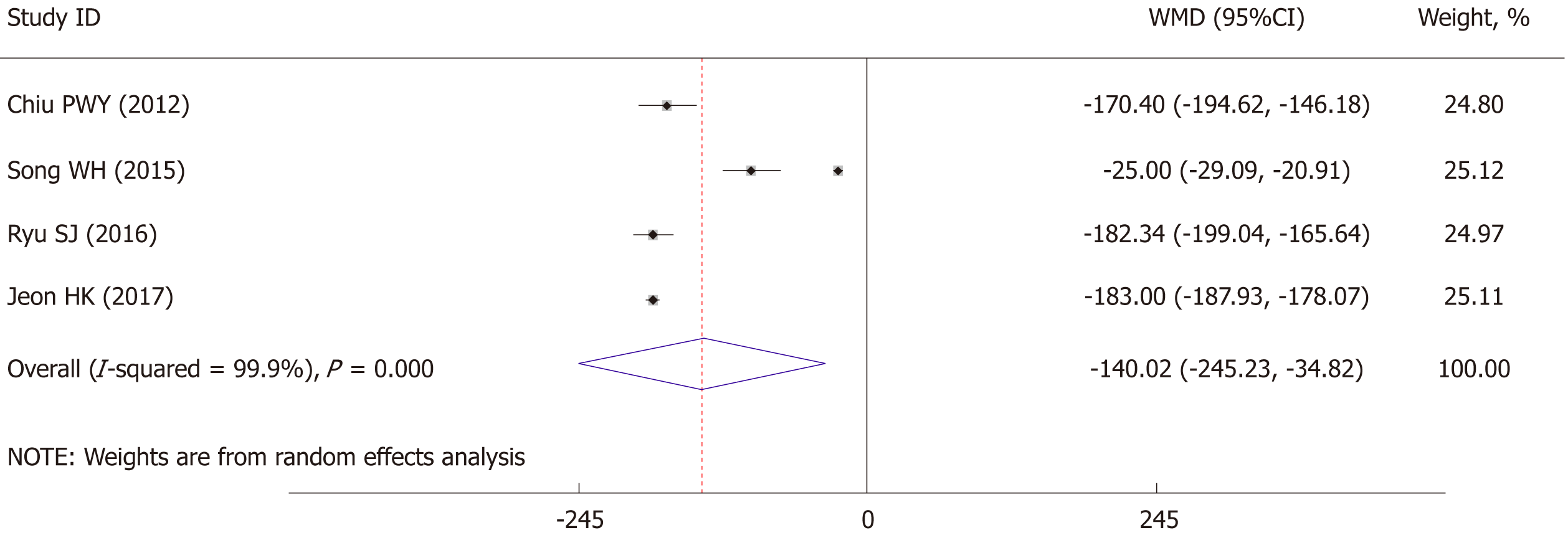
The length of operation time was significantly lower for EGC patients with ESD than for those who underwent radical operations (WMD: -140.02 min, 95%CI: -254.23 to -34.82 min, P = 0.009) (Figure 2). As significant heterogeneity was observed (I2 = 99.9%, P < 0.001), the analysis was performed using a random-effect model. Further sensitivity analysis showed the study by Song et al[17] might be the source of heterogeneity, therefore we excluded this study and the results showed a significant decrease in heterogeneity (I2 = 0%, P = 0.607) with still strong correlation (P < 0.001).
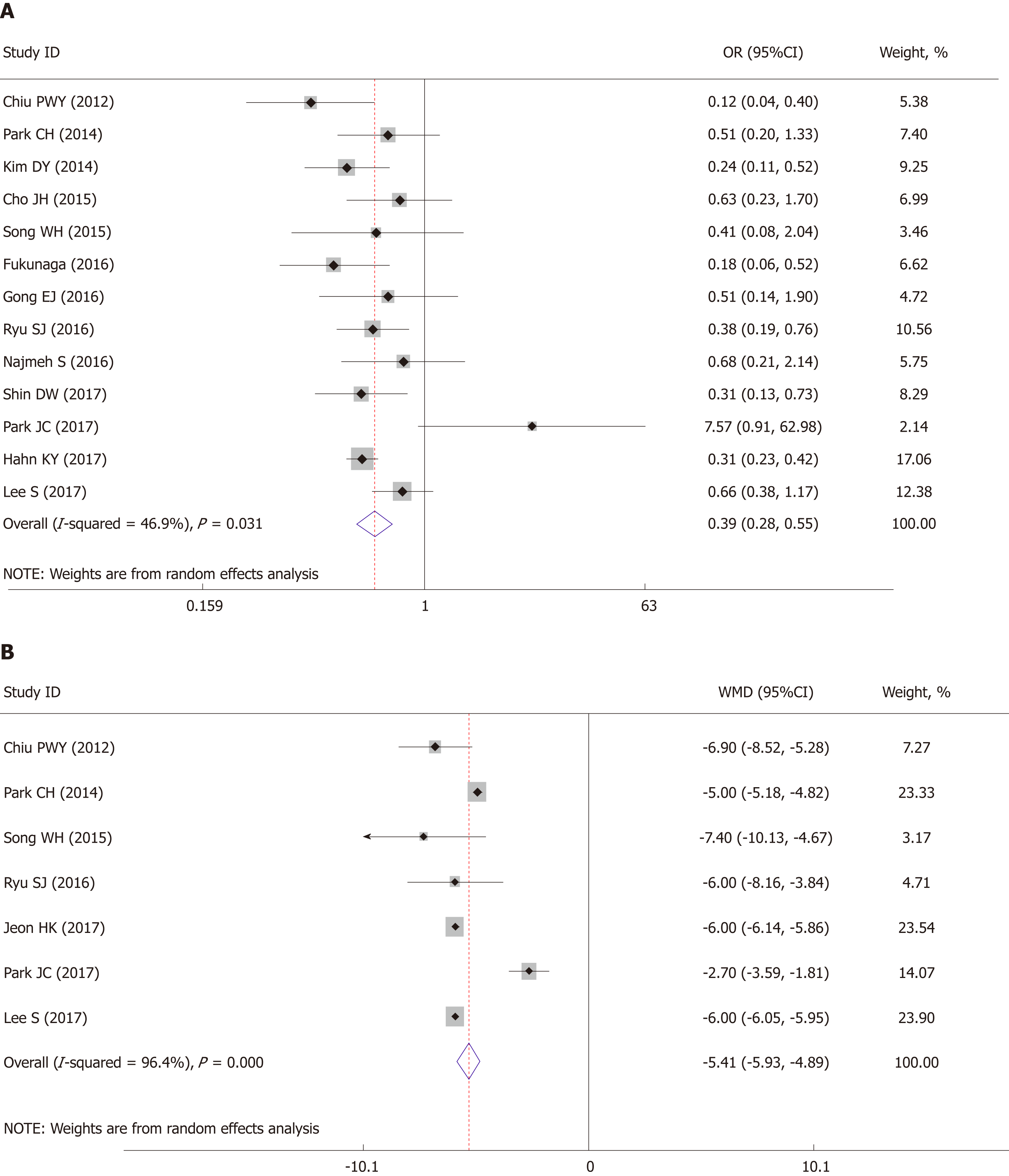
All fourteen studies reported postoperative complication rate. The pooled analysis showed that the ESD group had a lower postoperative complication rate than that in the surgery group (OR = 0.39, 95%CI: 0.28 to 0.55, P < 0.001) (Figure 3A). Moderate heterogeneity was detected within this comparison (I2 = 46.9%, P = 0.031) and a random-effect model was applied.
The pooled analysis of hospital stay showed that patients in the ESD group enjoyed a significant shorter hospital stay than those who underwent surgery (WMD: -5.41 d, 95%CI: -5.93 to -4.89 d, P < 0.001) (Figure 3B). There was significant heterogeneity within studies (I2 = 96.4%, P < 0.001) and therefore a random-effect model was applied.
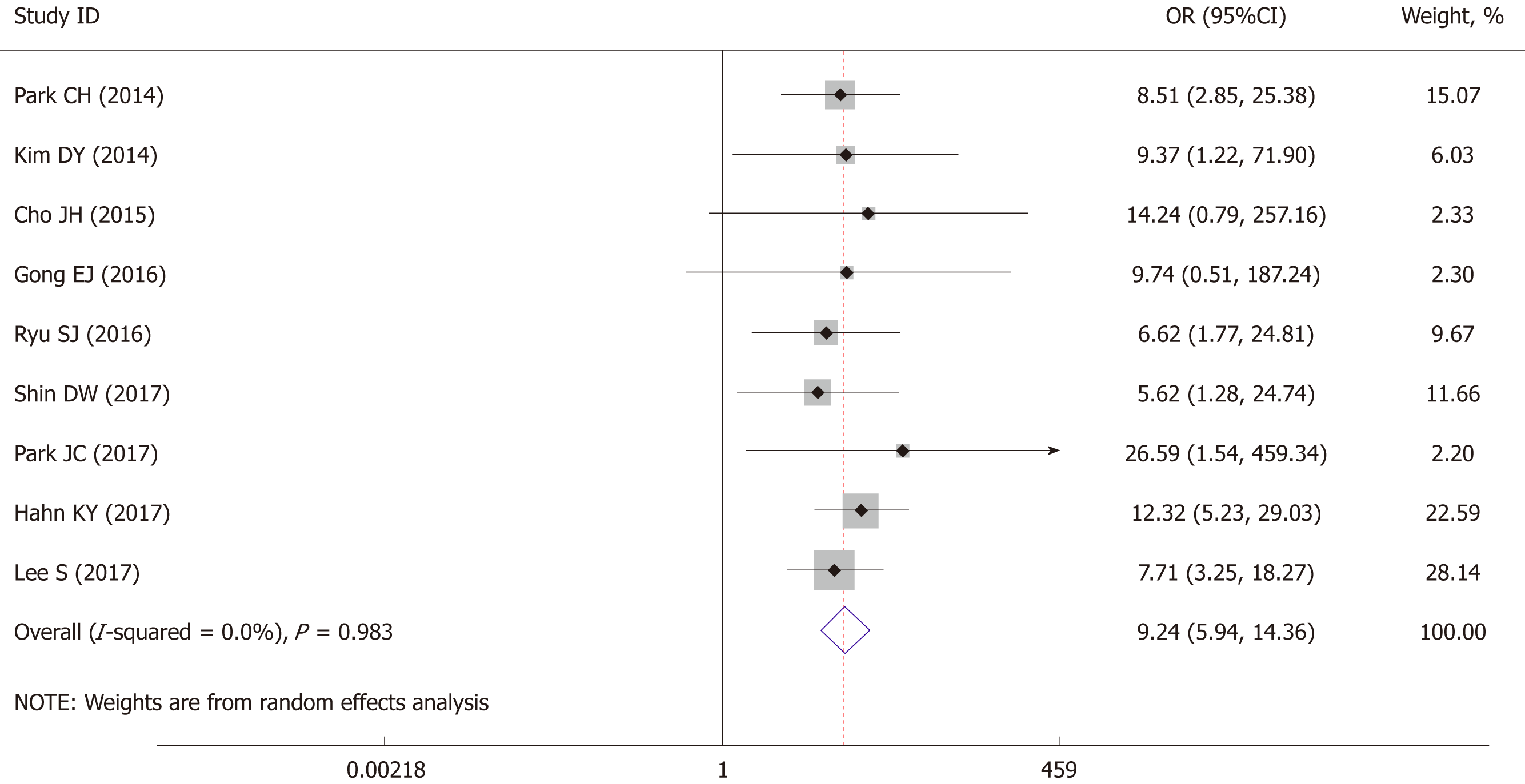
The postoperative cancer recurrence rate was reported by nine studies, pooled analysis showed a higher recurrence risk in ESD patients (OR = 9.24, 95%CI: 5.94 to 14.36, P < 0.001) with no obvious heterogeneity detected (I2 = 0%, P = 0.983) (Figure 4). Therefore, a fixed-effect model was applied in the pooled analysis.
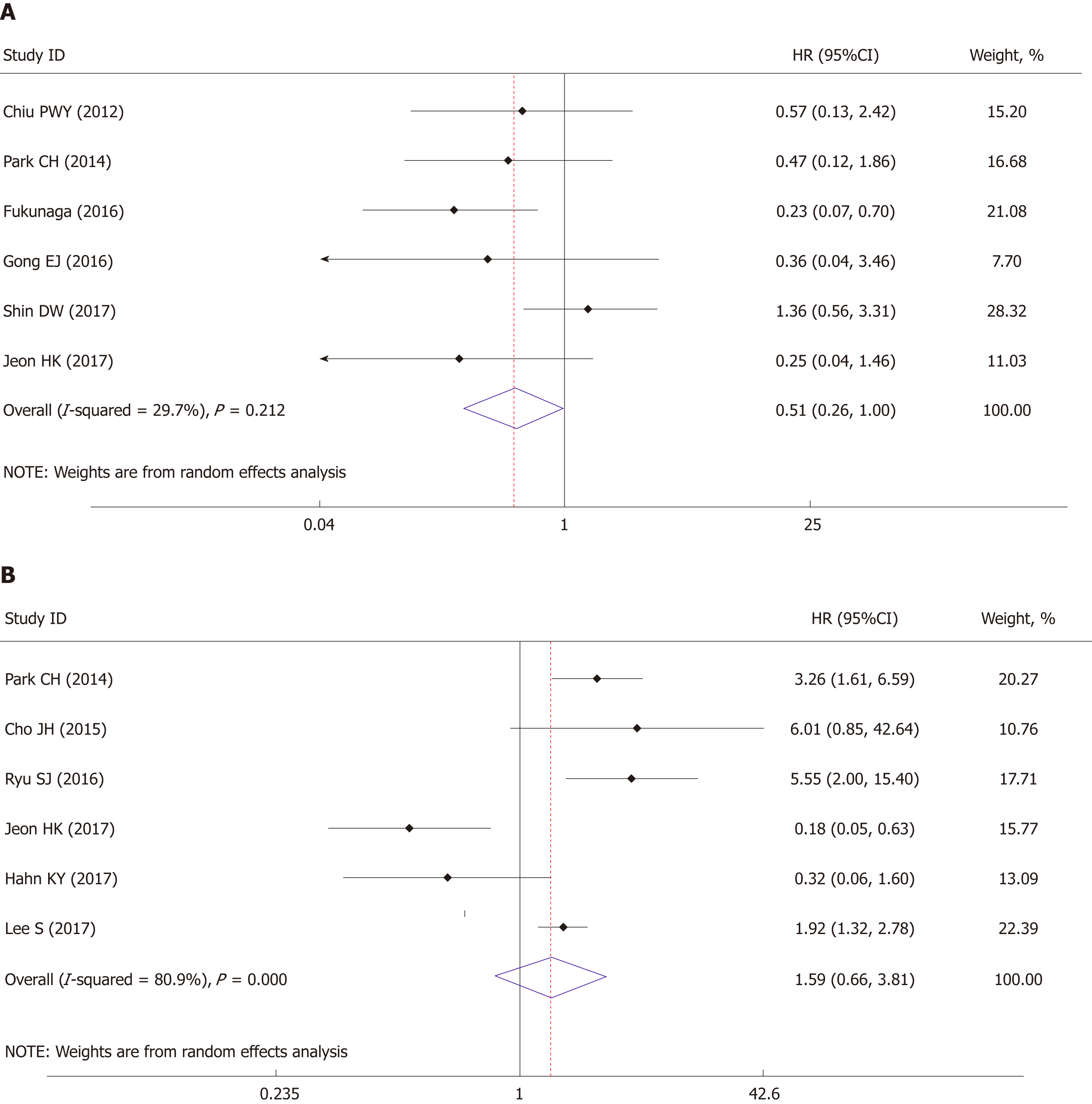
Long-time recurrence-free survival or disease-free survival was regarded as EFS. The results were provided by six studies, the pooled analysis of which showed no significant survival difference between these two groups (HR = 1.59, 95%CI: 0.66 to 9.81, P = 0.300) with obvious heterogeneity (I2 = 80.9%, P < 0.001) (Figure 5A).
The OS also demonstrated no significant difference between ESD and surgery (HR = 0.51, 95%CI: 0.26-1.00, P = 0.05) with trivial heterogeneity (I2 = 29.7%, P = 0.212) (Figure 5B).
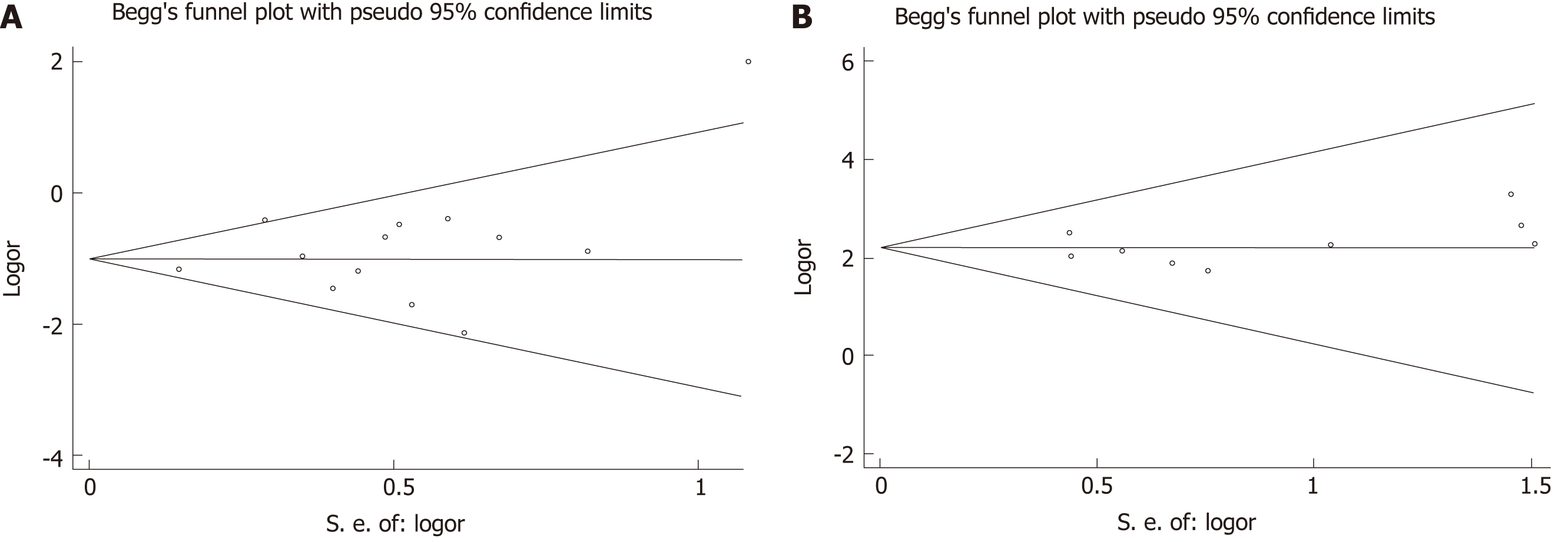
Publication bias was evaluated based on postoperative complication rate and recurrence rate (included in most of the eligible studies) using Begg’s test. No publication bias was observed in these analyses (Figure 6, complication rate: P = 0.502; Recurrence rate: P = 0.754).
Our meta-analysis demonstrated the short- and long-term oncological safety of ESD, as well as the potential superiority of ESD over surgery in treating EGC in regard to some clinical parameters. To the best of our knowledge, our study is the most comprehensive meta-analysis to date comparing clinical outcomes between ESD and surgery in treating EGC, involving more than 5000 patients.
The most important concern about ESD is oncological safety. In both ESD and radical surgery, complete resection should be confirmed by the lateral and vertical margin status[27]. The studies enrolled in our meta-analysis also demonstrated that compared with surgery, ESD enjoyed a similar curative resection rate (ranges from 86.7% to 99.4%).
The ESD procedure also calls for high levels of training and experience for endoscopy physicians or surgeons. However, the operation time will gradually decrease in line with accumulating experience of the endoscopist while maintaining an advantage over radical surgery in terms of surgical duration[28]. Another advantage of ESD procedure was fast recovery after operation than surgery, the hospital stays and medical costs thus greatly decreased since less trauma and less medical interventions caused by ESD procedure.
Postoperative complications might affect patients’ life quality and prolong hospital stay, leading to increased total medical cost. According to the enrolled studies, the most common complications occurred after ESD intervention were bleeding and perforation while bleeding and anastomotic leakage occurred more often in the surgery group. Some previous studies have reported that postoperative adverse events occurred less frequently in the ESD group than in surgery patients, while others found no significant difference[29,30]. After pooled analysis, our results supported that ESD might cause less trauma to EGC patients in the perspective of lower postoperative complication rate happened.
Our pooled analysis also found that recurrence rate in the ESD group was higher than in the surgery group. Theoretically, the ESD technique only allows the removal of the primary tumor along with the submucosal layer; therefore, the remnant surrounding mucosa still might carry the risk of developing cancer[31,32]. Although the event-free survival was similar between the two groups (P = 0.234), the differences in follow-up, surveillance strategy that adapted and sample scale might have led to this contradictory result. To manage this issue, regular (annual or biannual) endoscopic surveillance and abdominal computed tomography might be conducted for at least 5 years according to GC treatment guidelines[33,34].
This meta-analysis also has several limitations that should be addressed. First, the clinical heterogeneity of the included studies might affect the reproducibility of our results: the demographic characteristics of enrolled patients, the detailed procedure of ESD, the diagnosis technique of EGC might vary among different institutions. Second, the retrospective nature of enrolled studies limited the application of our results. Third, only one western study from Canada was enrolled and the conclusion might not apply in western countries.
In conclusion, this meta-analysis suggested that ESD is safe and feasible in comparison with resection surgery in treating EGC, with clinical advantages in operation time, hospital stay, postoperative complications. Although with some differences in tumor recurrence rate, the long-term survival also supported the safety of ESD compared with resection surgery. Moreover, further multi-center, prospective randomized controlled trials with longer and standard follow-up strategies are warranted to verify our findings.
There are several surgical options for treating early gastric cancers (EGCs), such as endoscopic resection, laparoscopic or open gastrectomy. The role of endoscopic submucosal dissection (ESD) in treating EGC is not well established, especially when compared with resection surgery.
In this study, the authors aim to compare the safety and efficacy of the short- and long-term outcomes between ESD and resection surgery.
The databases from January 1990 to June 2018 of PubMed, EMBASE, Web of Science, and the Cochrane Library were searched. The enrolling studies reporting short- or long-term outcomes of ESD in comparison with resection surgery for EGC. The quality of the studies was assessed by the Newcastle-Ottawa Quality Assessment Scale. By using either fixed- or random-effects models depending on heterogeneity across studies, the pooling analysis was conducted.
Fourteen studies comprising 5112 patients were eligible for analysis. This meta-analysis demonstrated that the ESD approach showed advantages through decreased operation time, shorter hospital stay, and lower postoperative complication rate. And the EGC patients who underwent ESD had higher recurrence rate than resection surgery patients. However, the long-term survival including overall survival and event-free survival showed no significant differences between these two groups.
This meta-analysis suggested that ESD is safe and feasible in comparison with resection surgery in treating EGC, with clinical advantages in operation time, hospital stay, and postoperative complications. The long-term survival also supported the safety of ESD compared with resection surgery, although with some differences in tumor recurrence rate.
The further multi-center and prospective randomized controlled trials with longer and standard follow-up strategies are warranted to verify the findings of the study.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amin S, Charco R, Takamatsu S S- Editor: Wang JL L- Editor: A E- Editor: Bian YN
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55699] [Article Influence: 7957.0] [Reference Citation Analysis (132)] |
| 2. | Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ; Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 647] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2865] [Article Influence: 204.6] [Reference Citation Analysis (0)] |
| 4. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1148] [Article Influence: 47.8] [Reference Citation Analysis (4)] |
| 5. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Kim YI, Kim YW, Choi IJ, Kim CG, Lee JY, Cho SJ, Eom BW, Yoon HM, Ryu KW, Kook MC. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy. 2015;47:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH, Kim JH, Park YS. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Kim MY, Cho JH, Cho JY. Ever-changing endoscopic treatment for early gastric cancer: yesterday-today-tomorrow. World J Gastroenterol. 2014;20:13273-13283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 10. | Hu J, Zhao Y, Ren M, Li Y, Lu X, Lu G, Zhang D, Chu D, He S. The Comparison between Endoscopic Submucosal Dissection and Surgery in Gastric Cancer: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2018;2018:4378945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Meng FS, Zhang ZH, Wang YM, Lu L, Zhu JZ, Ji F. Comparison of endoscopic resection and gastrectomy for the treatment of early gastric cancer: a meta-analysis. Surg Endosc. 2016;30:3673-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12554] [Article Influence: 836.9] [Reference Citation Analysis (0)] |
| 13. | Chiu PW, Teoh AY, To KF, Wong SK, Liu SY, Lam CC, Yung MY, Chan FK, Lau JY, Ng EK. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc. 2012;26:3584-3591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Park CH, Lee H, Kim DW, Chung H, Park JC, Shin SK, Hyung WJ, Lee SK, Lee YC, Noh SH. Clinical safety of endoscopic submucosal dissection compared with surgery in elderly patients with early gastric cancer: a propensity-matched analysis. Gastrointest Endosc. 2014;80:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Kim DY, Hong SJ, Cho GS, Jeong GA, Kim HK, Han JP, Lee YN, Ko BM, Lee MS. Long-term efficacy of endoscopic submucosal dissection compared with surgery for early gastric cancer: a retrospective cohort study. Gut Liver. 2014;8:519-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Cho JH, Cha SW, Kim HG, Lee TH, Cho JY, Ko WJ, Jin SY, Park S. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc. 2016;30:3762-3773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Song WC, Qiao XL, Gao XZ. A comparison of endoscopic submucosal dissection (ESD) and radical surgery for early gastric cancer: a retrospective study. World J Surg Oncol. 2015;13:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Fukunaga S, Nagami Y, Shiba M, Ominami M, Tanigawa T, Yamagami H, Tanaka H, Muguruma K, Watanabe T, Tominaga K, Fujiwara Y, Ohira M, Hirakawa K, Arakawa T. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc. 2017;85:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Gong EJ, Kim DH, Ahn JY, Jung KW, Lee JH, Choi KD, Song HJ, Lee GH, Jung HY, Kim HS, Lee IS, Kim BS, Yoo MW, Oh ST, Yook JH, Kim BS. Comparison of long-term outcomes of endoscopic submucosal dissection and surgery for esophagogastric junction adenocarcinoma. Gastric Cancer. 2017;20:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Ryu SJ, Kim BW, Kim BG, Kim JH, Kim JS, Kim JI, Park JM, Oh JH, Kim TH, Kim JJ, Park SM, Park CH, Song KY, Lee JH, Kim SG, Kim DJ, Kim W. Endoscopic submucosal dissection versus surgical resection for early gastric cancer: a retrospective multicenter study on immediate and long-term outcome over 5 years. Surg Endosc. 2016;30:5283-5289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Najmeh S, Cools-Lartigue J, Mueller C, Ferri LE. Comparing Laparoscopic to Endoscopic Resections for Early Gastric Cancer in a High Volume North American Center. J Gastrointest Surg. 2016;20:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Shin DW, Hwang HY, Jeon SW. Comparison of Endoscopic Submucosal Dissection and Surgery for Differentiated Type Early Gastric Cancer within the Expanded Criteria. Clin Endosc. 2017;50:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Jeon HK, Kim GH, Lee BE, Park DY, Song GA, Kim DH, Jeon TY. Long-term outcome of endoscopic submucosal dissection is comparable to that of surgery for early gastric cancer: a propensity-matched analysis. Gastric Cancer. 2018;21:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Park JC, Lee YK, Kim SY, Roh Y, Hahn KY, Shin SK, Lee SK, Lee YC, Kim HI, Cheong JH, Hyung WJ, Noh SH. Long-term outcomes of endoscopic submucosal dissection in comparison to surgery in undifferentiated-type intramucosal gastric cancer using propensity score analysis. Surg Endosc. 2018;32:2046-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Hahn KY, Park CH, Lee YK, Chung H, Park JC, Shin SK, Lee YC, Kim HI, Cheong JH, Hyung WJ, Noh SH, Lee SK. Comparative study between endoscopic submucosal dissection and surgery in patients with early gastric cancer. Surg Endosc. 2018;32:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, Lee JH, Kim DH, Song HJ, Lee GH, Yook JH, Kim BS, Jung HY. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2018;21:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Nagata K, Shimizu M. Pathological evaluation of gastrointestinal endoscopic submucosal dissection materials based on Japanese guidelines. World J Gastrointest Endosc. 2012;4:489-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 29. | Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, Ryu KW, Nam BH, Kook MC, Kim YW. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. 2015;81:333-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 30. | Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Ahn JH, Carriere KC, Kim JJ, Kim S. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am J Gastroenterol. 2016;111:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, Nishihara A, Nakanishi F, Nakahara M, Ogiyama H, Kinoshita K, Yamada T, Iijima H, Tsujii M, Takehara T. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 33. | Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH, Kim HI, Jung HY, Kim YS, Zang DY, Cho JY, Park JO, Lim DH, Jung ES, Ahn HS, Kim HJ. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 34. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1910] [Article Influence: 238.8] [Reference Citation Analysis (1)] |