Published online Dec 15, 2018. doi: 10.4251/wjgo.v10.i12.496
Peer-review started: August 8, 2018
First decision: October 4, 2018
Revised: October 19, 2018
Accepted: November 23, 2018
Article in press: November 24, 2018
Published online: December 15, 2018
Processing time: 129 Days and 20.1 Hours
To evaluate the efficacy of main portal vein stents combined with iodine-125 (125I) to treat main portal vein tumor thrombus.
From January 1, 2010 to January 1, 2015, 111 patients were diagnosed with liver cancer combined with main portal vein tumor thrombus. They were non-randomly assigned to undergo treatment with transarterial chemoembolization (TACE)/transarterial embolization (TAE) + portal vein stents combined with 125I implantation (Group A) and TACE/TAE + portal vein stents only (Group B). After the operation, scheduled follow-up was performed at 6, 12 and 24 mo. The recorded information included clinical manifestations, survival rate, and stent restenosis rate. Kaplan–Meier curves, log-rank test and Cox regression were used for data analyses.
From January 1, 2010 to January 1, 2015, 54 and 57 patients were allocated to Groups A and B, respectively. The survival rates at 6, 12 and 24 mo were 85.2%, 42.6% and 22.2% in Group A and 50.9%, 10.5% and 0% in Group B. The differences were significant [log rank P < 0.05, hazard ratio (HR): 0.37, 95%CI: 0.24-0.56]. The rates of stent restenosis were 18.5%, 55.6% and 83.3% in Group A and 43.9%, 82.5% and 96.5% in Group B. The differences were significant (log rank P < 0.05, HR: 0.42, 95%CI: 0.27-0.63). Cox regression identified that treatment was the only factor affecting survival rate in this study.
Main portal vein stents combined with 125I can significantly improve survival rate and reduce the rate of stent restenosis.
Core tip: This study evaluated the efficacy of stents combined with iodine-125 (125I) to treat main portal vein tumor thrombus and its complications. 125I was placed between the stent and tumor thrombus, and not in the form of particle strands. In this way, the quantity and position of 125I could be flexibly adjusted. Transarterial chemoembolization or transarterial embolization was used as basic treatment. Patients with liver cancer and main portal vein tumor thrombus were non-randomly assigned to undergo portal vein stents combined with 125I implantation or portal vein stents only. Portal vein stent combined with 125I significantly improved survival rate and reduced stent restenosis.
- Citation: Wu YF, Wang T, Yue ZD, Zhao HW, Wang L, Fan ZH, He FL, Liu FQ. Stents combined with iodine-125 implantation to treat main portal vein tumor thrombus. World J Gastrointest Oncol 2018; 10(12): 496-504
- URL: https://www.wjgnet.com/1948-5204/full/v10/i12/496.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i12.496
Liver cancer is a common malignant tumor[1], and it decreases patient quality of life[2,3]. Tumor thrombus in the main portal vein indicates late-stage disease. The treatment for portal vein tumor thrombus includes surgery and radiotherapy[4,5]. However, the overall effect is limited. In recent years, radioactive iodine-125 (125I) particles have been used to treat portal vein tumor thrombus to effectively decrease tumor thrombus volume and improve patient survival rates[6]. However, 125I was implanted in particle strands in those studies. This limits the amount of 125I implanted and the ability to reposition the 125I, which restrains the clinical use of 125I. We studied the clinical effect of 125I combined with main portal vein stents when the 125I was placed between the tumor thrombus and the stents. This method avoids the above disadvantages and has never been previously reported.
This was a nonrandomized controlled trial in which we compared transarterial chemoembolization (TACE)/transarterial embolization (TAE) + main portal vein stents combined with 125I implantation and TACE/TAE + main portal vein stents only for the treatment of liver cancer with main portal vein tumor thrombus and portal hypertension.
Inclusion criteria were as follows: (1) Liver cancer according to histological, cytological, or clinical diagnostic standards that conformed to the rules of diagnosis and treatment of primary hepatocellular carcinoma, 2011; (2) Main stem tumor thrombus of portal vein confirmed through biopsy (70%) or imaging, without tumor thrombus in the branches; (3) Clear indication of percutaneous liver puncture and main portal vein stent implantation; (4) Clear TACE or TAE treatment indication; (5) Age 18–70 years; and (6) No serious complications of portal hypertension, and only a small amount of ascites without bleeding or other complications. Exclusion criteria were: (1) Patients with serious disorders of the heart, lung, kidney, brain, or other important organs; (2) Active infection; (3) Women in pregnancy or lactation; (4) Life expectancy < 3 mo; and (5) Patients who could not cooperate with treatment and observation.
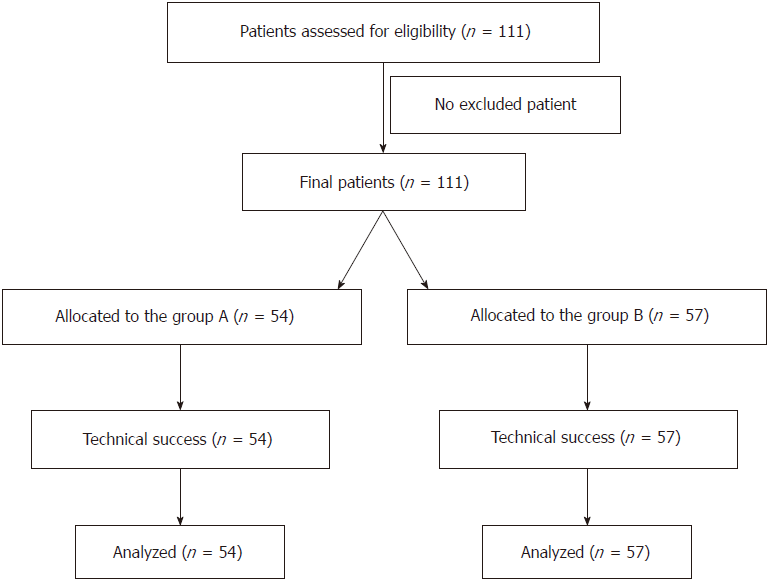
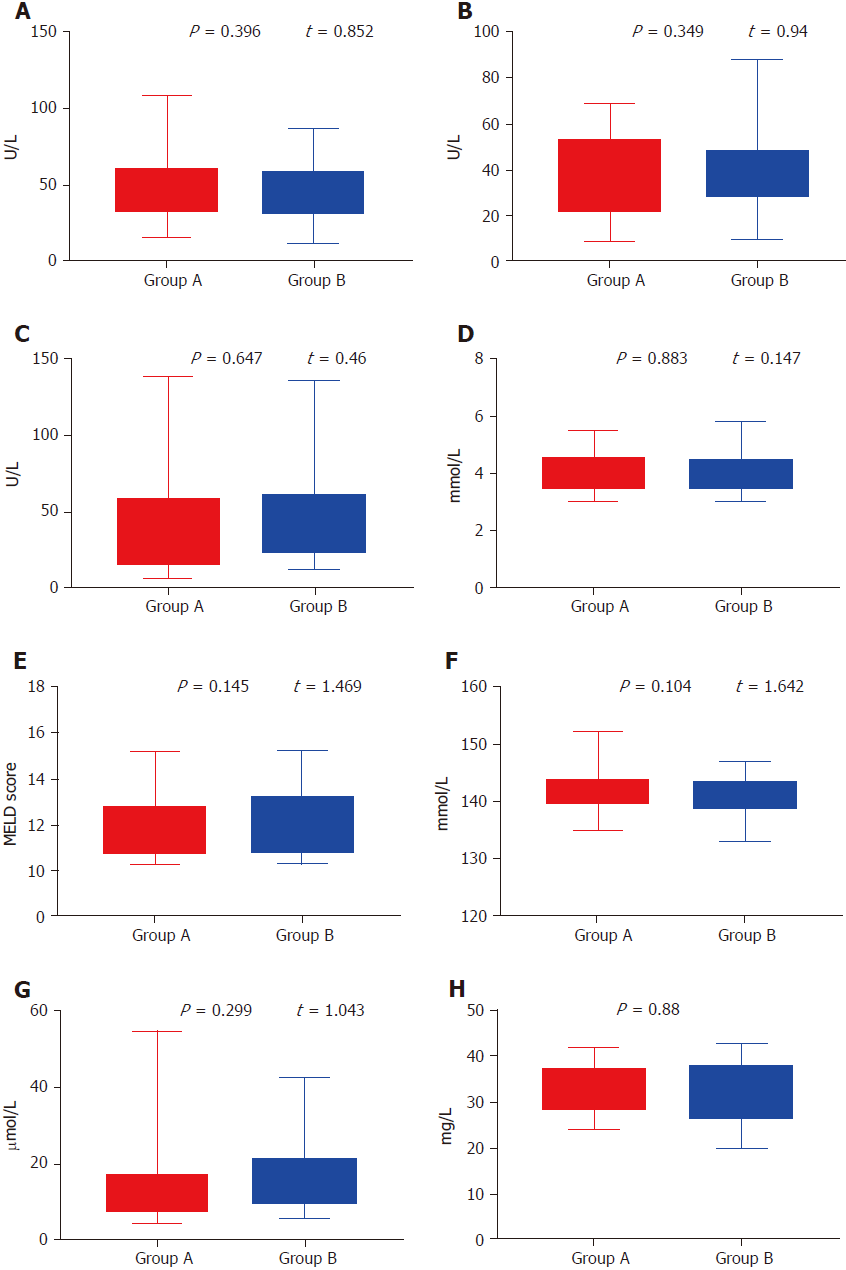
One hundred and eleven patients with main portal vein tumor thrombus were non-randomly assigned to undergo treatment with TACE/TAE + main portal vein stents combined with 125I implantation or TACE/TAE + main portal vein stents alone from January 1, 2010 to January 1, 2015. There were 73 cases of hepatitis B cirrhosis, 21 cases of hepatitis C cirrhosis, seven cases of alcoholic cirrhosis, three cholestatic cirrhosis cases, three autoimmune liver cirrhosis cases and four cases of cirrhosis from other causes. Twenty-three patients were diagnosed with primary carcinoma of the liver by percutaneous liver biopsy, whereas 88 patients were diagnosed by ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), serum α-fetoprotein levels and hepatic artery angiography. Imaging examination confirmed main portal vein tumor thrombus in all patients. According to the preoperative Child–Pugh classification, there were 49 cases of Class A, 62 cases of Class B, and zero class C cases. There were 20 patients with mild ascites and 91 without ascites. The flow chart is shown in Figure 1. Comparison between the two groups is shown in Table 1 and Figure 2.
| Group A (n = 54) | Group B (n = 57) | P value | |
| Gender | 0.693 | ||
| Male | 35 (64.8) | 39 (68.4) | |
| Female | 19 (35.2) | 18 (31.6) | |
| Average age (yr) | 43.6 ± 6.9 | 44.3 ± 5.2 | 0.697 |
| Pathogenesis | 0.788 | ||
| Hepatitis B | 35 (64.8) | 38 (66.7) | |
| Hepatitis C | 9 (16.7) | 12 (21) | |
| Alcoholic | 5 (9.3) | 2 (3.5) | |
| Cholestasis | 2 (3.7) | 1 (1.8) | |
| Autoimmunity | 1 (1.8) | 2 (3.5) | |
| Others | 2 (3.7) | 2 (3.5) | |
| Child–Pugh classification | 0.705 | ||
| A | 25 (46.3) | 24 (42.1) | |
| B | 29 (53.7) | 33 (57.9) | |
| C | 0 (0) | 0 (0) | |
| Albumin (g/L) | 34.5 ± 7.5 | 31.5 ± 11.5 | 0.880 |
| Alanine aminotransferase (U/L) | 62.5 ± 46.5 | 49.5 ± 37.5 | 0.396 |
| Glutamyl transpeptidase (U/L) | 73 ± 66 | 74 ± 62 | 0.647 |
| Na+ | 143.5 ± 8.5 | 140 ± 7 | 0.104 |
| K+ | 4.12 ± 1.08 | 4.75 ± 1.05 | 0.883 |
| Direct bilirubin (μmol/L) | 29.8 ± 25.2 | 24.5 ± 18.5 | 0.299 |
| Aspartate aminotransferase (U/L) | 39 ± 30 | 49 ± 39 | 0.349 |
| MELD score | 11.96 ± 1.68 | 12.76 ± 2.47 | 0.145 |
| Ascites | 0.624 | ||
| Yes | 11 (20.4) | 9 (15.8) | |
| No | 43 (79.6) | 48 (84.2) | |
| Size of liver cancer (cm) | 0.788 | ||
| ≤ 5 | 14 (25.9) | 17 (29.8) | |
| 5-8 | 31 (57.4) | 29 (50.9) | |
| > 8 | 9 (16.7) | 11 (19.3) | |
| No. of liver tumors | 0.834 | ||
| 1 | 28 (51.9) | 32 (56.1) | |
| 2 or 3 | 19 (35.2) | 17 (29.8) | |
| > 3 | 7 (12.9) | 8 (14.1) |
Before the operation, liver function tests, blood coagulation tests, routine blood tests, electrocardiography, CT, and/or MRI, color Doppler ultrasonography, and esophageal radiography were performed. In addition, gastroscopy was performed when necessary. Patients’ coagulation function was corrected to the normal range. The operation-related concerns were explained to the patients and their family members, and they were asked to give signed informed consent. This study was approved by the Institutional Review Board of Beijing Shijitan Hospital and conducted in accordance with all current ethical guidelines.
Patients were assigned to receive percutaneous transhepatic and portal vein covered stents (Fluency, Bard, Tempe, AZ, United States) with local anesthesia at the puncture site. A puncture device (NPAS-100; Cook, Indianapolis, IN, United States), which included a puncture needle, venous sheath and guide wire, was passed from the right hypochondriac region to the portal vein. After that, a pigtail catheter was advanced through the NPAS-100 to the distal end of the splenic vein or superior mesenteric vein to measure the portal vein pressure and conduct venography. The pigtail catheter was removed, and the stent was implanted through the vein sheath. A 10-mm covered stent was implanted. Portal vein pressure measurements before and after stent placement allowed assessment of the success of the procedure. The hepatic puncture passage was blocked during catheter removal to avoid intraperitoneal or thoracic hemorrhage.
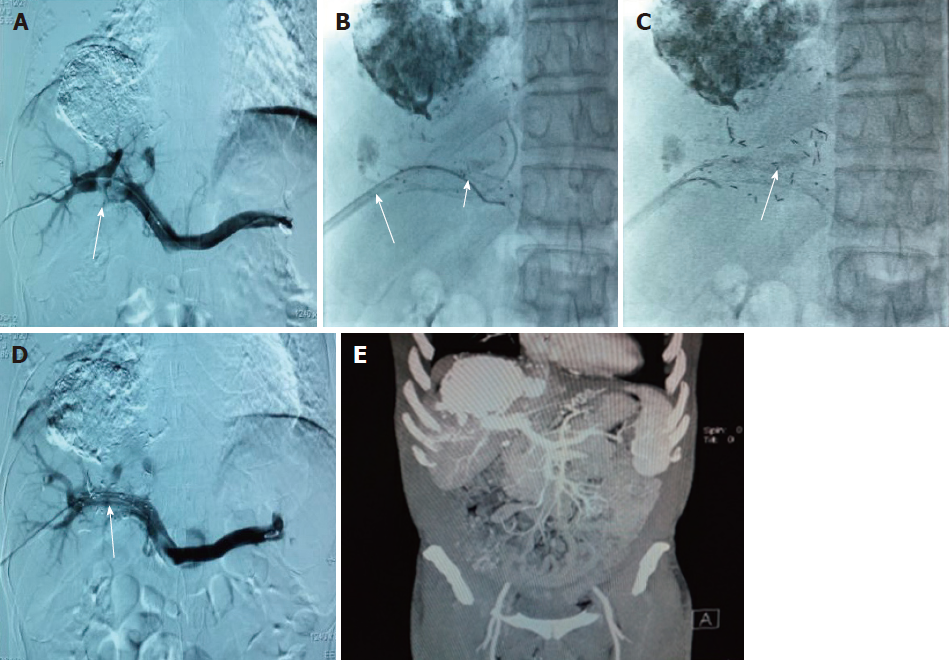
In Group A, the patients received treatment with percutaneous transhepatic and portal vein covered stent implantation, like the patients in Group B. After measurement of portal vein pressure and conduction of venography, the pigtail catheter was removed, and a guide wire was inserted through the venous sheath of the NPAS-100. Because the NPAS-100 had one guide wire, there were two guide wires in the main portal vein. The venous sheath was drawn out and inserted into the portal vein again along one of the guide wires. Another guide wire was placed between the tumor thrombus and venous sheath. Stents were implanted through the venous sheath. A catheter was inserted through the guide wire that was between the tumor thrombus and venous sheath. The catheter between the stent and the tumor thrombus was linked to the particle release gun. The catheter was slowly retracted, and 125I (Tong Fu, Beijing, China) was simultaneously released through the catheter up to the portal vein trunk and tumor thrombus. The radioactive particles were arranged as neatly as possible in all tumor thrombi. After implantation, portal vein pressure was measured and venography was conducted. These particles could emit characteristic electrons and photons through the recession of the electron capture surface. The electrons were absorbed by the titanium alloy wall of the sealed seeds of 125I. The photons mainly emitted X rays of 27.4 and 31.4 keV as well as γ rays of 35.5 keV. The pictures taken during the operation are shown in Figure 3.
Patients in Group A were treated with TACE according to the position of the lesion and its blood supply. The embolization agent was 3–30 mL iodinated oil. The chemotherapeutics included 10–20 mg pirarubicin and 5–15 mg hydroxycamptothecine. Patients in Group B were treated with TAE to reduce damage to liver function. The embolization agent was 5–25 mL iodinated oil.
For the basic technical operation, a needle was passed from the right or left femoral artery to the hepatic artery followed by hepatic arteriography. A catheter was placed in the direct blood supply artery of the tumor as close to the focus as possible, and embolization and infusion of chemotherapeutic drugs were performed. The interval and number of treatments depended on tumor size, arterial status and liver function status. The interval was usually once every 1–6 mo. In Group A, 25 and 29 patients were treated with TACE and TAE, respectively, while 24 and 33 patients in Group B were treated with TACE and TAE, respectively, with no significant difference in patient numbers (P = 0.705).
Patients with lesions ≤ 5 cm in size and with a rich blood supply underwent TACE or TAE first and radiofrequency ablation after 3–5 d. Patients whose lesion was > 5 cm underwent TACE or TAE once or several times and then radiofrequency ablation when the imaging showed that the lesions no longer had blood supply from the hepatic artery or when the catheter could not enter the artery supplying the lesion. Finally, all patients underwent radiofrequency ablation (WHK-IB; Weaver Electronics, Beijing, China).
Low molecular weight heparin (5000 IU, twice daily) was subcutaneously injected for 5 d, and then warfarin was administered for one year. Coagulation function of each patient was examined every 15 d to ensure that the International Normalized Ratio ranged from 2 to 3.
Scheduled follow-up was performed at 6, 12 and 24 mo postoperatively. The recorded information included clinical manifestations, survival rate, physical examination, stent restenosis evaluation (through ultrasound and endoscopy) and laboratory tests. Telephone follow-up was performed to record patient conditions and details of relevant clinical events.
Continuous variables are presented as mean ± median and were compared by independent-sample or paired-sample t test. Categorical and ordinal variables are presented as frequencies or percentages and compared using χ2 test. Time-to-event outcomes were evaluated with Kaplan–Meier curves and log-rank tests. Cox regression model was used to identify independent predictors. Unbalanced factors between groups were treated as covariates. Statistical analysis was performed using IBM SPSS Statistics version 22.0 (IBM, Chicago, IL, United States) and GraphPad Prism version 7.0 (GraphPad Software, La Jolla, CA, United States). Follow-up investigators and statisticians had access to all of the data and vouched for the integrity of the data analyses.
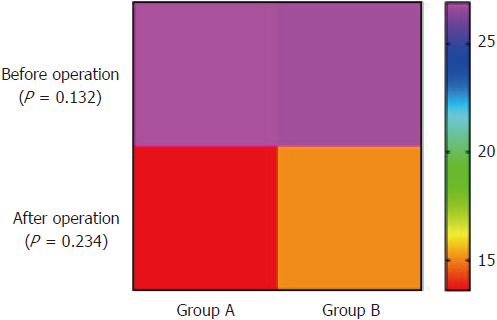
The portosystemic pressure gradient (PPG) in Group A decreased from 26.9 ± 6.22 to 13.6 ± 6.4 mmHg (t = 18.11, P < 0.05) after operation. The PPG before and after operation were significantly different. The PPG in Group B decreased from 26.77 ± 6.25 to 15.1 ± 7.2 mmHg (t = 17.1, P < 0.05). The PPG before and after operation were significantly different (Table 2). The pre-operative PPG was not significantly different between the two groups (t = 1.52, P = 0.132). The post-operative PPG was also not significantly different between the two groups (t = 1.20, P = 0.234) (Figure 4).
| PPG before operation (mmHg) | PPG after operation (mmHg) | t | df | P value | |
| Group A | 26.9 ± 6.22 | 13.6 ± 6.4 | 18.11 | 53 | < 0.0001 |
| Group B | 26.77 ± 6.25 | 15.1 ± 7.2 | 17.10 | 56 | < 0.0001 |
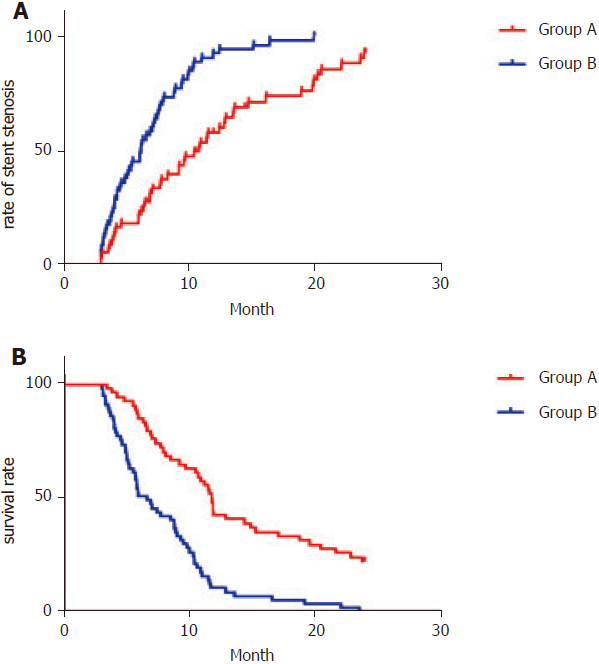
The rates of stent restenosis at 6, 12 and 24 mo were 18.5%, 55.6% and 83.3% in Group A and 43.9%, 82.5% and 96.5% in Group B, which differed significantly [log rank P < 0.05, hazard ratio (HR): 0.42, 95%CI: 0.27–0.63] (Figure 5A and Table 3). The rates of survival at 6, 12 and 24 mo were 85.2%, 42.6% and 22.2%, respectively in Group A and 50.9%, 10.5% and 0%, respectively in Group B, which differed significantly (log rank P < 0.05, HR: 0.37, 95%CI: 0.24–0.56) (Figure 5B and Table 3)
| Group A (n = 54) | Group B (n = 57) | P value | |
| Rate of stent stenosis | < 0.05 | ||
| 6 mo | 18.5 | 43.9 | |
| 12 mo | 55.6 | 82.5 | |
| 24 mo | 83.3 | 96.5 | |
| Survival rate | < 0.05 | ||
| 6 mo | 85.2 | 50.9 | |
| 12 mo | 42.6 | 10.5 | |
| 24 mo | 22.2 | 0 |
Cox regression showed that pathogenesis, tumor number and serum albumin had no significant effect on survival rate. Treatment was the only factor that affected survival rate (Table 4).
| Hazard ratio | 95%CI | P value | |
| Pathogenesis | 1.227 | 0.773–1.948 | 0.385 |
| Albumin (g/L) | 1.266 | 0.829–1.932 | 0.275 |
| Alanine aminotransferase (U/L) | 1.222 | 0.798–1.872 | 0.357 |
| Glutamyl transpeptidase (U/L) | 0.821 | 0.509–1.224 | 0.419 |
| Direct bilirubin (μmol/L) | 2.262 | 0.270–18.96 | 0.452 |
| Aspartate aminotransferase (U/L) | 1.270 | 0.800–2.017 | 0.311 |
| No. of liver tumors | 1.238 | 0.232–19.41 | 0.330 |
With a rapid increase in the number of patients with liver cancer, the incidence of portal vein tumor thrombus is gradually increasing. 125I was reported to have a good therapeutic effect[7]. However, 125I implantation has been in the form of particle strands. This has some disadvantages, such as implantation of a limited number of particles. In addition, the position of 125I cannot be adjusted. No one has studied the clinical effect of 125I combined with main portal vein stents in which the 125I is placed between the tumor thrombus and stents. This procedure could make it easier to adjust the position and amount of 125I. Thus, in the present study, we compared TACE/TAE + main portal vein stents combined with 125I implantation and TACE/TAE + main portal vein stents alone for the treatment of liver cancer patients with main portal vein tumor thrombus and portal hypertension. Overall, our study suggested a benign outcome: (1) 125I combined with stents implanted in the main portal vein significantly improved survival rate and reduced stent restenosis rate; (2) stents implanted in the main portal vein reduced portal vein pressure and relieved clinical symptoms; and (3) TACE/TAE + main portal vein stents combined with 125I implantation was safe and feasible.
The incidence of portal vein tumor thrombus is high, and its treatment includes surgical resection, chemotherapy, and stent[8-10]. Stent implantation of the main portal vein can quickly reduce portal vein pressure, relieve clinical symptoms and improve quality of life[11,12]. In recent years, portal vein stenting combined with 125I implantation has achieved significant effects in treating main portal vein tumor thrombus. Sun et al[13] conducted a study to evaluate the effect of 125I. In their study, the median survival was 147 d. The cumulative survival rates and stent patency rates at 90, 180, and 360 d were 94.1%, 61.8%, and 32.4% and 97.1% (33/34), 76.9% (24/34), and 29.4% (10/34), respectively[13]. However, in previous studies, 125I particles were implanted in the form of particle strands, which has some drawbacks. It is important to find a better method. In our study, the survival rate in Group A was higher than in Group B, and the stent restenosis rate was lower in Group A than in Group B. Cox regression was used to evaluate the effects of various factors on survival and stent restenosis. It showed that pathogenesis, tumor number and serum albumin had no significant effect on survival rate. Treatment was the only factor influencing survival rate. TACE/TAE + main portal vein stents combined with 125I implantation can improve patient survival rate and reduce stent restenosis rate.
Our study had several limitations. First, the radiation dose was not uniformly distributed. Second, the number of patients was small, which may have influenced the accuracy of the results.
In summary, TACE/TAE + main portal vein stents combined with 125I implantation is effective in treating main portal vein tumor thrombus and its complications, improving quality of life and reducing mortality.
Tumor thrombus in the main portal vein indicates late-stage disease. Treatment for portal vein tumor thrombus includes surgery, chemotherapy, radiotherapy, targeted therapy, and proton beam radiation. In recent years, radioactive iodine-125 (125I) particles have been used to treat portal vein tumor thrombus. However, seed implantation in recent studies had some disadvantages. We carried out the present study to explore a new method of seed implantation.
Previously, 125I was implanted in the form of particle strands. This limits the number of 125I particles implanted, and their position cannot be adjusted. In this study, we performed 125I seed implantation combined with stent implantation, placing the particles between the stent and tumor thrombus. The stent could hold the 125I particles, and the method can be widely used in clinical application.
125I has been shown to be effective in treating portal vein thrombosis. The main objective of this study was to determine the efficacy of stents combined with 125I implantation in the treatment of liver cancer accompanied by main portal vein tumor thrombus, as well as the technical feasibility of this method of seed implantation.
Patients were non-randomly assigned to undergo treatment with transarterial chemoembolization (TACE)/transarterial embolization (TAE) + portal vein stents combined with 125I implantation (Group A) or TACE/TAE + portal vein stents only (Group B). It could show differences in treatment and outcomes between the two groups. After operation, scheduled follow-up was performed at 6, 12 and 24 mo. Follow-up included postoperative and preoperative portosystemic pressure gradient, postoperative stenting stenosis rate, and survival rate. Time-to-event outcomes were evaluated with Kaplan–Meier curves and log-rank test. Cox regression model was used to identify independent predictors. Kaplan–Meier curves and log-rank test clearly demonstrated the differences in survival rate and restenosis rate between the two groups, as well as the efficacy of 125I in the treatment of main portal vein tumor thrombus. Cox analysis could take various factors into account to make the results more convincing.
Compared with stents alone, stents combined with 125I implantation had a good therapeutic effect in liver cancer with main portal vein tumor thrombus. This method reduced the restenosis rate and improved survival rate. Stents combined with 125I implantation were safe and reliable in clinical application. In this study, the 125I was placed between the stent and tumor thrombus and the stent could hold the particles. Using this method of 125I implantation, the number and position of the particles could be adjusted, which is more flexible in clinical application. However, as the size of the liver cancer shrinks, the particles may drift to other parts of the body via blood flow, and this needs further study.
Stents combined with 125I implantation have a good therapeutic effect in the treatment of liver cancer with main portal vein tumor thrombus. The 125I was placed between the stent and the tumor thrombus, and the stent could hold the particles. The new method can avoid the drawbacks of particle strands and can be widely used in the clinic. Stents combined with 125I implantation have a good therapeutic effect in the treatment of liver cancer with main portal vein tumor thrombus. The method is technically safe and reliable. Tumor thrombus in the main portal vein indicates late-stage disease. 125I is an effective treatment for main portal vein thrombosis. Compared with stents alone, stents combined with 125I implantation can reduce restenosis rates and improve survival rate. It is technically safe and reliable. 125I has made great achievements in the treatment of main portal vein tumor thrombus, but there are drawbacks in the method of 125I implantation, and new methods should be explored.
Liver cancer with portal vein thrombosis seriously affects patient quality of life and should be treated in a timely manner. Stents combined with 125I implantation have a good therapeutic effect in liver cancer with main portal vein tumor thrombus. Appropriate patients were selected for seed implantation treatment according to the inclusion criteria. The particle drift rate of the patients was followed up at 6, 12 and 24 mo after the operation.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciezki JP, Ward J S- Editor: Wang JL L- Editor: Filipodia E- Editor: Song H
| 1. | Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2016;379:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 2. | Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer. 2016;139:1534-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 3. | Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 693] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 4. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Matsuo Y, Yoshida K, Nishimura H, Ejima Y, Miyawaki D, Uezono H, Ishihara T, Mayahara H, Fukumoto T, Ku Y. Efficacy of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein tumor thrombosis/inferior vena cava tumor thrombosis: evaluation by comparison with conventional three-dimensional conformal radiotherapy. J Radiat Res. 2016;57:512-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Tan T, Xiao Y, Zhou S, Ma C, Zhang Z. Y-configuration stent combined with iodine-125 seeds strand for the treatment of hepatocellular carcinoma with tumor thrombosis in portal vein branches: A case report. Medicine (Baltimore). 2017;96:e8660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Sun H, Zhang M, Liu R, Liu Y, Hou Y, Wu C. Endovascular implantation of 125I seed combined with transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma. Future Oncol. 2018;14:1165-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Luo JJ, Zhang ZH, Liu QX, Zhang W, Wang JH, Yan ZP. Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int. 2016;10:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Han K, Kim JH, Ko GY, Gwon DI, Sung KB. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: A comprehensive review. World J Gastroenterol. 2016;22:407-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Zhang YF, Le Y, Wei W, Zou RH, Wang JH, OuYang HY, Xiao CZ, Zhong XP, Shi M, Guo RP. Optimal surgical strategy for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score analysis. Oncotarget. 2016;7:38845-38856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Higaki I, Hirohashi K, Kubo S, Tanaka H, Tsukamoto T, Omura T, Kinoshita H. Portal vein stenting to treat portal vein tumor thrombus in hepatocellular carcinoma. Osaka City Med J. 2000;46:99-104. [PubMed] |
| 12. | Lu J, Guo JH, Zhu HD, Zhu GY, Chen L, Teng GJ. Safety and Efficacy of Irradiation Stent Placement for Malignant Portal Vein Thrombus Combined with Transarterial Chemoembolization for Hepatocellular Carcinoma: A Single-Center Experience. J Vasc Interv Radiol. 2017;28:786-794.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Sun JH, Zhou T, Zhu T, Zhang Y, Nie C, Ai J, Zhou G, Zhang A, Dong MJ, Wang WL. Portal Vein Stenting Combined with Iodine-125 Seeds Endovascular Implantation Followed by Transcatheter Arterial Chemoembolization for Treatment of Hepatocellular Carcinoma Patients with Portal Vein Tumor Thrombus. Biomed Res Int. 2016;2016:3048261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |