Published online Nov 15, 2019. doi: 10.4251/wjgo.v11.i11.1054
Peer-review started: July 12, 2019
First decision: July 31, 2019
Revised: August 31, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: November 15, 2019
Processing time: 137 Days and 4.8 Hours
The fundus of the stomach is regarded as a difficult area for endoscopic resection of small tumors originating from the muscularis propria (MP tumors). Three endoscopic resection techniques have been developed to treat these tumors, including ligation-assisted endoscopic full-thickness resection (L-EFTR), snare-assisted EFTR (S-EFTR), and endoscopic submucosal dissection-assisted EFTR (E-EFTR). To date, no studies have compared these techniques.
We aimed to evaluate and compare S-EFTR with L-EFTR and E-EFTR for treating small MP tumors in the gastric fundus.
We retrospectively reviewed patients with primary small MP tumors in the gastric fundus and treated by these three techniques between January 2016 and December 2018 at Shengjing Hospital, China. Standard demographic and clinicopathologic data, including sex, age, tumor size, surgeon details, and pathological results, were collected. Data regarding operation duration, cost, en-bloc resection, and severe complications were also extracted and compared.
A total of 36 patients (27 women) with a mean age of 55.8 ± 10.20 years were included in this study. The mean tumor size was 9.0 ± 3.98 mm. All the methods showed a 100% en-bloc resection rate and 0% severe complication rate. There was no statistically significant difference among the three groups in the operation duration (P = 0.148). The cost comparison for the whole procedure was as follows: E-EFTR > L-EFTR > S-EFTR (5837.5 ± 7212.96 CNY, 5970.7 ± 3465.27 CNY, 5852.0 ± 6438.25 CNY, respectively, P < 0.001).
S-EFTR, L-EFT, and E-EFTR are all effective for resection of small MP tumors in the gastric fundus. S-EFTR is superior in terms of cost-effectiveness.
Core tip: Ligation-assisted endoscopic full-thickness resection (L-EFTR), snare-assisted EFTR (S-EFTR), and endoscopic submucosal dissection-assisted EFTR (E-EFTR) are all effective techniques for resection of small muscularis propria (MP tumors) in the gastric fundus. S-EFTR is superior in terms of cost-effectiveness of the treatment. S-EFTR can become the most efficient resection method for MP tumors in the gastric fundus.
- Citation: Ge N, Hu JL, Yang F, Yang F, Sun SY. Endoscopic full-thickness resection for treating small tumors originating from the muscularis propria in the gastric fundus: An improvement in technique over 15 years. World J Gastrointest Oncol 2019; 11(11): 1054-1064
- URL: https://www.wjgnet.com/1948-5204/full/v11/i11/1054.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i11.1054
In 2004, we reported the first endoscopic band ligation method (the tumor was first aspirated with a transparent cap and then ligated with the band) to treat small tumors originating from the muscularis propria (MP tumors), which proved to be simple and effective[1,2], except for the relatively high risk of post-ligation perforation of the gastric fundus[3]. In 2011, we reported the improved ligation technique in which we placed 4–5 hemoclips around the ligation band to prevent perforation[4]. This ligation technique effectively diminished small gastrointestinal stromal tumors (GISTs), but without recycling the tumors for pathological analysis.
From 2015, right after tumor ligation in the gastric fundus, we resected the ligated tumor and the band together with a snare. Further, we used several hemoclips or the over-the-scope-clip (OTSC) device to close the gastric wall defect (GWD). We named the method ligation-assisted endoscopic full-thickness resection (L-EFTR). In 2016, we performed direct tumor resection with a snare and closed the gastric defect with hemoclips or the OTSC device. We named it snare-assisted EFTR (S-EFTR). Additionally, we still used the traditional endoscopic submucosal dissection (ESD) technique to accomplish endoscopic full-thickness resection (E-EFTR). L-EFTR, S-EFTR, and E-EFTR are all available techniques for small MP tumor resection in the gastric fundus. However, to date, no studies have compared these techniques. Therefore, in this clinical study, we evaluated and compared the three techniques, S-EFTR, L-EFTR, and E-EFTR, for the treatment of small MP tumors in the gastric fundus.
We retrospectively reviewed and collected the medical records of patients diagnosed with primary MP tumors between January 2016 and December 2018 at Shengjing Hospital, China.
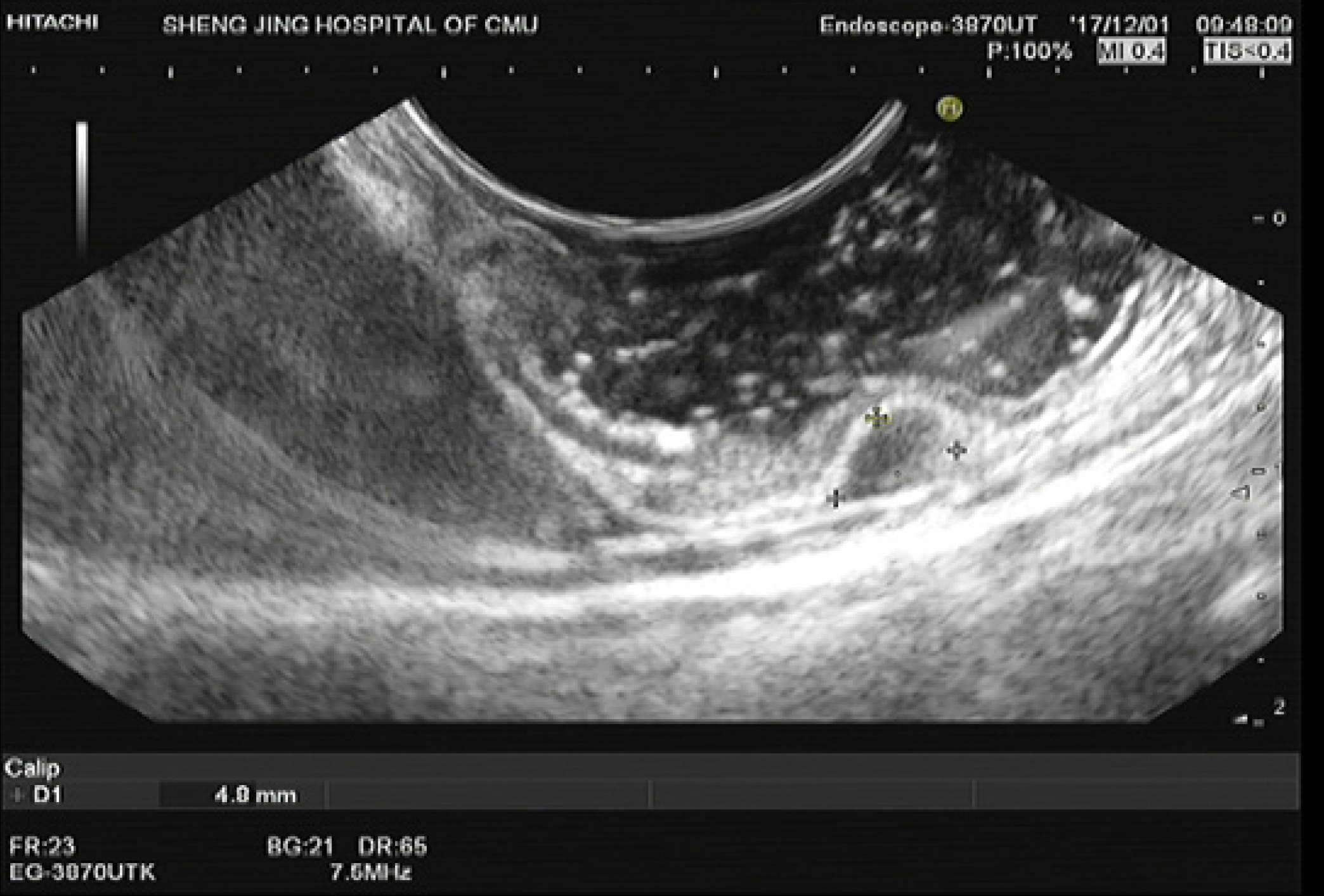
The definition of gastric fundus MP tumors is strictly limited to MP tumors in the fornix of the gastric fundus, which is not near the cardia or the body of the stomach. The abdominal structure outside the tumors was the diaphragm or edge of the liver, which can be confirmed using endoscopic ultrasound (EUS) (Figure 1). Among these patients, those who underwent endoscopic resection at our hospital through any of these three techniques were selected.
The inclusion criteria were as follows: Patients with gastric MP tumors diagnosed using EUS (Longitudinal echoendoscope, PENTAXEG3870UT, Pentax Corporation, Japan); patients with tumors located in the gastric fornix of the fundus confirmed by EUS; patients with tumors with a maximum diameter of 20 mm measured using EUS; patients who underwent S-EFTR, L-EFTR, or E-EFTR; and the patients with no severe comorbidities[5-7]. The exclusion criteria were as follows: Patients in whom the procedure was not performed by senior doctors and patients whose data were not completely recorded.
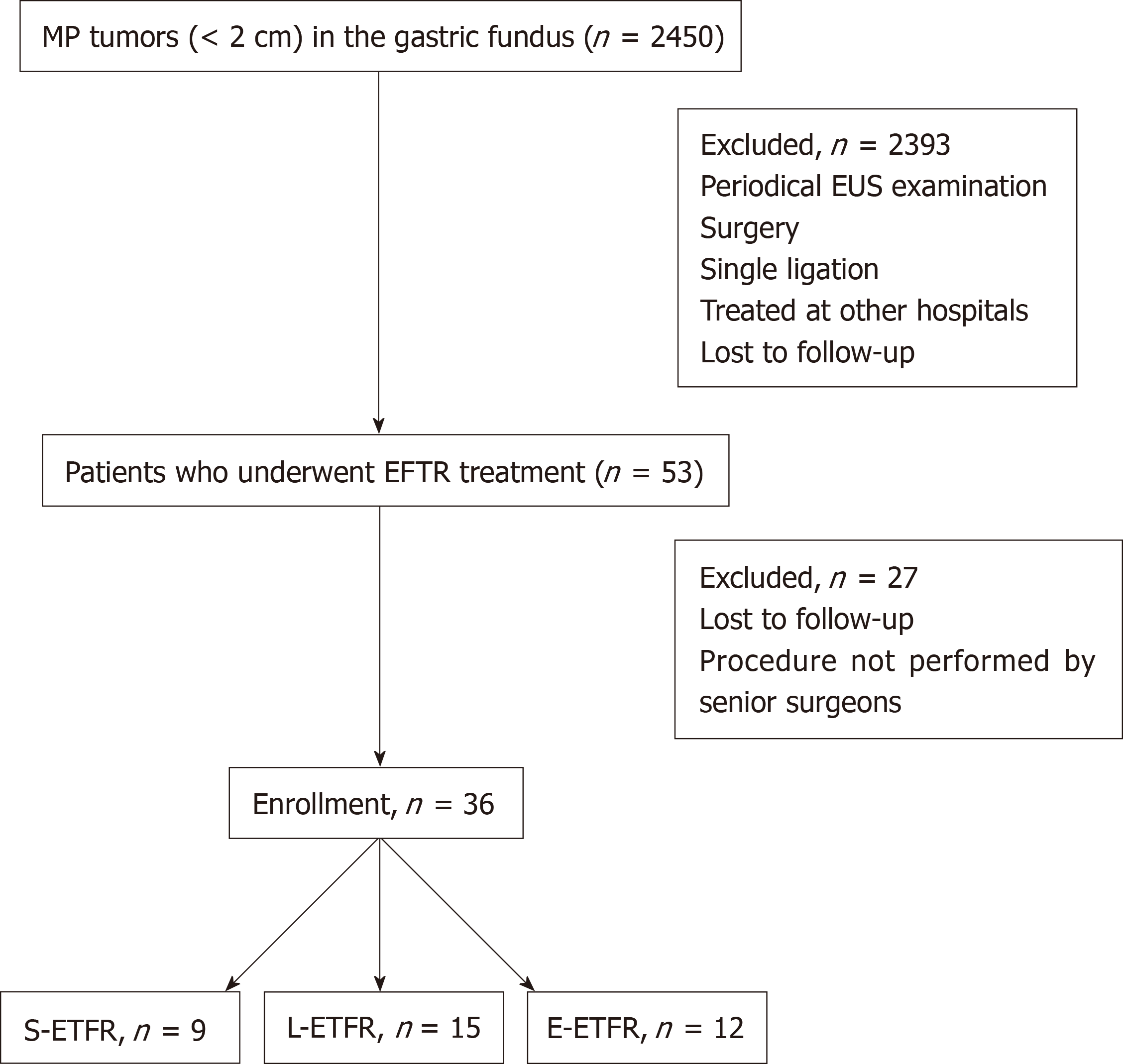
The flow diagram for screening and grouping of the study subjects is illustrated in Figure 2.
Standard demographic and clinicopathologic data including sex, age, tumor size, operator, and pathological results were collected. Data regarding total operation duration, tumor resection duration, GWD closure duration, total cost of the operation, cost of tumor resection, cost of defect closure, en-bloc resection, and severe complications were also extracted from the records.
All procedures were performed by four experienced senior endoscopists. All the patients were under general anesthesia during the procedure. After the EFTR, patients had to fast for 48 h. Antibiotic drugs and proton pump inhibitors were routinely administered intravenously. The patients were under observation for abdominal pain, fever, and signs of peritonitis or hemorrhage. If no complication occurred, the patients were discharged from the hospital 48 h postoperatively. Standard follow-up by esophagogastroduodenoscopy was recommended to patients.
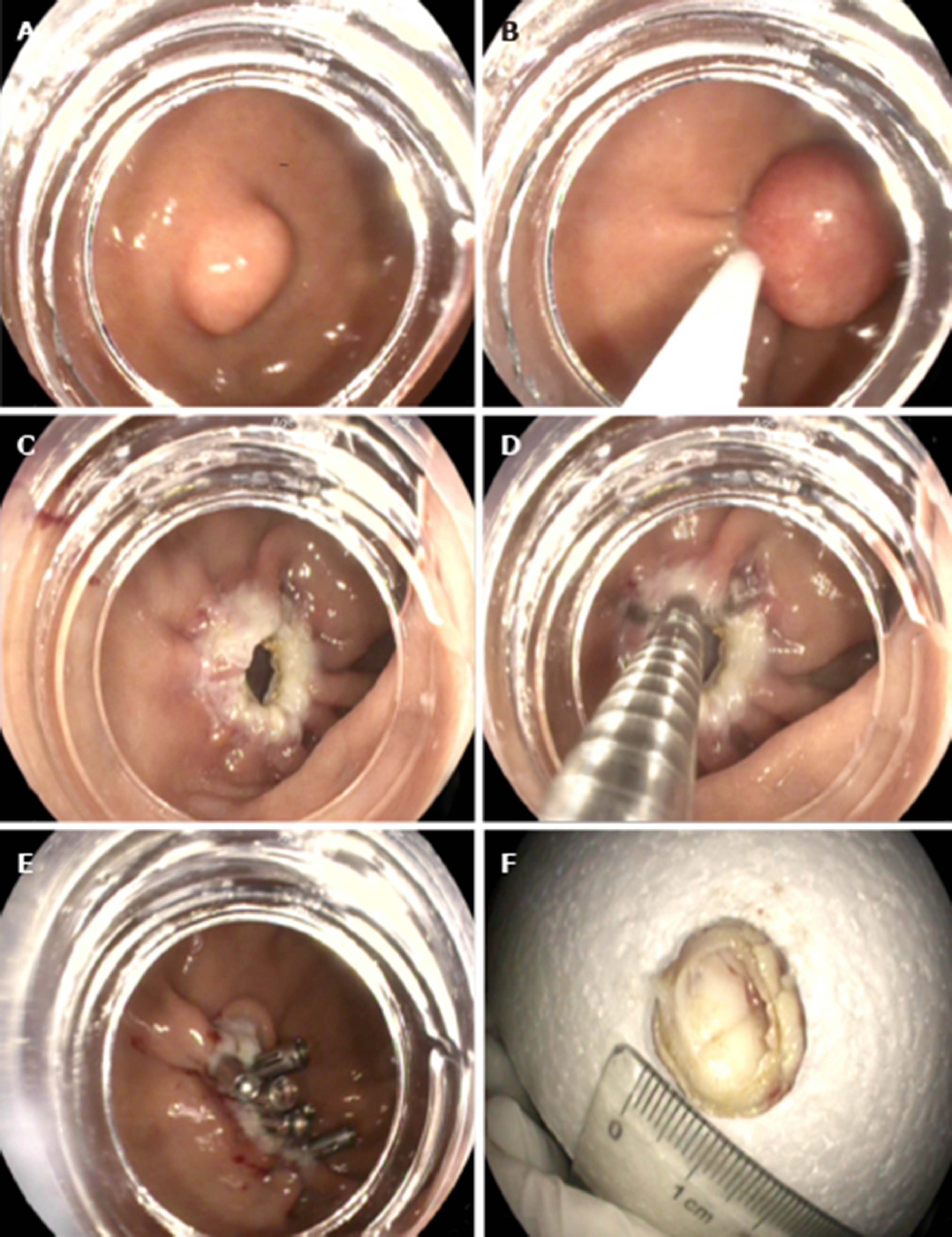
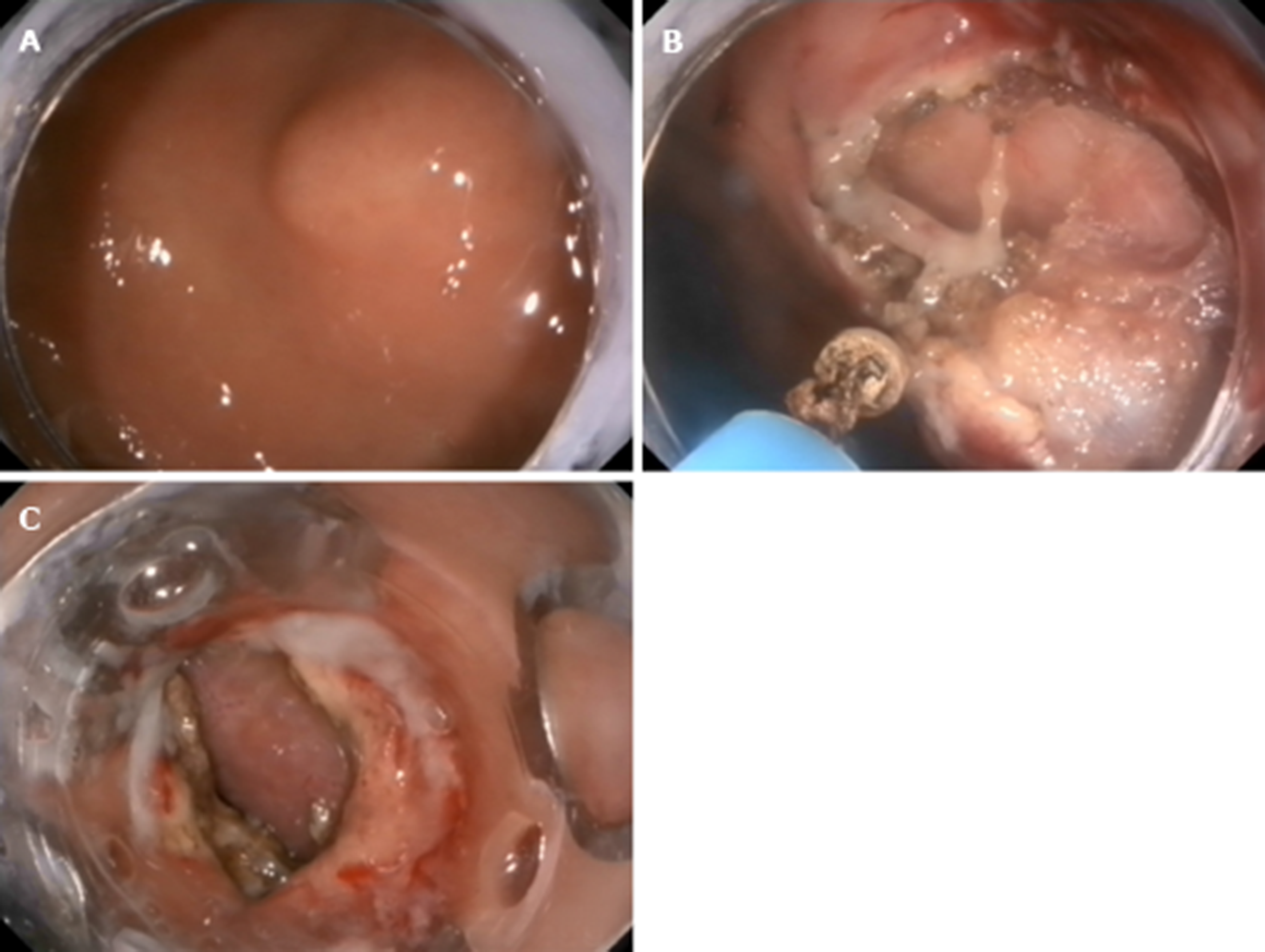
S-EFTR was performed using a standard high-definition, white-light gastroscope (EG29-i10, Pentax, Tokyo, Japan). A single-use polypectomy snare (ASM-1-S, Wilson-cook Medical Inc., USA) in combination with a standard high-frequency generator (ESG-400, Olympus Winter and Ibe GmbH, Germany) was used. The snare was placed around the lesion and mild suction was applied to loosen the gastric wall and ensure the complete grasp of the tumor. After the snare was tightened, the tumor presented as a Yamada type-III polyp. The standard polypectomy setting for gastric wall was used during cutting (Forced Coag 45, Effect 2) and was repetitively alternated with pulse-cut slow (45, Effect 2, Figure 3)
The resected specimen was retrieved using the snare. If bleeding occurred, electrosurgical hemostastic forceps (FD-410LR; Olympus, Japan) were applied. Several clips (POCC-C-26-230-C, Micro-tech, Nanjing, China) were used for the final closure of the gastric defect.
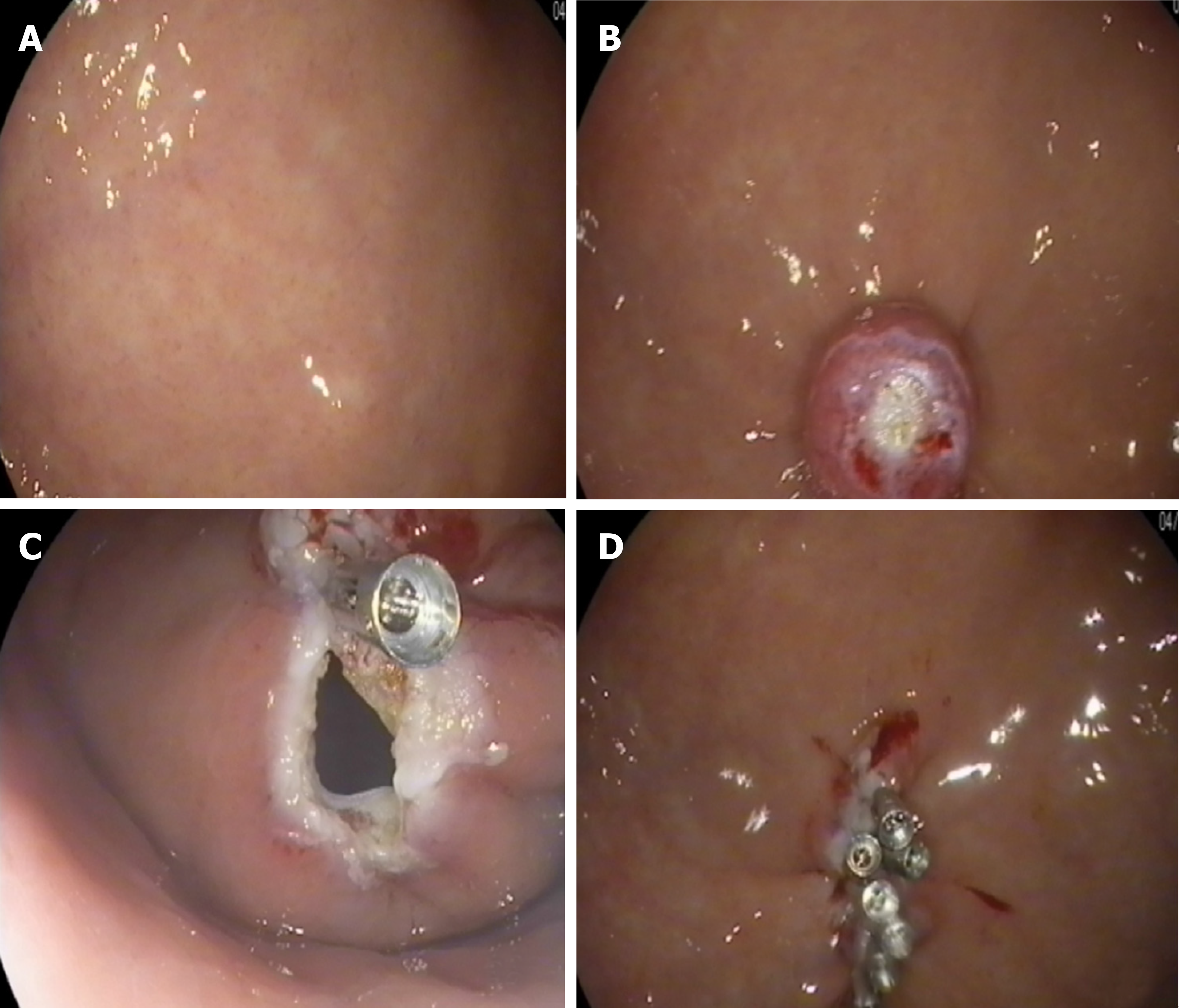
The lesion was fully aspirated into a transparent cap attached to an endoscope (EG29-i10, Pentax, Tokyo, Japan) that was introduced into the stomach before the rubber band (Sumitbe, Akita, Japan) was released. If aspiration was inadequate with only the mucosal and submucosal layers confined, the band was removed using foreign body forceps (JHY-FG-23-230-A2, JiuHong, China) before the lesion was religated. After the ligation, a snare was placed around the tumor and tightened under the ligated band. The standard polypectomy setting was used to cut the tumor and the band together. The band and tumor were retrieved with the snare. If bleeding occurred, hemostastic forceps were applied. Several clips were applied to close the defects. Occasionally, immediately after the ligation, we cut open the mucosal and submucosal layers to check whether the tumor was ligated (Figure 4).
The mucosal and submucosal layers around the tumor were precut with a triangle tip knife (KD-640, Olympus, Japan), and the submucosal layer was dissected until the tumor was exposed. Next, the tumor was dissected from the MP layer carefully to ensure complete en-bloc resection of the lesion. When dissecting the tumor, perforation and circumferential incision into the serous membrane around the tumor were performed with an IT-2 knife (KD-612L, Olympus, Japan). Finally, several clips were used to close the defect. If the defect was too large, we used the OTSC device (Ovesco Endoscopy AG, Tuebingen Germany)[8,9] (Figure 5).
Details of the surgeon, devices used for tumor resection, devices used for defect closure, cost of tumor resection, cost of defect closure, operation duration for tumor resection, operation duration for defect closure, severe complications, date of last follow-up, and recurrence were collected. Severe complications were defined as perforation or massive hemorrhage that required surgical intervention. The protocol to perform retrospective revision of the cases was approved by the Medical Ethics Committee. All patients were informed of the benefits and risks of the procedure; they signed the written informed consent document prior to each procedure.
Statistical analyses were performed with SPSS version 23.0 (SPSS Inc., Chicago, Ill, USA). Frequencies, percentages, means ± SD, and medians (range) are used, as appropriate, for descriptive analysis. Differences among groups were assessed by one-way analysis of variance. If P < 0.05, the comparisons were made between two groups out of three, at a time. For categorical variables, comparisons between groups were performed by the Fisher exact test(small sample). Continuous variables with a normal distribution were analyzed by the Student’s t-test; for those with an abnormal distribution, comparison was performed by the Mann-Whitney U test. All statistical analyses were two-sided. P < 0.05 was considered statistically significant.
Among the 2450 patients with MP tumors in the gastric fundus, 36 (27 women) were included in this study (Table 1). The mean age was 55.8 ± 10.20 years. The mean tumor size was 9.0 ± 3.98 mm. The pathological results showed GISTs with a very low risk in 28 patients (77.8%), GIST with a low risk in 1, leiomyoma in 3, tuberculosis in 1, hamartoma in 1, and inflammatory myofibroblastoma in 1. There was no difference in age or sex between the three groups.
| L-EFTR | S-EFTR | E-EFTR | P value | ||
| Female, n (%) | 27 (75) | 8 (29.6) | 8 (29.6) | 11 (40.7) | 0.554 |
| Age (yr) | 55.8 ± 10.20 | 57.7 ± 8.53 | 5.35 ± 8.91 | 57.2 ± 12.83 | 0.535 |
| Tumor size (mm) | 9.0 ± 3.98 | 6.5 ± 1.96 | 12.6 ± 4.42 | 9.6 ± 3.45 | 0.505 |
The operation time, cost, and en-bloc resection and complication rates are listed in Table 2. There was no significant difference among the three groups with respect to the total operation duration (P = 0.148), operation duration for tumor resection (P = 0.085), and duration of defect closure (P = 0.965). Therefore, the difference between two groups separately was not further calculated.
| L-EFTR | S-EFTR | E-EFTR | P value | |
| Total operation duration (min) | 27.2 ± 26.28 | 14.0 ± 8.19 | 33.0 ± 21.99 | 0.148 |
| Operation duration of tumor resection (min) | 18.4 ± 26.31 | 4.7 ± 3.39 | 23.6 ± 13.74 | 0.085 |
| Operation duration of defect closure (min) | 8.8 ± 4.39 | 9.3 ± 5.07 | 9.4 ± 9.10 | 0.965 |
| Total procedure cost (CNY) | 5970.7 ± 3465.27 | 5852.0 ± 6438.25 | 15837.5 ± 7212.96 | < 0.001 |
| Cost of tumor resection (CNY) | 3952.2 ± 1866.49 | 525.0 ± 0 | 5253.5 ± 2200.48 | < 0.001 |
| Cost of defect closure (CNY) | 2018.8 ± 3232.47 | 5237.0 ± 6438.25 | 10584.0 ± 5857.06 | 0.001 |
| En-bloc resection rate, % | 100 | 100 | 100 | |
| Complication rate, % | 0 | 0 | 0 |
The costs (total, tumor resection, and defect closure) among the three groups were different (Table 3). The cost comparison for the whole procedure was as follows: E-EFTR > L-EFTR > S-EFTR (15837.5 ± 7212.96 CNY, 5970.7 ± 3465.27 CNY, 5852.0 ± 6438.25 CNY, respectively, P < 0.001). We adjusted for sex, age, and tumor size as confirmed using EUS and compared between two groups separately, which still resulted in significant differences.
| Total procedure cost | |||||
| β | Standard error | Standardization coefficient | 95%CI | P value | |
| L-EFTR vs S-EFTR | 7220.69 | 2138.94 | 0.766 | (2743.83, 11697.54) | 0.003 |
| L-EFTR vs E-EFTR | -8556.61 | 2124.56 | -0.593 | (-12962.67, -4150.55) | 0.001 |
| S-EFTR vs E-EFTR | -14876.08 | 2282.57 | -0.896 | (-19714.91, -10037.26) | < 0.001 |
The costs for tumor resection using E-EFTR, L-EFTR, and S-EFTR were 5253.5 ± 2200.4 CNY, 3952.2 ± 1866.49 CNY, and 525.0 ± 0 CNY, respectively (P < 0.001). Therefore, we adjusted for sex, age, and tumor size as confirmed using EUS and compared between two groups separately. We found no significant difference between the E-EFTR and L-EFTR groups. We found that tumor resection using S-EFTR costed significantly less than that using E-EFTR and L-EFTR (P < 0.01 and P = 0.01, respectively) (Table 4).
| Cost of tumor resection | |||||
| β | Standard error | Standardization coefficient | 95%CI | P value | |
| L-EFTR vs S-EFTR | 3653.83 | 913.13 | 0.809 | (1742.63, 5565.02) | 0.001 |
| L-EFTR vs E-EFTR | -1277.22 | 905.56 | -0.310 | (-3155.24, 600.79) | 0.172 |
| S-EFTR vs E-EFTR | -4777.27 | 820.07 | -0.835 | (-6515.75, -3038.80) | < 0.001 |
The costs of the defect closure procedure for E-EFTR, L-EFTR, and S-EFTR were 10584.0 ± 5857.06 CNY, 2018.8 ± 3232.47 CNY, and 5237.0 ± 6438.25 CNY, respectively (P = 0.001). Therefore, we adjusted for sex, age, and tumor size as confirmed using EUS and compared between two groups separately. There were no significant differences between the S-EFTR and L-EFTR groups. E-EFTR costed significantly more than S-EFTR and L-EFTR (P < 0.01 and P = 0.01, respectively) (Table 5).
| Cost of defect closure | |||||
| β | Standard error | Standardization coefficient | 95%CI | P value | |
| L-EFTR vs S-EFTR | 3567.39 | 2099.27 | 0.364 | (-826.43, 7961.20) | 0.106 |
| L-EFTR vs E-EFTR | -7278.81 | 1843.43 | -0.591 | (-11101.85, -3455.76) | 0.001 |
| S-EFTR vs E-EFTR | -10098.81 | 1835.18 | -0.785 | (-13989.22, -6208.40) | < 0.001 |
All methods showed a 100% en-bloc resection rate and 0% severe complication rate. All the patients were discharged from hospital 48 h postoperatively, and no prolonged hospital stay was recorded.
Treatment of small GISTs or other lesions (< 2 cm) originating from the MP is controversial[10-13]. Previous studies have demonstrated that endoscopic full-thickness resection could be the diagnostic and definitive treatment for these tumors, with a high success rate of 90% and low incidence of complications. Therefore, for tumors < 2 cm, besides periodic follow-up using EUS, endoscopic resection may be another optimal option[14-17]. In our study, all the MP tumors in the gastric fundus could be resected using the three techniques, S-EFTR, L-EFTR, and E-EFTR. The overall en-bloc resection rate was 100% and severe complication rate was 0%, which confirmed that they are all effective and safe methods. Furthermore, the previously mentioned single ligation technique should not be applied in the gastric fundus, as it may still be associated with the unpredictable risk of delayed perforation.
Endoscopic resection of MP tumors in different locations of the gastrointestinal tract can encounter various difficulties; therefore, these tumors should be treated via different endoscopic approaches. Our previous study confirmed the difficulty in using the ESD/EFTR technique, and the complication rate varied among the different locations of the stomach[18]. Chiu et al[19] also confirmed that gastric subepithelial lesions located at the cardia, lesser curvature, and antrum could be treated by creating a submucosal tunnel. The fundus of the stomach is regarded as a difficult area for endoscopic resection[20,21].
En-bloc resection was performed in all the patients, which may be specific to the structure of the gastric fundus and tumor depth. In addition, we are not sure if S-EFTR can be used effectively in other locations of the stomach. For example, small MP tumors in the cardia or posterior wall of the stomach are seldom associated with the whole gastric layer defect or gastric cavity collapse by gas rush into the abdominal cavity[18,22]. Most of the resected MP tumors at this location are GISTs, which is similar to other reported studies[23]. EUS is still the most valuable tool for MP tumor diagnosis[24-27]. However, besides GISTs, there may be other tumors with similar echo morphology in this location[28,29]. Therefore, simple and safe resection methods with pathological results could still be the optimal treatment for these tumors.
There was no significant difference in operation duration (including total operation duration, operation duration of tumor resection, and defect closure) among the three groups. However, when we compared two techniques separately, S-EFTR had a markedly shorter tumor cutting duration than E-EFTR, which proved the efficiency of S-EFTR. However, this finding needs to be verified in a larger study sample.
When we compared the total cost of the three procedures, E-EFTR was found to be the most expensive method, followed by L-EFTR, with S-EFTR being the most cost-effective. Mostly, we just used a snare and 4–5 clips to complete the operation, which requires fewer devices than the standard endoscopic mucosal resection technique.
For lesion resection, there was no cost difference between E-EFTR and L-EFTR, and both procedures costed much more than S-EFTR, indicating that S-EFTR is a simplified technique. The ligator used in L-EFTR and the electrosurgery knives used in E-EFTR are all expensive devices.
For GWD closure, E-EFTR costed more than S-EFTR or L-EFTR, which indicated that the defect treated using E-EFTR may have been larger and may have needed more clips. There was no difference in the number of clips used between the S-EFTR and L-EFTR groups.
The main limitation of this study is its retrospective study design. Furthermore, other very newly developed techniques[30-33] have been published, which need to be evaluated in future studies.
In conclusion, S-EFTR, L-EFT, and E-EFTR are all effective for small MP tumor resection in the gastric fundus. S-EFTR is superior in terms of cost-effectiveness of the treatment. Our results suggest that S-EFTR can become the most efficient technique for MP tumor resection in the gastric fundus.
The fundus of the stomach is regarded as a difficult area for endoscopic resection of small tumors originating from the muscularis propria (MP tumors). Three endoscopic resection techniques have been developed to treat these tumors, including ligation-assisted endoscopic full-thickness resection (L-EFTR), snare-assisted EFTR (S-EFTR), and endoscopic submucosal dissection-assisted EFTR (E-EFTR).
To date, no studies have compared these techniques.
We aimed to evaluate and compare S-EFTR with L-EFTR and E-EFTR for treating small MP tumors in the gastric fundus.
We retrospectively reviewed patients with primary small MP tumors in the gastric fundus and treated them by the three techniques between January 2016 and December 2018 at Shengjing Hospital, China. Standard demographic and clinicopathologic data, including sex, age, tumor size, surgeon details, and pathological results, were collected. Data regarding operation duration, cost, en-bloc resection, and severe complications were also extracted and compared.
A total of 36 patients (27 women) with a mean age of 55.8 ± 10.20 years were included in this study. The mean tumor size was 9.0 ± 3.98 mm. All the methods showed a 100% en-bloc resection rate and 0% severe complication rate. There was no statistically significant difference among the three groups in the operation duration (P = 0.148). The cost comparison for the whole procedure was as follows: E-EFTR > L-EFTR > S-EFTR.
S-EFTR, L-EFT, and E-EFTR are all effective for small MP tumor resection in the gastric fundus. S-EFTR is superior in terms of cost-effectiveness of the treatment. S-EFTR can become the most efficient technique for MP tumor resection in the gastric fundus.
S-EFTR, L-EFT, and E-EFTR are all effective techniques for resection of small MP tumors in the gastric fundus. Other very newly developed techniques have been published, which need to be evaluated in future studies. Prospective and multicenter studies are needed.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Neri V, Nishida T S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Sun S, Ge N, Wang C, Wang M, Lü Q. Endoscopic band ligation of small gastric stromal tumors and follow-up by endoscopic ultrasonography. Surg Endosc. 2007;21:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Sun S, Jin Y, Chang G, Wang C, Li X, Wang Z. Endoscopic band ligation without electrosurgery: a new technique for excision of small upper-GI leiomyoma. Gastrointest Endosc. 2004;60:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Siyu S, Sheng W, Guoxin W, Nan G, Jingang L. Gastric perforations after ligation of GI stromal tumors in the gastric fundus. Gastrointest Endosc. 2010;72:615-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Nan G, Siyu S, Shiwei S, Sheng W, Xiang L. Hemoclip-reinforced and EUS-assisted band ligation as an effective and safe technique to treat small GISTs in the gastric fundus. Am J Gastroenterol. 2011;106:1560-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Laeeq SM, Tasneem AA, Hanif FM, Luck NH, Mandhwani R, Wadhva R. Upper Gastrointestinal Bleeding in Patients with End Stage Renal Disease: Causes, Characteristics and Factors Associated with Need for Endoscopic Therapeutic Intervention. J Transl Int Med. 2017;5:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Khalid MA, Iqbal J, Memon HL, Hanif FM, Butt MOT, Luck NH, Majid Z. Dyspepsia Amongst End Stage Renal Disease Undergoing Hemodialysis: Views from a Large Tertiary Care Center. J Transl Int Med. 2018;6:78-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Yamamichi N, Omata M. Feasibility of endoscopic submucosal dissection for patients with chronic renal failure on hemodialysis. Dig Endosc. 2010;22:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Dinelli M, Omazzi B, Andreozzi P, Zucchini N, Redaelli A, Manes G. First clinical experiences with a novel endoscopic over-the-scope clip system. Endosc Int Open. 2017;5:E151-E156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Fähndrich M, Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015;47:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Ojima T, Nakamura M, Nakamori M, Takifuji K, Hayata K, Katsuda M, Takei Y, Yamaue H. Laparoscopic and endoscopic cooperative surgery is a feasible treatment procedure for intraluminal gastric gastrointestinal stromal tumors compared to endoscopic intragastric surgery. Surg Endosc. 2018;32:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 11. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 822] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 12. | Theiss L, Contreras CM. Gastrointestinal Stromal Tumors of the Stomach and Esophagus. Surg Clin North Am. 2019;99:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Gluzman MI, Kashchenko VA, Karachun AM, Orlova RV, Nakatis IA, Pelipas IV, Vasiukova EL, Rykov IV, Petrova VV, Nepomniashchaia SL, Klimov AS. Technical success and short-term results of surgical treatment of gastrointestinal stromal tumors: an experience of three centers. Transl Gastroenterol Hepatol. 2017;2:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Yang Z, Feng X, Zhang P, Chen T, Qiu H, Zhou Z, Li G, Tao KX, Li Y; China Gastrointestinal Stromal Tumor Study Group (CN-GIST). Clinicopathological features and prognosis of 276 cases of primary small (≤ 2 cm) gastric gastrointestinal stromal tumors: a multicenter data review. Surg Endosc. 2019;33:2982-2990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Andalib I, Yeoun D, Reddy R, Xie S, Iqbal S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: methods and feasibility data. Surg Endosc. 2018;32:1787-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Tan Y, Tan L, Lu J, Huo J, Liu D. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol. 2017;2:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Joo MK, Park JJ, Kim H, Koh JS, Lee BJ, Chun HJ, Lee SW, Jang YJ, Mok YJ, Bak YT. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc. 2016;83:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Yang F, Wang S, Sun S, Liu X, Ge N, Wang G, Guo J, Liu W, Feng L, Ma W. Factors associated with endoscopic full-thickness resection of gastric submucosal tumors. Surg Endosc. 2015;29:3588-3593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Chiu PWY, Yip HC, Teoh AYB, Wong VWY, Chan SM, Wong SKH, Ng EKW. Per oral endoscopic tumor (POET) resection for treatment of upper gastrointestinal subepithelial tumors. Surg Endosc. 2019;33:1326-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Li B, Chen T, Qi ZP, Yao LQ, Xu MD, Shi Q, Cai SL, Sun D, Zhou PH, Zhong YS. Efficacy and safety of endoscopic resection for small submucosal tumors originating from the muscularis propria layer in the gastric fundus. Surg Endosc. 2019;33:2553-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Shi Q, Li B, Qi ZP, Yao LQ, Xu MD, Cai SL, Sun D, Zhou PH, Zhong YS. Clinical Values of Dental Floss Traction Assistance in Endoscopic Full-Thickness Resection for Submucosal Tumors Originating from the Muscularis Propria Layer in the Gastric Fundus. J Laparoendosc Adv Surg Tech A. 2018;28:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Kukreja K, Chennubhotla S, Bhandari B, Arora A, Singhal S. Closing the Gaps: Endoscopic Suturing for Large Submucosal and Full-Thickness Defects. Clin Endosc. 2018;51:352-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Pesenti C, Bories E, Caillol F, Ratone JP, Godat S, Monges G, Poizat F, Raoul JL, Ries P, Giovannini M. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: A retrospective study. Endosc Ultrasound. 2019;8:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Okasha HH, Naguib M, El Nady M, Ezzat R, Al-Gemeie E, Al-Nabawy W, Aref W, Abdel-Moaty A, Essam K, Hamdy A. Role of endoscopic ultrasound and endoscopic-ultrasound-guided fine-needle aspiration in endoscopic biopsy negative gastrointestinal lesions. Endosc Ultrasound. 2017;6:156-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Ignee A, Jenssen C, Hocke M, Dong Y, Wang WP, Cui XW, Woenckhaus M, Iordache S, Saftoiu A, Schuessler G, Dietrich CF. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc Ultrasound. 2017;6:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Antonini F, Delconte G, Fuccio L, De Nucci G, Fabbri C, Armellini E, Frazzoni L, Fornelli A, Magarotto A, Mandelli E, Occhipinti P, Masci E, Manes G, Macarri G. EUS-guided tissue sampling with a 20-gauge core biopsy needle for the characterization of gastrointestinal subepithelial lesions: A multicenter study. Endosc Ultrasound. 2019;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Lee HS, Cho CM, Kwon YH, Nam SY. Predicting Malignancy Risk in Gastrointestinal Subepithelial Tumors with Contrast-Enhanced Harmonic Endoscopic Ultrasonography Using Perfusion Analysis Software. Gut Liver. 2019;13:161-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Gottschalk U, Dietrich CF, Jenssen C. Ectopic pancreas in the upper gastrointestinal tract: Is endosonographic diagnosis reliable? Data from the German Endoscopic Ultrasound Registry and review of the literature. Endosc Ultrasound. 2018;7:270-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Flores A, Papafragkakis C, Uberoi AS, Thaiudom S, Bhutani MS. EUS of an atypical ectopic pancreas. Endosc Ultrasound. 2018;7:216-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Schmidt A, Beyna T, Schumacher B, Meining A, Richter-Schrag HJ, Messmann H, Neuhaus H, Albers D, Birk M, Thimme R, Probst A, Faehndrich M, Frieling T, Goetz M, Riecken B, Caca K. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018;67:1280-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (2)] |
| 31. | Dobashi A, Rajan E, Knipschield MA, Gostout CJ. Endoscopic full-thickness resection using suture loop needle T-tag tissue anchors in the porcine stomach (with video). Gastrointest Endosc. 2018;87:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Al-Bawardy B, Rajan E, Wong Kee Song LM. Over-the-scope clip-assisted endoscopic full-thickness resection of epithelial and subepithelial GI lesions. Gastrointest Endosc. 2017;85:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Bauder M, Schmidt A, Caca K. Non-Exposure, Device-Assisted Endoscopic Full-thickness Resection. Gastrointest Endosc Clin N Am. 2016;26:297-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |