Published online Dec 15, 2018. doi: 10.4251/wjgo.v10.i12.528
Peer-review started: August 27, 2018
First decision: September 11, 2018
Revised: September 17, 2018
Accepted: November 2, 2018
Article in press: November 2, 2018
Published online: December 15, 2018
Processing time: 109 Days and 19.5 Hours
A recent subgroup analysis of the TRIBE trial suggested that FOLFOXIRI plus bevacizumab may be a preferred option for the first-line treatment of only right-sided metastatic colorectal cancer (mCRC), regardless of RAS or BRAF status. Our subanalysis of a phase II trial of the FOLFOXIRI triplet regimen plus bevacizumab in patients with mCRC who had RAS mutant tumors showed that tumor shrinkage was better and the duration of treatment was longer in patients with left-sided tumors than in those with right-sided tumors, leading to a higher rate of conversion to surgery in mCRC patients with left-sided tumors. The early and deep responses to the triplet-regimen in patients with left-sided tumors might facilitate conversion treatment resulting in favorable survival. Our data suggest that the FOLFOXIRI plus bevacizumab might be a promising treatment for left-sided mCRC involving RAS mutant tumors.
Core tip: FOLFOXIRI plus bevacizumab regimen might be a preferred option for the first-line treatment of only right-sided metastatic colorectal cancer (mCRC) regardless of RAS or BRAF status. However, subanalysis of a phase II trial of the triplet plus bevacizumab in patients with RAS mutant mCRC demonstrated that more patients with left-sided tumors achieved good tumor shrinkage and long duration of treatment than did patients with right-sided tumors, leading to higher rate of conversion to surgery in mCRC patients with left-sided tumors. Our data suggest that FOLFOXIRI plus bevacizumab may be a promising treatment for left-sided mCRC associated with RAS mutant tumors.
- Citation: Sunakawa Y, Satake H, Ichikawa W. Considering FOLFOXIRI plus bevacizumab for metastatic colorectal cancer with left-sided tumors. World J Gastrointest Oncol 2018; 10(12): 528-531
- URL: https://www.wjgnet.com/1948-5204/full/v10/i12/528.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i12.528
A randomized study, the TRIBE trial, has demonstrated that FOLFOXIRI plus bevacizumab is more beneficial than FOLFIRI plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (mCRC). In addition, a number of clinical studies, including the STEAM and CHARTA trials, have shown similar clinical benefits of treatment with FOLFOXIRI plus bevacizumab[1-3]. Therefore, the triplet-regimen is considered one of the standard first-line treatments for mCRC in the National Comprehensive Cancer Network and European Society for Medical Oncology consensus guidelines[4,5]. Recently, a subgroup analysis of the TRIBE trial was performed to investigate the effect of upfront tumor sidedness on therapeutic effectiveness and whether this potentially heterogeneous effect differed according to RAS and BRAF mutational status in mCRC. The results indicated that patients who harbored right-sided tumors achieved an evident survival benefit from the triplet-regimen as backbone chemotherapy regardless of RAS and BRAF status. Namely, FOLFOXIRI plus bevacizumab can be considered to be a preferred treatment option in a first-line setting for only right-sided mCRC[6].
The location of the primary tumor has an impact on clinical behavior and has prognostic value in mCRC. A recent meta-analysis suggested that tumor sidedness is a predictive marker of the response to anti-epidermal growth factor receptor (EGFR) therapy in patients with RAS wild-type mCRC. Patients with left-sided tumors were shown to derive a greater benefit from chemotherapy plus an anti-EGFR antibody than from chemotherapy plus bevacizumab, whereas right-sided tumors were associated with trends toward detrimental effects of anti-EGFR therapy[7]. Therefore, anti-EGFR therapy with cetuximab or panitumumab is recommended for only RAS wild-type and left-sided tumors. A subanalysis of the TRIBE trial according to tumor sidedness showed no higher survival benefit from a triplet-regimen as compared with a doublet-regimen in patients with RAS wild-type mCRC who had left-sided tumors, suggesting that a doublet-regimen plus an anti-EGFR antibody is the preferred treatment for patients with left-sided RAS wild-type tumors. On the other hand, in patients with RAS mutant tumors, which do no benefit from anti-EGFR antibodies, the question remains whether intensification of the triplet regimen plus bevacizumab should be limited to patients who have mCRC with right-sided tumors.
In the subgroup analysis of the TRIBE trial, the objective response rate (ORR) of the triplet-arm was 65.0% for left-side tumors and 62.5% for right-side tumors in patients with RAS mutant tumors. The median progression-free survival (PFS) was 12.5 mo for patients with left-side tumors and 11.0 mo for those with right-side tumors. The efficacy of intensive chemotherapy with bevacizumab did not appear to differ significantly between tumor sidedness in patients with RAS mutant mCRC. Moreover, in patients who had left-sided tumors with RAS mutation, the ORR and PFS were slightly but not significantly higher in the triplet-arm than in the doublet-arm.
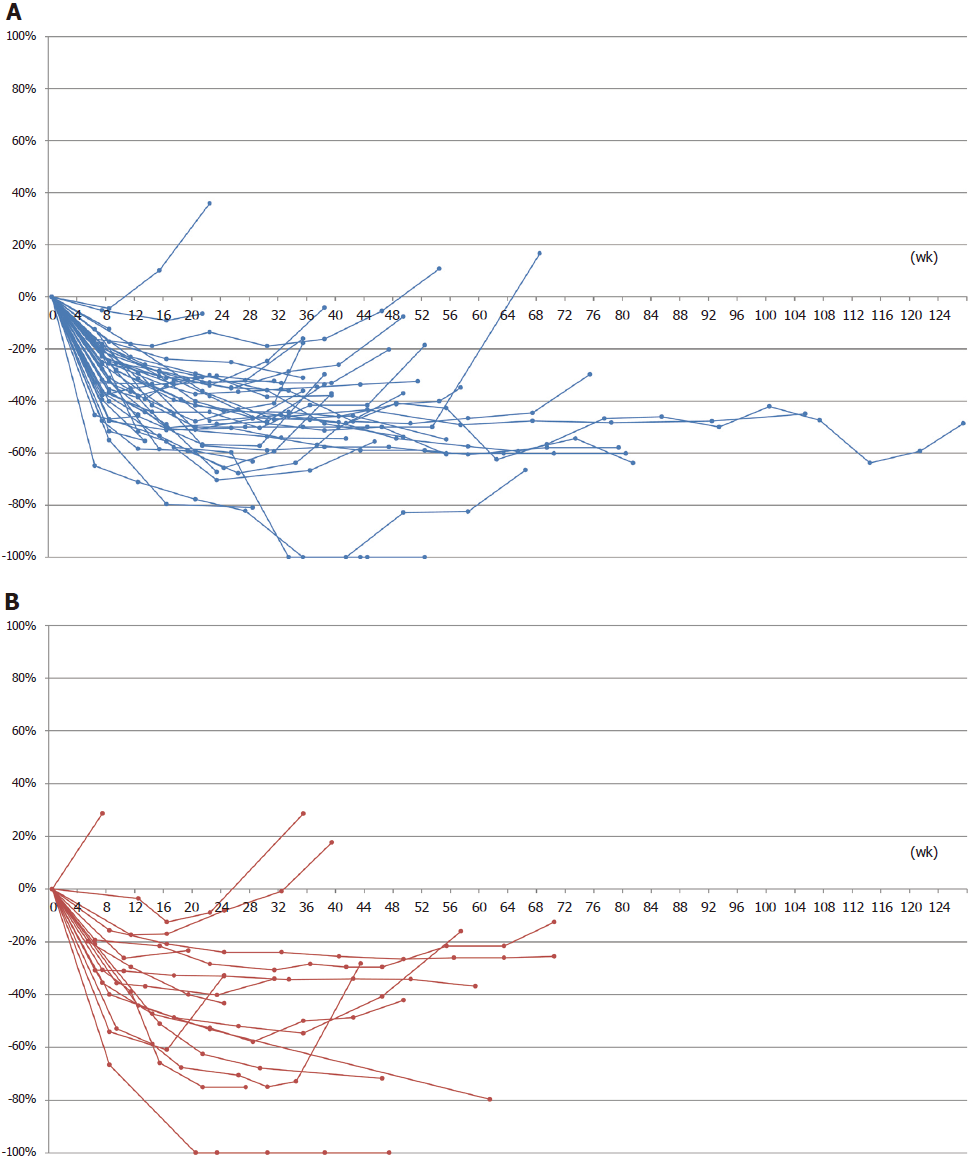
We have conducted a phase II trial of first-line FOLFOXIRI plus bevacizumab for mCRC with RAS mutant tumors. The ORR was the primary endpoint, and the secondary endpoints included PFS, early tumor shrinkage (ETS), and depth of response (DpR). The ORR and ETS rates in enrolled patients were 75.8% and 73.8%, respectively. According to primary tumor side, the ORR and ETS were both much better in patients with left-sided tumors than in patients with right-sided tumors (82.2% vs 58.8%, 77.3% vs 64.7%, respectively)[8]. Here, we performed an exploratory analysis of DpR using spider-plots in each tumor side. Interestingly, the spider-plots demonstrated that tumor shrinkage was better and the duration of treatment was longer in the patients with left-sided tumors than in those with right-sided tumors (Figure 1). One (6%) of 17 patients could undergo conversion surgery in the right-sided group, while 11 (28%) of 40 patients could receive conversion surgery in the left-sided group. The early and deep responses to the triplet regimen in the left-sided group may facilitate conversion treatment associated with favorable survival.
We showed an analysis of the evaluated radiographic responses to FOLFOXIRI plus bevacizumab for mCRC according to each tumor side using spider plots. Cremolini et al[9] has reported the association of ETS and DpR with clinical outcomes of triplet plus bevacizumab treatment using the TRIBE data; however, the results according to tumor sidedness have not been reported yet. Our phase II trial was a prospective study designed to evaluate the efficacy of FOLFOXIRI plus bevacizumab in molecular-selected patients with RAS mutation. Moreover, tumor response as a primary endpoint was evaluated prospectively by an external review board. Our findings for RAS mutant mCRC would be more reliable than the findings of the sub-analysis of the TRIBE trial. FOLFOXIRI plus bevacizumab was not beneficial in left-sided tumors in the TRIBE trial, while FOLFOXIRI plus bevacizumab appeared to be better compared to FOLFOX plus bevacizumab in left-sided tumors in other 2 trials[2,3]. Types of backbone chemotherapy may affect the results of sub-analyses by tumor sidedness. Although a recent study reported that FOLFOXIRI plus bevacizumab may be regarded as a preferred option for only right-sided mCRC[6], our data suggest that the triplet-regimen may be a promising treatment for left-sided mCRC with RAS mutant tumors.
We thank the patients, their families, and the investigators who participated in the Japan Clinical Cancer Research Organization (JACCRO) CC-11 trial. We also thank JACCRO and Masashi Fujii for trial support.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lin Q, Nahitendur MA, Saglam S S- Editor: Wang XJ L- Editor: A E- Editor: Song H
| 1. | Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 792] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 2. | Hurwitz H, Tan BR, Reeves JA, Xiong HQ, Somer BG, Lenz H-J, Hochster HS, Scappaticci F, Palma JF, Mancao C. Updated efficacy, safety, and biomarker analyses of STEAM, a randomized, open-label, phase II trial of sequential (s) and concurrent (c) FOLFOXIRI-bevacizumab (BV) vs FOLFOX-BV for first-line (1L) treatment (tx) of patients with metastatic colorectal cancer (mCRC). J Clin Oncol. 2017;35:657. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Schmoll HJ, Meinert FM, Cygon F, Garlipp B, Junghanss C, Leithäuser M, Vogel A, Schaefers M, Kaiser U, Hoeffkes H-G. “CHARTA”: FOLFOX/Bevacizumab vs FOLFOXIRI/Bevacizumab in advanced colorectal cancer—Final results, prognostic and potentially predictive factors from the randomized Phase II trial of the AIO. J Clin Oncol. 2017;35:3533. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2412] [Article Influence: 268.0] [Reference Citation Analysis (31)] |
| 5. | Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 6. | Cremolini C, Antoniotti C, Lonardi S, Bergamo F, Cortesi E, Tomasello G, Moretto R, Ronzoni M, Racca P, Loupakis F. Primary Tumor Sidedness and Benefit from FOLFOXIRI plus Bevacizumab as Initial Therapy for Metastatic Colorectal Cancer. Ann Oncol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 622] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 8. | Satake H, Sunakawa Y, Miyamoto Y, Nakamura M, Nakayama H, Shiozawa M, Makiyama A, Kobayashi K, Kubota Y, Mori M. A phase II trial of 1st-line modified-FOLFOXIRI plus bevacizumab treatment for metastatic colorectal cancer harboring RAS mutation: JACCRO CC-11. Oncotarget. 2018;9:18811-18820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, Cortesi E, Tomasello G, Spadi R, Zaniboni A. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |