Published online Dec 15, 2018. doi: 10.4251/wjgo.v10.i12.476
Peer-review started: September 14, 2018
First decision: October 3, 2018
Revised: October 15, 2018
Accepted: November 23, 2018
Article in press: November 24, 2018
Published online: December 15, 2018
Processing time: 91 Days and 9.7 Hours
To determine changes in the morphology and function of pancreatic cancer cells after irreversible electroporation (IRE) treatment, and to explore the clinical significance of IRE treatment for pancreatic cancer providing an experimental basis for the clinical application of IRE treatment.
IRE was carried out in an athymic nude mouse model of pancreatic carcinoma generated with human pancreatic cancer cells 1. In therapy groups, IRE electrodes were inserted with 90 pulses per second at 800 V/cm applied to ablate the targeted tumor tissues. Histological assessment of the affected tissue was performed by hematoxylin and eosin staining (HE). Quantification of cell proliferation and apoptosis was performed by evaluating Ki67 and caspase-3 levels, respectively. Flow cytometry was used to assess cell apoptosis. Ultrasound imaging was carried out to evaluate IRE treatment results. Pathological correlation studies showed IRE is effective for the targeted ablation of pancreatic tumors in an orthotopic mouse model.
IRE was efficacious in removing tumors in the orthotopic mouse model. The IRE-ablated zone displays characteristics of nude mouse models at different time-points as assessed by hematoxylin and eosin staining. Immunohistochemical analysis of samples from the pancreatic cancer models showed significantly enhanced caspase-3 cleavage and Ki67. Flow cytometry data corroborated the above findings that apoptosis in tumor cells was observed immediately on the first postoperative day, and with time the middle and late stages of apoptosis were observed. For ultrasound imaging studies, the IRE ablation zone became a hyperechoic area due to increasing inflammatory and immunologic cellular contents.
IRE is a promising new approach for pancreatic cancer, with many potential advantages over conventional ablation techniques.
Core tip: Patients with pancreatic cancer have a poor prognosis. It often quickly develops into locally advanced pancreatic cancer that is considered to be surgically unresectable. Irreversible electroporation represents a novel tumor ablation method that induces cell apoptosis with no thermal coagulation effects. This study aimed to assess the clinical significance of irreversible electroporation treatment in pancreatic cancer, and to provide an experimental basis for the clinical application of irreversible electroporation treatment.
- Citation: Su JJ, Xu K, Wang PF, Zhang HY, Chen YL. Histological analysis of human pancreatic carcinoma following irreversible electroporation in a nude mouse model. World J Gastrointest Oncol 2018; 10(12): 476-486
- URL: https://www.wjgnet.com/1948-5204/full/v10/i12/476.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i12.476
Immunodeficient animals are obtained from genetic mutations or by artificial methods that cause one or more genes of the immune system to be defective. Because such animals are immunodeficient, they are widely used in the fields of immunology, oncology, toxicology, and others. Currently, BALB/c mice are the most commonly used immunodeficient animals[1]. A recent xenograft pancreatic cancer model employing human pancreatic cells was adopted[2]. This constitutes a clinically relevant and reproducible animal model for assessing local and systemic treatments[3]. Indeed, orthotopic models of pancreatic carcinoma mimic the main features of human disease, and are excellent tools for the biological characterization of this malignancy.
Pancreatic carcinoma is a malignant tumor with the characteristics of insidious onset, fast progression, high postoperative recurrence and overall 5-year survival rate below 5%[4]. According to reports of the American Cancer Society, pancreatic carcinoma ranks fourth among deadliest cancers. Conventional therapeutic methods include surgical treatment and chemotherapy. However, the majority of cases cannot undergo surgery because they are diagnosed with advanced disease presenting distant metastasis; meanwhile, chemotherapeutics have low permeability and are limited by drug resistance[5].
Considering the limited therapeutic options, irreversible electroporation (IRE) has been developed in recent years for the treatment of locally advanced pancreatic cancer[6]. IRE represents a new modality that can be used independently for targeted tissue ablation, applying strong electrical fields instead of heat deposition or chemicals[7]. Because IRE has a non-thermal mechanism of action, it can be used to target malignancies adjacent to vital structures (e.g., major vessels)[8]. This study aimed to assess the efficacy of IRE in pancreatic cancer treatment using an orthotopic mouse model.
The human pancreatic cancer cells 1 (PANC-1) cell line was provided by American Type Culture Collection (Tumor Hospital of the Chinese Academy of Medical Sciences, China), and maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone) with 10% FBS (Sigma), 100 U/mL penicillin, and 100 mg/mL streptomycin in a humid environment containing 5% CO2. Cells were pelleted and re-suspended at 1 x 107/mL in phosphate buffer solution (PBS, pH = 7.4). Prior to implantation, cell viability was assessed by Trypan blue staining (assessing the viability of the cultured cells for each tumor implantation procedure, > 95%). The tumor cells were kept on ice prior to injection into the pancreas.
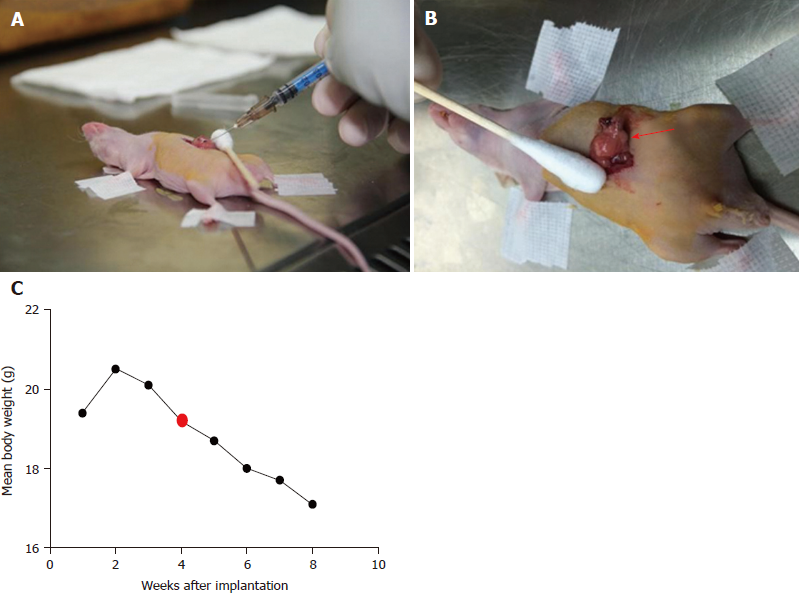
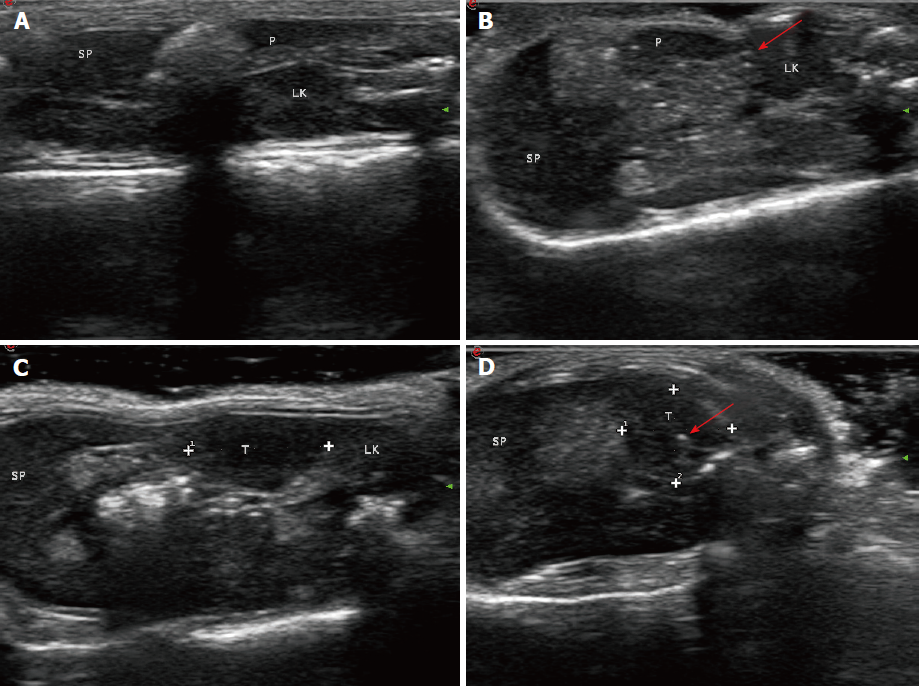
All animal experiments had approval from the Institutional Animal Care and Use Committee of Chinese People’s Liberation Army General Hospital. Fifty adult male BALB/c nude mice (Institute Of Medical Laboratory Animals, Chinese Academy Of Medical Sciences), initially weighing 18-20 g, were used in this study. The animals were housed in groups of five in facilities maintained at 22 °C ± 1 °C with 55 ± 10% relative humidity, under a 12 h – 12 h light/dark cycle for 1 wk before the experiments. Mice were anesthetized using inhaled 2%-3% isoflurane. Anesthesia depth was evaluated based on the lack of reflex to a toe pinch. The abdomen of each mouse was prepared with Anerdian skin disinfectant. Each mouse was turned on the side to raise the left side of the abdomen. Using a sterile scalpel, 1.5 cm skin incisions (about 1 cm left lateral from the midline) were made; 1.5 cm incisions in the underlying abdominal muscle were also made. Then, 50 μL of the cell suspension was injected into the pancreatic tail. These cells generated a bubble on the pancreatic surface (Figure 1A). The injection site was inspected to ensure that no leakage occurred. The abdominal musculature and the external skin in each mouse were separately closed with an absorbable braided suture using a continuous stitch. After wound healing (7 d), the mice were anesthetized, and the external sutures were removed. Following the initial implantation, approximately 5-10 d were required to allow sufficient tumor growth for pretreatment (diameter < 1.5 cm). The animals were externally examined frequently; in addition, ultra-high frequency, high-definition ultrasound (US) (Philips, CX50, Epiq 7, Seattle, WA, United States) assessment was performed every 3 d from 30 d after tumor cell injection (Figure 1B).
Forty-four nude mice from the initial 45 implanted animals produced pancreatic cancer (0.5 ± 1.5 cm in diameter) suitable for subsequent IRE treatment procedures. Forty-nine animals were assigned to three groups, including the normal (5 mice with no tumor cell implantation; Group 1), sham-operation (Group 2; n = 22), and IRE (Group 3; n = 22) groups. Mice in groups 2 and 3 were subsequently euthanized for histological examination at different time-points (1 d, 3 d, and 7 d) after the original baseline scan.
IRE tumor ablation was performed with an experimental IRE generator (Nanoknife; Angio Dynamics, Queensbury, NY, United States). IRE electrodes were positioned in parallel, with spacing set to 0.5 cm, and inserted into the diseased pancreas at a final depth of 0.5 cm. Ablation parameters included a voltage of 800 V/cm at 90 μs and a pulse length of 100 milliseconds. A total of 90 pulses were applied per minute, with an ablation rate of 90 pulses per second. The abdominal wall in mice was closed after the procedure. The abdominal cavity was opened, exposing the pancreas after 4 wk of modeling. The gross morphologies of the pancreas in various groups were: (1) control group, no obvious mass or tissue adhesion; and (2) IRE group, average tumor diameter of 0.8 cm, with the tumors having a hard texture. With regard to gross appearance, the tumors were round or nodular, and the tumor tissue was usually grayish white. Some of the tumors had mild adhesion to the surrounding tissue, while others invaded adjacent organs such as the stomach, duodenum and peritoneum. Early tumors showed no ascites (Figure 1B).
Tumor samples were harvested 1 d, 3 d, and 7 d after IRE from anesthetized animals, fixed with 10% formalin, and paraffin embedded. The sections were then submitted to hematoxylin and eosin (HE) staining for histopathological assessment on an Olympus BX43 microscope (Olympus, Japan). Histology slides were blinded and reviewed by a pathologist specialized in gastrointestinal oncology (> 10 years of experience). For immunohistochemistry, four-micron sections were incubated with antibodies against Ki67 (550609, 1:300, BD) and cleaved caspase-3 (Asp175) (9661S, 1:200, Cell Signaling Technology, United States). Ki67 staining was used as a marker of active proliferation, while caspase-3 signals reflected active apoptosis[9]. Tumor cell proliferation was assessed in five high power fields under a microscope in each slide; the Ki67 index was employed for quantitation. Immunohistochemistry (IHC) staining for Ki67 and cleaved caspase-3 was scored as the percentage of positive cells.
Statistical analysis was carried out with SPSS v.22 for Mac (SPSS, United States). Data were presented as mean ± SD. Groups were compared with variance tests (comparisons between untreated and treated mice). Differences were considered statistically significant with a P-value < 0.05.
A total of 44 of the 45 mice implanted with PANC-1 cells developed pancreatic cancer. One mouse was euthanized prior to the beginning IRE procedures because of suture failure, and one animal died after the IRE from improper operation. No severe postoperative complications occurred in the treated mice (Groups 2 and 3).
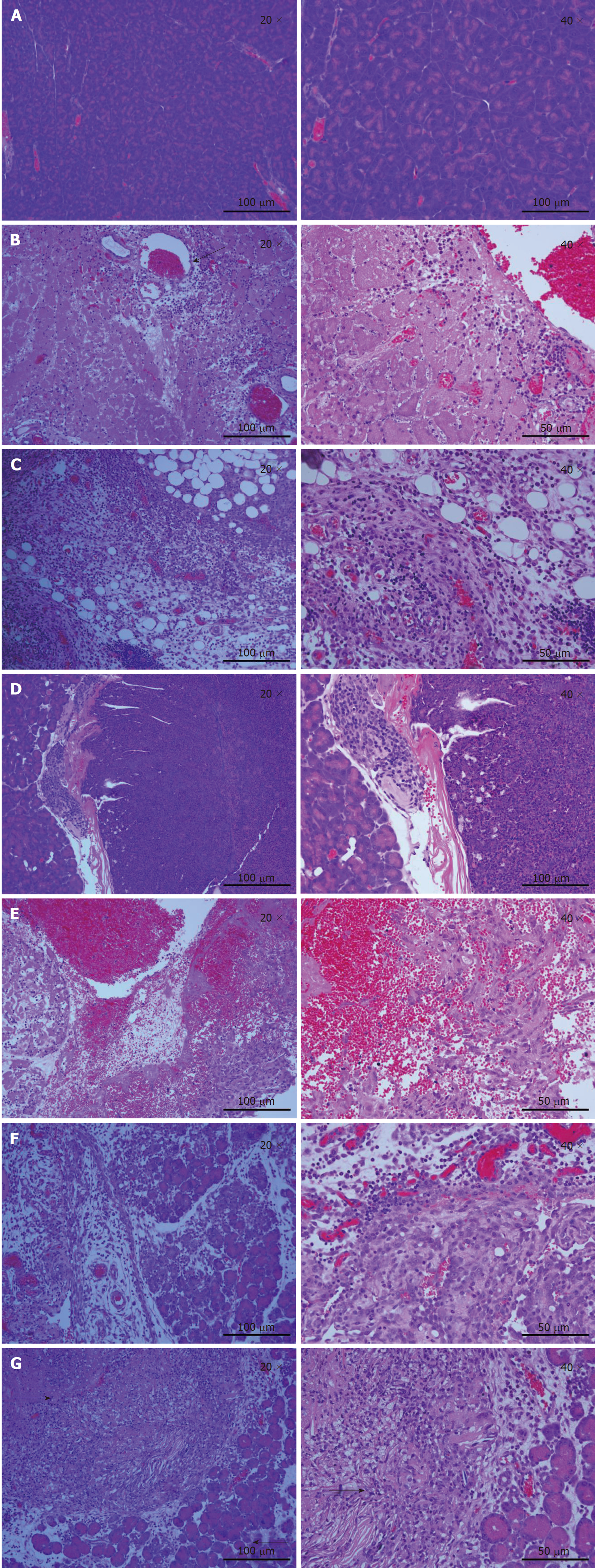
Histological examination of tumor tissues was performed by a pathologist. As shown in Figure 2A, pancreas cells in the normal mouse had large nuclei surrounded by well-demarcated cytoplasm and well-defined cytoplasmic membrane. A total of 3 d post-IRE (Figure 2B), the ablation zone showed areas of acute, extensive, and severe pancreatic cell death. A seepage zone of erythrocytes was observed around the ablation zone. However, larger vessels in the ablated area appeared to be structurally well preserved. The normal pancreatic architecture was preserved. At 7 d after IRE ablation (Figure 2C), erythrocyte leakage continued to decrease. As shown in Figure 2E, most tumor cells were deformed and melded together. Large numbers of inflammatory cells began to permeate into the ablated area. At 3 d after IRE (Figure 2F), the ablation zone was characterized by edematous swelling of the interstitium and tumor tissue necrobiosis. Eosinophilia increased continually, with marked ablation zone inflammation. At 1 d post-IRE treatment (Figure 2G), complete cell death was achieved in the ablation zone, with a sharply delineated margin between ablated and non-ablated surrounding tissues. The majority of tumor cells were displaced by fibrosis, and mononuclear cells and chronic inflammation were observed.
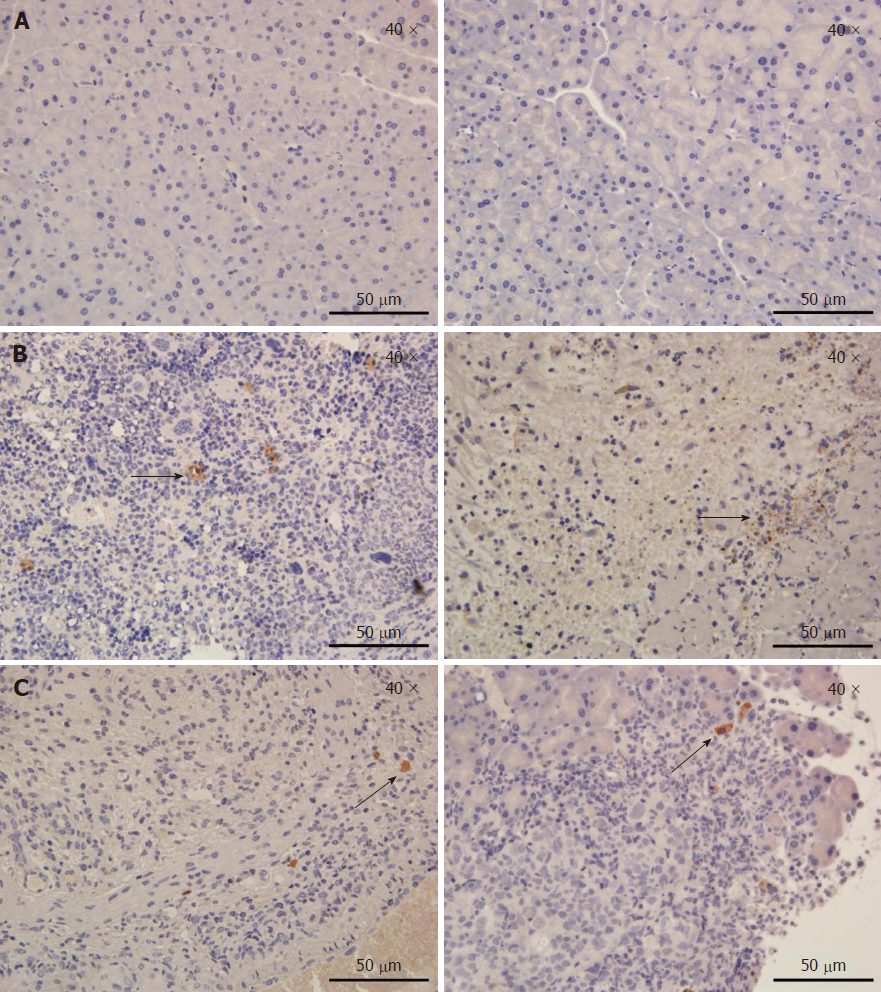
To evaluate the effect of IRE on tumor tissues at different time points, in vivo IHC experiments were performed. Staining with antibodies targeting Ki67 and cleaved caspase-3 was performed to assess cell proliferation and apoptosis, respectively. Figure 3A is a representative IHC image in an untreated pancreatic parenchyma. In tumor tissues (Figure 3B), extensive caspase-3 activation was observed on the first postoperative day. IRE significantly increased cell proliferation (Ki67 staining) at 1 d post-treatment, but cell proliferation was decreased at 7 d post-treatment. Limited caspase-3 staining at 7 d post-IRE treatment was found in treated tumors, while most of the viable tumor tissues showed no caspase-3 activation (Figure 3C). Our results fully demonstrated overt tumor necrosis in the IRE group, especially the first day after treatment.
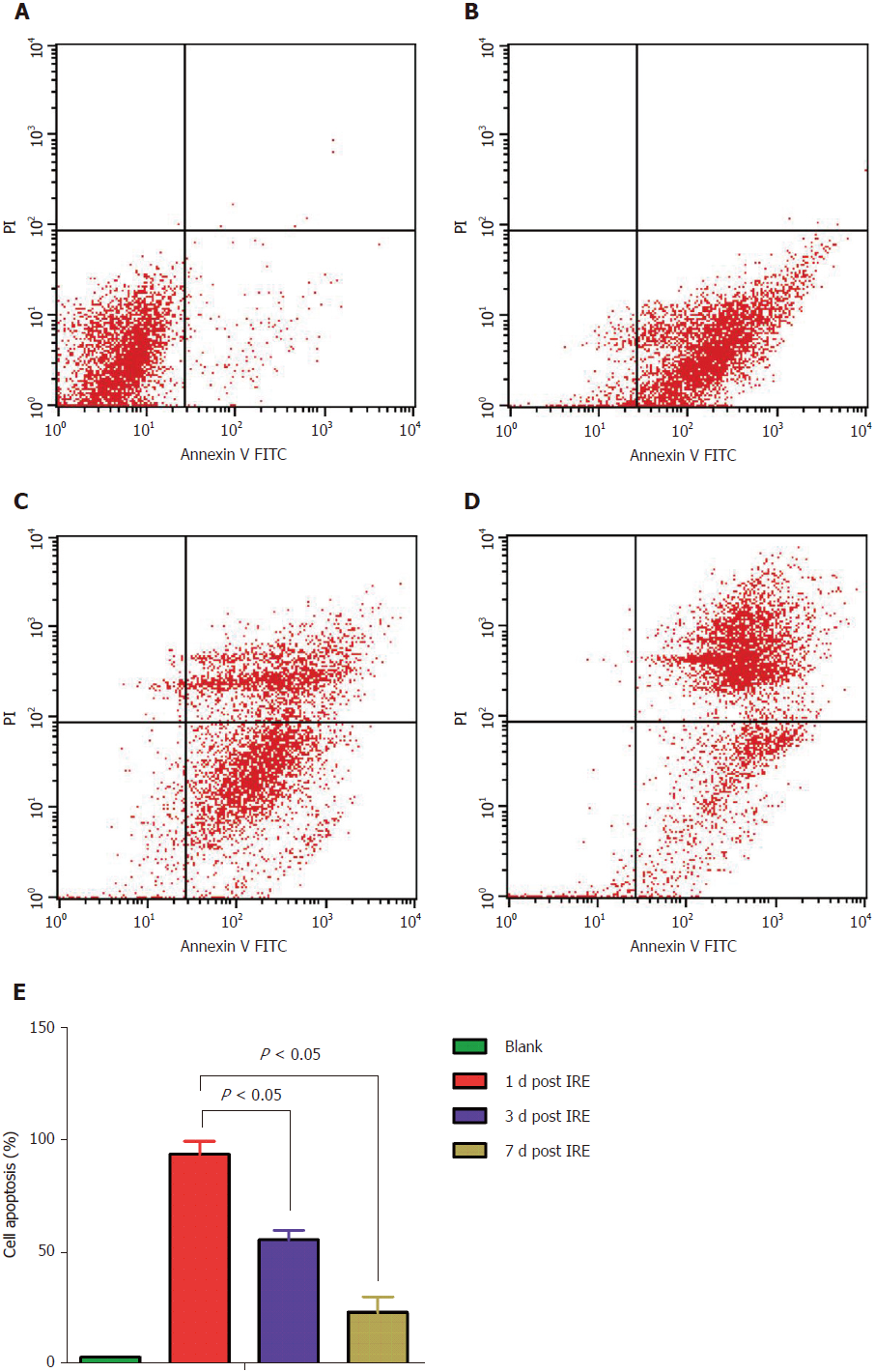
The propidium iodide staining assay was employed to assess cell cycle distribution. Tumor cells were fixed with 70% ethanol, washed, incubated in presence of 100 mg/mL RNase in PBS (30 min; 37 °C), and stained with 50 mg/mL propidium iodide in PBS. Cell cycle distribution was assessed on a Cell Lab Quanta SC flow cytometer (Beckman Coulter, United States). Meanwhile, Annexin V-fluorescein isothiocyanate Apoptosis Detection Kit (BioVision) was employed for apoptosis quantitation, as directed by the manufacturer. Spleens were extracted, weighed, and processed for analyses. For the cell cycle distribution assay, cells (5 × 105) were harvested, washed with PBS, and finally re-suspended in 500 mL binding buffer, followed by incubation with annexin V-fluorescein isothiocyanate (5 mL) and propidium iodide (5 mL) for 30 min at room temperature in the dark. After staining, cells were assessed on a flow cytometer. Flow cytometry data corroborated the above findings that apoptosis in tumor cells was observed immediately on the first postoperative day, and with time the middle and late stages of apoptosis were observed (Figure 4).
For US studies, pre-ablation US (Philips, CX50, Epiq 7, Seattle, WA, United States) was performed to visualize the normal pancreatic anatomy, and US imaging was carried out to evaluate IRE treatment results. Image analysis was carried out by two radiologists with ≥ 10 years of experience in pancreatic US. Consensus was based on post-ablation discussion. In the normal group (Figure 5A and B), the position of the normal pancreatic parenchyma was accurately detected before and after the IRE treatment by US examination. In the tumor group (Figure 5C and 5D), tumor size was determined by US before and after IRE. Upon IRE treatment, US was repeated to acquire post-IRE images. These experiments successfully showed that IRE induced rapid changes during ablation of the normal pancreatic tissue as well as tumor ablation, and these changes were apparent on US images[10]. In the tumor tissue, the IRE ablation zone became a hyperechoic area due to increasing inflammatory and immunologic cellular contents.
Unresectable lesions amount to roughly 80% of pancreatic cancers at the time of diagnosis, and show a 5-year overall survival below 5%[11]. This poor prognosis has historically reduced the enthusiasm for aggressive surgical resection[12]. Recently, alternative tools for local therapy (e.g., radiation and various thermal and non-thermal ablation methods) have been assessed ,but often produce discouraging outcomes[13]. IRE represents a promising novel tool for tissue and tumor ablation[6]. IRE destroys cells in the target region while preserving the collagen architecture of vascular, biliary, or neuronal structures[14]. High electric voltage generating a large potential gradient to cause IRE has been assessed in vitro and in vivo[8]. Such findings are interesting because IRE effectively causes cell death in the normal tissue as well as cancer cells[15]. The main advantage of IRE is in the conservation of blood vessel and bowel wall integrity[16]. The vascular structure in the ablation zone showed no damage and was only scarcely affected by the IRE treatment. In this study, we created a mouse model of orthotopic pancreatic carcinoma by treating BALB/c nude mice by transabdominal administration of PANC-1 cells. Orthotopic pancreatic cancer modeling was successfully achieved in forty-four nude mice. Studies on animal models demonstrated the efficacy of IRE for achieving anti-tumor effects in orthotopic mouse models of pancreatic cancer by HE staining, apoptosis-specific immunohistological analysis, flow cytometry, and US imaging.
HE staining (Figure 2) showed that the IRE-ablated zone (day 1) had an extensive necrotic area and the IRE ablation border zone had more infiltrated inflammatory cells compared with the ablation center zone. Large numbers of red blood cells were observed in the ablation area, which was probably due to IRE destroying microcirculation perfusion in the ablation area. Furthermore, there were large amounts of neutrophils with perivascular infiltration. Moreover, vessels in the ablation area showed an intact structure. We further demonstrated that extensive and severe cell death in the IRE ablation zone was completely different from that observed in the thermal ablation zone. Meanwhile, post-ablation inflammatory reactions were not the overall cause of necrosis resulting from the IRE treatment. At 7 d post-IRE, the tissue in the ablated area showed a uniform structure without cells. IRE caused an ablation of cells but left the cellular matrix intact, providing a good scaffold for new tissue formation[17]. Due to tissue necrosis and cellulose formation in the ablated area, the tissue structure was better visualized. We clearly observed that tissue dissolution and absorption occurred between the necrotic area and the lacunae.
To further assess tissue cell proliferation and apoptosis occurring after the IRE treatment, we next performed IHC to evaluate the effect of IRE on tumor tissues. This study evaluated Ki67 staining and caspase-3 activity, which measure cell proliferation and apoptosis, respectively, in the region between the two electrodes; as shown above, these two measurements showed a positive correlation. The results showed that cell death in the IRE-ablation area was reflected by increased amounts of caspase-3 compared with the adjacent normal tissue on the first day after IRE ablation (Figure 3B). At 7 d postoperatively, very few apoptotic cells were stained, indicating that IRE induced cell death by apoptosis rather than coagulative thermal necrosis. Figure 3B shows blood vessel cells that were not stained, suggesting that large blood vessels are not affected by IRE in the ablation area. This may be an advantage for IRE to create an effective ablation of the undesirable tumor without damaging the underlying architecture of the healthy pancreatic parenchyma.
Furthermore, we used flow cytometry to examine cell cycle distribution and the apoptotic rate. To assess cell apoptosis efficiency, mouse spleen cells in the treatment group were analyzed by flow cytometry at different time-points (Figure 4). No overt cell apoptosis in the control group (Figure 4A) was observed. However, cells treated by IRE (Figure 4B) had an apoptotic rate of 93.71%, which was markedly elevated compared with the control value (3.37%, P < 0.05), suggesting that the IRE treatment is highly effective. Cells in the middle and late stages of apoptosis increased with time (Figure 4C and 4D). As expected, cell apoptosis rates of the therapy groups were much higher than that of the control group. Meanwhile, significant differences were obtained in apoptotic rates at different time points (Figure 4E). These results indicated that the IRE treatment is effective for targeted ablation of pancreatic tumors in an orthotopic mouse model.
The above experiments successfully demonstrated that IRE induces changes detectable on US imaging. Pre-IRE US imaging showed the tumor appearing peripherally hyperechoic compared with the normal pancreatic parenchyma. This study showed that IRE ablation produced greater alterations to echogenicity in tumors compared with normal tissues. The above US findings demonstrated that ablated tissues in the normal pancreas and tumors became more hyperechoic. The US images obtained during IRE showed the hypoechoic ablation region as being mixed with the hyperechoic region in close proximity to the probes used. As shown in Figure 5B, both hyperechoic probe tips of the dual probe system in the ablated zone had a minimal amount of hyperechoic microbubbles. US images showed that the area of hypoechogenicity became largely hyperechogenic due to increased inflammatory and immunologic cellular contents in the ablated zone. We also demonstrated that the treated areas correlated with pathological measurements. Such changes in echogenicity provide strong evidence that perioperative US is feasible in monitoring IRE.
The efficacy of IRE treatment was also evaluated by monitoring body weights in mice for 7 d (Figure 1C). The therapy groups displayed more pronounced body weights over time compared with control mice, which may be due to decreased tumor sizes in the treatment groups.
Another significant finding was that IRE had a safer and shorter operation procedure compared with traditional techniques, such as the radiofrequency and microwave ablation methods. This compares to routine thermal ablation methods requiring > 30-60 min for ablating a tumor of comparable size[18]. A reduced operation time in IRE indicates less complications and improved safety for patients compared with conventional means.
In conclusion, this study systematically assessed the efficacy of IRE ablation, and demonstrated that the IRE-ablated zone displays characteristics of nude mouse models at different time-points as assessed by HE staining. IRE is a promising new approach for pancreatic cancer with many potential advantages over conventional ablation techniques. Follow-up US images demonstrated tumor size reduction suggesting that US may be used for ablation zone evaluation.
Irreversible electroporation (IRE) is a medical technique that utilizes high voltage pulses to create permanent nanopores in the cell membrane, which in turn induces apoptosis of the targeted cells. Although there are benefits of IRE, many adverse events should be taken into consideration before its use. We aimed to assess the efficacy of IRE ablation in nude mouse models providing an experimental basis for the clinical application of IRE treatment.
Animal models of pancreatic cancer were successfully established and were successfully treated by IRE treatment. Tumor cell proliferation and apoptosis were detected by different methods, which proved that this treatment was effective.
The main objectives aimed to determine changes in the morphology and function of pancreatic cancer cells after IRE treatment providing an experimental basis for the clinical application of IRE treatment.
Animal models of pancreatic cancer were successfully treated by IRE treatment. Histological assessment of the affected tissue was performed by hematoxylin and eosin staining. Quantification of cell proliferation and apoptosis was performed by evaluating Ki67 and caspase-3 levels, respectively. Flow cytometry was used to assess cell apoptosis. Ultrasound imaging was carried out to evaluate IRE treatment results. Pathological correlation studies showed IRE is effective for the targeted ablation of pancreatic tumors in an orthotopic mouse model. Ultrasound imaging was repeatedly carried out to evaluate IRE treatment results.
This study systematically assessed the efficacy of IRE ablation and demonstrated that the main advantage of IRE is in the conservation of blood vessel and bowel wall integrity. Clinical data of patients after the application of IRE treatment is needed to prove that IRE treatment is effective in treating patients with pancreatic cancer.
IRE ablation is safe and effective for treatment of pancreatic cancer in a mouse model. The implication of this study for future clinical practice is that advanced pancreatic cancer patients can use IRE ablation as an effective treatment.
The future direction of research is the extensive safety application of IRE ablation in patients. The best method for future research is to study the practical application of IRE ablation in patients.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Emi M, Hanaoka N, Park SJ S- Editor: Wang JL L- Editor: Filipodia E- Editor: Song H
| 1. | Ingman WV, Jones RL. Cytokine knockouts in reproduction: the use of gene ablation to dissect roles of cytokines in reproductive biology. Hum Reprod Update. 2008;14:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Philips P, Li Y, Li S, St Hill CR, Martin RC. Efficacy of irreversible electroporation in human pancreatic adenocarcinoma: advanced murine model. Mol Ther Methods Clin Dev. 2015;2:15001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Philips P, Li Y, Martin RC 2nd. Low-energy DC current ablation in a mouse tumor model. Methods Mol Biol. 2014;1121:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1543] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 5. | Czito BG, Willett CG, Clark JW, Fernandez Del Castillo C. Current perspectives on locally advanced pancreatic cancer. Oncology (Williston Park). 2000;14:1535-1545; discussion 1546, 1549-1552. [PubMed] |
| 6. | Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, Rubinsky B, Mir LM. Tumor ablation with irreversible electroporation. PLoS One. 2007;2:e1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 7. | Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007;6:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 335] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Yagi T, Hardin JA, Valenzuela YM, Miyoshi H, Gores GJ, Nyberg SL. Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology. 2001;33:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Appelbaum L, Ben-David E, Sosna J, Nissenbaum Y, Goldberg SN. US findings after irreversible electroporation ablation: radiologic-pathologic correlation. Radiology. 2012;262:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Linecker M, Pfammatter T, Kambakamba P, DeOliveira ML. Ablation Strategies for Locally Advanced Pancreatic Cancer. Dig Surg. 2016;33:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JD, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford). 2008;10:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Hajj C, Goodman KA. Pancreatic cancer and SBRT: A new potential option? Rep Pract Oncol Radiother. 2015;20:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Heger M, van der Wal AC, Storm G, van Gemert MJ. Potential therapeutic benefits stemming from the thermal nature of irreversible electroporation of solid cancers. Hepatobiliary Pancreat Dis Int. 2015;14:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Davalos RV, Msir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 887] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 16. | Leen E, Picard J, Stebbing J, Abel M, Dhillon T, Wasan H. Percutaneous irreversible electroporation with systemic treatment for locally advanced pancreatic adenocarcinoma. J Gastrointest Oncol. 2018;9:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Friedman M, Mikityansky I, Kam A, Libutti SK, Walther MM, Neeman Z, Locklin JK, Wood BJ. Radiofrequency ablation of cancer. Cardiovasc Intervent Radiol. 2004;27:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |