Published online Nov 15, 2018. doi: 10.4251/wjgo.v10.i11.370
Peer-review started: June 2, 2018
First decision: August 1, 2018
Revised: August 8, 2018
Accepted: October 8, 2018
Article in press: October 8, 2018
Published online: November 15, 2018
Processing time: 167 Days and 4.6 Hours
Ampulla of Vater is a peculiar anatomical structure, characterized by the crossroad of three distinct epithelia: Intestinal, ductal pancreatic and biliary. Adenocarcinomas arising in this area represent an opportunity to understand the comparative biology of all periampullary malignancies. These neoplasms can exhibit intestinal, pancreaticobiliary or mixed features, whereas the subclassification based on morphology and immunohistochemical features failed in demonstrating a robust prognostic reliability. In the last few years, the molecular landscape of this tumor entity has been uncovered, identifying alterations that may serve as prognostic and predictive biomarkers. In this review, the histological and genetic characteristics of ampullary carcinomas are discussed, taking into account the main clinical and therapeutic implications related to this tumor type as well.
Core tip: Ampulla of Vater carcinomas comprise tumors with intestinal and/or pancreaticobiliary differentiation, but such histotypical classification is of little help for their prognostic stratification. Integration of the recently reported molecular profiles with histopathological and clinical information furnishes novel keys for fostering the development of a more efficient prognostic stratification and the identification of novel therapeutic strategies.
- Citation: Pea A, Riva G, Bernasconi R, Sereni E, Lawlor RT, Scarpa A, Luchini C. Ampulla of Vater carcinoma: Molecular landscape and clinical implications. World J Gastrointest Oncol 2018; 10(11): 370-380
- URL: https://www.wjgnet.com/1948-5204/full/v10/i11/370.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i11.370
Ampullary neoplasms represent a wide array of tumors arising in the ampulla of Vater, the most common of which is represented by ampulla of Vater carcinoma (AVC), although other rare malignancies, such as neuroendocrine tumors, may be encountered in this location[1-3]. AVC comprises 30% of pancreatico-duodenectomies and 20% of all tumor-related obstructions of the common bile duct[4-6]. Data from the surveillance, epidemiology, and end results registries have indicated an increased number of new diagnoses in the last years, with the average age at diagnosis ranging from 60 to 70 years old[6-8]. The etiology of ampullary carcinoma has not been clearly defined and an association with a noninvasive component displaying the adenoma-to-carcinoma sequence similar to colorectal carcinoma may be present[9,10].
The ampulla of Vater region presents very peculiar histological aspects, as it represents a crossroad of three different epithelia: Intestinal, ductal pancreatic and biliary. This kind of structure characterizes this area, with a unique complexity and morphological heterogeneity[1]. From the histological point of view, coupling morphological and immunohistochemical analyses, AVCs have been subgrouped into intestinal and pancreatobiliary subtypes based on the epithelium of origin; in case of coexistence of aspects of both subtypes, the mixed category has been introduced for a more precise classification[1,11-14]. However, the former classification has been challenged by lines of evidence showing a significant interobserver variability upon the interpretation of these patterns, and the mixed subtype being the predominant subgroup of AVCs, representing up to 40% of cases[15-17]. In addition, poorly differentiated tumors can further confound the histological classification[1]. The prognostic significance of this histological classification has been subjected to investigation with inconsistent results[15-18] that will be briefly discussed in this review.
In recent years, much progress has been made in characterizing the molecular alterations underlying AVC tumorigenesis, showing a complex mutational spectrum that supports only in part the distinction in different histological subtypes[14,17]. Molecular analysis showed alterations in overlapping pathways that may serve as foundation for developing new therapeutic approaches and may improve early prognostication models. In this review, we will discuss the histological and genetic landscape of AVCs and its clinical implications, with a specific focus on the treatment of choice and on the future perspectives related to this important topic.
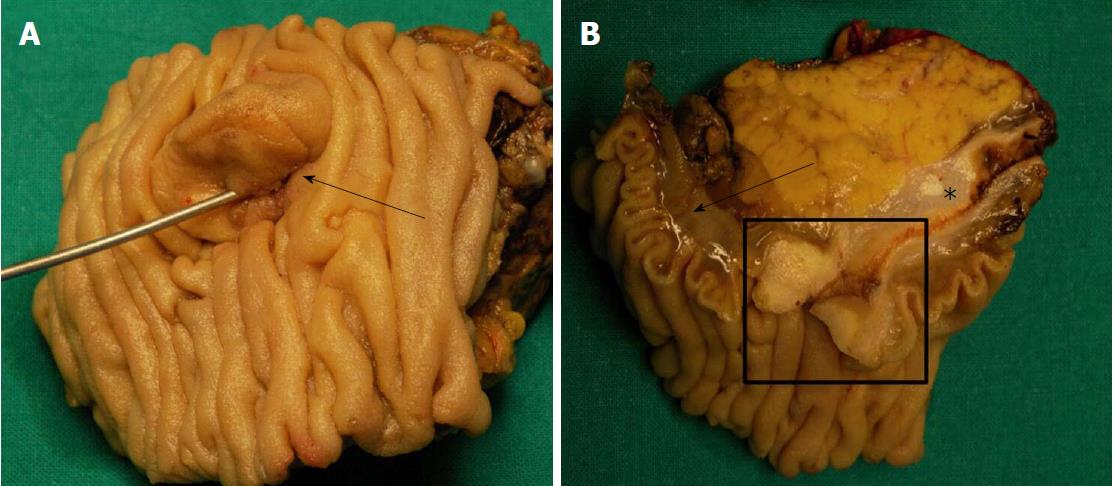
According to the gross appearance and location, AVCs can be divided into three different categories: (1) intraampullary neoplasms, characterized by a intraluminal growth pattern, without extension out of the Oddi’s sphincter; (2) periampullary neoplasms, with a significant vegetating component on the duodenal surface of the ampulla, usually adenomatous, noninvasive, and frequently characterized by an ulcerating part corresponding to the invasive component; and (3) mixed neoplasms, which show both intraampullary and vegetating growth[18-21]. In all of these cases, the ampullary region has a typical enlarged macroscopic appearance (Figure 1).
In 2010, the World Health Organization revised the criteria for the pathological diagnosis of ampullary carcinoma to include three distinct histopathological subtypes on the basis of morphology and immunohistochemical characteristics: (1) the intestinal-type AVCs; (2) the pancreatobiliary-type AVCs; and (3) the mixed-type AVCs[1].
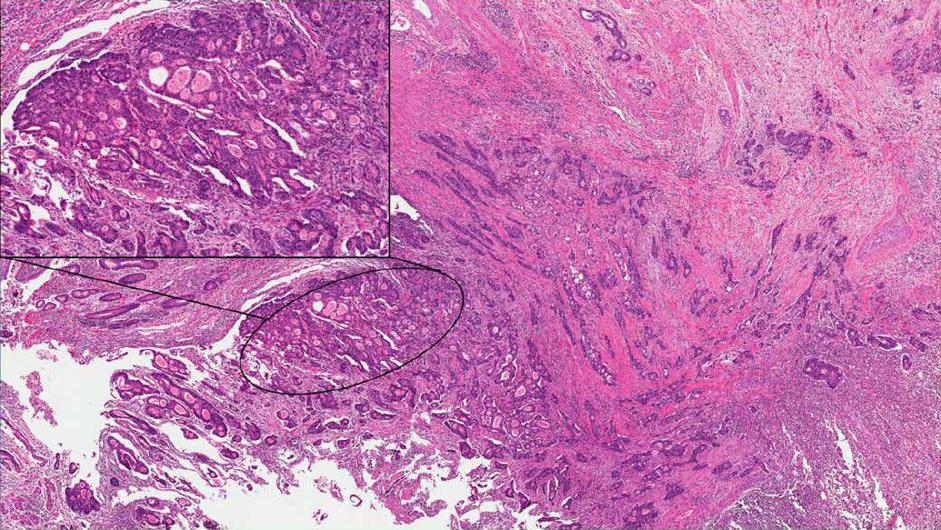
The intestinal type is frequently associated with a noninvasive component (duodenal adenoma). Its morphology is characterized by a colorectal-like architecture, with tubular or cribriform glands and central necrosis (Figure 2)[11,22]. The invasive component is usually smaller than in the pancreatobiliary type and less frequently exhibits adverse pathological factors and lymphovascular and perineural invasion[23-26]. This AVC subtype usually expresses intestinal immunomarkers, such as caudal-related homeodomain transcription factor 2 (CDX2), mucin2 (MUC2) and cytokeratin 20 (CK20)[27].
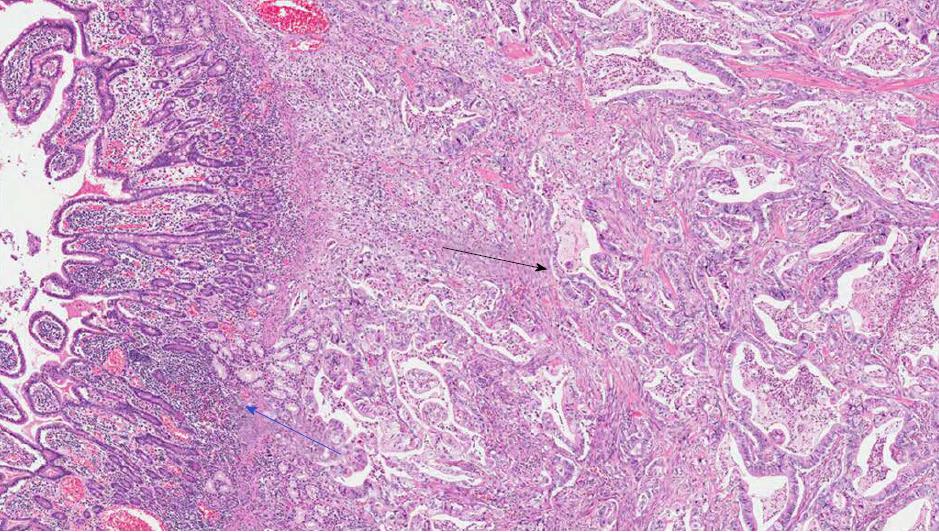
The pancreatobiliary type is morphologically similar to pancreatic ductal adenocarcinoma or to the cancer of the extra-pancreatic bile duct. Complex tubular glands composed of atypical cells and associated with a prominent desmoplastic stroma characterized this subtype (Figure 3)[11,22]. At immunohistochemistry, those cells stain positively for MUC1, MUC5AC and CK7[27].
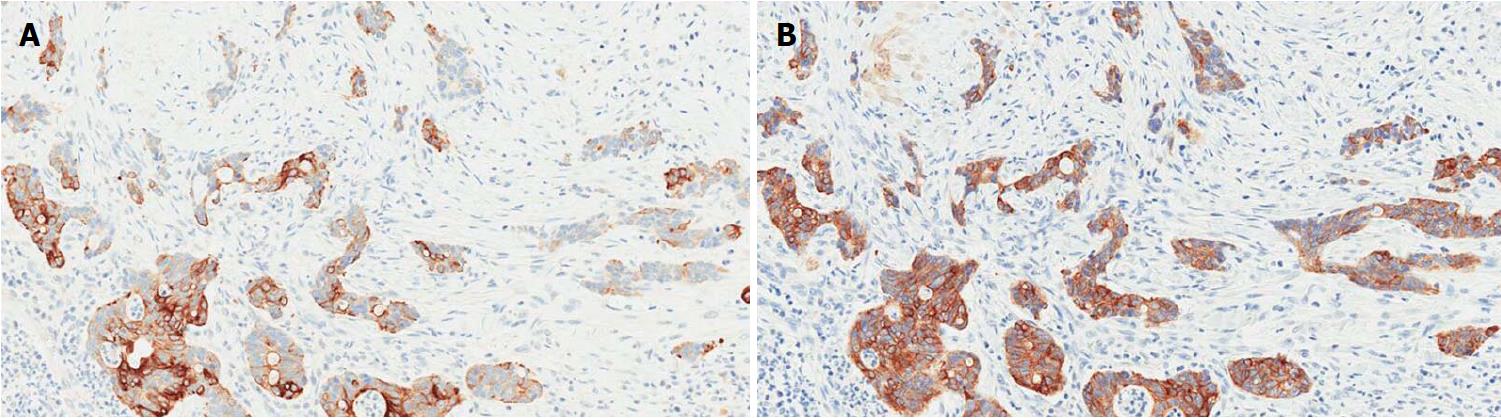
A significant proportion of AVCs, ranging between 18% and 40%, presents a hybrid phenotype characterized by overlapping intestinal and pancreatobiliary features[28,29] and frequently by a nondistinctive immunohistochemistry (Figure 4)[28]. These aspects partially explain the high interobserver variability among pathologists in classifying AVCs subtypes[15,16,28].
Different immunohistochemical panels have been suggested to overcome the difficulties in histological classification, also in order to stratify AVCs prognosis (Table 1)[12,13,15-17]. A 4-marker panel including MUC1, CK20, CDX2 and MUC2 has been proposed by Ang et al[12]. This panel has shown improved capacities in defining intermediate/mixed cases, although its correlation with clinical outcomes has not been evaluated. Chang et al[13] proposed a 2-marker panel, composed of CDX2 and MUC1, showing that the PB phenotype was associated with a poor prognosis. However, more recent studies questioning the accuracy and reproducibility of this method failed in identifying direct or significant prognostic correlations with the immunohistochemical patterns[15,16]. Notably, alterations in the “gastric” lineage marker MUC5AC have also been associated with poor outcome in AVCs, but further studies are needed to validate its prognostic role[15].
| Immunohistochemical marker criteria present | Intestinal type | Pancreatobiliary type positive | Mixed/Ambiguous type | Note |
| Ang et al[12] (MUC1, MUC2, CDX2, CK20) | Positive CK20 or CDX2 or MUC2, and negative MUC1 Positive CK20 and CDX2, and MUC2 and any MUC1 | Positive MUC1 and negative CDX2, and negative MUC2 and any CK20 | All other combinations | |
| Chang et al[13] (MUC1, CDX2) | Positive CDX2 or negative MUC1 | Negative CDX2 and positive MUC1 | Not applicable | CDX2 positivity based on H score (percentage of positive cells × intensity of staining) > 35 MUC1 positivity based on any staining |
| Gingras et al[17] (MUC1, CDX2) | Ratio of the CDX2/MUC1 H score ≥ 2 | Ratio of the CDX2/MUC1 H score < 0.5 | Ratio of CDX2/MUC1 H score ≥ 0.5 and < 2 | Use only MUC1 and CDX2 as per Chang et al[13], with H scores for both CDX2 and MUC1 |
| Mafficini et al[16] (MUC1, MUC2, CDX2, CK20) | Positive CK20 or CDX2 or MUC2, and negative MUC1 | Positive MUC1 and negative CDX2, and negative MUC2 and any CK20 | All other combinations |
The morphological heterogeneity that characterizes a significant proportion of AVCs and the lack of a prognostic reliability of the histological classification, either individually or within immunohistochemical panels, led to the integration of molecular alterations into clinical practice in order to better define AVCs prognosis and treatment.
Although AVCs are usually sporadic neoplasms, they can also arise in the context of familial syndromes. Particularly, patients with familiar adenomatous polyposis (FAP) frequently develop duodenal adenomas and have a 100- to 200-fold increased risk of developing AVCs[7,30,31]. A previous seminal manuscript has indicated that sporadic AVCs differ from those occurring in FAP, according to frequency (17% vs 64%), as well as in the site of APC somatic mutations, suggesting a different molecular pathogenesis for the two conditions[32]. The molecular basis for AVCs initially concentrated on chromosomal alterations, indicating chromosome 5 loss as an early event in AVC carcinogenesis, and chromosome 17p loss as a poor prognostic moderator[33,34].
Recent advances in sequencing technologies have permitted the in-depth characterization of the AVC molecular profile, providing important insights for the comprehension of the biology of this malignancy[14,16,17]. Particularly, two different whole exome sequencing analyses for a total of 240 patients have refined the knowledge about the mutational landscape of AVCs[14,17]. Both studies confirmed the presence of recurrent alterations in well-known AVC-related genes, including TP53, KRAS and those belonging to the Wnt-pathway, such as APC; at the same time, ELF3 has been indicated as a novel AVC driver gene in this kind of tumor[14].
The association between driver mutations and histological subtypes has been evaluated with conflicting results. The APC gene, an important actor of the Wnt-signaling pathway, is frequently mutated in the intestinal subtype (50%-65% of cases), similar to colorectal cancer[35], while the pancreatobiliary type exhibits a higher prevalence of mutations in the pancreatic driver genes KRAS, TP53 and SMAD4, with similar frequencies to pancreatic cancer[14,17,36].
Although histological subtypes show differences in prevalence for some genes (Table 2), important drivers, including KRAS, TP53 and ELF3, can be found mutated in all histotypes. The lack of a specific genetic signature for the histological types suggests the existence of common biological mechanisms in the development of ampullary carcinoma, highlighting the heterogeneity of AVCs from the morphological to the molecular levels. This further calls for a reconsideration of the utility of the histological classification, since the genetic landscape indicates the lack of a specific distinction corresponding to morphology[16].
| Yachida et al[14] | Gingras et al[17] | Biankin et al[36] Pancreatic carcinoma, % | Colorectal Carcinoma (TCGA), %[35] | |||
| Intestinal type, % | Pancreato-biliary type, % | Mixed type, % | Pancreato-biliary type, % | Intestinal type, % | ||
| APC (50) | KRAS (68) | KRAS (50) | TP53 (72) | TP53 (65) | KRAS (99) | APC (81) |
| TP53 (39) | TP53 (67) | APC (50) | KRAS (65) | KRAS (46) | TP53 (33) | TP53 (60) |
| KRAS (39) | SMAD4 (20) | TP53 (41) | SMAD4 (18) | APC (41) | SMAD4 (16) | KRAS (43) |
| CTNNB1 (26) | CTNNB1 (15) | SMARCA4 (27) | CDKN2A (16) | PIK3CA (26) | MLL3 (7) | TTN (31) |
| ARID2 (18) | ERBB3 (14) | PIK3CA (23) | PIK3CA (13) | SMAD4 (20) | ATM (5) | PIK3CA (18) |
| ERBB2 (14) | GNAS (12) | SMAD 4 (23) | ARID1A (13) | TGFBR2 (17) | NALCN (5) | FBXW7 (14) |
| ACVR2A (13) | CDH10 (12) | SOX 9 (23) | APC (11) | ARID2 (17) | ARID1A (4) | SMAD4 (10) |
| SMAD4 (13) | ELF3 (11) | CDKN2A (23) | ATM (10) | ELF3 (7) | SF3B1 (4) | NRAS (9) |
| GNAS (13) | CDKN2A (9) | ARID1A (18) | TGFBR2 (10) | CTNNB1 (17) | TGFBR2 (4) | TCF7L2 (9) |
| SOX9 (13) | TGFBR2 (14) | FBXW7 (8) | NF1 (15) | ARID2 (3) | FAM123B (7) | |
Both the recent whole-exome sequencing studies described inactivating mutations in the tumor-suppressor gene ELF3, in respectively 10% and 12% of cases[14,17]. In particular, Yachida et al[14] demonstrated with functional analyses a role of such a gene as an AVC driver. ELF3 encodes an ETS-domain transcription factor that is implicated in the regulation of epithelial differentiation. Using immortalized epithelial cell lines derived from the common bile duct and duodenal mucosa and knocked down for ELF3 expression, they demonstrated ELF3 to enhance proliferation, motility and invasion, associated with the concomitant up-regulation of markers of epithelial-to-mesenchymal transition, such as vimentin, matrix metalloproteinase-1 (MMP1) and MMP9[14]. However, the exact functional role of ELF3 as well as its potential role as a prognostic biomarker or target for therapy needs to be further investigated.
Interestingly, ERBB2 amplification has been demonstrated in up 23% of cases[16,37]. In a recent report, it was observed in 13% of AVCs regardless of histological subtype and was virtually mutually exclusive with downstream mutations in KRAS/NRAS/BRAF, that are responsible for resistance of therapies targeting ERBB2[37].
Molecular profiling of AVCs has recently demonstrated a higher prognostic reliability than the histological subclassification. Indeed, analyzing a cohort of 80 AVCs, Mafficini et al[16] showed that TP53 and KRAS, which were the most frequently mutated genes, were in respectively 41% and 35% of cases, were also independent prognostic predictors of survival regardless of histological subtypes. These data underline the importance of the mixed phenotype and the fact that the ampullary region is composed of various epithelia merging to form the complex epithelium of the ampulla. Common molecular alterations among different subtypes, such as TP53 and KRAS, may indeed represent drivers of tumor progression at an early stage of disease. Whereas other genetic alterations, such as those belonging to the Wnt-pathway and those characterizing the pancreatobiliary type, such as SMAD4 and CDKN2A, may occur at later stages of tumor growth[14,17].
Current treatment approaches do not distinguish patients based on subtypes[38,39], while molecular alterations may select patients that respond to different chemotherapeutic regimens, regardless of a clear histological differentiation[17]. In particular, clinical testing for Wnt-signaling and microsatellite instability (MSI) could be used to subclassify tumors for target therapies since therapies targeting the Wnt-pathway are in development and MSI-positive tumors may respond to immunotherapeutic approaches[17]. The detection of molecular alterations typical of late-stages may in the future support the choice of radical surgery with lymphadenectomy, rather than more conservative approaches. This highlights the importance of genetic analysis and the need of its future integration within the conventional pathology report.
The staging of AVCs is challenging due to the high complexity of this district and the three-dimensional spread pattern of tumors occurring in this region. In the new AJCC Cancer Staging System Manual, 8th edition[21], the pathological tumor (pT) stages have been reclassified, taking into account the degree of extensions and therefore improving the clinical and prognostic relevance of each pT stage (Table 3). In particular, new subsets for pT1, pT2 and pT3 have been introduced according to survival analyses and suggesting further prognostic variability[40]; the new pT4 stage comprises tumors involving peripancreatic arteries/axes, harmonizing with the exocrine pancreatic cancer staging system.
| Primary tumor (T) | |
| T category | T criteria |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to ampulla of Vater or sphincter of Oddi, or tumor invades beyond the sphincter of Oddi (perisphincteric invasion) and/or into the duodenal submucosa |
| T1a | Tumor limited to ampulla of Vater or sphincter of Oddi |
| T1b | Tumor invades beyond the sphincter of Oddi (perisphincteric invasion) and/or into the duodenal submucosa |
| T2 | Tumor invades into the muscularis propria of the duodenum |
| T3 | Tumor directly invades the pancreas (up to 0.5 cm) or tumor extends more than 0.5 cm into the pancreas, or extends into peripancreatic or periduodenal tissue or duodenal serosa without involvement of the celiac axis or superior mesenteric artery |
| T3a | Tumor directly invades pancreas, up to 0.5 cm |
| T3b | Tumor extends more than 0.5 cm into the pancreas, or extends into peripancreatic tissue or duodenal serosa without involvement of the celiac axis or superior mesenteric artery |
| T4 | Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery, irrespective of size |
| Regional lymph nodes (N) | |
| N category | N criteria |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to 1 to 3 regional lymph nodes |
| N2 | Metastasis in > 3 regional lymph nodes |
Metastatic lymph nodes are present in up to 60% of surgically resected AVCs[41-43], with a higher rate for pancreatobiliary than intestinal type carcinomas (55% vs 18%)[11]. The new staging system categorized the presence of nodal metastases in a three-tiered scale: N0 (no metastatic lymph node), N1 (one or two metastatic lymph nodes) and N2 (three or more metastatic lymph nodes); this subclassification has demonstrated a better predictive value in stratifying the prognosis than the previous dichotomous categories N0 (no metastatic lymph node) vs N1 (at least one metastatic lymph node). To reach a reliable value, the gross sampling of the surgical specimen should include a minimum of 12 lymph nodes[44]. However, since pancreatico-duodenal nodes are the most frequently involved and are usually resected within the specimen (pancreatico-duodenectomy), even if the minimum threshold of 12 is not met, pN0 should still be assigned. Notably, a preferential lymphatic spread from pancreatico-duodenal nodes to lymph nodes around the superior mesenteric artery has been suggested, highlighting the importance of a systemic and radical lymphadenectomy in this area[45].
The risk for lymph node metastases according to the T stage is clinically relevant since endoscopic ampullectomy has been proposed for early AVCs. Surgical series assessed a 8%-45% risk of lymph node metastases in tumors limited to ampulla of Vater and/or sphincter of Oddi (pT1a and pT1b, respectively, of the new staging system)[42,46-48]. The role of local excisions in surgically fit patients remains, being therefore controversial due to the relevant risk of lymph node metastases also in resected early cancers. Another pT-related issue regards the extra-nodal extension of nodal metastases, a histological feature indicating that the metastatic cells have reached the perinodal adipose tissue. In the new staging system, it has been not taken into account, whereas it has been demonstrated as an important prognostic factor in patients with AVC and other solid malignancies[49-57].
Other prognostic factors not included in the staging system but with a potential prognostic role are included among the histologic grading and the perineural invasion.
In the majority of cases, AVCs are present with obstructive jaundice, resulting in a high resectability rate at diagnosis[4,58,59]. Other symptoms, although less common, are upper gastrointestinal bleeding, pancreatitis and unspecific abdominal pain[60-62]. Ampullary tumors can even be incidentally discovered during endoscopic procedures or at cross-sectional imaging performed for other reasons. Despite the potentially high resectability rate, only up to 40% of patients undergo surgical resection[6], mostly due to the advanced age of presentation and the significant morbidity and mortality associated with pancreatic surgery.
The diagnostic work-up usually involves abdominal imaging using ultrasonography, computed tomography and/or magnetic resonance, aiming at excluding other causes of jaundice and at disease staging. Because of the anatomical location and the frequent small size of the tumor, an ampullary mass is often difficult to detect, but indirect signs such as pancreatic and/or bile duct obstruction/dilation can be observed[62,63].
Endoscopy plays a major role in the differential diagnosis of an altered papilla (either bulging or ulcerated) as well as in the local staging of the disease. Endoscopic biopsies are characterized by high false negative rates for adenocarcinoma, often underestimating the actual pathology[47,64], whereas endoscopic ultrasonography (EUS) guided-biopsies improve the diagnostic accuracy, assessing the correct pathology in almost 90% of the cases[65]. In the local staging of ampullary masses, EUS plays a primary role thanks to its capacities of estimating the depth of tumor infiltration within the duodenal wall and in predicting the presence of local node metastases[66-68], although their definitive demonstration is reserved for the histological examination.
Radical resection represents, to date, the only established curative option for AVCs, while an endoscopic papillectomy is indicated for noninvasive tumors. A radical resection with an adequate lymphoadenectomy is usually recommended for invasive tumors, even if very small, due to the nonnegligible risk of lymph node metastasization or of incomplete tumor resection. The correct local staging is essential to guide further treatment decisions.
Endoscopic papillectomy is the treatment of choice for benign or noninvasive ampullary lesions. When EUS shows a lesion confined within the mucosa, and there are not histological features of invasion or of high-grade dysplasia upon biopsy, endoscopic ampullectomy should be performed[69,70]. The following histological examination of the endoscopic specimen must report the status of the resection margins and consider the potential presence of an invasive component[66,71,72]. In the case of high-grade dysplasia determined by endoscopic biopsy, an underlying adenocarcinoma on definitive pathology is present in 50%–100% of patients and usually in the context of voluminous intestinal-like villous adenomas, usually larger than tubular adenomas, and for which a radical endoscopic ampullectomy may be difficult[66,73].
However, endoscopic ampullectomy should be considered part of the diagnostic process and potentially curative in cases of high-grade dysplasia and clear resection margins at the final pathological evaluation of the specimen. Considering the significant morbidity and mortality associated with pancreatic surgery, endoscopic papillectomy has also been suggested for early ampullary carcinoma, in particular for pT1 tumors[46,48,68]. However, to date, this indication remains to date controversial, mainly due to the clinically relevant risk of lymph node metastases and the high rate of positive resection margins, reserving this procedure for patients unfit for surgical resection[74]. Endoscopic ampullectomy is a safe procedure, characterized by a relatively low rate (about 10%) of postprocedural complications, the most common being acute pancreatitis, followed by papillary stenosis, cholangitis and bleeding[75-78]. Most of these complications can be prevented by the placement of temporary pancreatic and biliary stents[77,79,80].
Surgical ampullectomy has been proposed as an alternative to pancreaticoduodenectomy for selected patients with ampullary neoplasms[81]. This procedure is characterized by lower morbidity and mortality than major surgery, also allowing for performance of a partial lymphadenectomy (excluding the lymph nodes from the superior mesentery artery). However, its role in the treatment of AVCs is controversial, for the difficulties to obtain a radical resection[47,71,82]. Surgical ampullectomy shares the same complications of the endoscopic ampullectomy, with the risk of duodenal dehiscence and intra-abdominal collections as well as additional complications[71,83].
The current acceptable standard of care for resectable AVCs remains the pancreatico-duodenectomy, either with conventional or pylorus-preserving approach[42,46,47,84]. Surgery for AVCs is characterized by a high resectability rate, with close to 90% of cases undergoing laparotomy[7,24,85], but also by a higher rate of significant complications than pancreato-duodenectomies performed for pancreatic cancer. Such complications include pancreatic fistula, pneumonia, intra-abdominal infection, anastomotic leak, and delayed gastric emptying[86].
The histological subtypes have revealed major issues on both interobserver reproducibility and its prognostic reliability. Since the ampulla of Vater is the crossroad of three distinct epithelia, the study of the tumors arising in such a location represents a unique opportunity to better refine the knowledge about all periampullary cancers. The anatomical features of the ampulla of Vater may explain the histological heterogeneity of AVCs and the importance of also taking into account the mixed entity. Indeed, a significant part of this tumor type does not meet all the criteria for a definitive subclassification as intestinal or pancreaticobiliary-type. On the basis of such considerations, the integration of the molecular data appears as a fundamental step in understanding AVCs’ biology, helping in better stratifying the prognosis, and highlighting potential targets for tailored therapy. Future therapeutic research studies should investigate, more in-depth, the AVCs histological and molecular features, which may represent the key to resolving intestinal-pancreaticobiliary heterogeneity.
Manuscript source: Invited Manuscript
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent):
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Sun LM, Bhatti A S- Editor: Dou Y L- Editor: Filipodia E- Editor: Tan WW
| 1. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC 2010; . |
| 2. | Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Neuroendocrine tumors of the ampulla of Vater: biological behavior and surgical management. Arch Surg. 2009;144:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Noë M PA, Luchini C, Felsenstein M, Barbi S, Bhaijee B, Yonescu R, NingY , Adsay V, Zamboni G, Lawlor RT. Whole exome sequencing of ampullary somatostatinomas in patients with neurofibromatosis type 1. Modern Pathol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Neoptolemos JP, Talbot IC, Carr-Locke DL, Shaw DE, Cockleburgh R, Hall AW, Fossard DP. Treatment and outcome in 52 consecutive cases of ampullary carcinoma. Br J Surg. 1987;74:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Benhamiche AM, Jouve JL, Manfredi S, Prost P, Isambert N, Faivre J. Cancer of the ampulla of Vater: results of a 20-year population-based study. Eur J Gastroenterol Hepatol. 2000;12:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Talamini MA, Moesinger RC, Pitt HA, Sohn TA, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann Surg. 1997;225:590-599; discussion 599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Nakase A, Matsumoto Y, Uchida K, Honjo I. Surgical treatment of cancer of the pancreas and the periampullary region: cumulative results in 57 institutions in Japan. Ann Surg. 1977;185:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 156] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Baczako K, Büchler M, Beger HG, Kirkpatrick CJ, Haferkamp O. Morphogenesis and possible precursor lesions of invasive carcinoma of the papilla of Vater: epithelial dysplasia and adenoma. Hum Pathol. 1985;16:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 10. | Kozuka S, Tsubone M, Yamaguchi A, Hachisuka K. Adenomatous residue in cancerous papilla of Vater. Gut. 1981;22:1031-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kimura W, Futakawa N, Yamagata S, Wada Y, Kuroda A, Muto T, Esaki Y. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. 1994;85:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 163] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 12. | Ang DC, Shia J, Tang LH, Katabi N, Klimstra DS. The utility of immunohistochemistry in subtyping adenocarcinoma of the ampulla of vater. Am J Surg Pathol. 2014;38:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, Pinese M, Humphris JL, Jones MD, Toon C. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Yachida S, Wood LD, Suzuki M, Takai E, Totoki Y, Kato M, Luchini C, Arai Y, Nakamura H, Hama N. Genomic sequencing identifies ELF3 as a driver of ampullary carcinoma. Cancer Cell. 2016;29:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 15. | Xue Y, Reid MD, Balci S, Quigley B, Muraki T, Memis B, Xia J, Hacihasanoglu E, Bedolla G, Pehlivanoglu B. Immunohistochemical classification of ampullary carcinomas: critical reappraisal fails to confirm prognostic relevance for recently proposed panels, and highlights MUC5AC as a strong prognosticator. Am J Surg Pathol. 2017;41:865-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Mafficini A, Amato E, Cataldo I, Rusev BC, Bertoncello L, Corbo V, Simbolo M, Luchini C, Fassan M, Cantù C. Ampulla of Vater carcinoma: sequencing analysis identifies TP53 status as a novel independent prognostic factor and potentially actionable ERBB, PI3K, and WNT pathways gene mutations. Ann Surg. 2018;267:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Gingras MC, Covington KR, Chang DK, Donehower LA, Gill AJ, Ittmann MM, Creighton CJ, Johns AL, Shinbrot E, Dewal N. Ampullary cancers harbor ELF3 tumor suppressor gene mutations and exhibit frequent WNT dysregulation. Cell Rep. 2016;14:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Adsay V, Ohike N, Tajiri T, Kim GE, Krasinskas A, Balci S, Bagci P, Basturk O, Bandyopadhyay S, Jang KT. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36:1592-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Albores-Saavedra J HD, Klimstra DS. Tumors of the gallbladder, extrahepatic bile ducts, and ampulla of Vater - atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology 1998; . |
| 20. | Shirai Y, Tsukada K, Ohtani T, Koyama S, Muto T, Watanabe H, Hatakeyama K. Carcinoma of the ampulla of Vater: histopathologic analysis of tumor spread in Whipple pancreatoduodenectomy specimens. World J Surg. 1995;19:102-106; discussion 106-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Herman JM, Pawlik TM, Merchant NB, Tamm EP, Vauthey JN. Ampulla of Vater. 8th ed. AJCC Cancer Staging Manual. 2017;. [DOI] [Full Text] |
| 22. | Kimura W, Futakawa N, Zhao B. Neoplastic diseases of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 23. | Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, Clausen OP, Gladhaug IP. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 238] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Zhou H, Schaefer N, Wolff M, Fischer HP. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Lee JH, Lee KG, Ryou H, Jun YJ, Paik SS, Park HK, Lee KS. Significance analysis of histologic type and perineural invasion as prognostic factors after curative resection of ampulla of Vater carcinoma. Hepatogastroenterology. 2010;57:646-652. [PubMed] |
| 27. | Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol. 2005;29:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Reid MD, Balci S, Ohike N, Xue Y, Kim GE, Tajiri T, Memis B, Coban I, Dolgun A, Krasinskas AM. Ampullary carcinoma is often of mixed or hybrid histologic type: an analysis of reproducibility and clinical relevance of classification as pancreatobiliary versus intestinal in 232 cases. Mod Pathol. 2016;29:1575-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Morini S, Perrone G, Borzomati D, Vincenzi B, Rabitti C, Righi D, Castri F, Manazza AD, Santini D, Tonini G. Carcinoma of the ampulla of Vater: morphological and immunophenotypical classification predicts overall survival. Pancreas. 2013;42:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 292] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Church J, Simmang C; Standards Task Force; American Society of Colon and Rectal Surgeons; Collaborative Group of the Americas on Inherited Colorectal Cancer and the Standards Committee of The American Society of Colon and Rectal Surgeons. Practice parameters for the treatment of patients with dominantly inherited colorectal cancer (familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer). Dis Colon Rectum. 2003;46:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Achille A, Scupoli MT, Magalini AR, Zamboni G, Romanelli MG, Orlandini S, Biasi MO, Lemoine NR, Accolla RS, Scarpa A. APC gene mutations and allelic losses in sporadic ampullary tumours: evidence of genetic difference from tumours associated with familial adenomatous polyposis. Int J Cancer. 1996;68:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Achille A, Baron A, Zamboni G, Di Pace C, Orlandini S, Scarpa A. Chromosome 5 allelic losses are early events in tumours of the papilla of Vater and occur at sites similar to those of gastric cancer. Br J Cancer. 1998;78:1653-1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Scarpa A, Di Pace C, Talamini G, Falconi M, Lemoine NR, Iacono C, Achille A, Baron A, Zamboni G. Cancer of the ampulla of Vater: chromosome 17p allelic loss is associated with poor prognosis. Gut. 2000;46:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6665] [Article Influence: 512.7] [Reference Citation Analysis (0)] |
| 36. | Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1646] [Article Influence: 126.6] [Reference Citation Analysis (0)] |
| 37. | Hechtman JF, Liu W, Sadowska J, Zhen L, Borsu L, Arcila ME, Won HH, Shah RH, Berger MF, Vakiani E. Sequencing of 279 cancer genes in ampullary carcinoma reveals trends relating to histologic subtypes and frequent amplification and overexpression of ERBB2 (HER2). Mod Pathol. 2015;28:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Romiti A, Barucca V, Zullo A, Sarcina I, Di Rocco R, D’Antonio C, Latorre M, Marchetti P. Tumors of ampulla of Vater: A case series and review of chemotherapy options. World J Gastrointest Oncol. 2012;4:60-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, Carter R, Tebbutt NC, Dervenis C, Smith D. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 463] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 40. | You D, Heo J, Choi S, Choi D, Jang KT. Pathologic T1 subclassification of ampullary carcinoma with perisphincteric or duodenal submucosal invasion: Is it T1b? Arch Pathol Lab Med. 2014;138:1072-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Warren KW, Choe DS, Plaza J, Relihan M. Results of radical resection for periampullary cancer. Ann Surg. 1975;181:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Winter JM, Cameron JL, Olino K, Herman JM, de Jong MC, Hruban RH, Wolfgang CL, Eckhauser F, Edil BH, Choti MA. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg. 2010;14:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 43. | Hsu HP, Yang TM, Hsieh YH, Shan YS, Lin PW. Predictors for patterns of failure after pancreaticoduodenectomy in ampullary cancer. Ann Surg Oncol. 2007;14:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Adsay NV, Bagci P, Tajiri T, Oliva I, Ohike N, Balci S, Gonzalez RS, Basturk O, Jang KT, Roa JC. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Miyazaki I. Surgical strategy for carcinoma of the papilla of Vater on the basis of lymphatic spread and mode of recurrence. Surgery. 1997;121:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Yoon YS, Kim SW, Park SJ, Lee HS, Jang JY, Choi MG, Kim WH, Lee KU, Park YH. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg. 2005;242:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | de Castro SM, van Heek NT, Kuhlmann KF, Busch OR, Offerhaus GJ, van Gulik TM, Obertop H, Gouma DJ. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery. 2004;136:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Woo SM, Ryu JK, Lee SH, Lee WJ, Hwang JH, Yoo JW, Park JK, Kang GH, Kim YT, Yoon YB. Feasibility of endoscopic papillectomy in early stage ampulla of Vater cancer. J Gastroenterol Hepatol. 2009;24:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Luchini C, Veronese N, Pea A, Sergi G, Manzato E, Nottegar A, Solmi M, Capelli P, Scarpa A. Extranodal extension in N1-adenocarcinoma of the pancreas and papilla of Vater: a systematic review and meta-analysis of its prognostic significance. Eur J Gastroenterol Hepatol. 2016;28:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Luchini C, Fleischmann A, Boormans JL, Fassan M, Nottegar A, Lucato P, Stubbs B, Solmi M, Porcaro A, Veronese N. Extranodal extension of lymph node metastasis influences recurrence in prostate cancer: a systematic review and meta-analysis. Sci Rep. 2017;7:2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Luchini C, Wood LD, Cheng L, Nottegar A, Stubbs B, Solmi M, Capelli P, Pea A, Sergi G, Manzato E. Extranodal extension of lymph node metastasis is a marker of poor prognosis in oesophageal cancer: a systematic review with meta-analysis. J Clin Pathol. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Nottegar A, Veronese N, Senthil M, Roumen RM, Stubbs B, Choi AH, Verheuvel NC, Solmi M, Pea A, Capelli P. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory meta-analysis. Eur J Surg Oncol. 2016;42:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 53. | Veronese N, Luchini C, Nottegar A, Kaneko T, Sergi G, Manzato E, Solmi M, Scarpa A. Prognostic impact of extra-nodal extension in thyroid cancer: A meta-analysis. J Surg Oncol. 2015;112:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Veronese N, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, Sergi G, Manzato E, Fassan M, Wood LD. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol. 2016;27:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Luchini C, Nottegar A, Solmi M, Sergi G, Manzato E, Capelli P, Scarpa A, Veronese N. Prognostic implications of extranodal extension in node-positive squamous cell carcinoma of the vulva: A systematic review and meta-analysis. Surg Oncol. 2016;25:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Veronese N, Fassan M, Wood LD, Stubbs B, Solmi M, Capelli P, Pea A, Nottegar A, Sergi G, Manzato E. Extranodal extension of nodal metastases is a poor prognostic indicator in gastric cancer: a systematic review and meta-analysis. J Gastrointest Surg. 2016;20:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Luchini C, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, Sergi G, Manzato E, Fassan M, Wood LD. Extranodal extension is an important prognostic parameter for both colonic and rectal cancer. Ann Oncol. 2016;27:955-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Bakkevold KE, Arnesjø B, Kambestad B. Carcinoma of the pancreas and papilla of Vater--assessment of resectability and factors influencing resectability in stage I carcinomas. A prospective multicentre trial in 472 patients. Eur J Surg Oncol. 1992;18:494-507. [PubMed] |
| 59. | Walsh DB, Eckhauser FE, Cronenwett JL, Turcotte JG, Lindenauer SM. Adenocarcinoma of the ampulla of Vater. Diagnosis and treatment. Ann Surg. 1982;195:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg. 2008;207:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 61. | Petrou A, Bramis K, Williams T, Papalambros A, Mantonakis E, Felekouras E. Acute recurrent pancreatitis: a possible clinical manifestation of ampullary cancer. JOP. 2011;12:593-597. [PubMed] |
| 62. | Tsukada K, Takada T, Miyazaki M, Miyakawa S, Nagino M, Kondo S, Furuse J, Saito H, Tsuyuguchi T, Kimura F. Diagnosis of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:31-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Kim TU, Kim S, Lee JW, Woo SK, Lee TH, Choo KS, Kim CW, Kim GH, Kang DH. Ampulla of Vater: comprehensive anatomy, MR imaging of pathologic conditions, and correlation with endoscopy. Eur J Radiol. 2008;66:48-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Jung S, Kim MH, Seo DW, Lee SK. Endoscopic snare papillectomy of adenocarcinoma of the major duodenal papilla. Gastrointest Endosc. 2001;54:622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Defrain C, Chang CY, Srikureja W, Nguyen PT, Gu M. Cytologic features and diagnostic pitfalls of primary ampullary tumors by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2005;105:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Askew J, Connor S. Review of the investigation and surgical management of resectable ampullary adenocarcinoma. HPB (Oxford). 2013;15:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Chen CH, Yang CC, Yeh YH, Chou DA, Nien CK. Reappraisal of endosonography of ampullary tumors: correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound. 2009;37:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | ASGE Standards of Practice Committee. Chathadi KV, Khashab MA, Acosta RD, Chandrasekhara V, Eloubeidi MA, Faulx AL, Fonkalsrud L, Lightdale JR, Salztman JR, Shaukat A, Wang A, Cash BD, DeWitt JM. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 70. | van Stolk R, Sivak MV Jr, Petrini JL, Petras R, Ferguson DR, Jagelman D. Endoscopic management of upper gastrointestinal polyps and periampullary lesions in familial adenomatous polyposis and Gardner’s syndrome. Endoscopy. 1987;19 Suppl 1:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 197] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 72. | Panzeri F, Crippa S, Castelli P, Aleotti F, Pucci A, Partelli S, Zamboni G, Falconi M. Management of ampullary neoplasms: A tailored approach between endoscopy and surgery. World J Gastroenterol. 2015;21:7970-7987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 73. | Kim JH, Kim JH, Han JH, Yoo BM, Kim MW, Kim WH. Is endoscopic papillectomy safe for ampullary adenomas with high-grade dysplasia? Ann Surg Oncol. 2009;16:2547-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Kobayashi A, Konishi M, Nakagohri T, Takahashi S, Kinoshita T. Therapeutic approach to tumors of the ampulla of Vater. Am J Surg. 2006;192:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Catalano MF, Linder JD, Chak A, Sivak MV Jr, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 76. | Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | Bohnacker S, Seitz U, Nguyen D, Thonke F, Seewald S, deWeerth A, Ponnudurai R, Omar S, Soehendra N. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 78. | Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, Howard TJ, Lazzell-Pannell L, Lehman GA. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 79. | Yamao T, Isomoto H, Kohno S, Mizuta Y, Yamakawa M, Nakao K, Irie J. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 81. | Tien YW, Yeh CC, Wang SP, Hu RH, Lee PH. Is blind pancreaticoduodenectomy justified for patients with ampullary neoplasms? J Gastrointest Surg. 2009;13:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Lindell G, Borch K, Tingstedt B, Enell EL, Ihse I. Management of cancer of the ampulla of Vater: does local resection play a role? Dig Surg. 2003;20:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Sauvanet A, Regimbeau JM, Jaeck D. [Technique of surgical ampullectomy]. Ann Chir. 2004;129:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Yeh CC, Jeng YM, Ho CM, Hu RH, Chang HP, Tien YW. Survival after pancreaticoduodenectomy for ampullary cancer is not affected by age. World J Surg. 2010;34:2945-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 85. | Bettschart V, Rahman MQ, Engelken FJ, Madhavan KK, Parks RW, Garden OJ. Presentation, treatment and outcome in patients with ampullary tumours. Br J Surg. 2004;91:1600-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Pulvirenti A, Marchegiani G, Pea A, Allegrini V, Esposito A, Casetti L, Landoni L, Malleo G, Salvia R, Bassi C. Clinical Implications of the 2016 International Study Group on Pancreatic Surgery Definition and Grading of Postoperative Pancreatic Fistula on 775 Consecutive Pancreatic Resections. Ann Surg. 2017;268:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (1)] |