Published online Feb 15, 2018. doi: 10.4251/wjgo.v10.i2.62
Peer-review started: November 20, 2017
First decision: December 1, 2017
Revised: December 5, 2017
Accepted: December 13, 2017
Article in press: December 13, 2017
Published online: February 15, 2018
Processing time: 81 Days and 1.7 Hours
To perform automatic gastric cancer risk classification using photofluorography for realizing effective mass screening as a preliminary study.
We used data for 2100 subjects including X-ray images, pepsinogen I and II levels, PGI/PGII ratio, Helicobacter pylori (H. pylori) antibody, H. pylori eradication history and interview sheets. We performed two-stage classification with our system. In the first stage, H. pylori infection status classification was performed, and H. pylori-infected subjects were automatically detected. In the second stage, we performed atrophic level classification to validate the effectiveness of our system.
Sensitivity, specificity and Youden index (YI) of H. pylori infection status classification were 0.884, 0.895 and 0.779, respectively, in the first stage. In the second stage, sensitivity, specificity and YI of atrophic level classification for H. pylori-infected subjects were 0.777, 0.824 and 0.601, respectively.
Although further improvements of the system are needed, experimental results indicated the effectiveness of machine learning techniques for estimation of gastric cancer risk.
Core tip: We developed an automatic gastric cancer risk classification system that analyzes X-ray images as a preliminary study. To evaluate the effectiveness of our system, we performed a retrospective analysis of patients who underwent photofluorography and ABC (D) stratification by blood inspection. From the experimental results, we found that machine learning techniques might have a potential for extracting additional gastric cancer risk information. The collaborative use of image-based risk information and ABC (D) stratification will provide more reliable gastric cancer risk information.
- Citation: Togo R, Ishihara K, Mabe K, Oizumi H, Ogawa T, Kato M, Sakamoto N, Nakajima S, Asaka M, Haseyama M. Preliminary study of automatic gastric cancer risk classification from photofluorography. World J Gastrointest Oncol 2018; 10(2): 62-70
- URL: https://www.wjgnet.com/1948-5204/full/v10/i2/62.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i2.62
Gastric cancer remains the third leading cause of cancer mortality in the world, and East Asian countries, including China, South Korea and Japan, have the highest mortality rates[1,2]. In Japan, the number of gastric cancer-related deaths each year is approximately 50000, and there has been no change over the past several decades.
Many studies on gastric cancer have been carried out, and epidemiological studies have revealed that Helicobacter pylori (H. pylori) infection is a main cause of gastric cancer[3,4]. Consequently, in 1994, the International Agency for Research on Cancer (IARC) at the World Health Organization (WHO) declared that H. pylori infection can be classified as a group I carcinogen[5]. An animal experiment using Mongolian gerbils[6] and a prospective cohort study by Uemura et al[7] indicated that the main cause of gastric cancer is H. pylori infection. It has also been reported that about half of the world’s population is infected with H. pylori and that its prevalence is highly variable depending on age, geography and economic factors[8]. Although auto-immunization, drug-induced suffering and infectious diseases can cause gastritis and/or gastric cancer, most cases are due to H. pylori infection[9,10]. In Japan, the incidence of H. pylori-negative gastric cancer was reported to be 0.3%-0.6%[11,12], and almost all cases of gastric cancer are derived from H. pylori-induced gastritis. Moreover, H. pylori infection rates in Japan differ according to the year of birth, and generations born in the 1970s or later have extremely low infection rates[13]. Meanwhile, recent studies have shown that H. pylori eradication therapy reduces the risk for development of gastric cancer[14,15]. H. pylori eradication therapy for H. pylori-infected patients with gastritis has been covered by national health insurance since February 2013 in Japan, the first country in the world to do so. Hence, mass screening methods with consideration of gastric cancer risk are required[16,17].
ABC (D) stratification combining serum pepsinogen (PG) and H. pylori antibody has gradually been introduced for evaluation of gastric cancer risk[18]. It has been reported that the combination of these serum markers is effective for evaluating pre-malignant conditions of the gastric mucosa[19]. Since pre-malignant stages of atrophic gastritis, intestinal metaplasia and dysplasia, which can be detected from serum markers, lead to gastric adenocarcinoma, ABC (D) stratification is expected to become a new standard non-invasive inspection method for evaluation of gastric cancer risk[20]. On the other hand, the effectiveness of photofluorography and endoscopy for gastric cancer mass screening has also been evaluated. Hence, evaluation of gastric cancer risk from clinical image data is a crucial issue for the mass screening.
Recently, it has been reported that ABC (D) stratification and radiological findings of photofluorography have a good correlation with gastric cancer risk[21]. Since the main cause of gastric cancer and its risk factors have been clarified, a diagnostic technique for gastric cancer risk and/or H. pylori infection from photofluorography would play an important role in risk-based mass screening[22,23].
In this study, we performed a preliminary investigation of automatic gastric cancer risk classification using photofluorography for realizing effective risk-based mass screening.
We performed a preliminary study for classification of gastric cancer risk from photofluorography. Then we developed an automatic risk classification system utilizing machine learning techniques for achieving our objective.
Data for X-ray images (8-bit gray scale, 1024 × 1024 pixels), H. pylori antibody, pepsinogen I (PG I) level, pepsinogen II (PG II) level, PGI/PGII ratio, H. pylori eradication history and interview sheets were used in this study. These data were acquired at the Medical Examination Center of Yamagata City Medical Association that specializes in gastric cancer mass screening from April 2012 to March 2013. We used X-ray images of eight positions for each subject. H. pylori antibody titers were measured by enzyme-linked immunosorbent assay kits (E Plate Eiken H. pylori, Eiken Chemical Co., Ltd., Tokyo, Japan). PG I level and PG II level were measured by Auto pepsinogen I BML-2G and Auto pepsinogen II BML-2 (BML, Inc., Ltd., Saitama, Japan), respectively. The cut-off value of H. pylori antibody titers was 10 U/mL, and the cut-off values of PG levels were PG I < 70 ng/mL and PG I/PG II ratio < 3. Subjects in whom these serum markers were measured were categorized into three or four groups corresponding to their gastric cancer risk as shown in Table 1. In ABC (D) stratification, group A is defined as a very low gastric cancer risk group, group B is defined as a middle-risk group, and groups C and D are defined as high-risk groups, with group D generally being included in group C[21].
| A | B | C (D) | |
| H. pylori antibody level | - | + | + (-) |
| PG levels | - | - | + |
We developed an automatic gastric cancer risk classification system for identification of H. pylori infection status and atrophic level from photofluorography. In the first stage, H. pylori infection status classification was performed. In the second stage, atrophic level classification was applied to H. pylori-infected subjects. First, for gastric cancer risk classification, we derived image features from X-ray images for representing changes inside the stomach caused by H. pylori infection. In training procedures, we calculated more efficient image features that had high correlations with values of H. pylori antibody and serum markers. Specifically, we obtained new image features by projecting the original image features to a space that provided high correlations with values of PG levels and H. pylori antibody titers via Kernel Canonical Correlation Analysis (KCCA)[24]. Next, we classified these image features by a Support Vector Machine (SVM)[25]. An SVM technique is a machine learning technique that is often used for classification problems. Since multiple X-ray images were taken for each subject, the classification results of all X-ray images were integrated by an accuracy-based voting method. The values of H. pylori antibody and serum markers were used only in training procedures, and our system enabled classification of the risk of gastric cancer from only X-ray image information. Namely, if we want to estimate gastric cancer risk via our system, input data are only X-ray images, and calculated image features are automatically converted to new features considering PG levels and H. pylori antibody titers for the gastric cancer risk classification. A more detailed mathematical explanation of our system is given in[26].
The verification method was 15-fold cross-validation. The gold standard for evaluating our system was the result of ABC (D) stratification by blood inspection. Sensitivity, specificity and Youden index (YI) were used as evaluation criteria for each stage’s classification. A receiver operating characteristic (ROC) curve was generated based on each stage’s classification result. ROC curves were obtained by changing the threshold that determines gastric cancer risk. Accuracy, precision, false positive rate and false negative rate were calculated. We also utilized a confusion matrix for evaluation of our system. A confusion matrix is often used in the field of machine learning, and it represents information about actual and predicted classification results obtained by a classification system. In this study, Togo R, Ishihara K, Ogawa T and Haseyama M from the Graduate School of Information Science and Technology, Hokkaido University took charge of the statistical analysis since they have an advanced knowledge of statistical analysis.
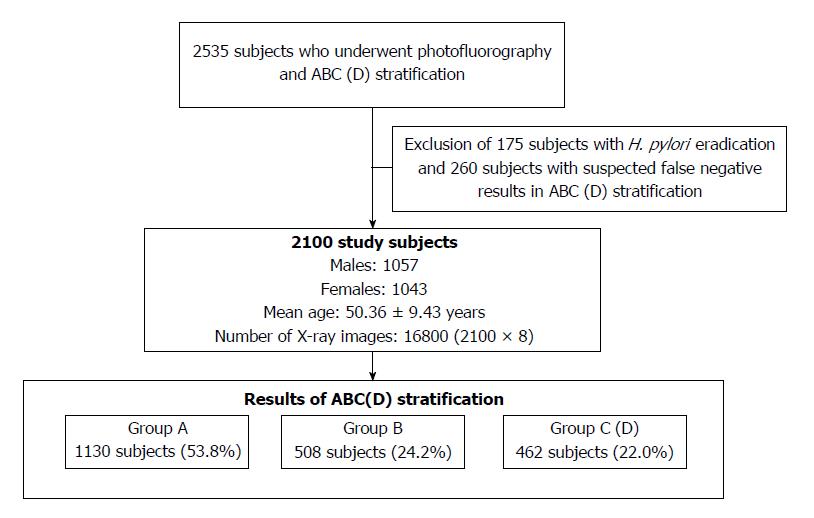
The total number of subjects was 2535, and subjects who had undergone H. pylori eradication therapy and had suspected false negative results in ABC (D) stratification were excluded as shown in Figure 1. Specifically, we excluded 175 subjects who had undergone H. pylori eradication therapy, and we excluded 260 subjects in group A with PG I levels ≤ 30 ng/mL, PG II levels ≥ 15 ng/mL or PG I/PG II ratio < 4. If the training data included data for such subjects, it would have caused classification performance degradation since the correlation between radiological findings and ABC (D) stratification results for them might be eliminated. Consequently, data for 2100 subjects (1057 males and 1043 females; mean age, 50.36 ± 9.43 years) were used for analysis. There were 1130 subjects (53.8%) in group A, 508 subjects (24.2%) in group B and 462 subjects (22.0%) in group C (D).
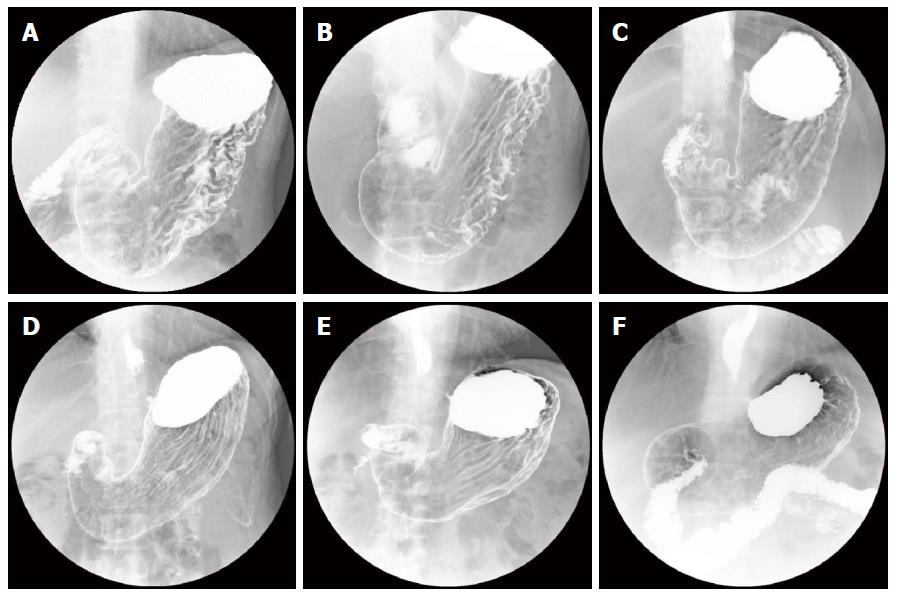
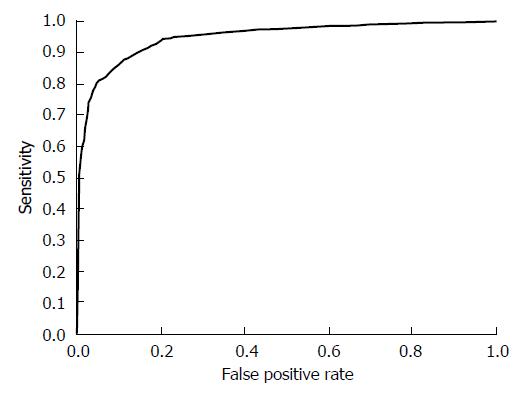
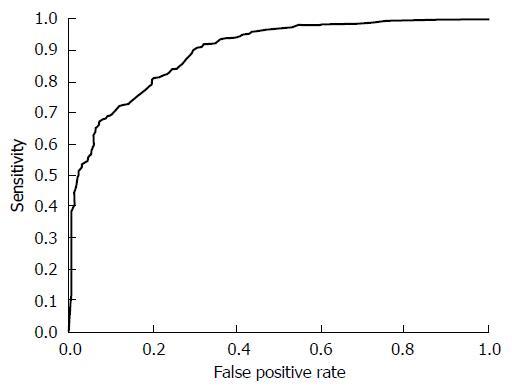
Our system was evaluated with 16800 X-ray images for 2100 subjects. In the first stage, we performed H. pylori infection status classification. The number of subjects classified into each class is shown as a confusion matrix in Table 2. Of the 970 subjects who belonged to groups B and C (D) in ABC (D) stratification, 868 were correctly classified into the high gastric cancer risk group (H. pylori infection) using only X-ray image information. Also, of the 1130 subjects who belonged to group A in ABC (D) stratification, 999 were correctly classified into the low gastric cancer group (H. pylori non-infection). On the other hand, 102 of the 2100 subjects (4.8%) were incorrectly classified into the H. pylori non-infection group in our system. Specifically, sensitivity (H. pylori infection), specificity (H. pylori non-infection) and YI were 0.884, 0.895 and 0.779, respectively. Other evaluation criteria were as follows: accuracy was 0.889, precision was 0.907, false positive rate was 0.105 and false negative rate was 0.116. Figure 2 shows examples of X-ray images correctly or incorrectly classified in the first stage. The ROC curve of the first stage that was obtained by changing the threshold determining H. pylori infection is shown in Figure 3.
| Predicted class | |||
| H. pylori non-infection | H. pylori infection | ||
| True class | Group A | 979 | 151 |
| Group B or C (D) | 102 | 868 | |
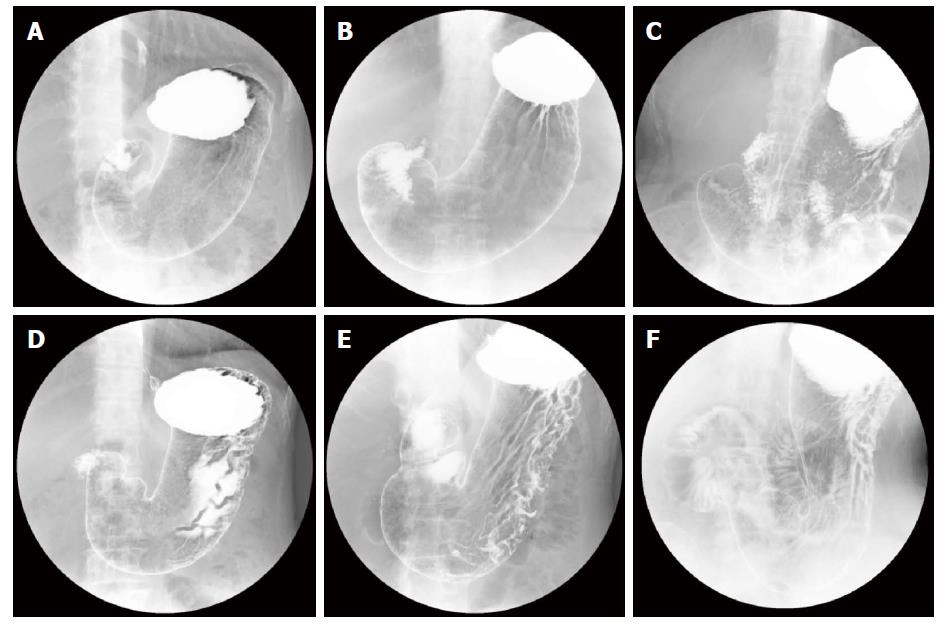
Next, we examined whether our system can be applied to more specific atrophic level classification. In the supplementary experiment of the second stage, we focused on H. pylori-infected subjects and applied atrophic level classification to them. The number of subjects classified into each class is shown as a confusion matrix in Table 3. The experimental results showed that 364 of the 462 subjects who belonged to group C (D) in ABC (D) stratification were correctly classified into the severe atrophic level group based on the condition of the stomach shown in X-ray images. Sensitivity (severe), specificity (non-severe) and YI in the second stage were 0.777, 0.824 and 0.601, respectively. Other evaluation criteria were as follows: accuracy was 0.800, precision was 0.809, false positive rate was 0.176 and false negative rate was 0.223. Figure 4 shows examples of X-ray images correctly or incorrectly classified in the second stage. The ROC curve of the second stage that was obtained by changing the threshold determining the severity of atrophic level is shown in Figure 5.
| Predicted class | |||
| Non-severe | Severe | ||
| True class | Group B | 331 | 177 |
| Group C (D) | 98 | 364 | |
It is a critical issue to evaluate gastric cancer risk for realizing effective gastric cancer mass screening[27]. H. pylori eradication therapy as primary prevention and early detection of gastric cancer as secondary prevention should be implemented more effectively. Concretely, it is necessary to identify individuals with a high gastric cancer risk for more detailed examination and continuous gastric cancer screening based on their H. pylori infection status and atrophic level.
ABC (D) stratification has already been introduced in some areas for gastric cancer risk screening. However, ABC (D) stratification may have a disadvantage for detecting individuals with high gastric cancer risk. Since individuals in whom H. pylori has been eradicated and individuals with a high atrophic level who have a high gastric cancer risk are often classified into group A in ABC (D) stratification[28-30], the false-negative rate is a problem. Thus, since even if individuals in group A in ABC (D) stratification can develop gastric cancer[31], the combined use of image-based inspection is mandatory for evaluation of gastric cancer risk[32]. Photofluorography or endoscopy remains the gold standard of gastric cancer mass screening in Japan since clinicians can examine conditions of the stomach through the images. Hence, supporting image-based inspections will lead to more efficient gastric cancer mass screening.
Endoscopy is superior to photofluorography for detection of cancerous lesions in image-based inspections[33]. In Japan, endoscopy has been recommended for gastric cancer mass screening programs in addition to photofluorography since 2016. Results of studies in South Korea have provided useful suggestions. In South Korea, a selective (i.e., photofluorography or endoscopy) gastric cancer mass screening program was started in 2002[34,35]. Lee et al[33] reported that the proportion of individuals who underwent endoscopic examination in the National Cancer Screening Program (NCSP) in South Korea increased greatly from 31.15% in 2002 to 72.55% in 2011. The NCSP provides biennial gastric cancer mass screening with either photofluorography or endoscopy for men and women over 40 years of age. On the other hand, the proportion of individuals who underwent photofluorography in the NCSP decreased from 68.85% in 2002 to 32.8% in 2011. Lee et al[33] also reported that the rate of participation in the NCSP increased from 7.40% in 2002 to 45.40% in 2011, and the number of individuals examined by photofluorography increased in accordance with an overall increase in the percentage of participants in the NCSP in South Korea. This indicates the importance of automatic gastric cancer risk classification systems for photofluorography even under the condition of selective gastric cancer mass screening. In Japan, it will take a long time to establish endoscopic examinations due to an insufficient number of medical specialists and regional disparities of clinicians. The uneven distribution of clinicians who have experience in endoscopy is a bottleneck for endoscopic mass screening. Furthermore, the number of individuals who can be examined in one day by endoscopy is much smaller than the number of individuals who can be examined by photofluorography. Although photofluorography involves radiation exposure, facilities have been constructed and inspection methods have been established. Each type of inspection has advantages and disadvantages, the above-described situation should be considered for establishing a gastric cancer risk classification system[36].
In this study, we developed an automatic gastric cancer risk classification system as a preliminary study. Our system analyzes X-ray images and provides H. pylori infection status or atrophic severity level. It should be noted that the most important classification is the first stage, and the second stage is a supplementary experiment to verify whether our system can perform more detailed atrophic level classification. Experimental results indicated that risk-based information can be provided by our system. In the first stage, the most important risk classification, 88.9% of the subjects were correctly classified into the low gastric cancer risk group (H. pylori non-infection) and the high risk group (H. pylori infection). The purpose of our system is to improve the final accuracy of clinicians’ diagnosis by providing risk-based information from image data. Gastric cancer risk information based on X-ray images is useful for identification of high-risk individuals and for reducing the burden on clinicians. Results of studies on identification of risk information for gastric cancer from photofluorography and examination of its application should be helpful for the future of gastric cancer mass screening. Moreover, the threshold determining each risk group in our system can be continuously changed depending on the demands of clinicians. Namely, it is possible to decrease false negative cases by enhancing sensitivity based on the threshold for gastric cancer mass screening. Therefore, the combination of the results of ABC (D) stratification and our system will provide more reliable information for clinicians.
As an example of gastric cancer mass screening using our system, more specific examinations can be performed for individuals who have positive results in the first stage and had not received H. pylori eradication therapy are led to more specific examinations. The Japanese national health insurance now covers H. pylori eradication therapy for H. pylori-infected patients with gastritis detected by endoscopic examination. If those patients have positive results in the examination, H. pylori eradication therapy will be conducted and they will be followed up by gastric cancer screening.
Our study has some limitations. First, although the gold standard of our system was ABC (D) stratification and H. pylori eradication history, there are often contain false negative or false positive cases. Ideally, H. pylori infection status and atrophic level should be evaluated by radiological findings of photofluorography or endoscopy, and these results should be used for the gold standard. However, since this preliminary study focused on mass screening data, we utilized ABC (D) stratification as the simplest inspection with a high objectivity. Secondly, the exclusion rule of this study is our limitation. The advantage of image-based risk information is that gastric cancer risk information can be estimated from individuals who have undergone H. pylori eradication therapy since the presence or absence of atrophy of the stomach remains a key factor for them. However, H. pylori-eradicated individuals and individuals with suspected false negative results of ABC (D) stratification were excluded from our study due to the lack of a gold standard of ABC (D) stratification. Instead, we performed a supplemental experiment for evaluation of stomach atrophy in this study. We will target H. pylori-eradicated individuals and suspected false negative individuals as a future work.
We presented a gastric cancer risk classification system using photofluorography as a preliminary study. The first step of our experimental results indicates that gastric cancer risk information can be provided by machine learning techniques. Although further investigation and improvements of the system are needed, it is expected that collaborative use of image-based risk information derived by our system and ABC (D) stratification will enable more accurate evaluation of gastric cancer risk.
Gastric cancer is one of the most common malignancies, and has the highest mortality rates in East Asian countries. Although ABC (D) stratification is effective method for evaluating gastric cancer risk, photofluorography still plays an important role in gastric cancer mass screening since image-based evaluation is mandatory.
If gastric cancer risk information can be provided automatically by analyzing X-ray images, it would be helpful for the future of gastric cancer mass screening.
The aim of this study was investigation of potential of machine learning techniques using photofluorography.
We developed an automatic gastric cancer risk classification system for identification of Helicobacter pylori infection status and atrophic level from photofluorography. All of 2100 patients’ data were acquired at the Medical Examination Center of Yamagata City Medical Association in Japan, from April 2012 to March 2013. From DICOM data, we extracted the image data while securing anonymity.
Experimental results suggested that image-based risk information can be calculated by our system.
Although further investigation and improvement of the system are needed, this retrospective study indicated that machine learning techniques analyzing X-ray images can provide effective gastric cancer risk information. Also, we discussed the potential of machine learning techniques and the future of gastric cancer mass screening.
In the field of breast cancer, computer-aided supporting systems have already become a part of routine clinical work for detection of breast cancer or abnormalities. Gastric cancer as well as breast cancer requires effective and highly accurate mass screening. We believe that this preliminary study will contribute the next step of the future of gastric cancer mass screening.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Muhammad JS, Wani IA S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20511] [Article Influence: 2051.1] [Reference Citation Analysis (20)] |
| 3. | Correa P, Fox J, Fontham E, Ruiz B, Lin YP, Zavala D, Taylor N, Mackinley D, de Lima E, Portilla H. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer. 1990;66:2569-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 924] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 5. | Vainio H, Heseltine E, Wilbourn J. Priorities for Future IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Environ Health Perspect. 1994;102:590-591. [PubMed] |
| 6. | Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 7. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3183] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 8. | Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 625] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Chronic disease management in ageing populations. Lancet. 2012;379:1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1718] [Article Influence: 132.2] [Reference Citation Analysis (1)] |
| 10. | Peleteiro B, Castro C, Morais S, Ferro A, Lunet N. Worldwide Burden of Gastric Cancer Attributable to Tobacco Smoking in 2012 and Predictions for 2020. Dig Dis Sci. 2015;60:2470-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter. 2011;16:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Ono S, Kato M, Suzuki M, Ishigaki S, Takahashi M, Haneda M, Mabe K, Shimizu Y. Frequency of Helicobacter pylori -negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion. 2012;86:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Ueda J, Gosho M, Inui Y, Matsuda T, Sakakibara M, Mabe K, Nakajima S, Shimoyama T, Yasuda M, Kawai T. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter. 2014;19:105-110. [PubMed] |
| 14. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 935] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 15. | Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 442] [Article Influence: 40.2] [Reference Citation Analysis (1)] |
| 16. | Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Kudo T, Kakizaki S, Sohara N, Onozato Y, Okamura S, Inui Y, Mori M. Analysis of ABC (D) stratification for screening patients with gastric cancer. World J Gastroenterol. 2011;17:4793-4798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Shimoyama T, Aoki M, Sasaki Y, Matsuzaka M, Nakaji S, Fukuda S. ABC screening for gastric cancer is not applicable in a Japanese population with high prevalence of atrophic gastritis. Gastric Cancer. 2012;15:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Yamaguchi Y, Nagata Y, Hiratsuka R, Kawase Y, Tominaga T, Takeuchi S, Sakagami S, Ishida S. Gastric Cancer Screening by Combined Assay for Serum Anti-Helicobacter pylori IgG Antibody and Serum Pepsinogen Levels--The ABC Method. Digestion. 2016;93:13-18. [PubMed] |
| 21. | Itoh T, Saito M, Marugami N, Hirai T, Marugami A, Takahama J, Tanaka T, Kichikawa K. Correlation between the ABC classification and radiological findings for assessing gastric cancer risk. Jpn J Radiol. 2015;33:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Yamamichi N, Hirano C, Shimamoto T, Minatsuki C, Takahashi Y, Nakayama C, Matsuda R, Fujishiro M, Konno-Shimizu M, Kato J. Associated factors of atrophic gastritis diagnosed by double-contrast upper gastrointestinal barium X-ray radiography: a cross-sectional study analyzing 6,901 healthy subjects in Japan. PLoS One. 2014;9:e111359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Yamamichi N, Hirano C, Takahashi Y, Minatsuki C, Nakayama C, Matsuda R, Shimamoto T, Takeuchi C, Kodashima S, Ono S. Comparative analysis of upper gastrointestinal endoscopy, double-contrast upper gastrointestinal barium X-ray radiography, and the titer of serum anti-Helicobacter pylori IgG focusing on the diagnosis of atrophic gastritis. Gastric Cancer. 2016;19:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Akaho S. A kernel method for canonical correlation analysis. ArXiv Comput Sci e-prints. 2007;40:263-269. |
| 25. | Chen HF. In silico log P prediction for a large data set with support vector machines, radial basis neural networks and multiple linear regression. Chem Biol Drug Des. 2009;74:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22481] [Cited by in RCA: 10757] [Article Influence: 672.3] [Reference Citation Analysis (0)] |
| 26. | Ishihara K, Ogawa T, Haseyama M. Classification of gastric cancer risk from X-ray images based on efficient image features related to serum Hp antibody level and serum PG Levels. ITE Trans Media Technol Appl. 2016;4:337-348. [DOI] [Full Text] |
| 27. | Compare D, Rocco A, Nardone G. Screening for and surveillance of gastric cancer. World J Gastroenterol. 2014;20:13681-13691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 29. | Mizuno S, Miki I, Ishida T, Yoshida M, Onoyama M, Azuma T, Habu Y, Inokuchi H, Ozasa K, Miki K. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci. 2010;55:3132-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Yoshida T, Kato J, Inoue I, Yoshimura N, Deguchi H, Mukoubayashi C, Oka M, Watanabe M, Enomoto S, Niwa T. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014;134:1445-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Miura K, Okada H, Kouno Y, Kanzaki H, Iwamuro M, Hori K, Kita M, Kawano S, Kawahara Y, Tanaka T. Actual Status of Involvement of Helicobacter pylori Infection That Developed Gastric Cancer from Group A of ABC (D) Stratification - Study of Early Gastric Cancer Cases That Underwent Endoscopic Submucosal Dissection. Digestion. 2016;94:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol. 2017;32:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Lee S, Jun JK, Suh M, Park B, Noh DK, Jung KW, Choi KS. Gastric cancer screening uptake trends in Korea: results for the National Cancer Screening Program from 2002 to 2011: a prospective cross-sectional study. Medicine (Baltimore). 2015;94:e533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Lee HY, Park EC, Jun JK, Choi KS, Hahm MI. Comparing upper gastrointestinal X-ray and endoscopy for gastric cancer diagnosis in Korea. World J Gastroenterol. 2010;16:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Choi KS, Jun JK, Park EC, Park S, Jung KW, Han MA, Choi IJ, Lee HY. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One. 2012;7:e50041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Hamashima C. Benefits and harms of endoscopic screening for gastric cancer. World J Gastroenterol. 2016;22:6385-6392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (3)] |