Published online Dec 15, 2018. doi: 10.4251/wjgo.v10.i12.522
Peer-review started: September 13, 2018
First decision: October 11, 2018
Revised: November 2, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 15, 2018
Processing time: 92 Days and 19 Hours
Mesenteric lymphangioma (ML) in adults is a very rare disease. We report six hospitalized adult patients with ML in our hospital between January 2013 and July 2018 to investigate the characteristics and prognosis of ML in adults.
The male-to-female ratio was 3:3, and the median age at diagnosis was 55.2 years. Clinical manifestations varied; however, most were acute cases (5/6). No history of trauma was reported. None (0/6) of the patients were accurately diagnosed with ML in the emergency and outpatient departments. Mesenteric cysts were identified in four patients (66.7%) by abdominal ultrasound and in five patients (83.3%) by computed tomography. ML was postoperatively confirmed by pathology. Most MLs (4/6) were associated with infection of other systems. ML was located in the mesentery of the small intestine (n = 4), ileum (n = 1) and rectum (n = 1). Cyst fluid was clear (n = 4), chylous (n = 1) and bloody (n = 1). Surgical procedures included complete tumor removal and partial intestinal excision (n = 6). Recurrence and adhesive intestinal obstruction were not observed during the 3-12 mo follow-up period.
ML in adults is a rare benign acquired disease that can be cured by surgical treatment. Infection may be a cause of ML.
Core tip: Mesenteric lymphangioma (ML) is a rare congenital lymphangioma that predominantly affects children. We reported six cases of adult patients with ML and reviewed the literature. The report is helpful in comprehensively understanding the characteristics and prognosis of ML in adults and arousing the clinician’s attention to this disease.
- Citation: Chen J, Du L, Wang DR. Experience in the diagnosis and treatment of mesenteric lymphangioma in adults: A case report and review of literature. World J Gastrointest Oncol 2018; 10(12): 522-527
- URL: https://www.wjgnet.com/1948-5204/full/v10/i12/522.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i12.522
Mesenteric lymphangioma (ML) is a rare congenital lymphangioma of uncertain etiology that predominantly occurs in children. ML lacks specific clinical signs and symptoms, and patients are often admitted to the hospital due to complications, such as abdominal pain, abdominal distension, intestinal obstruction and other acute abdominal manifestations. In adults, it is often found coincidentally during auxiliary examinations or even exploratory abdominal laparotomy, leading to passive surgery or surgical preparation, sometimes missing the best surgical opportunity due to delayed diagnosis. Cases and misdiagnosed cases have been reported in previous studies[1-3]. However, these studies mainly focused on imaging. Here, we retrospectively analyzed six adults with ML confirmed by pathological examination and admitted between January 2013 and July 2018. Our findings may improve our understanding of this disease and provide more clinical references for early and correct treatment.
Case 1: A 45-year-old man with abdominal pain for 16 h.
Case 2: A 59-year-old man with severe abdominal pain, no defecation and exhaustion for 3 d.
Case 3: A 62-year-old man with abdominal pain, nausea and fever for 3 h.
Case 4: A 71-year-old woman with abdominal pain, fever, diarrhea, and gastrointestinal bleeding for 1 wk.
Case 5: A 42-year-old woman with abdominal distension for 2 years that had recurred for 1 d.
Case 6: A 52-year-old woman with mild abdominal pain and distension, nausea and fever for more than 1 d.
None of the patients had a significant history of trauma. Most MLs (4/6) were associated with infection of other systems. Case 1: cholecystitis; Case 2: no other systemic co-infection was found; Case 3: cholangitis; Case 4: urinary tract infection; Case 5: no other systemic co-infection was found; Case 6: Gastrointestinal tract infection, acute gastritis and colitis.
The history of symptoms ranged from 3 h to 2 yr. Case 3 had a medical history of cholangiolithiasis and endoscopic retrograde cholangiopancreatography, Case 5 had an untreated abdominal cyst 2 years previously and diabetes for more than 5 years and was admitted to our hospital due to acute abdominal distension. All other cases were admitted to the emergency department for acute abdominal pain without history of other chronic diseases.
Case 2: middle and upper abdominal tenderness and rebound tenderness, no muscle guarding; bowel sound was high pitched tinkling. Case 4: periumbilical tenderness, no rebound tenderness and muscle guarding, a palpable mass of about 10 cm in diameter was detected when abdominal pain occurred, but disappeared when abdominal pain was relieved. All other cases showed tenderness in different parts of the abdomen, no rebound tenderness and muscle guarding, and bowel sounds were normal.
Tumor markers were normal in all cases, and laboratory tests indicated increased white blood cell to different degrees. In addition, Case 2 and 3 showed a slight increase in alanine aminotransferase and γ-glutamyl transpeptidase. Case 4 and Case 6 indicated fecal occult blood (+), and fecal bacteria cultures were negative.
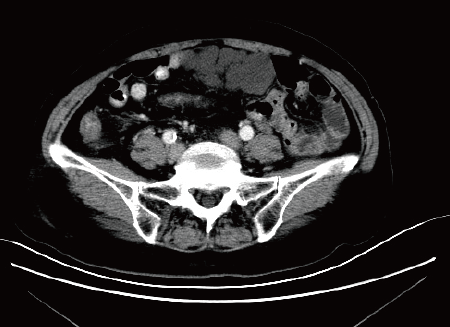
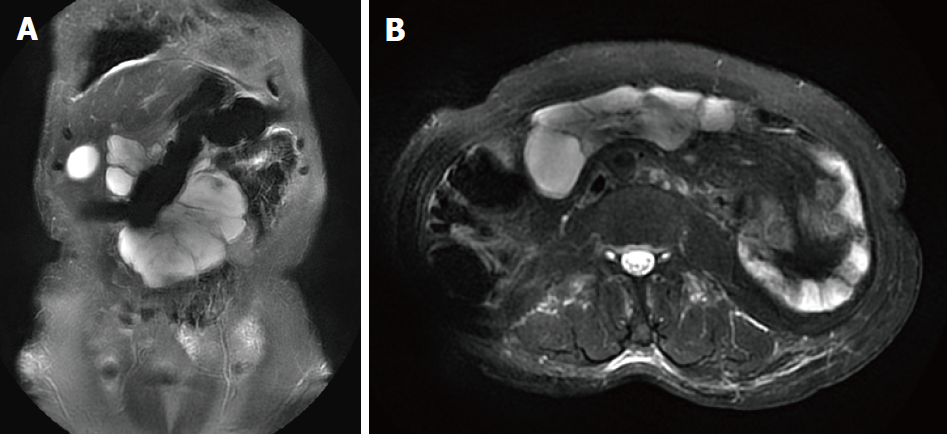
All patients underwent abdominal ultrasound and abdominal computed tomography/magnetic resonance imaging (commonly known as CT/MRI) examination after admission (Figures 1 and 2), and some patients had abdominal X-ray examination. In one patient with acute intussusception and one patient with diarrhea, abdominal X-rays showed fluid and an incomplete intestinal obstruction. In other patients with acute abdominal pain, abdominal X-ray showed no obvious abnormalities. Five patients were found to have an intraperitoneal cystic or solid cystic mass by CT/MRI. Abdominal ultrasonography failed to detect an abdominal cyst in Case 3 and Case 4. Preoperative ultrasound and CT failed to detect an abdominal cyst in Case 3.
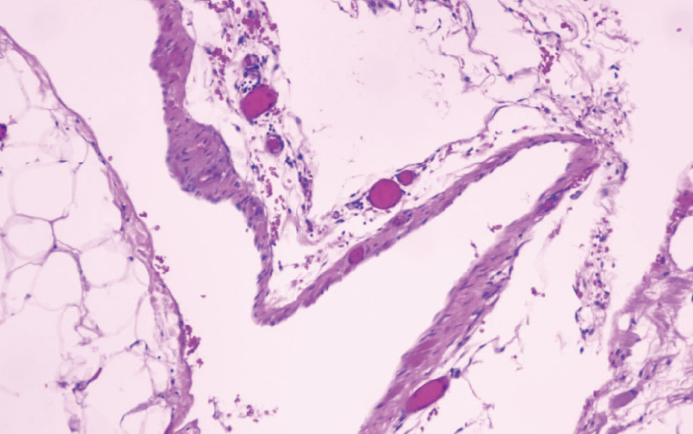
All cysts were examined by pathology after operation, and all were ML (Figure 3).
Case 6 of a suspected rectal tumor and Case 5 of an intra-abdominal benign cyst chose elective surgery, and the remaining four patients underwent emergency surgery within 48 h of hospital admission. Intraoperative ML was identified in the jejunum in four cases, the ileum in one case, and the rectum in one case. Four cases had clear cystic fluid, one case had chylous fluid and one case had bloody fluid. Tumor invasion was noted in the bile duct in one case, the duodenum in one case, the transverse colon in one case, and the rectum in one case. All six cases underwent complete removal of the tumor and partial intestinal excision, ranging from 6 to 50 cm.
Six patients were included in this study. Patient information and clinical manifestations are shown in Table 1. Of the six patients, three were male and three were female, aged 42-71 years, with an average age of 55.2 years. The clinical manifestations of ML included acute abdominal pain, acute intestinal obstruction (vomiting, abdominal distension, no defecation and exhaustion), fever, diarrhea, and gastrointestinal bleeding. The initial diagnoses included: one case of acute intussusception, three cases of abdominal tumors (mesenteric lipoma, duodenal and rectum tumor), one case of acute cholangitis, and one case of acute hemorrhagic enteritis. All six patients were diagnosed with ML by pathology following surgery. The accuracy of initial diagnosis was zero (0/6). The diagnostic accuracy of ultrasound for mesenteric cyst was 66.7% (4/6). The diagnostic accuracy of CT/MRI for mesenteric cyst was 83.3% (5/6).
| Cases | 1 | 2 | 3 | 4 | 5 | 6 |
| Gender | M | M | M | F | F | F |
| Age, yr | 45 | 59 | 62 | 71 | 42 | 52 |
| Abdominal pain | + | + | + | + | - | + |
| Nausea | - | - | + | - | - | + |
| Vomiting | - | + | - | - | - | - |
| Diarrhea | - | - | - | + | - | - |
| GI bleeding | - | - | - | + | - | - |
| Fever | - | - | + | + | - | + |
| Concurrent infection | Cholecystitis | UK | Cholangitis | Urinary tract | UK | GI tract |
| Abdominal distension | - | - | + | + | + | + |
| Intestinal obstruction | - | + | - | - | - | - |
| Medical history | - | - | Cholangiolithiasis, ERCP | - | Diabetes | - |
| Trauma | - | - | - | - | - | - |
All of the patients had good postoperative recovery, the abdominal mass disappeared, appetite, defecation and urine output became normal. All patients were followed up for 3 mo by abdominal CT, and no recurrence or adhesive intestinal obstruction occurred. Five cases (83.3%) were followed for up to one year, and no recurrences were observed.
Lymphangiomas are uncommon congenital malformations of the lymphatic system. They can occur at any site in the body, but are most commonly found in the neck and head area as well as the abdominal wall, but rarely in the mesentery[4]. It is reported that the incidence of ML is approximately 5%, and the male-female ratio is about 1.5-3:1[5,6]. In addition, ML has been described in less than 1% of all lymphangiomas[1,7,8].
The exact etiology of ML is unknown. It is likely to be a developmental anomaly of the lymphatic system, as 65% of MLs are present at birth and 90% of all patients are diagnosed before the age of 2[7]. However, they can also develop due to an inflammatory process, lymphatic obstruction, surgery, radiation and abdominal trauma[9,10]. ML is rarely seen in adults. Therefore, it is not clear what the incidence of ML is in adults. Only a few case reports of ML in adults are available in the published literature[11]. In adults, lymphangiomas primarily occur on the body surface or in the abdominal cavity, and the incidence of ML is 1/100000[2], mostly in the small intestine, followed by the omentum majus, mesentery and retroperitoneum. ML is a benign lesion, with a relatively asymptomatic onset, slow growth and a long disease course.
Most MLs are initially asymptomatic, and are therefore usually discovered incidentally. However, when the tumor is large, it can compress the surrounding viscera or block the intestine, producing corresponding symptoms. The clinical symptoms of ML vary depending on location. ML can manifest as abdominal pain, abdominal distension, diarrhea, hematochezia, constipation, hypoproteinemia, intussusception, and decreased physical quality[12]. Due to the lack of specific clinical signs and symptoms, ML is easily missed and misdiagnosed. Abdominal ultrasonography or CT can be used to identify an early abdominal cystic mass, especially in patients with recurrent abdominal pain, abdominal distension, and stubborn constipation. Emergency doctors should be vigilant, and early abdominal ultrasound or CT examination should be carried out to detect this disease as soon as possible.
Ultrasonography is of high diagnostic value in detecting the location, size, division of the cyst, cyst fluid, cyst wall and its relation to surrounding tissues[13]. In small cysts, abdominal CT is more sensitive and helps to differentiate from other related abdominal pelvic cysts, such as greater omentum cysts, intestinal repetitive malformations, ovarian cysts, common bile duct cysts, and kidney cysts. CT is useful for further understanding the relationship between the cyst and the surrounding tissues and organs, especially large blood vessels and the bowel, which ultimately aids treatment decisions and surgical approaches in these patients[14]. MRI is more sensitive in patients with intracavitary hemorrhage. In the case of intrathecal hemorrhage, the imaging may show solid cystic signs[15,16].
Pathological examination is the gold standard for the diagnosis of ML, and it provides strong evidence for postoperative identification of other types of cysts. Microscopy shows dilated lymphatics, and the thin wall lining epithelial cells in the lymphatic cavity gap and a small amount of smooth muscle tissue can be seen. During infection, the infiltration of lymphocytes, plasma cells, eosinophils and other inflammatory cells are also visible. MLs are classified as simple, cavernous and cystic. The simple type of ML is primarily situated superficially in the skin and is composed of small thin-walled lymphatic vessels. The cavernous type has dilated lymphatic vessels and has connections with normal adjacent lymphatics. Cystic lymphangioma is composed of large macroscopic lymphatic spaces surrounded by collagen and smooth muscle and does not have connections with adjacent normal lymphatics (CD34 and CD31 positive)[17].
ML needs to be differentiated from peritoneal abscess, hematoma, malignant tumor center liquefaction necrosis, malignant tumor cystic adenoma and some solid masses derived from mesenchymal tissue such as sarcomas. Simple abdominal ultrasonography may be difficult to distinguish, and abdominal CT/MRI can provide more information. However, the diagnosis of ML primarily depends on pathological diagnosis. When necessary, ultrasound-guided diagnostic puncture can further differentiate between cyst, abscess and hematoma. Ultrasound-guided needle aspiration cytology can identify whether the mass is benign or malignant, providing a basis for differential diagnosis.
To treat ML, most doctors recommend radical surgical excision, as ML can grow very large and invade adjacent structures, develop complications and the risk of sarcoma transformation upon irradiation[9]. After excision of a mass that involves the whole mesentery, internal herniation is likely to occur due to the presence of skeletonized vessels. A biological collagen implant can be used to repair the mesenteric defect after excision of a large ML, and monitoring for recurrence during follow-up is necessary[18]. However, opinions differ about the course of treatment.
A previous study[19] showed that for asymptomatic or mild lymphangioma patients, conservative treatment and close follow-up are recommended. In a case report, colorectal lymphangioma spontaneously disappeared. We suggest that if the ML is relatively large, it is possible to infiltrate the surrounding organs and cause tissue ischemic necrosis, causing potentially life-threatening complications, such as traumatic rupture, anemia secondary to intraabdominal or intra-cavitary bleeding, intestinal gangrene secondary to volvulus and intermittent intestinal obstruction[2,16]. It is better to remove it as soon as possible.
In the present study, the ratio of men to women was 3:3, with no significant gender difference. The rate of accurate initial diagnosis was zero. This indicated that clinicians lack awareness of ML in adults, and the average age at diagnosis in these patients was 55.2 years. All of these patients had received routine physical examination in the past few years, but no abdominal cysts were found. Five of these six cases had no previous history of abdominal cysts, but all patients had acute onset, mainly presenting with acute abdominal pain, incomplete intestinal obstruction, and mucinous bloody diarrhea. The remaining patient was found to have abdominal cysts two years previously, which were untreated. Acute abdominal distension was not treated with anti-inflammatory therapy and was diagnosed and cured after surgery.
Following symptom onset, the abdominal cysts were not identified by abdominal color Doppler ultrasound or CT. However, the diagnosis of abdominal cysts was confirmed during laparotomy. It is speculated that adult ML may be an acquired disease and that infection may be a risk factor. Of these six cases, two had biliary tract infection, one had urinary tract infection, one had digestive tract infection, and two patients had no obvious infection. The pathogenesis of ML may include the formation of secondary cysts caused by lymphatic obstruction due to infection. Moreover, we found that the detection rate of abdominal cysts by ultrasound was lower than that of abdominal CT (66.7% vs 88.3%). This may have been due to the following possibilities: (1) due to the rarity of ML in adults, ultrasound clinicians lacked awareness of ML, resulting in misdiagnosis; (2) misdiagnosed cystic lesions such as ascites or dilated bowel may be caused by intestinal obstruction; and (3) ML may be an acquired disease.
As clinicians are accustomed to prescribing abdominal ultrasound first and then CT, an abdominal cyst may not yet have formed when ultrasonography is performed. Pathological examination is the gold standard in the diagnosis of ML. An accurate and thorough surgical technique is an effective method of treating ML. No recurrence was found during the follow-up period of 3-12 mo in the six patients included in this study. However, timely surgery is essential. In a patient with mucinous diarrhea, infiltration of the transverse colon was found during surgery, leading to transverse colonic ischemic necrosis, and partial transverse colectomy was performed.
In summary, ML in adults is an extremely rare benign, potentially acquired disease distinct from ML in children, which is due to congenital lymphatic dysplasia. Infection may be involved in the pathogenesis of ML in adults. However, the exact etiology should be confirmed in a large sample study. In some cases, ML can be fatal, as it may cause tissue ischemic necrosis due to infiltration of surrounding viscera, compression of the intestine or peritoneal vessels, resulting in serious complications. Timely and effective radical surgery is necessary, which requires increased awareness of the disease in clinicians to avoid misdiagnosis and missed diagnosis.
Although ML occurs more frequently in children, it also occurs in adults, but the exact etiology in adults requires further study. Although ML is benign, it can also lead to serious and fatal consequences. Timely and effective radical surgery can cure this disease. Clinicians should raise awareness of ML in adults.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aykan NF, Ciocalteu A S- Editor: Dou Y L- Editor: Filipodia E- Editor: Song H
| 1. | Suthiwartnarueput W, Kiatipunsodsai S, Kwankua A, Chaumrattanakul U. Lymphangioma of the small bowel mesentery: a case report and review of the literature. World J Gastroenterol. 2012;18:6328-6332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Losanoff JE, Kjossev KT. Mesenteric cystic lymphangioma: unusual cause of intra-abdominal catastrophe in an adult. Int J Clin Pract. 2005;59:986-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Chen CW, Hsu SD, Lin CH, Cheng MF, Yu JC. Cystic lymphangioma of the jejunal mesentery in an adult: a case report. World J Gastroenterol. 2005;11:5084-5086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Okazaki T, Iwatani S, Yanai T, Kobayashi H, Kato Y, Marusasa T, Lane GJ, Yamataka A. Treatment of lymphangioma in children: our experience of 128 cases. J Pediatr Surg. 2007;42:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Fernández Ibieta M, Rojas Ticona J, Martinez Castaño I, Reyes Rios P, Villamil V, Giron Vallejo O, Mendez Aguirre N, Sanchez Morote J, Aranda Garcia MJ, Guirao Piñera MJ. [Mesenteric cysts in children]. An Pediatr (Barc). 2015;82:e48-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Huis M, Balija M, Lez C, Szerda F, Stulhofer M. Mesenteric cysts. Acta Med Croatica. 2002;56:119-124. [PubMed] |
| 7. | Geraci G, Sciumè C, Pisello F, Volsi FL, Facella T, Tinaglia D, Arnone E, Modica G. Mesenteric cyst lymphangioma; a case report and literature review. Ann Ital Chir. 2006;77:521-527; discussion 528. [PubMed] |
| 8. | Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH Jr, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics. 2011;31:949-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Losanoff JE, Richman BW, El-Sherif A, Rider KD, Jones JW. Mesenteric cystic lymphangioma. J Am Coll Surg. 2003;196:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Iwabuchi A, Otaka M, Okuyama A, Jin M, Otani S, Itoh S, Sasahara H, Odashima M, Kotanagi H, Satoh M. Disseminated intra-abdominal cystic lymphangiomatosis with severe intestinal bleeding. A case report. J Clin Gastroenterol. 1997;25:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Wani I. Mesenteric lymphangioma in adult: a case series with a review of the literature. Dig Dis Sci. 2009;54:2758-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Kim TO, Lee JH, Kim GH, Heo J, Kang DH, Song GA, Cho M. Adult intussusception caused by cystic lymphangioma of the colon: a rare case report. World J Gastroenterol. 2006;12:2130-2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Zhu LX, Guan BY, Yu XL, He XH, Fang Q. Ultrasonographic characteristics of abdominal cystic lymphangioma in Children. Nanchang Daxue Xuebao (Yixueban). 2014;54:59-61. |
| 14. | Mao XN, Lu ZM, Liao W, Wen F, Guo QY. CT findings of intra-abdominal lymphangioma in children. Zhongguo Linchuang Yixue Yingxiang Zazhi. 2013;24:485-488. |
| 15. | Pampal A, Yagmurlu A. Successful laparoscopic removal of mesenteric and omental cysts in toddlers: 3 cases with a literature review. J Pediatr Surg. 2012;47:e5-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Kim SH, Kim HY, Lee C, Min HS, Jung SE. Clinical features of mesenteric lymphatic malformation in children. J Pediatr Surg. 2016;51:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Enzinger FM, Weiss SW. Tumors of the lymph vessels. Enzinger FM, Weiss SW, eds . Soft Tissue Tumors. St. Louis, MO: Mosby Publishers 1994; 679-700. |
| 18. | Kim SH, Yoon KC, Lee W, Kim HY, Jung SE. Result of using a biologic collagen implant (Permacol) for mesenteric defect repair after excision of a huge mesenteric lymphangioma in a child. Ann Surg Treat Res. 2015;89:330-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Lee JM, Chung WC, Lee KM, Paik CN, Kim YJ, Lee BI, Cho YS, Choi HJ. Spontaneous resolution of multiple lymphangiomas of the colon: a case report. World J Gastroenterol. 2011;17:1515-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |