Published online Oct 15, 2018. doi: 10.4251/wjgo.v10.i10.351
Peer-review started: June 2, 2018
First decision: July 10, 2018
Revised: July 17, 2018
Accepted: August 26, 2018
Article in press: August 26, 2018
Published online: October 15, 2018
Processing time: 135 Days and 23.2 Hours
To assess the long-term prognostic value of vascular endothelial growth factor receptor 1 (VEGFR1) and class III β-tubulin (TUBB3) mRNA expression in non-metastatic rectal cancer.
A total of 75 consecutive patients with non-metastatic rectal cancer from March 2004 to November 2008 were analyzed retrospectively at our institute. The mRNA expressions of VEGFR1 and TUBB3 were detected by multiplex branched DNA liquid-chip technology. The Cutoff Finder application was applied to determine cutoff point of mRNA expression. SPSS software version 22.0 was used for analysis.
The median follow-up was 102.7 mo (range, 6-153.6). The χ2 and Fisher’s exact tests showed that VEGFR1 expression was related to lymph node metastasis (P = 0.013), while no relationships between TUBB3 and clinicopathological features were observed. Univariate analysis showed that T stage, lymph node metastasis, tumor differentiation, VEGFR1 and TUBB3 mRNA expression were correlated to overall survival (OS) (P = 0.048, P = 0.003, P = 0.052, P = 0.003 and P = 0.015, respectively). Also, lymph node metastasis and VEGFR1 expression independently influenced OS by multivariate analysis (P = 0.027 and P = 0.033). VEGFR1 expression was positively correlated with TUBB3 (P = 0.024). The patients with low expression of both TUBB3 and VEGFR1 presented a better OS (P = 0.003). In addition, the receiver operating characteristic analysis suggested that the combination of lymph node metastasis and VEGFR1 had a more favorable prognostic value (P < 0.001).
VEGFR1 expression and lymph node metastasis independently and jointly affect survival. Moreover, low expression of VEGFR1 and TUBB3 presented a better OS in patients with non-metastatic rectal cancer, which might serve as a potential prognostic factor.
Core tip: Nowadays, personalized and precision medicine becomes vital in cancer treatment. Herein, we focus on the long-term prognostic value of vascular endothelial growth factor receptor 1 (VEGFR1) and class III β-tubulin (TUBB3) mRNA expression in non-metastatic rectal cancer. In the 75 consecutive patients enrolled, we found that VEGFR1 expression and lymph node metastasis were independent factors influencing overall survival, and the combination of them showed a favorable prognostic value. Also, VEGFR1 expression was significantly related to lymph node metastasis. In addition, VEGFR1 expression was positively correlated with TUBB3 expression.
- Citation: Kong XQ, Huang YX, Li JL, Zhang XQ, Peng QQ, Tang LR, Wu JX. Prognostic value of vascular endothelial growth factor receptor 1 and class III β-tubulin in survival for non-metastatic rectal cancer. World J Gastrointest Oncol 2018; 10(10): 351-359
- URL: https://www.wjgnet.com/1948-5204/full/v10/i10/351.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i10.351
Rectal cancer is one of the most diagnosed malignancies among both males and females worldwide with worse outcomes than colon cancer[1,2]. Clinically, patients showed various outcomes to multimodality therapies. Nowadays, personalized and precision medicine has become essential in the treatment of rectal cancer. Recent studies conducted gene expression profiling to predict the response and long-term prognosis of malignancies[3,4]; however, no consensus was achieved on prognostic gene profiling for rectal cancer.
Vascular endothelial growth factor (VEGF) possesses a significant role in angiogenesis by binding to VEGFR1 and VEGFR2, which is required for cancer progression and metastasis[5,6]. A phase II trial indicated that VEGF could predict the pathological response to locally advanced rectal cancer patients treated with neoadjuvant cetuximab-based chemoradiation[7]. In addition, class III β-tubulin (TUBB3) has been reported to play a critical role in tumor development and malignant transformation as a β-tubulin isotype. The variable levels of expression of the gene have been reported in colon, lung, ovary, kidney, prostate, and throat cancer with solid tumors[8-10]. However, only a few studies focused on its role in rectal cancer.
Herein, our study attempted to explore the potential prognostic value of VEGFR1 and TUBB3 for long-term survival in non-metastatic rectal cancer.
Eighty cases of well-preserved formalin-fixed and paraffin embedded tumor tissue specimens that had undergone total mesorectal excision (TME) at the Fujian Cancer Hospital from March 2004 to November 2008 were retrospectively examined. Among these, two patients with previous malignancy and three with distant metastasis were excluded. Finally, 75 patients who fulfilled the following inclusion criteria were enrolled in the study: (1) Pathologically confirmed as primary rectal adenocarcinoma; (2) underwent TME; (3) no evidence of distant metastasis; (4) no previous or concurrent malignancy; and (5) complete follow-up information was obtained.
The variables such as gender, age, preoperative carcino-embryonic antigen (pre-CEA), pre-operative hemoglobin (pre-Hb), distance to the verge, T stage, lymph node metastasis, venous invasion, and tumor differentiation were considered. The T stage and lymph node metastasis were re-diagnosed based on the 8th Edition of the American Joint Committee on Cancer (AJCC)[11].
All patients underwent TME, including abdominoperineal resection and low anterior resection. Of these, eight cases received neoadjuvant chemoradiotherapy followed by TME. A total of 66 cases received 5-fluorouracil (5-FU)-based chemotherapy. The overall survival (OS) was defined as the duration from the date of diagnosis to the last follow-up or the date of death due to any cause, which was obtained from the medical records and telephonic interviews.
The formalin-fixed and paraffin embedded (FFPE) tumor tissue specimens containing more than 70% of tumor cells were selected. The Multiplex branched DNA liquidchip (MBL) technology (Guangzhou SurExam Bio-Tech Co., Ltd., China) was implemented to determine the mRNA expression levels of VEGFR1 and TUBB3. The FFPE tissue samples were lysed in the presence of proteinase K, at 56°C for 2 h. Then, the lysate was transferred to a 96-well plate containing the blocking reagent, capture beads with probes for VEGFR1 and TUBB3, and target gene-specific probe sets. The sandwich nucleic acid hybridization was carried out for 16 h. The unbound RNA was removed by three washes with buffer under a vacuum system. The signal bound to the target mRNA was amplified with a streptavidin-conjugated phycoerythrin solution at 50°C for 30 min. The fluorescence values of the samples were identified and analyzed using Luminex 200 system (Luminex, Austin, TX, United States), which were regarded as the RNA expression levels of each gene. The cutoff point of mRNA expression affecting the survival was determined by the Cutoff Finder application[12].
The end point of our analysis was OS. The association of gene expression level and clinicopathological features was studied by the χ2 and Fisher’s exact tests. The association between the mRNA expressions of VEGFR1 and TUBB3 was studied by the Spearman correlation test. The Kaplan–Meier test was used to analyze the OS, and Cox regression model (LR forward) was employed for univariate and multivariate analysis. Receiver operating characteristic (ROC) analysis was employed for assessing the specificity as well as the sensitivity of predicting OS by specific parameters. The statistical significance of area under the ROC (area under curve, AUC) was calculated by Delong’s test[13]. P-values < 0.05 were deemed significant. The statistical analysis was conducted by SPSS version 22.0 (IBM Corporation, Armonk, NY, United States). The statistical methods of our study were reviewed by Qian-yu Ni from The First Affiliated Hospital of Fujian Medical University.
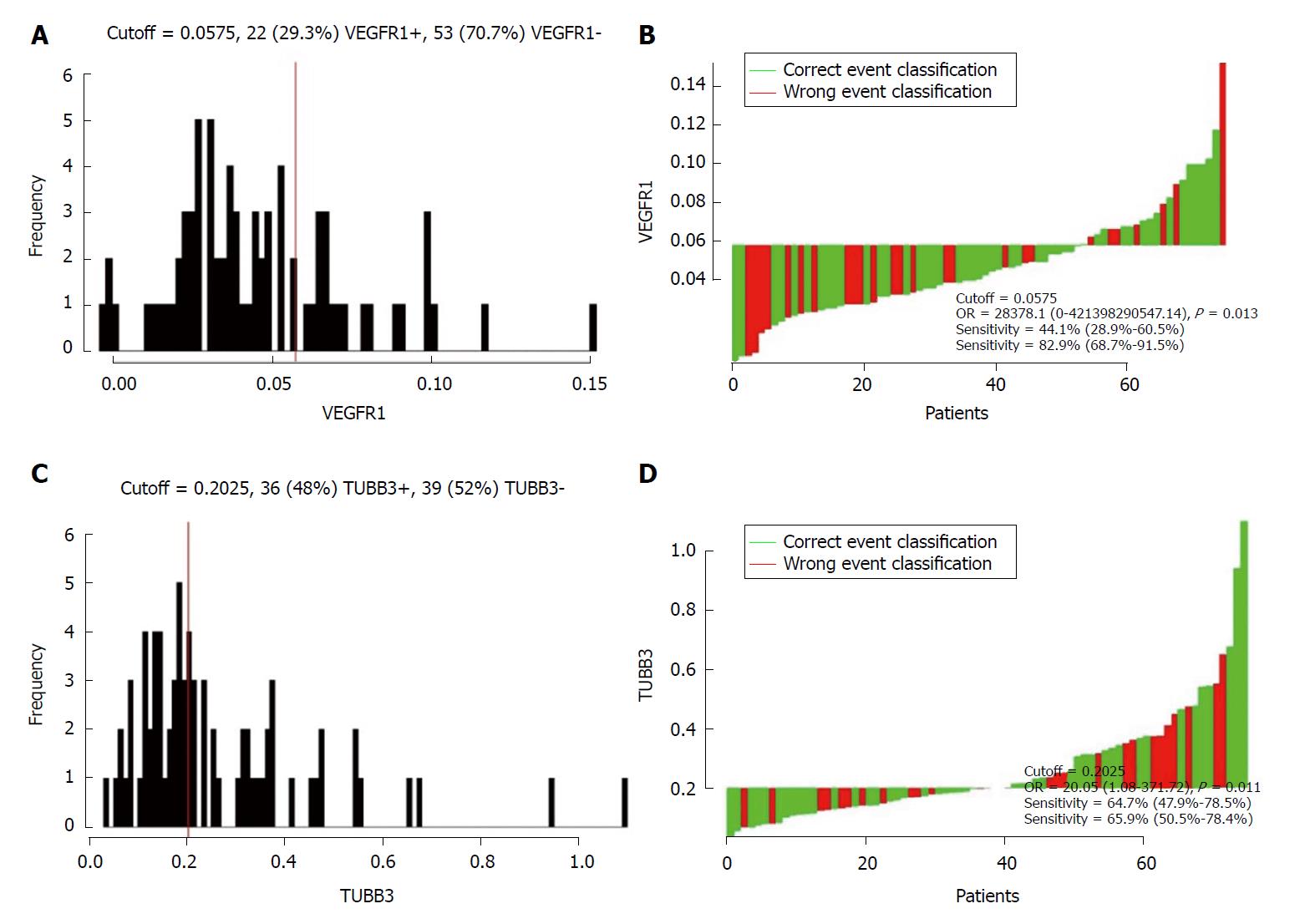
A total of 75 patients were enrolled in the present study. The characteristics of non-metastatic patients are summarized in Table 1. Median follow-up time was 102.7 mo (range: 6.0-153.6). The cohort comprised of 39 (52%) male and 36 (48%) female cases with the median age 52 years (range, 29-74). Among these patients, 21 (36.8%) cases presented pre-CEA records that were higher than 5 ng/mL, while they could not be accessed for 18 cases. In the case of pre-Hb, 26 (34.7%) patients were ≤ 120 g/L and the remaining were > 120 g/L. In terms of the tumor location, 46 (61.3%) patients had low rectal cancer (0-5 cm distance to verge), while the other 29 (38.7%) patients were > 5 cm. In all, 22 (29.3%) with lymph node metastasis positive and 53 (70.6%) were negative. Twenty (26.7%) patients were identified as poorly differentiated and 55 (73.3%) as moderate-to-well differentiated. According to the Cutoff Finder software, 0.0575 and 0.2025 were considered as the optimal cutoff point for the VEGFR1 and TUBB3 expression value, respectively (Figure 1). In addition, 36 (48%) and 22 (29.3%) patients showed a high expression of VEGFR1 and TUBB3, respectively.
| Characteristics | Data, n (%) |
| Gender | |
| Female | 36 (48) |
| Male | 39 (52) |
| Age (yr) | |
| median (range) | 52 (29-74) |
| ≤ 60 | 58 (77.3) |
| > 60 | 17 (22.7) |
| Pre-CEA (ng/mL) | |
| ≤ 5 | 36 (63.2) |
| > 5 | 21 (36.8) |
| Pre-Hb (g/L) | |
| ≤ 120 | 26 (34.7) |
| > 120 | 49 (65.3) |
| Distance to verge (cm) | |
| ≤ 5 | 46 (61.3) |
| > 5 | 29 (38.7) |
| T stage | |
| T1 + T2 | 13 (17.3) |
| T3 + T4 | 63 (82.6) |
| Lymph node metastasis | |
| Negative | 22 (29.3) |
| Positive | 53 (70.6) |
| Venous invasion | |
| Negative | 68 (90.7) |
| Positive | 7 (9.3) |
| Tumor differentiation | |
| Poorly differentiated | 20 (26.7) |
| Moderately-well differentiated | 55 (73.3) |
| Chemotherapy | |
| No | 9 (12) |
| Yes | 66 (88) |
| TUBB3 expression | |
| Low-expression | 39 (52) |
| High-expression | 36 (48) |
| VEGFR1 expression | |
| Low-expression | 53 (70.7) |
| High-expression | 22 (29.3) |
| TUBB3 and VEGFR1 | |
| Both low expression | 32 (42.6) |
| Others | 43 (57.3) |
The correlations between VEGFR1/TUBB3 mRNA expression and clinicopathological features were analyzed (Table 2). A majority of the patients displayed positive lymph node metastasis in the high-expression group of VEGFR1 (P = 0.013). However, no significant difference was found between the expression level of TUBB3 expression and clinicopathological features (gender, age, pre-CEA, pre-Hb, distance to the verge, T stage, lymph node metastasis and venous invasion, all P > 0.05).
| TUBB3 | VEGFR1 | |||||
| Parameter | Low (n) | High (n) | P | Low (n) | High (n) | P |
| Gender | 0.426 | 0.081 | ||||
| Female | 17 | 19 | 22 | 14 | ||
| Male | 22 | 17 | 31 | 8 | ||
| Age (yr) | 0.31 | 1 | ||||
| ≤ 60 | 32 | 26 | 41 | 17 | ||
| > 60 | 7 | 10 | 12 | 5 | ||
| Pre-CEA | 0.203 | 0.244 | ||||
| ≤ 5 | 20 | 16 | 26 | 10 | ||
| > 5 | 8 | 13 | 12 | 9 | ||
| Pre-Hb | 0.801 | 0.206 | ||||
| ≤ 120 | 13 | 13 | 16 | 10 | ||
| > 120 | 26 | 23 | 37 | 12 | ||
| Distance to verge (cm) | 0.608 | 0.792 | ||||
| ≤ 5 | 25 | 21 | 32 | 14 | ||
| > 5 | 14 | 15 | 21 | 8 | ||
| T stage | 0.883 | 0.744 | ||||
| T1 + T2 | 7 | 6 | 10 | 3 | ||
| T3 + T4 | 32 | 30 | 43 | 19 | ||
| Lymph node metastasis | 0.071 | 0.013 | ||||
| Negative | 15 | 7 | 20 | 2 | ||
| Positive | 24 | 29 | 33 | 20 | ||
| Tumor thrombus | 0.25 | 1 | ||||
| Negative | 37 | 31 | 48 | 20 | ||
| Positive | 2 | 5 | 5 | 2 | ||
| Tumor differentiation | 0.754 | 0.939 | ||||
| Poorly | 11 | 9 | 14 | 6 | ||
| Moderately-well | 28 | 27 | 39 | 16 | ||
| Chemotherapy | 0.156 | 0.051 | ||||
| No | 7 | 2 | 9 | 0 | ||
| Yes | 32 | 34 | 44 | 22 | ||
| VEGFR1 | 0.024 | |||||
| Low | 32 | 21 | ||||
| High | 7 | 15 | ||||
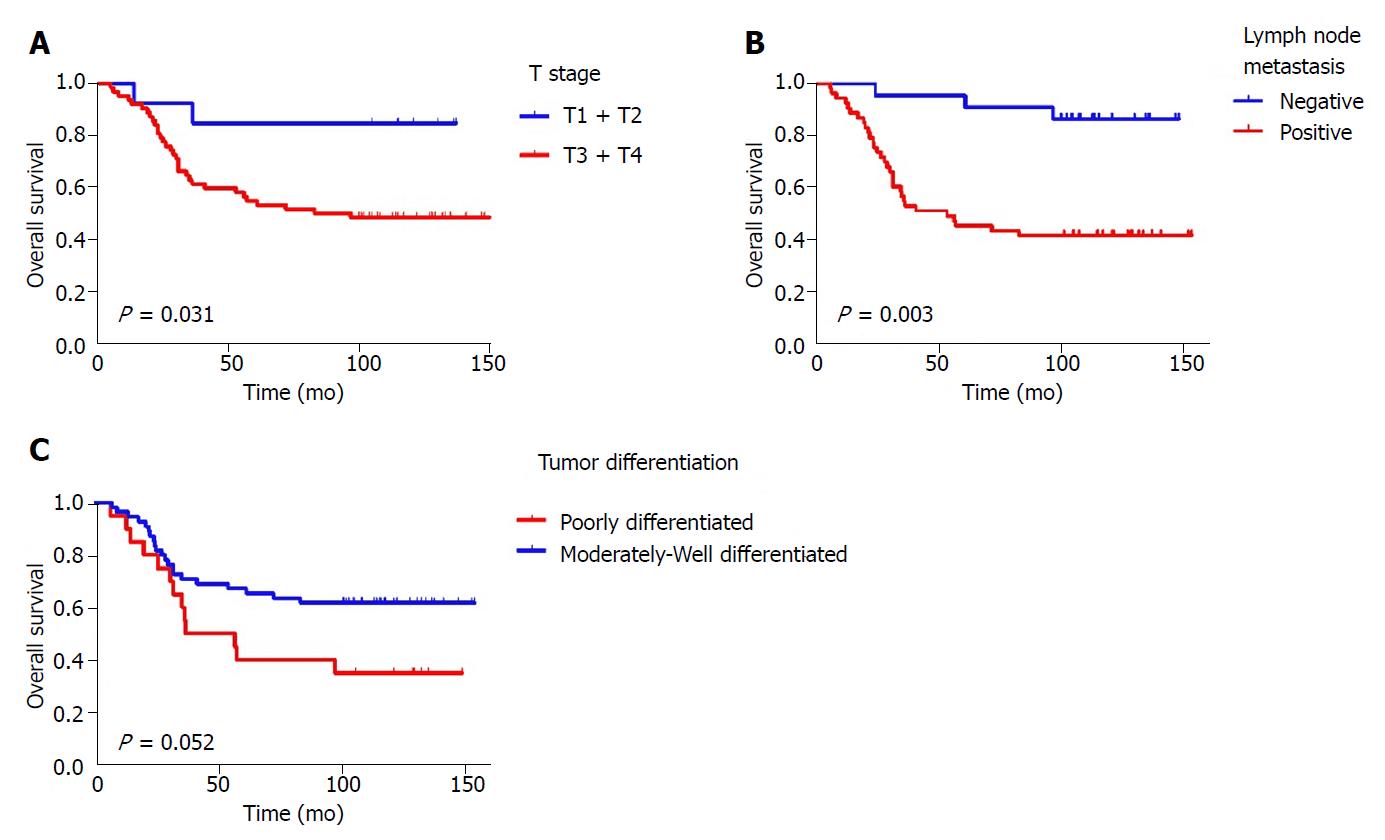
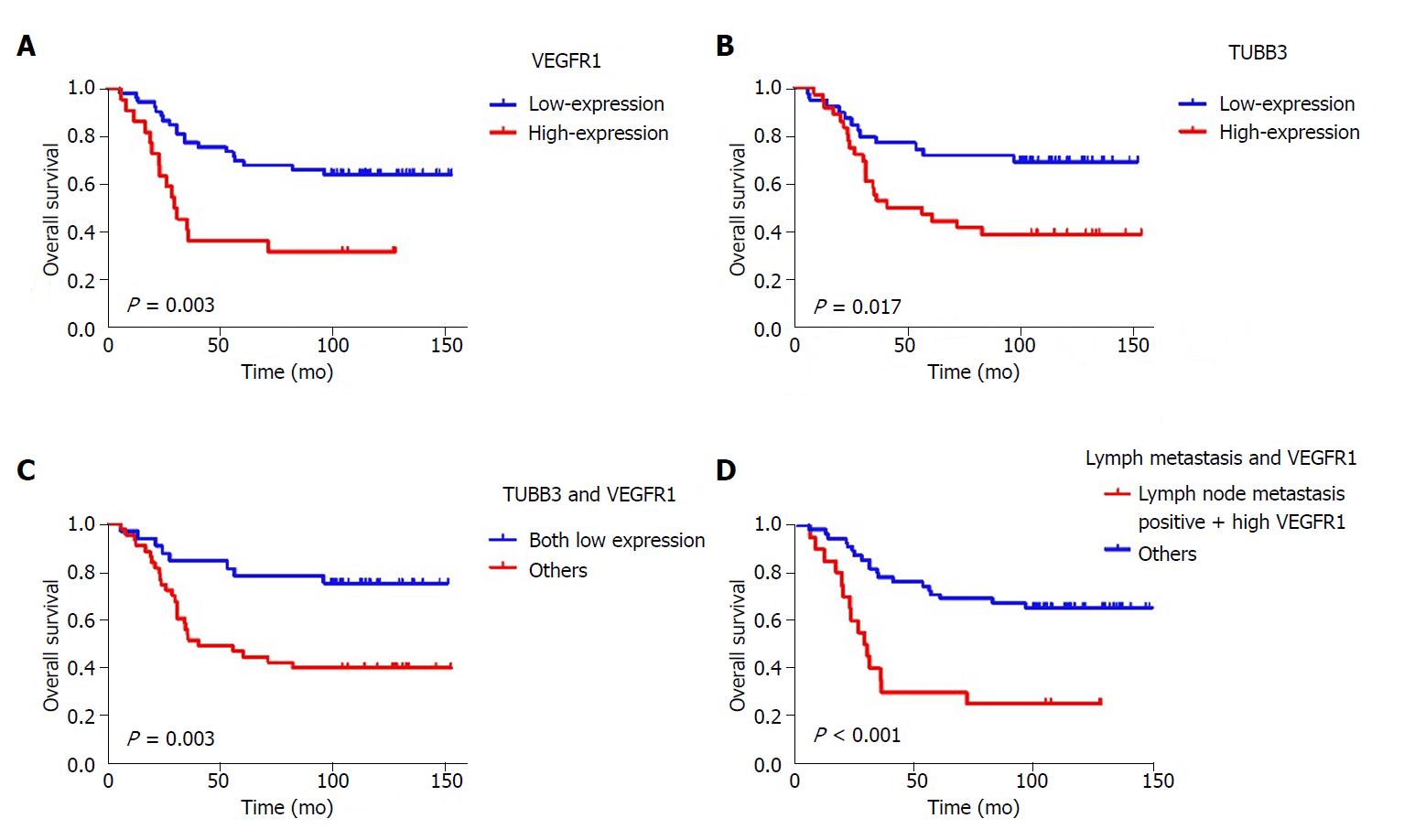
The Cox regression analysis of OS influencing factors was shown in Table 3. Univariate analysis showed that T stage, lymph node metastasis, tumor differentiation, and VEGFR1 and TUBB3 expression were significantly related to OS (P = 0.048, P = 0.003, P = 0.052, P = 0.003 and P = 0.015, respectively) (Figures 2, 3 A and B). Moreover, Kaplan-Meier analysis showed that the rates of 1-, 3-, and 5-year OS in the TUBB3 low- and high-expression groups were 94.9% vs 94.4%, 76.9% vs 52.8%, and 71.8% vs 47.2%, respectively (P = 0.017). The rates of OS in the VEGFR1 low- and high-expression groups were 98.1% vs 86.4%, 77.4% vs 36.4%, and 69.8% vs 36.4%, respectively (P = 0.003). Moreover, lymph node metastasis (HR = 3.042, 95%CI: 1.137-8.142, P = 0.027) and VEGFR1 (HR = 2.151, 95%CI: 1.062-4.355, P = 0.033) were independent factors influencing OS, as evaluated by the multivariate Cox regression model.
| Variables | Univariate | Multivariate | ||||
| HR | 95%CI | P | HR | 95%CI | P | |
| Gender | ||||||
| Female/male | 1.018 | 0.519-1.997 | 0.958 | |||
| Age | ||||||
| ≤ 60/> 60 | 1.175 | 0.548-2.518 | 0.679 | |||
| Pre-CEA | ||||||
| ≤ 5/> 5 | 1.067 | 0.496-2.298 | 0.868 | |||
| Pre-Hb | ||||||
| ≤ 120/> 20 | 0.651 | 0.328-1.290 | 0.219 | |||
| Distance to verge (cm) | ||||||
| ≤ 5/> 5 | 1.265 | 0.642-2.491 | 0.497 | |||
| T stage | ||||||
| T1 + T2/T3 + T4 | 4.221 | 1.011-17.632 | 0.048 | 4.05 | 0.968-116.93 | 0.055 |
| Lymph node metastasis | ||||||
| Negative/positive | 6.247 | 1.905-20.491 | 0.003 | 3.042 | 1.137-8.142 | 0.027 |
| Tumor thrombus | ||||||
| Negative/positive | 1.303 | 0.458-3.705 | 0.62 | |||
| Tumor differentiation | ||||||
| Poorly/moderately-well | 0.503 | 0.251-1.006 | 0.052 | - | - | 0.18 |
| Chemotherapy | ||||||
| No/yes | 1.407 | 0.430-4.605 | 0.572 | |||
| TUBB3 expression | ||||||
| Low/high | 2.407 | 1.188-4.877 | 0.015 | - | - | 0.1 |
| VEGFR1 expression | ||||||
| Low/high | 2.817 | 1.424-5.570 | 0.003 | 2.151 | 1.062-4.355 | 0.033 |
VEGFR1 and TUBB3 expression were positively correlated (P = 0.006, r = 0.315) by the Spearman’s correlation test. Both low expression of VEGFR1 and TUBB3 were observed in 32 (42.6%) cases. Moreover, the Kaplan-Meier analysis showed that the 1-, 3-, and 5-year OS of both low-expression patients vs others were 96.9% vs 93.0%, 84.4% vs 53.5%, and 78.1% vs 46.5%, respectively (P = 0.003, Figure 3C). Meanwhile, Kaplan-Meier analysis showed that the rates of 1-, 3-, and 5-year OS in positive lymph node metastasis patients with high expression of VEGFR1 vs others were 90.0% vs 98.2%, 35.0% vs 78.2%, and 30.0% vs 70.9%, respectively (P < 0.001) (Figure 3D).
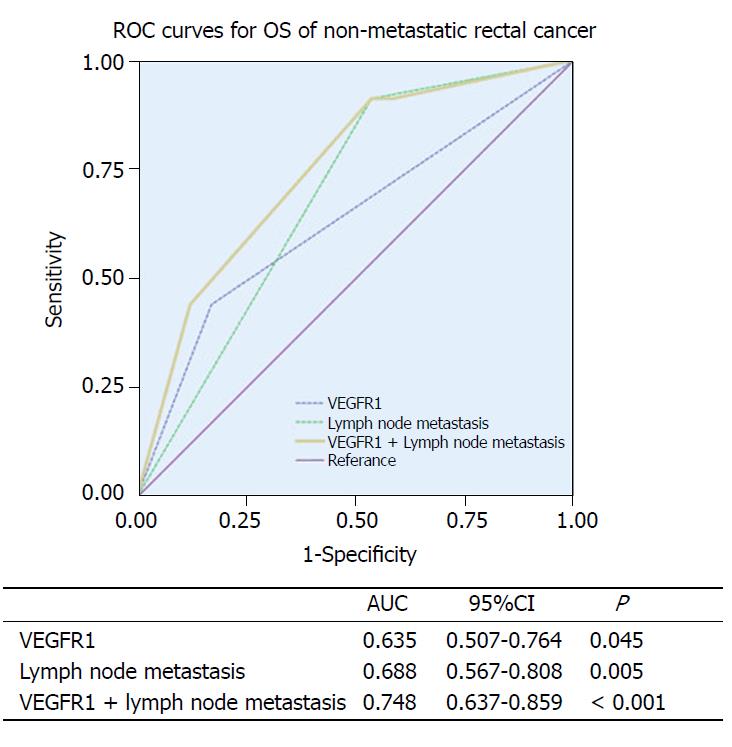
Finally, we combined the two independent prognostic factors, lymph node metastasis and VEGFR1 expression, to construct a prognostic model and supplemented the VEGFR1 expression to the lymph node metastasis by ROC analysis to assess the improvement of the model for OS. The lymph node metastasis (AUC: 0.688, 95%CI: 0.567–0.808, P = 0.005) showed a better prognostic value than VEGFR1 expression (AUC: 0.635, 95%CI: 0.507–0.764, P = 0.045). Furthermore, a better prognostic value was shown when combining the lymph node metastasis and VEGFR1 expression (AUC: 0.748, 95%CI: 0.637–0.859, P < 0.001) (Figure 4).
Firstly, we evaluated the long-term prognostic value of VEGFR1 and TUBB3 expression after the diagnosis of non-metastatic rectal cancer with a median follow-up of 102 mo. Here, we found that VEGFR1 and TUBB3 expression affected OS in non-metastatic rectal cancer by univariate analysis. Moreover, a favorable OS in both low expression of VEGFR1 and TUBB3 was noted as compared to others. In addition, the association between VEGFR1 expression and lymph node metastasis was also assessed. The combination of lymph node metastasis and VEGFR1 expression might also provide a promising tool for the prognosis of non-metastatic rectal cancer.
Reportedly, VEGFR correlates with poor prognosis, metastasis, and recurrence in various tumor types, including breast and lung cancers[14,15]. Moreover, previous studies demonstrated that VEGF plays a crucial role as a potent angiogenic factor in both experimental and human studies with respect to colorectal cancer progression and metastasis[16-18]. The co-expression of VEGF and VEGFR1/2 in the nucleus stimulates the proliferation and migration of endothelial cells, thereby providing nutrition for growing tumors and establishing a continuity between tumor cells and host vasculature[19].
VEGFR1 is primarily localized in the nucleus of endothelial cells; As the predominant receptor of the tumor microenvironment, it is essential for the survival of endothelial cells[20]. Tsai et al[21] reported that the overexpression of VEGF is a significant positive predictor for early postoperative relapse in stage I-III colorectal cancer patients, leading to poor OS (P = 0.002). Similarly, Nriagu et al[22] reported that the overexpression of VEGF mRNA was an independent factor affecting OS as assessed by multivariate analysis (HR = 1.94, P = 0.005). Herein, we found that the low expression of VEGFR1 might positively affect OS with a 5-year OS of 69.8% for low vs 36.4% for the high-expression group (HR = 2.151, P = 0.033). These results indicated that VEGFR1 functions as a positive regulator of angiogenesis[23], which might lead to poor survival in cancer patients.
A previous study evaluated VEGF expression in 117 colorectal adenocarcinoma patients, and confirmed that lymph node metastasis (positive vs negative, P < 0.001) and TNM stage (stage III vs I/II, P < 0.001) were related to increased VEGF expression. Moreover, the mean number of metastatic nodes was significantly associated with VEGF expression (1.06 ± 2.84 for low expression vs 2.45 ± 4.03 for high expression, P = 0.031)[24]. Similarly, our study implied that VEGFR1 expression was related to lymph node metastases (P = 0.013). However, whether the function of VEGF/VEGFR1 affects lymph node metastasis is yet unclear. Nagy et al[25] hypothesized that tumor cells in the circulation directly reached the regional lymph nodes through the supply vessels or blood vessel-lymph vessel junctions.
A retrospective study reported that VEGF expression could identify an unfavorable subgroup of patients with stage II colon cancer for optimal treatment strategy (the recurrence rate was 50% for VEGF-positive vs 11.7% for VEGF-negative, P = 0.001)[26]. As shown by ROC curves in our analysis, though low sensitivity of VEGFR1 (44.1%), the specificity was high with 82.9%, which exerted a similar effect on prognosis as lymph node metastasis. Moreover, the sensitivity increased when combined with lymph node status, and a superior prognostic value was noted for the combination. Further identification of a group of lymph node metastasis-positive with high VEGFR1 expression allows for selective treatment with adjuvant chemotherapy using antiangiogenic therapy, including VEGFR1 antisense and monoclonal antibodies, as well as postoperative follow-up.
Several clinical studies demonstrated that the increased expression of TUBB3 in various human malignancies was related to low response rate and poor survival in patients treated with taxane-based chemotherapies[27-30]. However, studies focusing on the relationship between TUBB3 and non-metastatic rectal cancer are limited. The current study showed that the low expression of TUBB3 had better OS in non-metastatic rectal cancer patients as assessed by univariate analysis (5-year OS, 71.8% vs 47.2%), although no significant difference was observed by multivariate analysis.
Furthermore, Makarchenko et al[31] and Widow et al[32] reported that VEGFR1 regulated the chemo-resistant genes such as TUBB3, which might result in the poor prognosis of lung and gastroesophageal cancers. The current study established a positive correlation between VEGFR1 and TUBB3 (r = 0.315, P = 0.006), and a favorable OS was observed in both low expression groups (P = 0.003). Paradiso et al[33] had investigated the combination of TUBB3 and VEGFR1 in advanced breast cancer. Hypoxia in the tumor microenvironment promotes angiogenesis, and VEGFR1 is known to be related to angiogenesis[23]. TUBB3 was found to be involved in an adaptive response to low oxygen levels and poor nutrient supply in solid tumors[34,35]. Therefore, we speculate that the underlying mechanism of the two correlations might be related to anoxic environments.
Notably, this study was limited to a small-sample retrospective analysis. Thus, additional mRNA expression data might help to establish a superior predictor. Finally, prospective data and large sample size are essential for further substantiation of the results.
We confirmed that the increased expression of VEGFR1 and TUBB3 might be negatively correlated with long-term prognosis of non-metastatic rectal cancer. Furthermore, VEGFR1 expression and lymph node metastasis affected the survival independently as well as synergistically. These results might provide additional prognostic information compared to the conventional tumor histopathological factors.
Rectal cancer is one of the most common form of cancer in both men and women. Gene expression profiling for predicting the response and long-term prognosis of malignancies has been reported in recent decades. Vascular endothelial growth factor (VEGF) and class III β-tubulin (TUBB3) have been reported to play a vital role in cancer progression. However, few studies focused on their role in rectal cancer.
We try to explore the potential prognostic value of VEGFR1 and TUBB3 for long-term survival in non-metastatic rectal cancer.
A total of 75 patients diagnosed with primary rectal adenocarcinoma without metastases were retrospectively analyzed.
Multiplex branched DNA liquidchip technology was applied to detected mRNA expressions of VEGFR1 and TUBB3. The cutoff point of mRNA expression was determined by Cutoff Founder.
VEGFR1 expression was positively correlated to TUBB3. Patients with both low expression of TUBB3 and VEGFR1 presented a better overall survival (OS). In addition, VEGFR1 and lymph node metastasis had potential as prognostic factors for OS in non-metastatic rectal cancer patients, and the combination of them showed a favorable prognostic value.
We confirmed that the increased expression of VEGFR1 and TUBB3 might be negatively correlated with long-term prognosis of non-metastatic rectal cancer. Furthermore, VEGFR1 expression and lymph node metastasis affected the survival independently, as well as synergistically. These results might provide additional prognostic information compared to the conventional tumor histopathological factors.
VEGFR1 has the potential to contribute to decision making regarding individual treatment in rectal cancer. A larger sample size and additional mRNA expression data are warranted to establish a superior prognosis model.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Jorgensen JT, Shu X, Soh JS S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Tan WW
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12186] [Article Influence: 1523.3] [Reference Citation Analysis (3)] |
| 2. | Barr RD. A two-year prospective analysis of emergency admissions to an adult medical unit at the Kenyatta National Hospital, Nairobi. East Afr Med J. 1972;49:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 777] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 3. | Wang H, Yang B, Geng T, Li B, Dai P, Chen C. Tissue-specific selection of optimal reference genes for expression analysis of anti-cancer drug-related genes in tumor samples using quantitative real-time RT-PCR. Exp Mol Pathol. 2015;98:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Banerjee CM. Dr. K.C. Chaudhuri--reminiscences. Indian J Pediatr. 1974;41:89-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5899] [Article Influence: 109.2] [Reference Citation Analysis (1)] |
| 6. | Goto Y. Experimental animal models of diabetes mellitus. Nihon Rinsho. 1991;49 Suppl:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Grimminger PP, Danenberg P, Dellas K, Arnold D, Rödel C, Machiels JP, Haustermans K, Debucquoy A, Velenik V, Sempoux C. Biomarkers for cetuximab-based neoadjuvant radiochemotherapy in locally advanced rectal cancer. Clin Cancer Res. 2011;17:3469-3477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Katsetos CD, Herman MM, Mörk SJ. Class III beta-tubulin in human development and cancer. Cell Motil Cytoskeleton. 2003;55:77-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Leandro-García LJ, Leskelä S, Landa I, Montero-Conde C, López-Jiménez E, Letón R, Cascón A, Robledo M, Rodríguez-Antona C. Tumoral and tissue-specific expression of the major human beta-tubulin isotypes. Cytoskeleton (Hoboken). 2010;67:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Tsourlakis MC, Weigand P, Grupp K, Kluth M, Steurer S, Schlomm T, Graefen M, Huland H, Salomon G, Steuber T. βIII-tubulin overexpression is an independent predictor of prostate cancer progression tightly linked to ERG fusion status and PTEN deletion. Am J Pathol. 2014;184:609-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Yao H, Wu H, Liu Y. [Improvement of prognostic and predictive network of colorectal cancer based upon the 8th edition of AJCC colorectal cancer staging system]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:24-27. [PubMed] |
| 12. | Meneilly GS, Elahi D, Minaker KL, Rowe JW. The dawn phenomenon does not occur in normal elderly subjects. J Clin Endocrinol Metab. 1986;63:292-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 783] [Cited by in RCA: 1015] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 13. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13220] [Cited by in RCA: 13268] [Article Influence: 358.6] [Reference Citation Analysis (0)] |
| 14. | Brancatisano A, Amis TC, Tully A, Engel LA. Blood flow distribution within the rib cage muscles. J Appl Physiol (1985). 1991;70:2559-2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Fontanini G, Lucchi M, Vignati S, Mussi A, Ciardiello F, De Laurentiis M, De Placido S, Basolo F, Angeletti CA, Bevilacqua G. Angiogenesis as a prognostic indicator of survival in non-small-cell lung carcinoma: a prospective study. J Natl Cancer Inst. 1997;89:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 208] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Sky-Peck HH. Distribution of trace elements in human hair. Clin Physiol Biochem. 1990;8:70-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Chin KF, Greenman J, Gardiner E, Kumar H, Topping K, Monson J. Pre-operative serum vascular endothelial growth factor can select patients for adjuvant treatment after curative resection in colorectal cancer. Br J Cancer. 2000;83:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Tamura M, Oda M, Tsunezuka Y, Matsumoto I, Kawakami K, Watanabe G. Vascular endothelial growth factor expression in metastatic pulmonary tumor from colorectal carcinoma: utility as a prognostic factor. J Thorac Cardiovasc Surg. 2004;128:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Grau U. Chemical stability of insulin in a delivery system environment. Diabetologia. 1985;28:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Gandolfi SA, Maier JA, Petronini PG, Wheeler KP, Borghetti AF. Multicomponent analysis of amino acid transport System L in normal and virus-transformed fibroblasts. Biochim Biophys Acta. 1987;904:29-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 142] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Tsai HL, Yang IP, Lin CH, Chai CY, Huang YH, Chen CF, Hou MF, Kuo CH, Juo SH, Wang JY. Predictive value of vascular endothelial growth factor overexpression in early relapse of colorectal cancer patients after curative resection. Int J Colorectal Dis. 2013;28:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Nriagu JO. Eric I. Hamilton. Sci Total Environ. 1991;100:viii-vxvi. [PubMed] [DOI] [Full Text] |
| 23. | Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203-212. [PubMed] |
| 24. | Zafirellis K, Agrogiannis G, Zachaki A, Gravani K, Karameris A, Kombouras C. Prognostic significance of VEGF expression evaluated by quantitative immunohistochemical analysis in colorectal cancer. J Surg Res. 2008;147:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Nagy JA, Brown LF, Senger DR, Lanir N, Van de Water L, Dvorak AM, Dvorak HF. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta. 1989;948:305-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 87] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Cascinu S, Staccioli MP, Gasparini G, Giordani P, Catalano V, Ghiselli R, Rossi C, Baldelli AM, Graziano F, Saba V. Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res. 2000;6:2803-2807. [PubMed] |
| 27. | DeCamp MM, Demling RH. Posttraumatic multisystem organ failure. JAMA. 1988;260:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 122] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Galmarini CM, Treilleux I, Cardoso F, Bernard-Marty C, Durbecq V, Gancberg D, Bissery MC, Paesmans M, Larsimont D, Piccart MJ. Class III beta-tubulin isotype predicts response in advanced breast cancer patients randomly treated either with single-agent doxorubicin or docetaxel. Clin Cancer Res. 2008;14:4511-4516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Ferrandina G, Zannoni GF, Martinelli E, Paglia A, Gallotta V, Mozzetti S, Scambia G, Ferlini C. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res. 2006;12:2774-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Li WJ, Zhong SL, Wu YJ, Xu WD, Xu JJ, Tang JH, Zhao JH. Systematic expression analysis of genes related to multidrug-resistance in isogenic docetaxel- and adriamycin-resistant breast cancer cell lines. Mol Biol Rep. 2013;40:6143-6150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Makarchenko OF. The state and prospectives of physiology in the Unkrainian SSR. Fiziol Zh. 1972;18:435-445. [PubMed] |
| 32. | Widow W. Treatment situation in bronchial carcinoma. Z Erkr Atmungsorgane Folia Bronchol. 1971;134:57-65. [PubMed] |
| 33. | Paradiso A, Mangia A, Chiriatti A, Tommasi S, Zito A, Latorre A, Schittulli F, Lorusso V. Biomarkers predictive for clinical efficacy of taxol-based chemotherapy in advanced breast cancer. Ann Oncol. 2005;16 Suppl 4:iv14-iv19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Raspaglio G, De Maria I, Filippetti F, Martinelli E, Zannoni GF, Prislei S, Ferrandina G, Shahabi S, Scambia G, Ferlini C. HuR regulates beta-tubulin isotype expression in ovarian cancer. Cancer Res. 2010;70:5891-5900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Raspaglio G, Filippetti F, Prislei S, Penci R, De Maria I, Cicchillitti L, Mozzetti S, Scambia G, Ferlini C. Hypoxia induces class III beta-tubulin gene expression by HIF-1alpha binding to its 3’ flanking region. Gene. 2008;409:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |