Published online Nov 15, 2019. doi: 10.4251/wjgo.v11.i11.1021
Peer-review started: May 23, 2019
First decision: July 31, 2019
Revised: September 3, 2019
Accepted: September 12, 2019
Article in press: September 13, 2019
Published online: November 15, 2019
Processing time: 179 Days and 4.1 Hours
There is no standard therapy for second-line treatment of gemcitabine-refractory pancreatic cancer patients with poor performance status. A combination of chemotherapy drugs 5-fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) or 5-fluorouracil/leucovorin plus nanoliposomal irinotecan can be considered as second-line treatment for such patients; however, due to toxicity, none of the regimens are recommended for patients with poor performance. Capecitabine or S-1 has relatively low toxicity and can be considered a treatment option for gemcitabine-refractory pancreatic cancer.
To investigate the efficacy and toxicity of oral chemotherapy as second-line treatment in patients with pancreatic cancer.
Patients who had progressive disease after first-line gemcitabine-based chemotherapy were retrospectively analyzed between January 2011 and December 2018. They were treated with capecitabine or S-1 as the second-line treatment. Capecitabine was administered as a 2500 mg/m2 divided dose on days 1-14, followed by a 1-wk rest. S-1 was taken orally based on the patient’s body surface area for 28 d, followed by 2-wk of rest. Progression-free survival and overall survival were used to compare efficacy of capecitabine and S-1.
Of the 81 patients, 41 were treated with capecitabine and 40 with S-1. The median time to treatment failure in both groups was 1.5 mo (P = 0.425). The objective response rate was similar in the two groups: 9.8% with capecitabine and 2.5% with S-1 (P = 0.359). Median progression-free survival was longer in the S-1 group than in the capecitabine group (S-1 2.7 mo, capecitabine 2.0 mo, P = 0.003). There was no significant difference in the median overall survival between the capecitabine and S-1 groups (4.3 mo vs 5.0 mo, P = 0.092). Grade 3 or 4 hand-foot syndrome was significantly more common in the capecitabine group than in the S-1 group (14.6% vs 0%, P = 0.026).
Capecitabine or S-1 can be used as a second-line treatment for patients with advanced pancreatic cancer with poor performance status after progression to a gemcitabine-based regimen.
Core tip: To date, there is no standard regimen for patients with gemcitabine-refractory pancreatic cancer with poor performance status. In this study, we compared the efficacy and toxicity of capecitabine and S-1 for such patients. The median progression free survival was longer in the S-1 group than in the capecitabine group; however, there were no statistical differences in their overall survival. Among grade 3 or 4 toxicity, hand-foot syndrome was significantly more common in the capecitabine group than in the S-1 group. Thus, oral chemotherapy can be considered as a second-line treatment in gemcitabine-refractory pancreatic cancer patients with poor performance status.
- Citation: Park SJ, Kim H, Shin K, Lee MA, Hong TH. Oral chemotherapy for second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. World J Gastrointest Oncol 2019; 11(11): 1021-1030
- URL: https://www.wjgnet.com/1948-5204/full/v11/i11/1021.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i11.1021
Pancreatic cancer is one of the leading causes of cancer-related deaths worldwide, with an overall 5-year survival rate of < 5%. More than 80% of patients present with advanced or metastatic disease[1,2]. For patients with locally advanced or metastatic disease, gemcitabine plus nab-paclitaxel therapy has improved overall survival (OS) of patients compared with gemcitabine monotherapy and is therefore the currently recommended first-line treatment (median OS 8.7 mo vs 6.6 mo, respectively, P < 0.0001; hazard ratio (HR) 0.72)[3]. A combination of chemotherapy drugs 5-fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) therapy also showed improved progression-free survival (PFS) and OS compared with gemcitabine monotherapy in patients with metastatic pancreatic cancer with good performance status (PS) (median PFS 6.4 vs 3.3 mo, P < 0.001; median OS 11.1 vs 6.8 mo, P < 0.001)[4]. This regimen is therefore considered as an alternative first-line treatment option for patients with pancreatic cancer, as it is associated with good PS.
As a second-line treatment after progressive disease following gemcitabine-based chemotherapy, the 5-FU/leucovorin + nanoliposomal irinotecan treatment showed promising clinical outcomes in the NAPOLI-1 trial; however, it is considered only for patients with Eastern Cooperative Oncology Group (ECOG) PS 0-1, due to treatment-related toxicity[5,6]. To date, treatment with fluoropyrimidine-based combination regimens in gemcitabine-refractory pancreatic cancer patients with poor PS, is controversial.
Capecitabine, a prodrug of 5-FU, is one of the options as a second-line agent after gemcitabine failure in patients with pancreatic cancer and poor PS[7]. Capecitabine has shown a relatively good response as a first-line treatment in patients with metastatic pancreatic cancer, with a response rate of 24%[7,8]. S-1 is a fourth-generation oral fluoropyrimidine that combines tegafur (5-FU prodrug) with two modulators, 5-chloro-2,4-dihydroxypyridine (gimeracil) and potassium oxonate (oteracil) in a molar ratio of 1 : 0.4 : 1. According to some randomized studies, S-1 as second-line chemotherapy in gemcitabine-pretreated advanced pancreatic cancer showed a relatively high disease control rate, and was well tolerated with acceptable toxicity[9,10].
In this study, we retrospectively analyzed the comparable efficacy and toxicity of capecitabine or S-1 as a second-line treatment in patients with advanced or metastatic pancreatic cancer.
From January 2011 to December 2018, we analyzed the medical records of patients diagnosed with pancreatic cancer in our Department of Oncology. Patients aged at least 19 years, with histologically confirmed, locally advanced, recurrent or metastatic pancreatic adenocarcinoma, who were previously treated with gemcitabine-based first-line chemotherapy, were eligible for this study if they met the following inclusion criteria: ECOG PS 0-2; measurable or evaluable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.0) criteria; progression of disease with gemcitabine-based chemotherapy as first-line therapy; adequate hematological, liver, and renal functions (hemoglobin > 9.0 g/dL, white blood cell count > 4000/mm3, absolute neutrophil count > 1000/mm3, platelet count > 100000/mm3, total bilirubin < 1.5-fold higher than the upper normal limit, serum transaminase < 3-fold higher than the upper normal limit, creatinine < 1.5-fold higher than the upper normal limit).
Capecitabine was given orally as a 2500 mg/m2 divided dose for 14 d, followed by a 7-d rest. S-1 was taken orally based on the patient’s body surface area (BSA) (60 mg twice daily for a BSA > 1.5, 50 mg twice daily for a BSA 1.25-1.5, and 40 mg twice daily for a BSA < 1.25) for 28 days, followed by a 14-d rest. This treatment course was repeated until disease progression, unacceptable toxicities, or patient’s refusal to continue. Chemotherapy dose adjustments were allowed.
Tumor responses to treatment were evaluated every 3 cycles in the capecitabine group and every 2 cycles in the S-1 group. Toxicity was assessed after chemotherapy for every cycle. Imaging evaluations were performed with computed tomography and magnetic resonance imaging at the discretion of the attending physician. Imaging tests were analyzed by radiologists at our institution according to RECIST version 1.0.
Toxicities were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0. Chemotherapy dose intensity was the total amount of drug given in a fixed unit of time (cumulative dose/treatment duration).
This study was approved by the Institutional Review Board of The Catholic University of Korea, Seoul St. Mary’s Hospital (KC18RESI0535).
Responses were assessed objectively according to RECIST version 1.0. The objective response rate represented the percentage of patients with a complete response (CR) or partial response (PR) among those with measurable lesions. The primary endpoint was PFS, defined as the time interval between capecitabine or S-1 treatment initiation and time of disease progression based on imaging studies or death, whichever occurred first. OS was estimated from the date of capecitabine or S-1 initiation to the date of death or last follow-up visit. Time to treatment failure (TTF) was measured from the date of capecitabine or S-1 initiation to the date of last administration due to disease progression, unacceptable toxicity, or cancer-related complications.
Kaplan-Meier analysis was performed for both treatment groups to obtain median OS and median PFS. The HR and 95% confidence intervals (CIs) for OS and PFS were estimated using a stratified Cox regression model. Relative dose intensity was calculated as the ratio between administered dose and planned dose, expressed as a percentage. Statistical significance was considered when P values < 0.05 (two-sided). Statistical analyses were performed using IBM SPSS for Windows version 24.0 (IBM SPSS Inc., Armonk, New York, United States) and GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA, United States).
From January 1, 2011 to December 31, 2018, a total of 81 patients were found to be eligible for this study. Forty-one patients were treated with capecitabine and 40 were treated with S-1. Patient baseline characteristics in the two groups were well balanced (Table 1). Median age was 61 years (range 39-77 years) in the capecitabine group and 63 years in the S-1 group (range 41-78 years). Nearly one-quarter of the patients in both groups exhibited ECOG PS 2. Although not statistically significant, the capecitabine group had more patients with lesions that were initially unresectable compared with the S-1 group. (56% vs 35%, respectively, P = 0.057). As first-line chemotherapy, gemcitabine plus erlotinib was given to most patients in the capecitabine group (76%), and gemcitabine plus nab-paclitaxel was given to most patients in the S-1 group (60%). Overall, 1 (2%) of 41 patients in the capecitabine group and 4 (10%) of 40 patients in the S-1 group had received two or more previous lines of therapy.
| Total (n = 81) | Capecitabine | S-1 | P value |
| (n = 41) | (n = 40) | ||
| Age | |||
| Median (Range) | 61 (39-77) | 63 (41-78) | 0.175 |
| Gender, n (%) | |||
| Male | 24 (59) | 27 (67) | 0.404 |
| Female | 17 (41) | 13 (33) | |
| ECOG performance status, n (%) | |||
| 0-1 | 30 (73) | 29 (72) | 0.946 |
| 2 | 11 (27) | 11 (28) | |
| Disease status at diagnosis, n (%) | |||
| Resectable | 15 (36) | 26 (65) | |
| Locally advanced | 4 (10) | 2 (5) | |
| Metastatic | 19 (46) | 12 (30) | |
| Missing data | 3 (7) | 0 | |
| Overall disease classification, n (%) | |||
| Initially unresectable | 23 (56) | 14 (35) | 0.057 |
| Locally advanced | 4 (9) | 2 (5) | |
| Distant metastasi | 19 (46) | 12 (30) | |
| Recurrent | 18 (44) | 26 (65) | |
| Prior treatment, n (%) | |||
| Surgery | 18 (44) | 26 (65) | 0.057 |
| Radiotherapy | 17 (41) | 11 (28) | 0.186 |
| Adjuvant chemotherapy | 14 (34) | 19 (48) | 0.221 |
| Number of previous regimens received, n (%) | |||
| 1 | 40 (98) | 36 (90) | 0.201 |
| > 2 | 1 (2) | 4 (10) | |
| Subsequent chemotherapy after oral chemotherapy, n (%) | 12 (29) | 17 (43) | 0.214 |
| Prior gemcitabine-based regimen, n (%) | |||
| Gemcitabine | 8 (20) | 9 (22) | |
| Gemcitabine/erlotinib | 31 (76) | 7 (18) | |
| Gemcitabine/cisplatin | 2 (5) | 0 | |
| Gemcitabine/nab-paclitaxel | 0 | 24 (60) | |
| 5-Fluorouracil exposure, n (%) | 13 (31) | 19 (50) | 0.146 |
The median duration of treatment for capecitabine was 1.7 mo and for S-1 it was 2.0 mo. Median relative dose intensity was 0.92 in the capecitabine group and 1.0 in the S-1 group, with no statistically significant difference (P = 0.986, Table 2). The median TTF in both groups was 1.5 mo (range: Capecitabine 0.5-6.7 mo, S-1 0.3-5.7 mo, P = 0.425). As of April 2019, the median duration of follow-up was 2.8 mo (1.1-12.1 mo) in the capecitabine group and 4.8 mo (0.6-14.2 mo) in the S-1 group.
| Capecitabine (n = 41) | S-1 (n = 40) | P value | |
| Duration of treatment, months | |||
| Median | 1.7 | 2.0 | 0.120 |
| Range | 0.7-6.9 | 0.8-6.2 | |
| Dose intensity | |||
| Median | 0.92 | 1.00 | 0.986 |
| Range | 0.70-1.00 | 0.50-1.00 | |
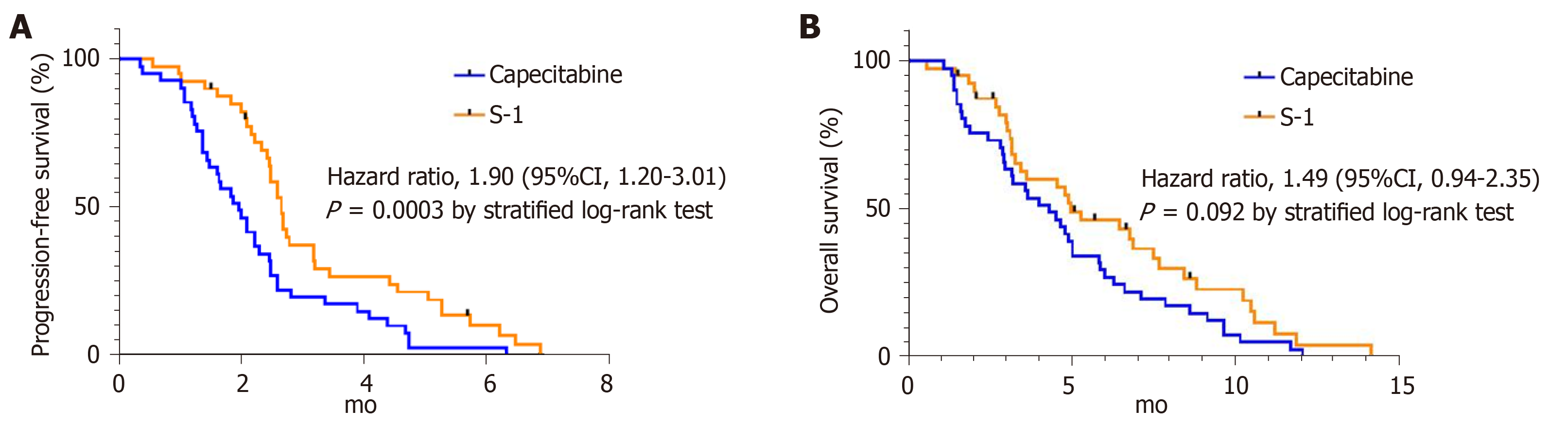
The objective response rate was 9.8% (PR = 4) in the capecitabine group and 2.5% (PR = 1) in the S-1 group with no statistical difference between the two groups (P = 0.359, Table 3). PFS was shorter in the capecitabine group (median 2.0 mo; 95%CI 1.5-2.4) than in the S-1 group (median 2.7 mo; 95%CI 2.4-2.8). The stratified HR for disease progression or death was 1.90 (95%CI 1.20-3.01; P = 0.003). (Figure 1A). There were no statistical differences in OS between the two treatment groups. The median OS was 4.3 mo (95%CI 2.9-5.7) in the capecitabine group compared with 5.0 mo (95%CI 2.8-7.1) in the S-1 group (HR for death 1.49; 95%CI 0.94-2.35, P = 0.092) (Figure 1B).
| Capecitabine (n = 41) | S-1 (n = 40) | P value | |
| Complete response | 0 | 0 | |
| Partial response | 4 (9.8) | 1 (2.5) | |
| Stable disease | 5 (12.2) | 10 (25.0) | |
| Progressive disease | 32 (78.0) | 27 (67.5) | |
| Not available | 0 | 2 (5.0) | |
| Objective response rate, n (%) | 4 (9.8) | 1 (2.5) | 0.359 |
| Disease control rate, n (%) | 8 (19.5) | 11 (21.5) | 0.396 |
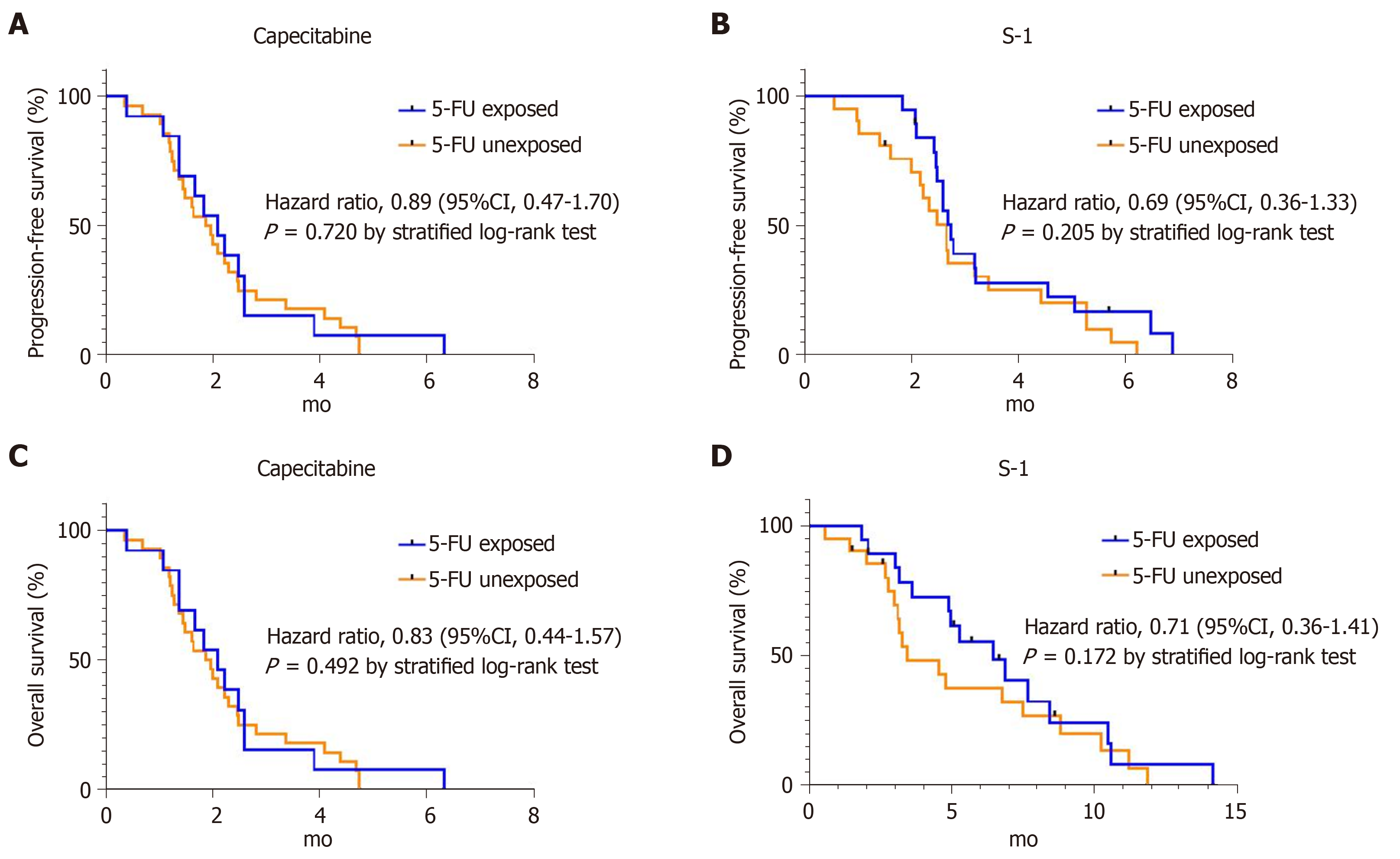
In the capecitabine group, median PFS was 2.1 mo (95%CI 1.4-2.8) for the 5-FU-exposed group and 1.9 mo (95%CI 1.4-2.4) for the 5-FU-unexposed group (HR for disease progression 0.89; 95%CI 0.47-1.7, P = 0.720) (Figure 2A). Median OS was 5.0 mo (95%CI 1.6-8.4) for the 5-FU-exposed group, and 3.6 mo (95%CI 1.9-5.3) for the 5-FU-unexposed group (HR for death 0.83; 95%CI 0.44-1.57, P = 0.492) (Figure 2C). In the S-1 group, median PFS was 2.8 mo (95%CI 2.4-3.1) for the 5-FU-exposed group and 2.7 mo (95%CI 2.2-3.1) for the unexposed group (HR for disease progression 0.69; 95%CI 0.36-1.33, P = 0.205) (Figure 2B). Median OS for the 5-FU-exposed group was 6.5 mo (95%CI 3.9-9.0) and for the 5-FU-unexposed group it was 3.4 mo (95%CI 1.5-5.4) (HR for death 0.71; 95%CI 0.36-1.41, P = 0.172) (Figure 2D). In both groups, 5-FU exposure did not affect statistical differences in median PFS and OS.
Table 4 summarizes the treatment-related toxicity profiles of each treatment group. There were no treatment-related deaths. There was no grade 3 or 4 neutropenia in either group. Hand-foot syndrome (HFS) was the most common grade 3 or 4 toxicity in the capecitabine group (14.6%, 6 of 41 patients); however, in the S-1 group, stomatitis and nausea were the most common grade 3 or 4 toxicities (2.5%, 1 of 40 patients for each). Occurrence of most adverse events were similar in both groups, with the exception of grade 3 or 4 HFS, which was significantly more common in the capecitabine group than in the S-1 group (14.6% vs 0%, respectively, P = 0.026).
| Adverse event n, (%) | Capecitabine (n = 41) | S-1 (n = 40) | |||
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | P value1 | |
| Nausea | 11 (26.8) | 1 (2.4) | 13 (32.5) | 1 (2.5) | 1.000 |
| Vomiting | 4 (9.8) | 1 (2.4) | 5 (12.5) | 0 | 1.000 |
| Diarrhea | 4 (9.8) | 0 | 3 (7.5) | 0 | |
| Stomatitis | 7 (17.1) | 0 | 6 (15.0) | 1 (2.5) | 0.494 |
| Hand-foot syndrome | 13 (31.7) | 6 (14.6) | 2 (5.0) | 0 | 0.026 |
| Anemia | 12 (29.3) | 0 | 9 (22.5) | 0 | |
| Neutropenia | 7 (17.1) | 0 | 3 (7.5) | 0 | |
The results of our study showed that median OS did not differ statistically in the two groups; however, median PFS was statistically longer in the S1 group than in the capecitabine group. The difference in PFS results in the two groups may be due to differences in duration of response evaluation. In the capecitabine group, 23 (58%) patients were evaluated for treatment response earlier than the scheduled assessment, while in the S-1 group, 17 (43%) patients were evaluated earlier (P = 0.221). In addition, the proportion of initially unresectable patients at diagnosis was higher in the capecitabine group than in the S-1 group, although not statistically significant (56% vs 35%, respectively, P = 0.057). This imbalance in patient characteristics may have resulted in a more favorable outcome in the S-1 group.
Pancreatic cancer remains a deadly disease that is rarely cured, except in cases of complete resection. The cancer carries a dismal prognosis among patients with locally advanced or metastatic disease[11]. Gemcitabine plus nab-paclitaxel or FOLFIRINOX are recommended as first-line therapy for advanced pancreatic cancer, but FOLFIRINOX is generally favored in patients with good PS[3,4].
Patients with advanced pancreatic cancer who are refractory to gemcitabine-based therapy have a dismal prognosis and limited therapeutic options, consisting of 5FU/leucovorin plus nanoliposomal irinotecan or FOLFIRINOX. Despite the good response rate of 5-FU/leucovorin plus nanoliposomal irinotecan or FOLFIRINOX, these regimens are not considered as optimal treatment options for elderly patients or those with poor PS due to the associated toxicity. Assessment of a patient’s symptom burdens, PS, and associated comorbidities are important considerations in selecting the most appropriate chemotherapy. Therefore, for elderly patients or those with a poor PS, chemotherapy regimens with less toxicity should be considered.
Immune checkpoint inhibitors are also known to be less toxic and can be used in such patients. As a single agent, immune checkpoint inhibitors have shown limited response in patients with pancreatic cancer[12-14]. However, immune checkpoint inhibitors such as pembrolizumab may be effective in tumors, including pancreatic cancer, with mismatch repair deficiency (dMMR) or high microsatellite instability (MSI)[15,16]. Hence, there is a need to identify dMMR or MSI status in patients with pancreatic cancer and poor PS after gemcitabine failure, to confirm whether the immune checkpoint inhibitor will be effective. However, immune checkpoint inhibitors are limited because they can be used only in a few selected patients.
Oral fluoropyrimidine treatment, such as capecitabine or S-1, can be a second-line treatment option for patients with poor PS or those who are elderly, due to less severe hematologic or non-hematologic adverse events compared with intravenous cytotoxic agents[7,9]. Capecitabine has shown favorable efficacy and tolerable toxicity as a first-line treatment for metastatic pancreatic cancer according to a previous clinical study, and can be considered in patients with poor PS, due to the convenience of oral administration[7]. S-1 is another prodrug of 5-FU that can reduce gastrointestinal toxicity, and has been shown to have a relatively favorable response rate as a second-line treatment for patients with advanced pancreatic cancer[9,10]. However, no studies have compared the efficacy and toxicity of the two regimens as second-line chemotherapy treatments for patients with advanced pancreatic cancer.
In the current study, capecitabine and S-1 showed similar response rates, that were not significantly different from those reported in previous studies. Also, there was no statistically significant difference in median OS. The safety profile for both regimens was consistent with those reported previously[7,9]. Prior clinical studies have suggested that 5-FU prodrugs containing dihydropyrimidine dehydrogenase inhibitors such as S-1 or uracil/tegafur can reduce the incidence of HFS[17]. In this study, HFS was significantly less common in the S-1 group than in the capecitabine group, consistent with previous studies[18,19]. Although HFS is not life-threatening, it can significantly impair a patient’s daily activities and decrease their quality of life. Therefore, S-1 could be beneficial for patients who experience intolerable HFS while on capecitabine treatment.
Previous 5-FU exposure may affect the efficacy of capecitabine or S-1; however, in this study 5-FU exposure did not affect median PFS and OS. This result may due to the fact that most 5-FU exposure was from adjuvant chemotherapy and therefore patients did not develop resistance to 5-FU.
At approximately 1.5 mo, median TTF did not differ significantly between the capecitabine and S-1 groups (P = 0.425). However, both agents showed shorter TTF compared to other chemotherapy regimens (gemcitabine: 3.6 mo, gemcitabine plus nab-paclitaxel: 5.1 mo, 5FU/leucovorin plus nanoliposomal irinotecan: 2.3 mo)[3,5]. The causes of TTF were progressive disease (56.8%), cancer-related complications (24.7%), intolerance (16.0%), and toxicity (2.5%). Gastrointestinal obstruction (11.1%) and cholangitis (8.6%) were the main causes of cancer-related complications. Because treatment failure was more likely to be caused by cancer-related complications than by intolerance or toxicity, it may be important to continue chemotherapy with appropriate management of cancer-related complications.
Our study has some limitations, the first being the non-randomized, retrospective nature of evaluation. The small sample size from a single-center study is another limitation. Third, patients in the capecitabine group generally underwent response evaluation every 9 wk, while those in the S-1 group were evaluated for response at various intervals (every 6 or 12 wk). In addition, the proportion of patients in whom response was evaluated earlier than scheduled was higher in the capecitabine group than in the S-1 group; results for PFS may therefore not be comparable due to this different timing.
In conclusion, this is the first retrospective study to compare the efficacy and safety of capecitabine and S-1 as second-line therapy in patients with gemcitabine-refractory pancreatic cancer. Although the retrospective nature of the study and the small number of patients are major limitations, capecitabine and S-1 showed similar efficacy and safety for patients with gemcitabine treatment failure. However, HFS was significantly more common in the capecitabine group. This study can act as a pilot study for initiation of a large sample, multicenter study. To confirm our preliminary results, we need a randomized study to compare the efficacy of capecitabine and S-1 for gemcitabine-refractory pancreatic cancer patients with poor PS.
Pancreatic adenocarcinoma is one of the leading causes of death from cancer in the world, and it carries a grim prognosis. Most patients are diagnosed with advanced disease, thus systemic chemotherapy plays a key role in treatment. 5-fluorouracil combination regimens may be considered in patients who have failed first-line gemcitabine-based chemotherapy. However, due to toxicity, these regimens are not considered for elderly patients or those with poor performance status.
Capecitabine has shown activity as a first-line treatment in patients with metastatic pancreatic cancer, with a relatively good response. Also, S-1 has shown favorable antitumor activity in several phase II studies in patients with metastatic pancreatic cancer. Thus, oral chemotherapy, such as capecitabine or S-1, can be a second-line treatment option for patients with poor performance status, due to less toxicity. However, until recently, few studies have compared the efficacy and toxicity of these two drugs.
This study investigated the efficacy and toxicity of oral chemotherapy, with capecitabine or S-1 as the second-line treatment in patients with pancreatic cancer who have failed to gemcitabine-based therapy.
This study used a retrospective cohort analysis to compare efficacy and toxicity between capecitabine and S-1 in patients with gemcitabine-refractory pancreatic cancer. The survival outcomes of the two groups were compared through a Cox regression model, and displayed using Kaplan-Meier curve.
The objective response rates were similar in both groups with no statistical difference. The objective response rate in this study was consistent with the results of previous studies. There was no significant difference in the median overall survival between the two groups. Median progression-free survival was longer in the S-1 group than in the capecitabine group, however, differences in duration of response assessment of the two regimens may affect the results. Grade 3 or 4 toxicity was similar in both groups; however, hand-foot syndrome was more frequently observed in the capecitabine group.
Capecitabine and S-1 showed relatively favorable efficacy and low toxicity, which can be considered as a second-line treatment option for gemcitabine-refractory pancreatic cancer patients with poor performance status. Hand-foot syndrome was significantly less common in the S-1 group, thus S-1 may be considered in patients who have experienced intolerable hand-foot syndrome during capecitabine treatment.
Our study showed that oral chemotherapy can be considered as second-line treatment in patients with pancreatic cancer after gemcitabine failure. This study is a retrospective analysis with small sample size, thus further randomized prospective study are needed to confirm our preliminary results.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hourneaux de Moura DT, Sun XY S-Editor: Zhang L L-Editor: A E-Editor: Qi LL
| 1. | Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694-9705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1003] [Cited by in RCA: 957] [Article Influence: 106.3] [Reference Citation Analysis (24)] |
| 2. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2205] [Article Influence: 147.0] [Reference Citation Analysis (2)] |
| 3. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4883] [Article Influence: 406.9] [Reference Citation Analysis (0)] |
| 4. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5634] [Article Influence: 402.4] [Reference Citation Analysis (1)] |
| 5. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 829] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 6. | Wang-Gillam A, Li C-P, Bodoky G, Dean A, Shan Y-S, Jameson GS, Lee KH, Cunningham D, Blanc JF, Hubner R, Chiu CF, Schwartsmann G, Siveke JT, Braiteh FS, Moyo VM, Belanger B, Bayever E, Von Hoff DD, Chen LT. Updated overall survival (OS) analysis of NAPOLI-1: Phase III study of nanoliposomal irinotecan (nal-IRI, MM-398), with or without 5-fluorouracil and leucovorin (5-FU/LV), vs 5-FU/LV in metastatic pancreatic cancer (mPAC) previously treated with gemcitabine (gem)-based therapy. J Clin Oncol. 2016;34:417-417. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Almhanna K, Kim R. Second-line therapy for gemcitabine-refractory pancreatic cancer: is there a standard? Oncology (Williston Park). 2008;22:1176-83; discussion 1190, 1192, 1196. [PubMed] |
| 8. | Cartwright TH, Cohn A, Varkey JA, Chen YM, Szatrowski TP, Cox JV, Schulz JJ. Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol. 2002;20:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Morizane C, Okusaka T, Furuse J, Ishii H, Ueno H, Ikeda M, Nakachi K, Najima M, Ogura T, Suzuki E. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2009;63:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Sudo K, Nakamura K, Yamaguchi T. S-1 in the treatment of pancreatic cancer. World J Gastroenterol. 2014;20:15110-15118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2011, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. Bethesda, MD: National Cancer Institute; 2014. Bethesda, MD: National Cancer Institute; 2014; Available from: https://seer.cancer.gov/archive/csr/1975_2014. |
| 12. | Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 964] [Cited by in RCA: 977] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 13. | Mohindra NA, Kircher SM, Nimeiri HS, Benson AB, Rademaker A, Alonso E, Blatner N, Khazaie K, Mulcahy MF. Results of the phase Ib study of ipilimumab and gemcitabine for advanced pancreas cancer. J Clin Oncol. 2015;33:e15281. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6283] [Article Influence: 483.3] [Reference Citation Analysis (0)] |
| 15. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4945] [Article Influence: 618.1] [Reference Citation Analysis (0)] |
| 16. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7239] [Article Influence: 723.9] [Reference Citation Analysis (0)] |
| 17. | Yen-Revollo JL, Goldberg RM, McLeod HL. Can inhibiting dihydropyrimidine dehydrogenase limit hand-foot syndrome caused by fluoropyrimidines? Clin Cancer Res. 2008;14:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Zheng D-X, Jin J-H, Liu Y-J. Comparison of capecitabine and tegafur/gimeracil/oteracil (S-1) in the treatment of advanced breast carcinoma in the elderly. Trop J Pharm Res. 2018;17:543-547. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Kwakman JJM, Baars A, Boot H, Pruijt JFM, Winther SB, Pfeiffer P, Punt CJA. Tolerability of the oral fluoropyrimidine S-1 after hand-foot syndrome-related discontinuation of capecitabine in western cancer patients. Acta Oncol. 2017;56:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |