Published online Sep 15, 2018. doi: 10.4251/wjgo.v10.i9.231
Peer-review started: March 27, 2018
First decision: April 10, 2018
Revised: June 13, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: September 15, 2018
Processing time: 172 Days and 8.2 Hours
Helicobacter pylori (H. pylori) infection is a well-established risk factor for the development of gastric cancer (GC), one of the most common and deadliest neoplasms worldwide. H. pylori infection induces chronic inflammation in the gastric mucosa that, in the absence of treatment, may progress through a series of steps to GC. GC is only one of several clinical outcomes associated with this bacterial infection, which may be at least partially attributed to the high genetic variability of H. pylori. The biological mechanisms underlying how and under what circumstances H. pylori alters normal physiological processes remain enigmatic. A key aspect of carcinogenesis is the acquisition of traits that equip preneoplastic cells with the ability to invade. Accumulating evidence implicates H. pylori in the manipulation of cellular and molecular programs that are crucial for conferring cells with invasive capabilities. We present here an overview of the main findings about the involvement of H. pylori in the acquisition of cell invasive behavior, specifically focusing on the epithelial-to-mesenchymal transition, changes in cell polarity, and deregulation of molecules that control extracellular matrix remodeling.
Core tip:Helicobacter pylori (H. pylori) infection induces chronic inflammation in the gastric mucosa that, in the absence of treatment, may progress through a series of steps to gastric cancer (GC). GC is only one of several clinical outcomes associated with this bacterial infection, which may be at least partially attributed to the high genetic variability of H. pylori. Accumulating evidence implicates H. pylori in the manipulation of cellular and molecular programs that are crucial for conferring the cells with invasive capabilities, including reprograming of the epithelial-to-mesenchymal transition signaling programs, changing of the cell apicobasal polarity, and remodeling of the extracellular matrix.
- Citation: Molina-Castro S, Ramírez-Mayorga V, Alpízar-Alpízar W. Priming the seed: Helicobacter pylori alters epithelial cell invasiveness in early gastric carcinogenesis. World J Gastrointest Oncol 2018; 10(9): 231-243
- URL: https://www.wjgnet.com/1948-5204/full/v10/i9/231.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i9.231
Persistent Helicobacter pylori (H. pylori) infection induces chronic inflammation in the gastric mucosa, which in susceptible individuals may progress to gastric cancer (GC)[1,2]. The final clinical outcome of the infection depends on complex interactions among the infecting strain of the bacterium, the host, and the environment[3]. The biological mechanisms underlying how and under what circumstances H. pylori alters normal physiological processes in such a way that sequential events culminate in the development of GC remain largely unknown.
A key feature of malignant transformation and progression is the invasion of malignant cells locally and then to distant sites (metastasis)[4]. Invasion and metastasis occur through a series of events in which several processes take place, including reprograming of signaling pathways that drive the epithelial-derived malignant cells into a mesenchymal-like phenotype, the so-called epithelial-to-mesenchymal transition (EMT), changing of the cell polarity, and remodeling of the extracellular matrix (ECM)[5,6]. Several of these events are activated in gastric epithelial cells by H. pylori directly or as a result of the inflammatory reaction mounted in response to this bacterial infection. This review summarizes the current evidence implicating H. pylori in the activation of molecular and cellular mechanisms related to invasion in the early stages of the pathogenic series of events leading to GC. Specifically, we address the role of H. pylori in the deregulation of molecules that control EMT, cell polarity, and ECM remodeling.
GC is the fifth most common and the third death-causing cancer worldwide[7]. Incidence rates vary considerably depending on age and sex; however, the most substantial variation is connected to geographic location, with very well-established high- and low-risk areas across the world[8,9]. GC incidence is steadily declining worldwide; and although the reasons are not clear, this may be at least partially linked to the concomitant decrease in H. pylori prevalence[8]. The decrease, however, is not of the same magnitude in GC of different histological subtype or anatomical location[10]. Similarly, mortality rate varies geographically, being particularly high in developing countries but declining globally[8,9]. The 5-year survival rate remains below 30% in most countries, which is mainly connected to the fact that most of the cases are diagnosed at advanced stages, when therapeutic interventions are likely to fail.
Several schemes are used for classifying GC according to microscopic and histological characteristics. The Lauren classification system is probably the most commonly used[11,12]. The Lauren system divides GC into intestinal, diffuse and mixed subtypes, with important differences at the epidemiological, pathological and molecular levels[11,13].
Marked epidemiological and etiological differences have been revealed for malignant tumors located in the distal part of the stomach and those of the proximal region[14,15]. Therefore, anatomical location of the lesions is regarded as an important parameter in the classification of GC.
The pathogenesis of GC is a complex and multifactorial process in which environment and lifestyle, host genetics, and H. pylori infection play a role[2,16-21]. As already mentioned, the pathogenesis of GC substantially differs depending on the histological and anatomical subtype. The intestinal subtype of GC, for instance, arises through a sequential series of steps known as the Correa cascade[22], in which H. pylori plays a pivotal role. The infection is usually established early in life and persists lifelong in the absence of treatment, which in combination with environmental factors leads to sustained chronic inflammation characterized by infiltration of inflammatory cells in the gastric mucosa and expression of inflammatory mediators.
Intriguingly, most of the infected individuals remain asymptomatic, while others develop pathologies that are not related to GC. In a minority of infected people, the inflammation evolves into a chronic atrophic gastritis, which is regarded as a pre-neoplastic lesion[22,23]. This may subsequently progress to intestinal metaplasia, dysplasia, and invasive carcinoma[22]. Much less is known about the pathogenesis of the diffuse subtype of GC[24,25] and the malignant lesions arising in the most proximal segment of the stomach[26].
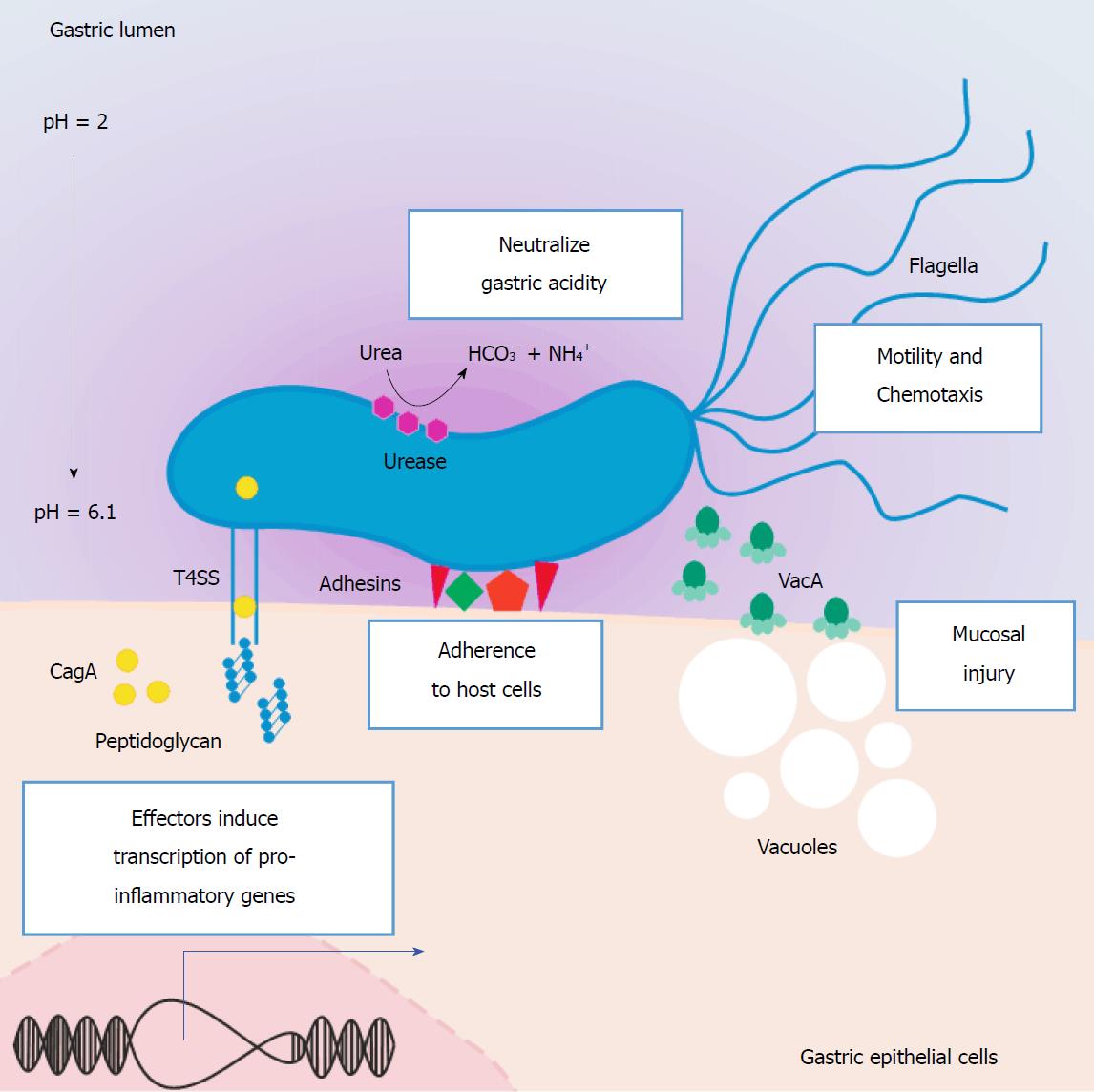
Infection with H. pylori is one of the most prevalent bacterial infections worldwide[27]. This bacterium utilizes several strategies for colonizing and surviving in the hostile environment of the stomach. Some of these are common bacterial mechanisms of acid resistance, such as proton pump activation, decarboxylases, and membrane lipid modification[28]. More specific adaptations to the acidic environment include the enzyme urease, which is encoded by the ure gene cluster and catalyzes the conversion of urea into ammonium and carbon dioxide. Urease was, in fact, the first protein identified in H. pylori with a role in neutralizing gastric acid, and it is considered a virulence factor[29] since it has proven essential to the survival of the bacterium in the gastric mucosa[30,31]. Besides the ure gene cluster, transcriptional regulation in response to acid extends to other genes related to motility, chemotaxis, and virulence[32].
The genetic variability of H. pylori is high, and it probably explains in part the association of this infection with several gastric and extra-gastric pathologies, in addition to GC. Some strains, however, are more strongly associated with GC, namely those harboring particular polymorphic variants in the gene encoding the vacuolating cytotoxin A (VacA) and the ones expressing the Cag pathogenicity island (Cag-PAI)[3,33-36]. Despite no physical or functional relation known for vacA and cag-PAI loci, strains that express virulent VacA usually contain functional Cag-PAI[35,37]. In addition to VacA and Cag-PAI, other virulence factors of H. pylori have been associated with gastric pathology, including BabA, SabA, OipA, and DupA (Figure 1)[3,36].
The dissemination of cancer cells from primary lesions to form new tumor colonies at distant sites is a key feature of cancer[4]. This occurs in a multistep process, termed the invasion-metastasis cascade: cancer cells locally invade, intravasate into the vascular system, travel in the circulation, extravasate at distant sites, form micrometastatic nodules of cancer cells, and, finally, grow into overt metastatic lesions[5,6]. Importantly, early in this series of events, malignant cells acquire traits that equip them with the ability to invade, leave, and travel to distant tissues. A centrally important process that confers epithelial-derived malignant cells with increased motility and invasiveness is the EMT program[38,39].
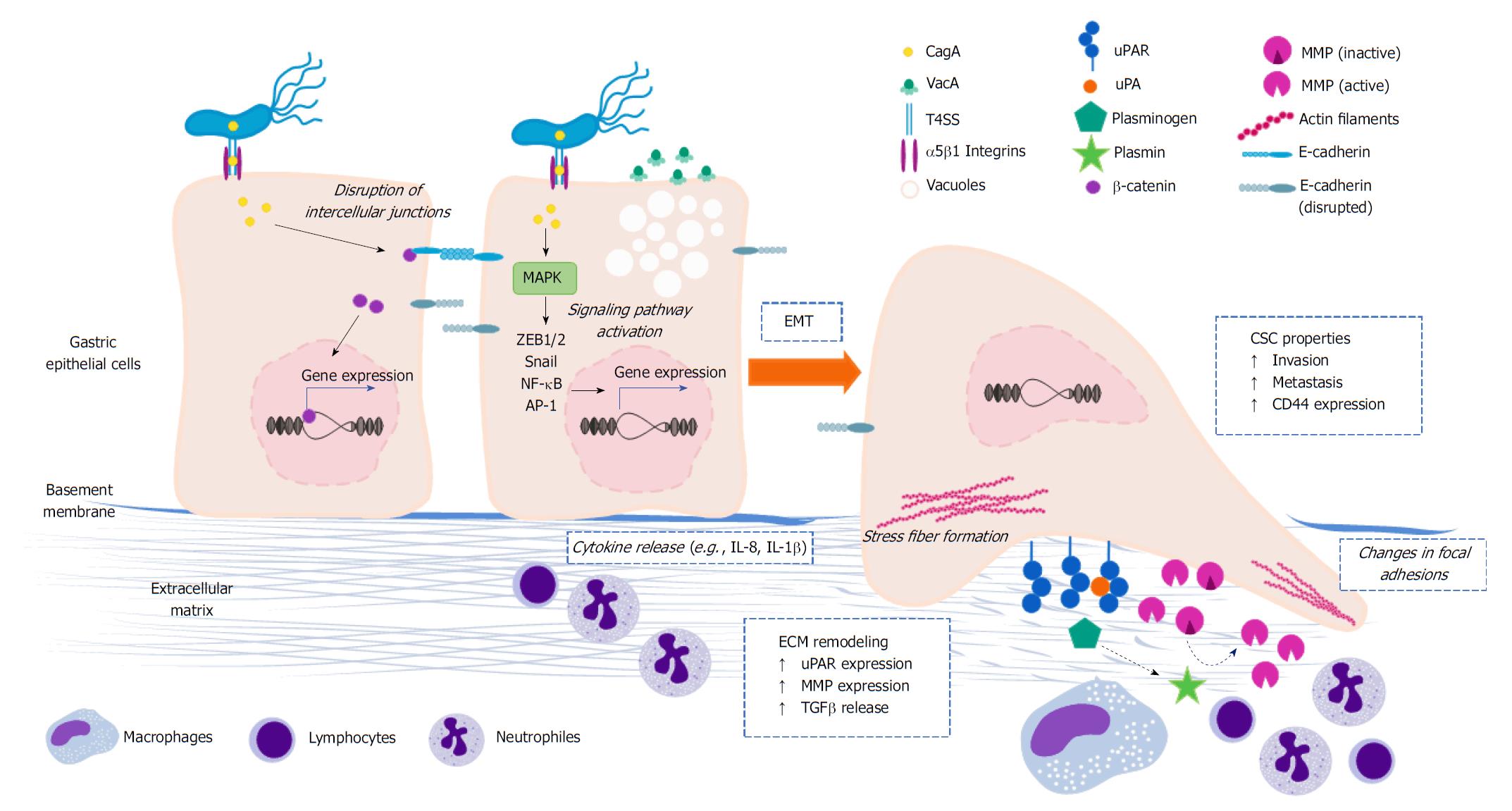
In order to become invasive, cells commonly lose their apico-basal polarity due to rearrangements in the cytoskeleton, which maintains the shape and internal organization of the cells, and modifications in the intercellular unions that hold them together[39,40]. Also, the degradation of ECM components is essential in several phases of the invasion-metastasis cascade. ECM remodeling is primarily mediated by proteases that belong to the plasminogen activation (PA) system and the matrix metalloproteinase (MMP) family[41,42]. The cellular and molecular mechanisms underlying these processes, as well as their regulation, have been reviewed in depth[39,40,43-45]. Accumulating experimental evidence has implicated H. pylori in all these aspects (Figure 2), as discussed below.
EMT is an evolutionary conserved, reversible process in which polarized epithelial cells acquire a mesenchymal phenotype through phenotypical and biochemical changes, thereby resulting in increased capacities of migration, invasion, and apoptosis resistance as well as ECM production and remodeling[38]. Transcription factors such as Snail, Slug, zinc-finger E-box binding (ZEB1/2) and FOXC2 are activated at the beginning of the process. This is accompanied by the expression of specific microRNAs (miRs), for instance the miR-200 family, changes in the expression of particular cell surface proteins, cytoskeletal reorganization, and activation of Wnt/β-catenin and Notch signaling[38,46,47].
A critical feature of EMT is the down-regulation of E-cadherin[48], a surface glycoprotein expressed in epithelial cells that is a key component of the adherent junctions in epithelial tissues[49]. Expression of E-cadherin can be repressed directly or indirectly by multiple transcription factors, including ZEB1/2, Snail, Slug, nuclear factor-kappa B (NF-κB), E47 and KLF8, but also by the proteins SIX1 and FOXC2[50-52]. Furthermore, various signaling pathways can influence the expression of E-cadherin, including TGFβ, hypoxia-induced response, Wnt/β-catenin, Notch and PI3K/Akt, and therefore play a role in EMT[53,54]. Although EMT is usually depicted as a binary switch that shifts cells from a fully epithelial to a fully mesenchymal state, this is a misrepresentation of this process. Frequently, the EMT program drives cells from a fully epithelial state to a partially mesenchymal one in which some epithelial markers are retained. Nonetheless, this subset of mesenchymal traits has profound effects on the cell biology[55].
Activation of EMT programs in neoplastic cells is usually connected to their dedifferentiation and acquisition of stem cell-like properties[56]. The existence of cancer cells with stem-like properties, the so-called cancer stem cells (CSCs), was first described in breast cancer and subsequently documented in various malignancies, including GC. One the first studies about CSCs in GC showed that these cells have enhanced capability of invasion and tumorsphere formation. Also, it was found that CSCs have distinctive features of the EMT, such as reduced expression of E-cadherin and increased levels of vimentin and MMP2[57]. In primary GC tissue, it was demonstrated by immunohistochemistry that the combination of Snail-1, vimentin, E-cadherin and CD44 predicts tumor aggressiveness[58]. Furthermore, it has been reported that MKN7 GC cells undergoing Wnt5a-induced EMT acquire CSC properties[59], similar to what has been observed in hypoxia-driven EMT in vitro models with the BGC823 and SGC7901 GC cell lines[60].
Using in vitro systems, it was revealed that H. pylori infection results in the activation of EMT programs and the emergence of CD44high cell populations with CSC properties in the AGS, MKN45 and MKN74 GC cell lines[61]. These cells acquire elongated shape and show enhanced expression of mesenchymal markers (i.e., Snail1, ZEB1, and vimentin). Compared to the CD44low cells, the CD44high GC cell population gained the ability to migrate and invade and was better at forming tumorspheres in vitro and tumors in immunodeficient mice. According to that study, the induction of the EMT and CD44high cell population was dependent on the CagA oncoprotein. CagA induces the EMT in a number of ways, as exemplified by a recent in vitro study showing that this bacterial protein up-regulates MMP3, which is also part of the EMT program, through EPIYA motifs in a phosphorylation-dependent manner[62]. Immunohistochemistry staining of human and murine gastric tissue have confirmed that H. pylori infection is correlated with high expression of CD44 and EMT markers[61]. Presumably, ERK and JNK are involved in the described EMT-like changes, CD44 overexpression and the ability to form tumorspheres in vitro that is triggered by H. pylori cagA-positive strains[61].
Other studies addressing the potential induction of CSC-like properties by H. pylori concluded that Wnt/β-catenin activation in response to this bacterial infection is a necessary event for the acquisition of such properties in GC cell lines[63]. Finally, observations on patients with gastric dysplasia and early GC, before and after eradication of H. pylori, showed a connection between the mRNA expression levels of TGF-β1, EMT markers, and immunohistochemical expression of CD44, suggesting that H. pylori infection may trigger a TGF-β1-induced EMT and the emergence of CSCs[64].
Epithelial cells in the gastrointestinal tract are normally found in organized layers or epithelia. Their polygonal shape and functional organization in an apico-basal polarized manner allow them to lay in an orderly fashion, and the unions they form with each other and with the basal membrane give the epithelium a barrier function. As already mentioned, invasiveness is enhanced when cells lose their polarity due to alterations in the cytoskeleton and intercellular unions. H. pylori has been implicated in changing the polarity of the gastric epithelial cells, which may have important consequences in the context of gastric carcinogenesis. Of the three types of filaments that compose the cytoskeleton, the actin microfilaments are the most affected during H. pylori infection.
The actin cytoskeleton is a very dynamic structure whose assembly is finely tuned by complex signaling networks and involves numerous regulatory proteins. Actin microfilaments form a wide variety of structures: contractile rings, phagocytosis- and endocytosis-related structures, microvilli, cortex, adherens belts (associated with adherens junctions), filopodia, lamellipodia, and stress fibers. The ability of H. pylori to promote rearrangements of the actin cytoskeleton is well-established[65-67]. The most evident demonstration of this is the so-called hummingbird phenotype, comprising a change in the epithelial cell shape to the characteristic elongated morphology of H. pylori-infected cells in vitro. This phenotype is thought to be linked to cancer cell migration and invasive growth in vivo[68]. The hummingbird phenotype involves the formation of stress fibers and protrusions, the disruption of cell-to-cell adhesions, and the deregulation of focal adhesions between the cell and the ECM.
The basic mechanisms by which H. pylori changes the dynamics of the actin cytoskeleton during cell migration have been reviewed in depth by Wessler et al[69]. Briefly, H. pylori, via cag-PAI type-4 secretion system (T4SS; especially CagL) and CagA, is able to modify the host cell’s signaling networks. On the one hand, CagL binds β1 integrins, thereby stimulating the focal adhesion kinases (FAKs) and the Src-family kinases (SFKs); meanwhile, CagA activates the Abl-kinase. FAK, SFK, and Abl activate Crk, which in turn activates Rac1, which then promotes the assembly of actin filaments via activation of the Arp2/3 complex, contributing to cell motility. On the other hand, upon injection into the host-cell cytosol, CagA is phosphorylated by SFK (c-Src) and binds Shp-2 and Csk, which then inhibit SFK in a negative feedback loop. Inhibition of SFK induces dephosphorylation of actin regulatory proteins, such as ezrin, vinculin, and cortactin. Cortactin stimulates the actin nucleation activity of Arp2/3 and, upon H. pylori-induced dephosphorylation, accumulates at the tip of the cellular protrusions and colocalizes with F-actin[70].
The serine/threonine kinase polarity-regulating kinase partitioning-defective 1b (PAR1) participates in the CagA-mediated remodeling of the actin cytoskeleton. PAR1 inhibits the formation of stress fibers and cortical actin in the cell periphery. Kikuchi et al[71] showed that the physical interaction between the CagA multimerization sequence and PAR1b, the isoform present in gastric epithelial cells, is crucial for the stable binding of CagA and Shp-2. In fact, a second study found that CagA indirectly activates RhoA-dependent formation of stress fibers by impairing PAR1b-mediated inhibition of RhoA[72]. These results were elegantly combined in a model that proposes a link among cell polarity regulation, the hummingbird phenotype, and actin cytoskeleton[73]. More specifically, upon cell polarity loss of the epithelial cell, PAR1b and aPKC are relocated, resulting in the establishment of a front-to-rear polarity in which these two molecules are asymmetrically distributed, with PAR1b localized in the rear part of the migrating cell.
The binding of CagA to PAR1b modifies this program by perturbing PAR1b localization, which translates into loss of its kinase activity, lifting of the repression of RhoA, and formation of stress fibers; the salient manifestation of this is the hummingbird phenotype[73]. The affinity of CagA for PAR1b and formation stress fibers increases proportionally to the number copies of the CagA-multimerization (CM) domain present in CagA, which is seemingly higher in East Asian CM than in Western CM and differs in five amino acid residues[74].
Podosomes are dot-like structures of densely packed F-actin and serve as regulatory proteins by their capacity to degrade ECM components due to the presence of MMPs within. It has been shown in a model of primary hepatocytes and hepatoma cell lines that H. pylori can enhance the formation of podosomes by the induction of inflammatory cytokines such as TGFβ[75], thus providing additional evidence of the capacity of H. pylori to modify actin structures. Actin-remodeling activity has also been described in another Helicobacter species, H. pullorum. Its cytolethal distending toxin, responsible for the cytopathological effects observed upon infection, induces actin cytoskeleton remodeling that is accompanied by delocalization of vinculin and up-regulation of cortactin in large, cortical actin-rich lamellipodia[76].
Another important component of the cellular cytoskeleton is the microtubules. Structurally, they are formed by tubulins and regulated by microtubule-associated proteins. The microtubular network organizes the cell movement of organelles and is part of specialized structures such as cilia, flagella, mitotic spindles, centrosomes, and basal bodies. During cell migration, the small Rho-GTPase protein Cdc42 controls the actin microfilaments in the cell migration front and binds PAR6. The Cdc42-PAR6 dimer recruits the microtubule-regulating dynein/dynactin complex, which directs the machinery of the secretory pathway to the migration front. Importantly, this facilitates the delivery of integrins and other proteins that mediate the interaction with the ECM to the sites of migration.
Although not many, some studies have addressed the role of H. pylori infection in microtubule regulation in the context of cell migration. Slomiany and colleague[77], for example, concluded that H. pylori lipopolysaccharide induced the secretion of MMP9 in a primary culture of murine gastric mucosal cells. In that study, the authors also found an accompanying increase of microtubule stabilization. Presumably, those changes are modulated by ghrelin and involve the activation of PKCδ and SFK.
Focal adhesions provide the structural link between the stress fibers and the ECM. They need to be dynamically assembled and disassembled in order to allow cell migration. H. pylori impairs focal adhesion release during cell migration, which leads to the characteristic elongation of infected cells[68]. Paxillin, a multidomain protein that acts as an adaptor between the cytoplasmic tail of integrins and the actin cytoskeleton, has been designated as the convergent point of the epithelial growth factor receptor, FAK/Src, and PI3K/Akt signaling pathways in the context of H. pylori infection[78]. Paxillin phosphorylation is dependent on the presence of a functional Cag-PAI or OipA. The phosphorylated paxillin was localized along the elongations, suggesting a role in the formation of stress fibers[78].
The PA system comprises a few proteins that, by acting in sequence, lead to the conversion of zymogenic plasminogen into its active enzymatic form, plasmin. Extravascular activation of plasminogen is controlled by the urokinase-type plasminogen activator (uPA), its receptor (uPAR), its inhibitor PAI-1, and α2-antiplasmin. Besides degrading major ECM proteins (e.g., fibrin fibronectin, laminins, and vitronectin), the generated plasmin also releases latent growth and angiogenic factors sequestered in the matrix[79,80]. The expression of uPA, uPAR, and PAI-1 under normal homeostatic conditions is almost undetectable; however, in cancer and other pathologies, their expressions increase significantly[81]. An important body of evidence correlates uPAR expression in cancer lesions with invasive and metastatic disease. Accordingly, high levels of uPAR in tissue and plasma are associated with poor patient survival in various types of cancer, including GC[82-84]. Most of these reports have focused on uPAR, since this receptor is crucial for the initiation of the sequential series of events that ultimately result in the activation of plasminogen.
As already mentioned, H. pylori has been linked to the induction of members of the PA system, primarily uPAR. Part of the experimental evidence supporting this phenomenon comes from in vitro studies, for example global gene-expression analyses ranking uPAR among the top up-regulated genes in AGS and T84 cell lines, when co-cultured with H. pylori[85-87]. This has been confirmed by more specific in vitro studies, showing that in co-cultures, the bacterium rapidly induces uPAR expression in GC cell lines[88-91]. A few of these reports indicate that uPAR induction is predominantly linked to CagA-positive strains[87,89]. The potential connection between H. pylori and uPAR induction has also been documented in non-neoplastic tissue adjacent to GC lesions[92] and in gastric biopsies from healthy patients who are infected with the bacterium[93]. Interestingly, it has been reported that the expression of uPAR in neoplastic tissue may be correlated with the presence of H. pylori in adjacent non-neoplastic tissue[94].
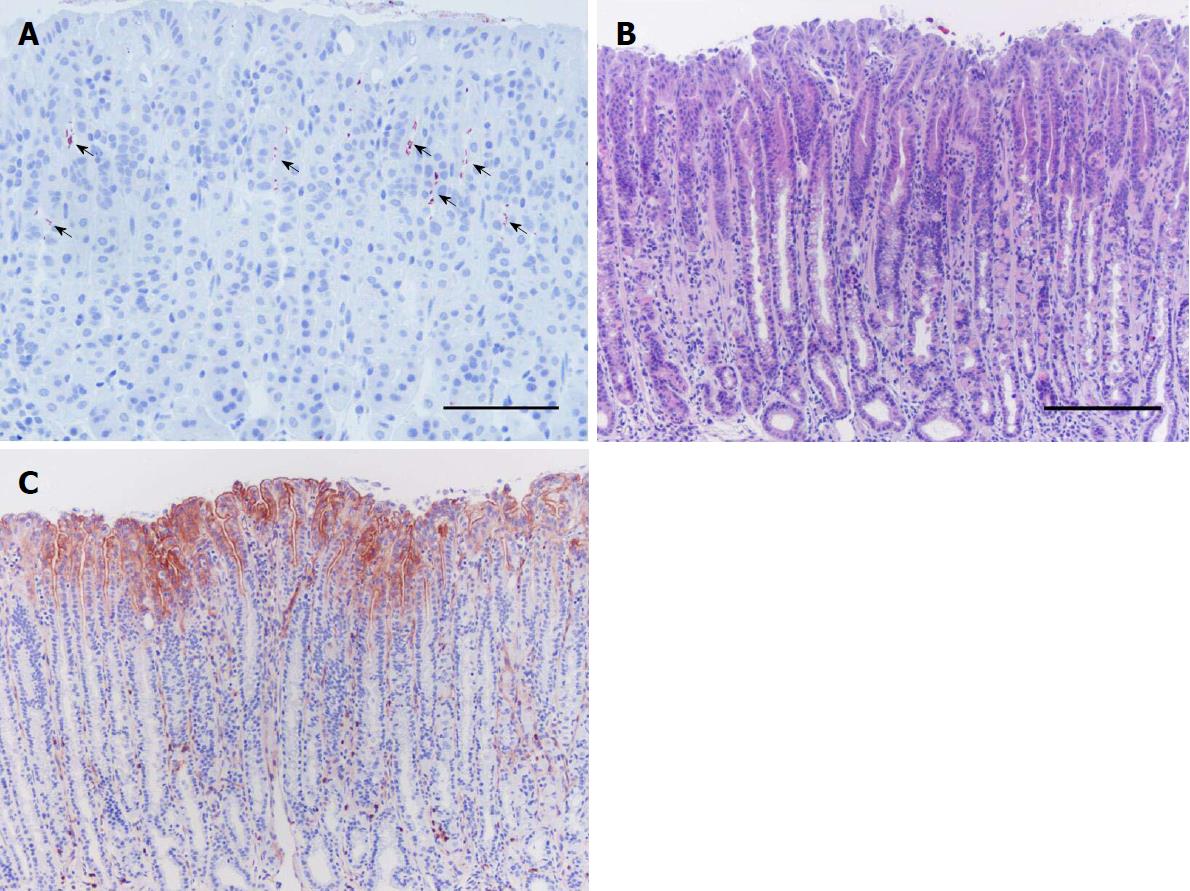
The link between H. pylori and uPAR has been systematically investigated in a mouse model of H. pylori-induced gastritis (Figure 3)[95]. In this model, uPAR expression is up-regulated very early in response to the infection and increases progressively during the course of infection, and this is reverted to its physiological baseline levels if H. pylori is eradicated by antimicrobial therapy[95]. Additional experiments in this model suggest that uPAR expression is directly induced by the bacterium (and Alpízar-Alpízar, unpublished results)[95]. It is not possible to rule out, however, that uPAR induction in murine gastric epithelium is a consequence of the inflammatory reaction against H. pylori.
A few signaling pathways and transcription factors have been proposed as potential inducers of uPAR in cancer; however, much less is known about the mechanisms of induction in response to H. pylori. Studies in cancer cell lines have found that the NF-κB can drive uPAR expression by direct binding to specific sequences within the regulatory region of the gene encoding uPAR[96] or indirectly via HIF1α activation[97]. It is well known that H. pylori infection can lead to the activation of NF-κB[36,98]. Therefore, NF-κB is a likely transcriptional inducer of uPAR in epithelial cells of H. pylori-colonized mucosa, both in human and mouse. In vitro evidence supports this idea[88], but no experimental data have been generated in vivo.
AP-1 is another transcriptional regulator that is activated by H. pylori infection[36] and has been implicated in the induction of uPAR in cancer[99,100]. Thus, AP-1 may explain the potential connection between these two parameters[90]. Both NF-κB and AP-1 can be activated via the Ras–ERK MAPK signaling pathway[99,101]. This pathway is often manipulated by H. pylori[36], which makes it an interesting study target to gain further insight about the mechanism of induction of uPAR in the gastric epithelium colonized with H. pylori.
The MMP family comprises more than 23 zinc-dependent endopeptidases, subdivided into eight groups according to structural characteristics[42,102]. MMPs are synthesized in the form of zymogens (pro-MMPs) by several cell types of the tumor microenvironment; and, when released to the extracellular space, they become activated by other proteases, including MMPs themselves and plasmin[42]. Besides their role in invasion and metastasis, MMPs are involved in other aspects of tumor biology. The degradation of ECM constituents results in the liberation of sequestered growth, proliferative and angiogenic factors but also in the generation of ECM-derived peptides with similar biological properties to those factors. Some MMPs can cleave membrane-bound growth factor precursors, thus releasing their active form, for example TGFβ[42]. Elevated expression of several MMPs has been consistently correlated with poor cancer patient survival in several types of cancer, including GC. Of note, a few MMPs actually inhibit malignant transformation and tumor growth, including MMP8, MMP12, and MMP26[103-107].
The possible connection between H. pylori infection and induction of MMPs (e.g., MMP2, MMP3 and MMP9) in gastric epithelial cells has been suggested; however, the most compelling evidence is probably for MMP7. MMP7 enhances tumor formation in rodents[108], and it is particularly interesting in the context of the gastric carcinogenesis because its expression is increased in human GC lesions[109,110]. In human gastric cell lines co-cultured with H. pylori, it was found that cag-PAI-positive strains augment the levels of MMP7 up to 7-fold compared to uninfected controls or to cells incubated with specific isogenic mutant strains[111]. According to that report, the induction of MMP7 in the in vitro system was dependent on the activation of ERK 1/2 and required an active interplay between viable bacteria and epithelial cells[111]. That study also evaluated the expression of MMP7 in gastric biopsies of human patients and found that it was over-expressed in epithelial cells of gastritis-affected individuals infected with CagA-positive strains[111], which has also been previously documented by an independent report[112].
These observations served as the driving force for conducting subsequent investigations in MMP7 knockout mouse models. Such studies concluded that gastric inflammation and epithelial cellular turnover are substantially increased in MMP7-deficient mice infected with H. pylori, compared to their wild-type counterparts[113,114]. It is speculated that over-expression of MMP7 in response to H. pylori colonization may be a mechanism to protect the gastric mucosa from damage and development of lesions that could ultimately result in GC[112,113]. Nevertheless, it is also proposed that sustained expression of MMP7 in the gastric epithelium could lead to malignant transformation[112]. In fact, a few studies suggest that MMP7 proteolytically cleaves specific pro-apoptotic molecules, such as Fas ligand, from tumors cells, thus promoting tumor survival[115,116].
H. pylori is a determining factor in the development of GC, due to the multiple ways in which it manipulates the host gastric epithelial cells. A key aspect of carcinogenesis is the acquisition of invasive capacities, and H. pylori could modulate several factors associated with invasion. A number of bacterial virulence factors may be of relevance in the manipulation of cellular and molecular programs that lead to increased invasive behavior; however, Cag-PAI stands out as a major orchestrator in hijacking these host cell pathways.
A number of key effectors of the EMT and cell polarity are deregulated in response to H. pylori infection. The two processes enhance cell motility and regulate the attachment of preneoplastic cells to the ECM and to other cells. This finally translates into an increased versatility of the cells to initiate the invasive process and adapt to the physiological changes suffered by the cell through the dedifferentiation induced by EMT. The acquisition of stem-like properties is a pivotal event that results from the activation of the EMT programs in response to H. pylori, since it confers the cells with augmented capability of survival and proliferation.
The induction of members of the PA system and MMPs by H. pylori could have important implications in the genesis of GC, given the wide array of aspects in which these molecules participate. Particularly interesting is the fact that this bacterium up-regulates the expression in non-neoplastic gastric mucosa of the uPAR, a protein that until now has been implicated in processes related to late stages of cancer development and progression, and has been correlated with the prognosis of cancer patients in general.
Altogether, the findings reviewed here show that H. pylori alters a fundamental process in gastric malignant transformation and invasiveness. Although we have discussed aspects related to EMT, cell polarity and ECM remodeling as independent processes, there are several points of interconnection among them (Figure 2). For instance, some factors implicated in the activation of EMT programs that are deregulated by H. pylori lead to the induction of MMPs and changes in cytoskeletal reorganization. Some of these proteinases, in the meantime, are capable of activating mediators of the EMT and cell polarity programs.
Therefore, the link between H. pylori and cell invasive properties is complex and an exciting open area of research where many aspects remain far from being clear. For instance, there is a need to gain further insight on how and under what circumstances H. pylori manipulates regulatory networks controlling the EMT and stem-cell programs. Also, it is important to unravel the cellular and molecular mechanisms underlying the induction of members of the PA system and MMPs in H. pylori-colonized gastric epithelium.
Elucidation of the key orchestrators governing these invasion-related programs is crucial to understanding the implications that these processes may have for the survival of the bacterium or in the pathological context. All this information could be of relevance for identifying individuals with an increased risk of GC, who may require H. pylori eradication therapy, especially in countries with limited resources and high prevalence of this bacterial infection. Finally, this may contribute to prediction of pre-neoplastic lesions that are more likely to progress in the pathogenic series of steps to malignancy, which may be of relevance to reducing GC burden.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Costa Rica
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Caboclo JF, Ding SZ, Kang W S- Editor: Ma YJ L- Editor: A E- Editor: Tan WW
| 1. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3183] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 2. | Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 505] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 3. | Cover TL. Helicobacter pylori Diversity and Gastric Cancer Risk. MBio. 2016;7:e01869-e01815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 4. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47109] [Article Influence: 3364.9] [Reference Citation Analysis (5)] |
| 5. | Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 2961] [Article Influence: 211.5] [Reference Citation Analysis (0)] |
| 6. | Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell. 2017;168:670-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2147] [Cited by in RCA: 2190] [Article Influence: 273.8] [Reference Citation Analysis (0)] |
| 7. | Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 2960] [Article Influence: 370.0] [Reference Citation Analysis (0)] |
| 8. | Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 9. | Sierra MS, Cueva P, Bravo LE, Forman D. Stomach cancer burden in Central and South America. Cancer Epidemiol. 2016;44 Suppl 1:S62-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2645] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 11. | Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4322] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 12. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 13. | Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North Am. 2013;42:261-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 15. | Huang Q, Sun Q, Fan XS, Zhou D, Zou XP. Recent advances in proximal gastric carcinoma. J Dig Dis. 2016;17:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 414] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 17. | Müller A, Oertli M, Arnold IC. H. pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell Commun Signal. 2011;9:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15:1998-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Larsson SC, Orsini N, Wolk A. Processed meat consumption and stomach cancer risk: a meta-analysis. J Natl Cancer Inst. 2006;98:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 696] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 21. | Alpízar-Alpízar W, Pérez-Pérez GI, Une C, Cuenca P, Sierra R. Association of interleukin-1B and interleukin-1RN polymorphisms with gastric cancer in a high-risk population of Costa Rica. Clin Exp Med. 2005;5:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 23. | Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Petrovchich I, Ford JM. Genetic predisposition to gastric cancer. Semin Oncol. 2016;43:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015;1:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 26. | Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ, Wang TC. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer. 2016;16:305-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9 Suppl 2:33-39. [PubMed] |
| 28. | Wen Y, Marcus EA, Matrubutham U, Gleeson MA, Scott DR, Sachs G. Acid-adaptive genes of Helicobacter pylori. Infect Immun. 2003;71:5921-5939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Dunn BE, Campbell GP, Perez-Perez GI, Blaser MJ. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464-9469. [PubMed] |
| 30. | Eaton KA, Brooks CL, Morgan DR, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470-2475. [PubMed] |
| 31. | Tsuda M, Karita M, Mizote T, Morshed MG, Okita K, Nakazawa T. Essential role of Helicobacter pylori urease in gastric colonization: definite proof using a urease-negative mutant constructed by gene replacement. Eur J Gastroenterol Hepatol. 1994;6 Suppl 1:S49-S52. [PubMed] |
| 32. | Scott DR, Marcus EA, Wen Y, Oh J, Sachs G. Gene expression in vivo shows that Helicobacter pylori colonizes an acidic niche on the gastric surface. Proc Natl Acad Sci USA. 2007;104:7235-7240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 459] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 34. | Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 880] [Cited by in RCA: 857] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 35. | Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 36. | Naumann M, Sokolova O, Tegtmeyer N, Backert S. Helicobacter pylori: A Paradigm Pathogen for Subverting Host Cell Signal Transmission. Trends Microbiol. 2017;25:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 37. | Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 38. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6575] [Cited by in RCA: 7906] [Article Influence: 494.1] [Reference Citation Analysis (0)] |
| 39. | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4715] [Cited by in RCA: 6228] [Article Influence: 566.2] [Reference Citation Analysis (0)] |
| 40. | Halaoui R, McCaffrey L. Rewiring cell polarity signaling in cancer. Oncogene. 2015;34:939-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 41. | Danø K, Rømer J, Nielsen BS, Bjørn S, Pyke C, Rygaard J, Lund LR. Cancer invasion and tissue remodeling--cooperation of protease systems and cell types. APMIS. 1999;107:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 212] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 42. | Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3948] [Cited by in RCA: 3795] [Article Influence: 253.0] [Reference Citation Analysis (0)] |
| 43. | Skrypek N, Goossens S, De Smedt E, Vandamme N, Berx G. Epithelial-to-Mesenchymal Transition: Epigenetic Reprogramming Driving Cellular Plasticity. Trends Genet. 2017;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 44. | Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 3032] [Article Influence: 303.2] [Reference Citation Analysis (0)] |
| 45. | Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 711] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 46. | Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, Wang AM, Kuo ML, Chi CW. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res. 2009;69:5039-5048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 47. | Mongroo PS, Rustgi AK. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol Ther. 2010;10:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 48. | Sun D, Mcalmon KR, Davies JA, Bernfield M, Hay ED. Simultaneous loss of expression of syndecan-1 and E-cadherin in the embryonic palate during epithelial-mesenchymal transformation. Int J Dev Biol. 1998;42:733-736. [PubMed] |
| 49. | Rodriguez FJ, Lewis-Tuffin LJ, Anastasiadis PZ. E-cadherin’s dark side: possible role in tumor progression. Biochim Biophys Acta. 2012;1826:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 505] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 51. | Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2496] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 52. | Lin Y, Dong C, Zhou BP. Epigenetic regulation of EMT: the Snail story. Curr Pharm Des. 2014;20:1698-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Theys J, Jutten B, Habets R, Paesmans K, Groot AJ, Lambin P, Wouters BG, Lammering G, Vooijs M. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol. 2011;99:392-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 54. | Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene. 2005;24:7443-7454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 952] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 55. | Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 56. | Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 700] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 57. | Yang L, Ping YF, Yu X, Qian F, Guo ZJ, Qian C, Cui YH, Bian XW. Gastric cancer stem-like cells possess higher capability of invasion and metastasis in association with a mesenchymal transition phenotype. Cancer Lett. 2011;310:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Ryu HS, Park DJ, Kim HH, Kim WH, Lee HS. Combination of epithelial-mesenchymal transition and cancer stem cell-like phenotypes has independent prognostic value in gastric cancer. Hum Pathol. 2012;43:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Kanzawa M, Semba S, Hara S, Itoh T, Yokozaki H. WNT5A is a key regulator of the epithelial-mesenchymal transition and cancer stem cell properties in human gastric carcinoma cells. Pathobiology. 2013;80:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Guo J, Wang B, Fu Z, Wei J, Lu W. Hypoxic Microenvironment Induces EMT and Upgrades Stem-Like Properties of Gastric Cancer Cells. Technol Cancer Res Treat. 2016;15:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Bessède E, Staedel C, Acuña Amador LA, Nguyen PH, Chambonnier L, Hatakeyama M, Belleannée G, Mégraud F, Varon C. Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene. 2014;33:4123-4131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 62. | Sougleri IS, Papadakos KS, Zadik MP, Mavri-Vavagianni M, Mentis AF, Sgouras DN. Helicobacter pylori CagA protein induces factors involved in the epithelial to mesenchymal transition (EMT) in infected gastric epithelial cells in an EPIYA- phosphorylation-dependent manner. FEBS J. 2016;283:206-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 63. | Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, Hu CJ, Dong H, Yang SM. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016;374:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 64. | Choi YJ, Kim N, Chang H, Lee HS, Park SM, Park JH, Shin CM, Kim JM, Kim JS, Lee DH. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis. 2015;36:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 65. | Segal ED, Falkow S, Tompkins LS. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 66. | Pai R, Cover TL, Tarnawski AS. Helicobacter pylori vacuolating cytotoxin (VacA) disorganizes the cytoskeletal architecture of gastric epithelial cells. Biochem Biophys Res Commun. 1999;262:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Su B, Ceponis PJ, Sherman PM. Cytoskeletal rearrangements in gastric epithelial cells in response to Helicobacter pylori infection. J Med Microbiol. 2003;52:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Schneider S, Weydig C, Wessler S. Targeting focal adhesions:Helicobacter pylori-host communication in cell migration. Cell Commun Signal. 2008;6:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Wessler S, Gimona M, Rieder G. Regulation of the actin cytoskeleton in Helicobacter pylori-induced migration and invasive growth of gastric epithelial cells. Cell Commun Signal. 2011;9:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Selbach M, Moese S, Hurwitz R, Hauck CR, Meyer TF, Backert S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003;22:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 71. | Kikuchi K, Murata-Kamiya N, Kondo S, Hatakeyama M. Helicobacter pylori stimulates epithelial cell migration via CagA-mediated perturbation of host cell signaling. Microbes Infect. 2012;14:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Yamahashi Y, Saito Y, Murata-Kamiya N, Hatakeyama M. Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization. J Biol Chem. 2011;286:44576-44584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Yamahashi Y, Hatakeyama M. PAR1b takes the stage in the morphogenetic and motogenetic activity of Helicobacter pylori CagA oncoprotein. Cell Adh Migr. 2013;7:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Nishikawa H, Hatakeyama M. Sequence Polymorphism and Intrinsic Structural Disorder as Related to Pathobiological Performance of the Helicobacter pylori CagA Oncoprotein. Toxins (Basel). 2017;9:pii: E136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Le Roux-Goglin E, Varon C, Spuul P, Asencio C, Mégraud F, Génot E. Helicobacter infection induces podosome assembly in primary hepatocytes in vitro. Eur J Cell Biol. 2012;91:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Varon C, Mocan I, Mihi B, Péré-Védrenne C, Aboubacar A, Moraté C, Oleastro M, Doignon F, Laharie D, Mégraud F. Helicobacter pullorum cytolethal distending toxin targets vinculin and cortactin and triggers formation of lamellipodia in intestinal epithelial cells. J Infect Dis. 2014;209:588-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Slomiany BL, Slomiany A. Helicobacter pylori-induced changes in microtubule dynamics conferred by α-tubulin phosphorylation on Ser/Tyr mediate gastric mucosal secretion of matrix metalloproteinase-9 (MMP-9) and its modulation by ghrelin. Inflammopharmacology. 2016;24:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Tabassam FH, Graham DY, Yamaoka Y. Paxillin is a novel cellular target for converging Helicobacter pylori-induced cellular signaling. Am J Physiol Gastrointest Liver Physiol. 2011;301:G601-G611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Lamarre J, Vasudevan J, Gonias SL. Plasmin cleaves betaglycan and releases a 60 kDa transforming growth factor-beta complex from the cell surface. Biochem J. 1994;302:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry (Mosc). 2002;67:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Kriegbaum MC, Persson M, Haldager L, Alpízar-Alpízar W, Jacobsen B, Gårdsvoll H, Kjær A, Ploug M. Rational targeting of the urokinase receptor (uPAR): development of antagonists and non-invasive imaging probes. Curr Drug Targets. 2011;12:1711-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Lund IK, Illemann M, Thurison T, Christensen IJ, Høyer-Hansen G. uPAR as anti-cancer target: evaluation of biomarker potential, histological localization, and antibody-based therapy. Curr Drug Targets. 2011;12:1744-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Alpízar-Alpízar W, Christensen IJ, Santoni-Rugiu E, Skarstein A, Ovrebo K, Illemann M, Laerum OD. Urokinase plasminogen activator receptor on invasive cancer cells: a prognostic factor in distal gastric adenocarcinoma. Int J Cancer. 2012;131:E329-E336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Alpízar-Alpízar W, Malespín-Bendaña W, Une C, Ramírez-Mayorga V. Relevance of the plasminogen activator system in the pathogenesis and progression of gastric cancer. Rev Biol Trop. 2018;66:28-47. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 85. | Sepulveda AR, Tao H, Carloni E, Sepulveda J, Graham DY, Peterson LE. Screening of gene expression profiles in gastric epithelial cells induced by Helicobacter pylori using microarray analysis. Aliment Pharmacol Ther. 2002;16 Suppl 2:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Guillemin K, Salama NR, Tompkins LS, Falkow S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc Natl Acad Sci USA. 2002;99:15136-15141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | El-Etr SH, Mueller A, Tompkins LS, Falkow S, Merrell DS. Phosphorylation-independent effects of CagA during interaction between Helicobacter pylori and T84 polarized monolayers. J Infect Dis. 2004;190:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Kim MH, Yoo HS, Kim MY, Jang HJ, Baek MK, Kim HR, Kim KK, Shin BA, Ahn BW, Jung YD. Helicobacter pylori stimulates urokinase plasminogen activator receptor expression and cell invasiveness through reactive oxygen species and NF-kappaB signaling in human gastric carcinoma cells. Int J Mol Med. 2007;19:689-697. [PubMed] |
| 89. | Iwamoto J, Mizokami Y, Takahashi K, Nakajima K, Ohtsubo T, Miura S, Narasaka T, Takeyama H, Omata T, Shimokobe K. Expressions of urokinase-type plasminogen activator, its receptor and plasminogen activator inhibitor-1 in gastric cancer cells and effects of Helicobacter pylori. Scand J Gastroenterol. 2005;40:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Kim MH, Yoo HS, Chang HJ, Hong MH, Kim HD, Chung IJ, Shin BA, Cho MJ, Ahn BW, Jung YD. Urokinase plasminogen activator receptor is upregulated by Helicobacter pylori in human gastric cancer AGS cells via ERK, JNK, and AP-1. Biochem Biophys Res Commun. 2005;333:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Iwamoto J, Mizokami Y, Takahashi K, Matsuoka T, Matsuzaki Y. The effects of cyclooxygenase2-prostaglandinE2 pathway on Helicobacter pylori-induced urokinase-type plasminogen activator system in the gastric cancer cells. Helicobacter. 2008;13:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Alpízar-Alpízar W, Nielsen BS, Sierra R, Illemann M, Ramírez JA, Arias A, Durán S, Skarstein A, Ovrebo K, Lund LR. Urokinase plasminogen activator receptor is expressed in invasive cells in gastric carcinomas from high- and low-risk countries. Int J Cancer. 2010;126:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Kenny S, Duval C, Sammut SJ, Steele I, Pritchard DM, Atherton JC, Argent RH, Dimaline R, Dockray GJ, Varro A. Increased expression of the urokinase plasminogen activator system by Helicobacter pylori in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G431-G441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Beyer BC, Heiss MM, Simon EH, Gruetzner KU, Babic R, Jauch KW, Schildberg FW, Allgayer H. Urokinase system expression in gastric carcinoma: prognostic impact in an independent patient series and first evidence of predictive value in preoperative biopsy and intestinal metaplasia specimens. Cancer. 2006;106:1026-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Alpízar-Alpízar W, Skindersoe ME, Rasmussen L, Kriegbaum MC, Christensen IJ, Laerum OD, Lund IK, Krogtelt KA, Andersen LP, Ploug M. Helicobacter pylori colonization drives urokinase receptor expression in murine gastric epithelium during early pathogenesis. In: Mégraud F, Malfertheiner P, editors. Abstracts of the European Helicobacter Study Group XXVIth International Workshop on Helicobacter and Related Bacteria in Chronic Digestive Inflammation and Gastric Cancer. Helicobacter. 2013;18 Suppl S1:77-154. [DOI] [Full Text] |
| 96. | Wang Y, Dang J, Wang H, Allgayer H, Murrell GA, Boyd D. Identification of a novel nuclear factor-kappaB sequence involved in expression of urokinase-type plasminogen activator receptor. Eur J Biochem. 2000;267:3248-3254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1217] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 98. | Rieke C, Papendieck A, Sokolova O, Naumann M. Helicobacter pylori-induced tyrosine phosphorylation of IKKβ contributes to NF-κB activation. Biol Chem. 2011;392:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Baek MK, Kim MH, Jang HJ, Park JS, Chung IJ, Shin BA, Ahn BW, Jung YD. EGF stimulates uPAR expression and cell invasiveness through ERK, AP-1, and NF-kappaB signaling in human gastric carcinoma cells. Oncol Rep. 2008;20:1569-1575. [PubMed] |
| 100. | Lengyel E, Wang H, Stepp E, Juarez J, Wang Y, Doe W, Pfarr CM, Boyd D. Requirement of an upstream AP-1 motif for the constitutive and phorbol ester-inducible expression of the urokinase-type plasminogen activator receptor gene. J Biol Chem. 1996;271:23176-23184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Lengyel E, Gum R, Stepp E, Juarez J, Wang H, Boyd D. Regulation of urokinase-type plasminogen activator expression by an ERK1-dependent signaling pathway in a squamous cell carcinoma cell line. J Cell Biochem. 1996;61:430-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4447] [Cited by in RCA: 4499] [Article Influence: 195.6] [Reference Citation Analysis (0)] |
| 103. | Balbín M, Fueyo A, Tester AM, Pendás AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, López-Otín C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 104. | Gutiérrez-Fernández A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL, Jones JL, Span PN. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 105. | Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149-6155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 106. | López-Otín C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 606] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 107. | López-Otín C, Palavalli LH, Samuels Y. Protective roles of matrix metalloproteinases: from mouse models to human cancer. Cell Cycle. 2009;8:3657-3662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 108. | Rudolph-Owen LA, Chan R, Muller WJ, Matrisian LM. The matrix metalloproteinase matrilysin influences early-stage mammary tumorigenesis. Cancer Res. 1998;58:5500-5506. [PubMed] |
| 109. | Honda M, Mori M, Ueo H, Sugimachi K, Akiyoshi T. Matrix metalloproteinase-7 expression in gastric carcinoma. Gut. 1996;39:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 110. | Yamashita K, Azumano I, Mai M, Okada Y. Expression and tissue localization of matrix metalloproteinase 7 (matrilysin) in human gastric carcinomas. Implications for vessel invasion and metastasis. Int J Cancer. 1998;79:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 111. | Crawford HC, Krishna US, Israel DA, Matrisian LM, Washington MK, Peek RM Jr. Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology. 2003;125:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 112. | Wroblewski LE, Noble PJ, Pagliocca A, Pritchard DM, Hart CA, Campbell F, Dodson AR, Dockray GJ, Varro A. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci. 2003;116:3017-3026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 113. | Ogden SR, Noto JM, Allen SS, Patel DA, Romero-Gallo J, Washington MK, Fingleton B, Israel DA, Lewis ND, Wilson KT. Matrix metalloproteinase-7 and premalignant host responses in Helicobacter pylori-infected mice. Cancer Res. 2010;70:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | Krakowiak MS, Noto JM, Piazuelo MB, Hardbower DM, Romero-Gallo J, Delgado A, Chaturvedi R, Correa P, Wilson KT, Peek RM Jr. Matrix metalloproteinase 7 restrains Helicobacter pylori-induced gastric inflammation and premalignant lesions in the stomach by altering macrophage polarization. Oncogene. 2015;34:1865-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 115. | Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577-581. [PubMed] |
| 116. | Vargo-Gogola T, Fingleton B, Crawford HC, Matrisian LM. Matrilysin (matrix metalloproteinase-7) selects for apoptosis-resistant mammary cells in vivo. Cancer Res. 2002;62:5559-5563. [PubMed] |