Published online Jan 15, 2018. doi: 10.4251/wjgo.v10.i1.23
Peer-review started: October 9, 2017
First decision: November 3, 2017
Revised: November 10, 2017
Accepted: December 5, 2017
Article in press: December 5, 2017
Published online: January 15, 2018
Processing time: 95 Days and 11.9 Hours
Vitamin D has emerged as a promising anti-cancer agent due to its diverse biological effects on tumor differentiation, apoptosis and suppression of cellular proliferation. Current evidence suggests a protective role of vitamin D in colon cancer. The effect of vitamin D on esophageal cancer remains controversial. Multiple studies investigated the association between vitamin D and esophageal cancer, employing different modes of assessment of vitamin D status such as serum 25-hydroxyvitamin D levels, vitamin D dietary intake or exposure to ultraviolet B (UVB) radiation. Genetic variations of the vitamin D receptor (VDR) gene and VDR expression in esophageal specimens have also been investigated. Ecological studies evaluating exposure to UVB radiation yielded an inverse correlation with esophageal cancer. When vitamin D dietary intake was assessed, direct association with esophageal cancer was observed. However, circulating 25-hydroxyvitamin D concentrations showed inconsistent results. In this review article, we present a detailed summary of the current data on the effects of vitamin D on various histological subtypes of esophageal cancer and their precursor lesions. Well-powered prospective studies with accurate measurement of vitamin D status are needed before chemoprevention with vitamin D is recommended, as current evidence does not support a chemopreventive role of vitamin D against esophageal cancer. Future studies looking at the incidence of esophageal cancer in patients with pre-cancerous lesions (Barrett's esophagus and squamous cell dysplasia) receiving vitamin D supplementation are needed.
Core tip: Vitamin D has emerged as a promising anti-cancer agent due to its diverse biological effects on tumor differentiation, apoptosis and suppression of cellular proliferation. Ecological studies evaluating exposure to ultraviolet B radiation yielded an inverse correlation with esophageal cancer. When vitamin D dietary intake was assessed, direct association with esophageal cancer was observed. However, circulating 25-hydroxyvitamin D concentrations showed inconsistent results.
- Citation: Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: Is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1): 23-30
- URL: https://www.wjgnet.com/1948-5204/full/v10/i1/23.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i1.23
« Sol est remediorum maximum » (The sun is the best remedy)--Pliny, the Elder.
This remark, attributed to Pliny, exemplifies the healing properties of sunlight known since ancient times[1]. The fact that most of the beneficial effects of sunlight are mediated by vitamin D came to light by experimental studies on Rickets in the 1930s[1,2]. Epidemiologic research in the 1980s showed that incidence and death rates for certain cancers were lower among individuals with higher exposure to sunlight[3]. Researchers hypothesized that variation in vitamin D levels might account for this association. Since then, laboratory studies elucidated several antineoplastic properties of vitamin D such as its role in promoting cellular differentiation, decreasing cancer cell growth, stimulating apoptosis, and inhibiting angiogenesis[4,5].
Vitamin D appears to have a protective role in colorectal and breast cancers but confirmatory data for cancers of other organs such as prostate or esophagus remains lacking[6-9]. Esophageal cancer is a major public health concern due to increasing incidence and poor survival rates after diagnosis. Numerous studies investigated the association between vitamin D status and esophageal cancer with inconsistent results.
The aim of this review is to present the available scientific evidence for the role of vitamin D in esophageal squamous cell cancer (ESCC), esophageal adenocarcinoma (EAC) and their precursor lesions- squamous cell dysplasia and Barrett’s esophagus (BE) respectively.
A PubMed search of all studies published in English from 2006 to 2016 was performed. Medical subject headings (MeSH terms) used were “vitamin D”, “calcitriol”, “vitamin D receptor”, “sun”, “sunlight”, “esophageal neoplasm”, “esophageal adenocarcinoma”, “Barrett’s esophagus”, and “esophageal squamous cell carcinoma”. References of relevant articles were also reviewed and selected.
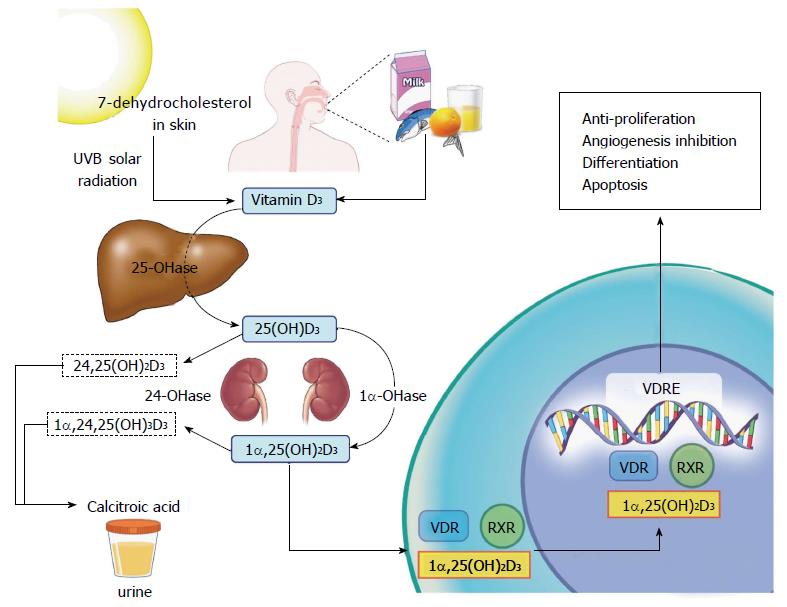
The two main sources of vitamin D are diet and solar radiation. Provitamin D in the skin is converted to previtamin D by ultraviolet B (UVB) radiation, which is then converted to vitamin D3 (cholecalciferol) through isomerization. Vitamin D3 is hydroxylated in the liver to form 25-hydroxycholecalciferol [25(OH)D3]. Another hydroxylation reaction occurs in the kidneys, where 25(OH)D3 is converted to the biologically active form 1α,25(OH)2D3 (calcitriol), involved in bone and calcium metabolism[4]. Calcitriol also regulates its own catabolic cascade: it induces the expression of the CYP24A1 gene, which encodes the 24-hydoxylase enzyme. The latter converts 25(OH)D3 and 1α,25(OH)2D3 to the less active metabolites 24,25(OH)D3 and 1α,24,25(OH)3D3 respectively. This is the rate-limiting step of vitamin D catabolism[4].
Calcitriol, thought to be the metabolite involved in the anticancer properties of vitamin D, binds to the vitamin D receptor (VDR). The calcitriol-VDR complex binds to the retinoid X receptor (RXR), forming the heterodimer VDR-RXR, which translocates to the nucleus and binds to the vitamin D response element (VDRE) on a particular gene, with subsequent transcription and translation of various proteins, including the ones involved in the vitamin D anti-carcinogenic properties, i.e., anti-proliferation, apoptosis, differentiation, and angiogenesis inhibition[4,5] (Figure 1). Calcitriol inhibits proliferation by inducing cell cycle arrest at the G0/G1 phase. Cyclins and cyclin-dependent kinase inhibitors regulate cell cycle progression and induce G1 cell-cycle arrest. Interestingly, cyclin-dependent kinase inhibitor 1A contains a VDRE, which accounts for the anti-proliferative effects of vitamin D[4,10]. Apoptosis is another key mechanism in inhibiting carcinogenesis. Calcitriol induces the expression of pro-apoptotic proteins and activates caspase, a cysteine protease that mediates apoptosis. In addition to its apoptotic and anti-proliferative effects, vitamin D inhibits angiogenesis. In prostate cancer, vitamin D interrupts signaling of an angiogenic factor, interleukin 8, leading to decreased endothelial cell migration and possibly metastasis[4].
Osteopontin and E-cadherin are two proteins induced by vitamin D with antagonistic growth regulatory activity. While osteopontin promotes cellular invasion[11], E-cadherin suppresses cell growth by inhibiting the transcriptional activity of β-catenin, a protein that induces genes involved in promoting cell growth and proliferation[12]. In colon adenocarcinoma, for instance, E-cadherin is preserved as opposed to low osteopontin levels[13]. Subsequently, high levels of calcitriol would lead to further E-cadherin-induced tumor suppression with low osteopontin levels, and subsequent cell growth inhibition[14].
From an immunologic perspective, multiple cells are known to be involved in EAC and its precursor lesions, BE and reflux esophagitis: In addition to the dendritic cells and CD4 T cells, signaling pathways involved include NFk-B, Wnt and Hegdehog pathways. Immunologically, the role of vitamin D in esophageal cancer remains inconclusive and unclear. For instance, vitamin D was shown to inhibit the Hedgehog signaling cascade which is overexpressed in BE. Similarly dendritic cells, increased in BE and EAC, are maintained in an immature form by vitamin D. On the other hand, BE is characterized by a Th2-predominant response and data suggests that 1α,25-hydroxyvitamin D promotes the Th2 response. In addition, vitamin D was shown to increase interleukin-4 cytokine production, which has been implicated in BE. In view of these multiple contradictory effects on neoplastic progression, the role of vitamin D in esophageal cancer needs to be evaluated[15].
Serum concentration of vitamin D seems to be the most accurate indicator of a patient’s vitamin D status and is usually monitored to treat vitamin D deficiencies. More than 50 vitamin D metabolites have been identified over the past years but only two gained particular attention: 1α,25(OH)2D3 and 25(OH)D3. While 1α,25(OH)2D3’s half-life is around 4 h and levels are widely dependent on an individual’s calcium needs, 25(OH)D3 has a half-life of around 3 wk, reflecting more accurately a patient’s vitamin D stores, and therefore widely accepted as an indicator of an individual’s vitamin D status[16]. The normal levels are considered to be 10-68 ng/mL (24.9-169.5 nmol/L) with different cut-offs in various assays and laboratories[17]. Sunlight is a major contributor to vitamin D status. Many studies attempted to validate different UVB exposure questionnaires and found correlations ranging between 0.16 and 0.4 for vitamin D serum concentration and reported UVB exposure[18-21]. Correlations noted were not strong however, raising the hypothesis that sun exposure alone does not explain serum vitamin D levels[19]. Multiple studies also used dietary vitamin D intake as a surrogate of vitamin D status and showed a good correlation between dietary vitamin D intake and serum vitamin D levels. This correlation could be stronger for instance, in wintertime, when exposure to UVB radiation is reduced[19].
Taking vitamin D dietary intake, lifetime UVB exposure and vitamin D serum concentrations into account seems to be the most accurate method to assess an individual’s vitamin D status[19]. As a matter of fact, Giovannucci et al[22] built a predictor score to assess long-term vitamin D status using multiple determining factors of vitamin D exposure including dietary and supplementary vitamin D intake, geographic residence, race, physical activity and body mass index[22].
On another note, two genome wide association studies of vitamin D levels have been conducted and common genetic variants of genes involved in vitamin D metabolism pathways were identified[23,24]. Subsequently, multiple single nucleotide polymorphisms (SNPs) were investigated in an attempt to find correlations with esophageal cancer[24,25].
Esophageal cancer encompasses two histological subtypes: ESCC and EAC, which differ epidemiologically, by risk factors and outcomes. ESCC is the most common esophageal cancer worldwide with an increased incidence in developing countries. Esophageal squamous dysplasia is the histologic precursor of ESCC. Developed countries witness a higher prevalence of EAC[26], which is commonly related to chronic acid reflux exposure, with BE being the main risk factor for EAC. Potential associations of vitamin D have been investigated in both histological subtypes of esophageal cancer as presented below.
Only one study based on the Linxian population in China investigated the role of vitamin D in esophageal squamous cell dysplasia and found a linear association between vitamin D levels and development of squamous dysplasia: 230 out of 724 patients had esophageal squamous dysplasia. Patients diagnosed with esophageal squamous dysplasia had higher median levels of 25(OH)D3 levels compared to controls (36.5 nmol/L vs 31.5 nmol/L, P = 0.0004)[27].
Three studies evaluated vitamin D status and ESCC with diverging results depending on mode of assessment of Vitamin D status[28-30] (Table 1). While one study in China concluded a direct correlation between ESCC and measured serum 25(OH)D3 concentrations[28], another study conducted in Italy noted an inverse association between increased dietary vitamin D intake and ESCC[29]. The third one, done in Australia, found no association between ESCC and lifetime UVB radiation exposure[30].
| Ref. | Study design/location | Vitamin D exposure/status/genetics studies | Statistical correlation |
| Abnet et al[27] | Cross-sectional study | 25-hydroxyvitamin D serum level | RR = 1.86, 95%CI: 1.35-2.62 |
| China | |||
| Chen et al[28] | Prospective study | 25-hydroxyvitamin D serum level | ESCC in men: |
| China | HR = 1.77, 95%CI: 1.16-2.70 | ||
| Lipworth et al[29] | Case-control study | Vitamin D dietary intake | ESCC: |
| Italy | OR = 0.58, 95%CI: 0.39-0.86 | ||
| Tran et al[30] | Case-control study | Ultraviolet B radiation | ESCC: No association |
| Australia | |||
| Wang et al[24] | Case-control study | Genetic polymorphisms | ESCC: No association |
| China |
The study from China was population-based and included 2018 participants, out of which 545 developed ESCC, with an overall trend towards higher concentrations in serum 25(OH)D3 in those who developed cancers. Multivariate analysis demonstrated increased risk with higher 25(OH)D3 values (4th quartile hazard ratio (HR): 1.30, 95%CI: 0.97-1.73, P = 0.013). When stratified by gender, ESCC risk remained increased in men with higher vitamin D levels (4th quartile HR = 1.77, 95%CI: 1.16-2.70, P = 0.003) but not in women. These conclusions could not be extrapolated to other populations due to overall low vitamin D levels and high rate of exposure to polycyclic aromatic hydrocarbons in this study population, with the latter factor placing them at higher risk for neoplasia[28]. It is worthwhile noting however, that pre-neoplastic lesions with squamous cell dysplasia were also found to have an E-cadherin/osteopontin disequilibrium, with E-cadherin suppression and osteopontin up-regulation leading to increased risk of cell growth, proliferation and subsequently malignant transformation with higher calcitriol levels[14].
The study from Italy was a case-control study with 304 patients and investigated the association between dietary vitamin D intake over the prior two years and ESCC[29]. In ESCC patients, an inverse relationship was noted between vitamin D intake and esophageal neoplasia. The highest tertile corresponded to > 3.5 μg/d with a risk reduction of around 40% compared to lowest tertile (< 2.51 μg/d).
The last case-control study from Australia assessed UVB exposure and prevalence of ESCC. No relationship was observed between lifetime UVB radiation and ESCC (OR = 0.94, 95%CI: 0.82-1.09) in contrast to EAC and esophago-gastric junction adenocarcinoma[30].
An association between SNPs in the genes involved in vitamin D pathway and ESCC was also evaluated: Wang et al[24] investigated 12 SNPs in four genes known to be part of the vitamin D pathway: vitamin D binding protein, 7-dehydrocholesterol reductase, 25-hydroxylase and 24-hydroxylase or CYP24A1. SNPs related to vitamin D levels were not found to be associated with ESCC risk.
The rate-limiting step of vitamin D synthesis was also investigated in regards to ESCC. In one study of 42 patients with esophageal cancer of which 39 had ESCC, CYP24 gene expression was assessed by semi-quantitative RT-PCR assay. Cases with lower CYP24 expression (n = 25) had significantly higher survival rate compared to patients with increased CYP24 expression (n = 17, P < 0.05), making of CYP24 a “candidate oncogene” that might serve as a biomarker of increased ESCC risk[31].
Vitamin D dietary intake and supplementation have been studied with regards to BE. An Irish study evaluated the association between vitamin D intake assessed via food questionnaires, among patients with BE (n = 224), reflux esophagitis (n = 230) and EAC (n = 227), compared to 260 healthy controls[32]. Vitamin D intake was not found to be associated with reflux esophagitis or BE. After adjusting for reflux symptoms however, a positive correlation emerged between patients with BE and the highest tertile of dairy products intake (≥ 493.2 g/d) (OR = 1.94, 95%CI: 1.01-3.71). This could imply that patients are consuming dairy products to treat their symptoms, rather than an actual association with BE, as proposed by the authors[32]. In a clinical trial studying the effect of vitamin D supplementation on BE, 3 of the first 10 evaluable patients had BE with high-grade dysplasia. After 2 wk of vitamin D supplementation (50000 units weekly), 2 out of 3 patients with BE had regression to low-grade dysplasia on pathology, suggesting a potential benefit of vitamin D in BE[33].
Three studies assessed VDR expression in BE[25,34,35]. Trowbridge et al[34] compared VDR expression in normal esophagus, BE and normal gastric tissue, by immunofluorescent staining. No VDR expression was detected in normal squamous mucosa in contrast to normal gastric mucosa and BE mucosa. This suggests a restriction of VDR expression to columnar epithelium and glandular structures, as well as potential chemopreventive effects of vitamin D in patients with BE. Those findings were reproducible in a Dutch study where VDR mRNA had a 2-fold higher expression in BE epithelium compared to squamous epithelium[25]. In another study comprising 37 patients with BE and 107 with EAC, VDR expression was found to be increased in both BE (95%) and EAC (79%), but significantly higher in BE[35]. This implies that VDR might be involved early on in EAC development.
To date, the studies that examined the association between vitamin D status and EAC showed inconsistent results[30,32,36-38]. Several of these studies were either population-based or ecologic studies with lack of information on 25(OH)D3 levels either before or after EAC diagnosis, and therefore relied on various other measures of vitamin D status such as sunlight exposure or dietary vitamin D intake.
The studies that examined the association between vitamin D and EAC are summarized in Table 2. Only 2 studies evaluated the association of serum 25(OH)D3 concentrations and EAC. Abnet et al[36], in a nested case-control study, examined the relationship between upper gastrointestinal cancers and circulating serum 25(OH)D3 levels. No significant association was noted with EAC when comparing patients with highest and lowest categories of 25(OH)D3 levels (50-75 nmol/L vs < 25 nmol/L, OR = 1.63, 95%CI: 0.25-2.12)[36]. Another US-based study also did not show any association between 25(OH)D3 levels and incidence or prevalence of EAC among patients with BE[38]. Giovannucci et al[22] used a predicted 25(OH)D3 level derived by modeling various factors that can affect vitamin D status such as UVB, dietary vitamin d intake, supplementation, skin pigmentation and body mass index. A 25nmol/L increment in predicted vitamin D resulted in 17% reduction in total cancer incidence and 29% reduction in cancer mortality. However, the study did not mention the rates of EAC in particular, although there was an inverse association with esophageal cancer incidence (RR = 0.37, 95%CI: 0.17-0.80)[22].
| Ref. | Study design/location | Vitamin D exposure/status/genetics studies | Statistical correlation | Other |
| Tran et al[30] | Case-control study | Cumulative ambient ultraviolet B radiation | EAC risk: OR = 0.59, 95%CI: 0.35-0.99 | |
| Australia | EAC risk for every 107 J/m2 increase in radiation: OR = 0.82, 95%CI: 0.72-0.93 | |||
| Mulholland et al[32] | Case-control study | Vitamin D dietary intake via food questionnaire | EAC risk: OR = 1.99, 95%CI: 1.03-3.86 | |
| Ireland | BE risk: no association | |||
| Mayne et al[37] | Case-control study | Vitamin D dietary intake | EAC: no association | |
| United States | ||||
| Thota et al[38] | Retrospective study of a prospectively collected database | 25-hydroxyvitamin D serum levels | EAC: no association | |
| United States | BE with HGD: no association | |||
| Abnet et al[36] | Nested case-control study | 25-hydroxyvitamin D serum levels | EAC: no association | |
| United States, Finland, China | ||||
| Trowbridge et al[43] | Retrospective study | Vitamin D receptor expression | Not assessed | VDR expression decreased with tumor dedifferentiation |
| United States | VDR expression lower in neoadjuvant therapy responders | |||
| Trowbridge et al[34] | Retrospective study | Vitamin D receptor expression | Not assessed | VDR expression increased in Barrett’s esophagus |
| United States | ||||
| Zhou et al[35] | Descriptive | Vitamin D receptor expression | Not assessed | VDR expressed in 95% of BE (35/37) |
| United States | VDR expressed in 78% of EAC (86/109) | |||
| Janmaat et al[25] | Cohort study | Vitamin D receptor polymorphisms | EAC: 2 GT copies: | VDR expression is 2 fold higher in BE as compared to normal esophagus |
| Netherlands | OR = 0.50, 95%CI: 0.27-0.96 | |||
| BE: 2 GT copies: | ||||
| OR = 0.46, 95%CI: 0.26-0.80 | ||||
| Chang et al[45] | Case- control study | Vitamin D receptor polymorphisms | EAC: rs2238139 TT: | |
| Ireland | OR 0.26, 95% CI: 0.07-0.93 | |||
| EAC: rs2107301 TT: | ||||
| OR = 0.19, 95%CI: 0.06-0.67 | ||||
| Zgaga et al[5] | Meta-analysis | Ultraviolet B radiation | Vitamin D level and overall esophageal cancer: | |
| United States | Vitamin D intake | OR = 1.39, 95%CI: 1.03-1.74 | ||
| Vitamin D serum levels | Vitamin D intake and EAC: | |||
| OR = 1.45; 95%CI: 0.65-2.24 |
Data from animal models have shown that dietary vitamin D is associated with tumor inhibition and reduction of tumor growth, especially in colorectal cancer and breast cancer[39-41]. However, the epidemiologic studies for EAC have been contradictory[32,37,42]. In fact, in an Irish study, patients with the highest tertile of vitamin D intake had increased risk of EAC compared to the lowest tertile (OR = 1.99, 95%CI: 1.03-3.86)[42]. In another population-based study in the US, no association was found between vitamin D intake and EAC (OR = 1.10, 95%CI: 0.86-1.40)[37]. Similar results were found in a meta-analysis that concluded that higher intake of vitamin D results in a non-significant increase in the risk of EAC (OR = 1.45, 95%CI: 0.65-2.24)[5]. The current evidence hence fails to establish a relationship between vitamin D intake and EAC.
The other significant contributor of vitamin D status is sunlight exposure. To date only one study examined UVB exposure as a risk factor for EAC[30]. Patients with EAC were 41% less likely to have high levels of lifetime ambient UVB radiation compared to population controls (OR = 0.59, 95%CI: 0.35-0.99). Although the study did not check serum vitamin D levels to establish the diagnosis of vitamin D deficiency, the study results were adjusted for several potential confounders such as body mass index, reflux symptoms, education, smoking, alcohol and Helicobacter pylori infection, following which the inverse association remained between UVB and EAC. The same inverse association was seen between number of nevi, which is a surrogate marker of sun exposure, and EAC, further supporting the hypothesis of sun exposure and tumor inhibition[30].
In an attempt to find biomarkers predicting the malignant potential of an esophageal lesion, response to treatment and prognosis, investigators have evaluated the genetics involved in the vitamin D pathway in regards to EAC. The focus has mainly been on the VDR expression in different tissues as well as SNPs of some of the genes in the vitamin D signaling pathway.
Trowbridge et al[43] looked at VDR expression using immunofluorescence in 15 biopsy specimens of patients with EAC. Greater average mean fluorescence, a reflection of higher VDR expression, was observed for moderately and well-differentiated tumors (111.7) compared to poorly differentiated tumors (98.7), which highlights the anti-carcinogenic properties of vitamin D through VDR, particularly differentiation. This was also established in colon adenocarcinoma where decreased VDR expression was noted with progressive de-differentiation[44].
Apart from assessing VDR expression level, VDR polymorphisms in EAC have also been investigated. Vitamin D exerts many of its biological effects by binding to VDR and VDR gene polymorphisms may alter mRNA stability and transcriptional activity.
In an Irish population-based case-control study, 224 cases of EAC were identified and 256 controls were selected[45]. Variants in the VDR gene were explored and TT homozygotes at rs2238139 and rs2107301 SNPs seemed to have a reduced risk of EAC compared to individual with CC alleles at those sites (OR = 0.26, 95%CI: 0.007, 0.93 and OR = 0.19, 95%CI: 0.06-0.67, respectively). However when permutation analyses were done, there was no significant association between EAC and VDR polymorphisms[45]. A later study identified two SNPs of the VDR gene associated with reduced risk of reflux esophagitis, BE and EAC[25]. Patients with the rs1989969 T/rs2238135 G haplotype had a lower risk for reflux esophagitis (OR = 0.48, 95%CI: 0.28-0.81), BE (OR 0.46, 95%CI: 0.26-0.80) as well as EAC (OR = 0.50, 95%CI: 0.27-0.96). Both of these haplotypes appear to be associated with reduced VDR expression. The authors studied the mechanism by which those SNPs work and discovered that the rs1989969 T allele lead to the appearance of a GATA-1 transcription factor binding site, which is known to be a negative transcriptional regulator. This haplotype could be exerting its direct biological effects on the rate of reflux esophagitis with a subsequent decreased rates of BE and EAC[25]. Those findings could have significant clinical implications in terms of identifying patients who would benefit from vitamin D chemoprevention.
In summary, data continues to be inconsistent and firm conclusions regarding the chemopreventive role of vitamin D in esophageal cancer cannot be made. While vitamin D studies struggle with measuring the combined influences of dietary vitamin D intake and sunlight, vitamin D serum levels are a single point measure in time, and levels are known to change throughout the year. As a matter of fact, while an inverse association exists between UVB radiation and EAC, this was not observed with vitamin D intake. Serum 25(OH) D3 levels appear to be associated with higher risk of ESCC especially in Chinese population. No association was noted however between vitamin D serum levels and EAC. Studies have been population-specific making it difficult to apply findings to other populations. Multiple genetic studies provided new grounds for future investigations such as SNPs leading to the appearance of transcription sites with known negative regulatory roles. VDR expression is increased in BE as compared to EAC or normal squamous epithelium, making of VDR a potential biomarker in selecting those who could benefit from vitamin D as a chemopreventive agent. Well-powered prospective studies with accurate measurement of vitamin D status are needed before chemoprevention with vitamin D is recommended, as current evidence does not support a chemopreventive role of vitamin D against esophageal cancer. Future studies looking at the incidence of esophageal cancer in patients with pre-cancerous lesions (BE and squamous cell dysplasia) receiving vitamin D supplementation are needed.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hashimoto N, Nagahara H, Su CC S- Editor: Gong ZM L- Editor: Logan S E- Editor: Wang CH
| 2. | The Photo-activity of substances curative of Rickets-A remarkable discovery. JAMA. 1924;83:1169-1170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 541] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1015] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 5. | Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of Vitamin D Exposure and Esophageal Cancer Risk: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25:877-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Meyer HE, Robsahm TE, Bjørge T, Brustad M, Blomhoff R. Vitamin D, season, and risk of prostate cancer: a nested case-control study within Norwegian health studies. Am J Clin Nutr. 2013;97:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Giovannucci E. Epidemiological evidence for vitamin D and colorectal cancer. J Bone Miner Res. 2007;22 Suppl 2:V81-V85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | van der Rhee H, Coebergh JW, de Vries E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur J Cancer. 2013;49:1422-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Bilinski K, Boyages J. Association between 25-hydroxyvitamin D concentration and breast cancer risk in an Australian population: an observational case-control study. Breast Cancer Res Treat. 2013;137:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Moreno J, Krishnan AV, Feldman D. Molecular mechanisms mediating the anti-proliferative effects of Vitamin D in prostate cancer. J Steroid Biochem Mol Biol. 2005;97:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Kurisetty VV, Johnston PG, Johnston N, Erwin P, Crowe P, Fernig DG, Campbell FC, Anderson IP, Rudland PS, El-Tanani MK. RAN GTPase is an effector of the invasive/metastatic phenotype induced by osteopontin. Oncogene. 2008;27:7139-7149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J Cell Biol. 2001;154:1185-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Rohde F, Rimkus C, Friederichs J, Rosenberg R, Marthen C, Doll D, Holzmann B, Siewert JR, Janssen KP. Expression of osteopontin, a target gene of de-regulated Wnt signaling, predicts survival in colon cancer. Int J Cancer. 2007;121:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Campbell FC, Xu H, El-Tanani M, Crowe P, Bingham V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem Pharmacol. 2010;79:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Trowbridge R, Kizer RT, Mittal SK, Agrawal DK. 1,25-dihydroxyvitamin D in the pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Expert Rev Clin Immunol. 2013;9:517-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S-1091S. [PubMed] |
| 17. | Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med. 2004;351:1548-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 404] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 18. | van der Mei IA, Blizzard L, Ponsonby AL, Dwyer T. Validity and reliability of adult recall of past sun exposure in a case-control study of multiple sclerosis. Cancer Epidemiol Biomarkers Prev. 2006;15:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Millen AE, Bodnar LM. Vitamin D assessment in population-based studies: a review of the issues. Am J Clin Nutr. 2008;87:1102S-1105S. [PubMed] |
| 20. | Jones G, Dwyer T, Hynes KL, Parameswaran V, Greenaway TM. Vitamin D insufficiency in adolescent males in Southern Tasmania: prevalence, determinants, and relationship to bone turnover markers. Osteoporos Int. 2005;16:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sørensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86 Suppl 1:S97-S103. [PubMed] |
| 22. | Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 740] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 23. | Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739-2745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 584] [Cited by in RCA: 597] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 24. | Wang JB, Dawsey SM, Fan JH, Freedman ND, Tang ZZ, Ding T, Hu N, Wang LM, Wang CY, Su H. Common genetic variants related to vitamin D status are not associated with esophageal squamous cell carcinoma risk in China. Cancer Epidemiol. 2015;39:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Janmaat VT, Van De Winkel A, Peppelenbosch MP, Spaander MC, Uitterlinden AG, Pourfarzad F, Tilanus HW, Rygiel AM, Moons LM, Arp PP. Vitamin D Receptor Polymorphisms Are Associated with Reduced Esophageal Vitamin D Receptor Expression and Reduced Esophageal Adenocarcinoma Risk. Mol Med. 2015;21:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 499] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (4)] |
| 27. | Abnet CC, Chen W, Dawsey SM, Wei WQ, Roth MJ, Liu B, Lu N, Taylor PR, Qiao YL. Serum 25(OH)-vitamin D concentration and risk of esophageal squamous dysplasia. Cancer Epidemiol Biomarkers Prev. 2007;16:1889-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Chen W, Dawsey SM, Qiao YL, Mark SD, Dong ZW, Taylor PR, Zhao P, Abnet CC. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97:123-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Lipworth L, Rossi M, McLaughlin JK, Negri E, Talamini R, Levi F, Franceschi S, La Vecchia C. Dietary vitamin D and cancers of the oral cavity and esophagus. Ann Oncol. 2009;20:1576-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Tran B, Lucas R, Kimlin M, Whiteman D, Neale R; Australian Cancer Study. Association between ambient ultraviolet radiation and risk of esophageal cancer. Am J Gastroenterol. 2012;107:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Mimori K, Tanaka Y, Yoshinaga K, Masuda T, Yamashita K, Okamoto M, Inoue H, Mori M. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann Oncol. 2004;15:236-241. [PubMed] |
| 32. | Mulholland HG, Murray LJ, Anderson LA, Cantwell MM; FINBAR study group. Vitamin D, calcium and dairy intake, and risk of oesophageal adenocarcinoma and its precursor conditions. Br J Nutr. 2011;106:732-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Cummings LC, Willis J, Cooper GS, Bednarchik B, Markowitz S, Chak A. Mo1926 Effects of Vitamin D supplementation on Barrett’s esophagus. Gastroenterology. 2013;144:S696. [DOI] [Full Text] |
| 34. | Trowbridge R, Mittal SK, Sharma P, Hunter WJ, Agrawal DK. Vitamin D receptor expression in the mucosal tissue at the gastroesophageal junction. Exp Mol Pathol. 2012;93:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Zhou Z, Xia Y, Bandla S, Zakharov V, Wu S, Peters J, Godfrey TE, Sun J. Vitamin D receptor is highly expressed in precancerous lesions and esophageal adenocarcinoma with significant sex difference. Hum Pathol. 2014;45:1744-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Abnet CC, Chen Y, Chow WH, Gao YT, Helzlsouer KJ, Le Marchand L, McCullough ML, Shikany JM, Virtamo J, Weinstein SJ. Circulating 25-hydroxyvitamin D and risk of esophageal and gastric cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:94-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055-1062. [PubMed] |
| 38. | Thota PN, Kistangari G, Singh P, Cummings L, Hajifathalian K, Lopez R, Sanaka MR. Serum 25-Hydroxyvitamin D Levels and the Risk of Dysplasia and Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus. Dig Dis Sci. 2016;61:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871-1875. [PubMed] |
| 41. | Xue L, Lipkin M, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. J Natl Cancer Inst. 1999;91:176-181. [PubMed] |
| 42. | O’Brien MM, Kiely M, Harrington KE, Robson PJ, Strain JJ, Flynn A. The North/South Ireland Food Consumption Survey: vitamin intakes in 18-64-year-old adults. Public Health Nutr. 2001;4:1069-1079. [PubMed] |
| 43. | Trowbridge R, Sharma P, Hunter WJ, Agrawal DK. Vitamin D receptor expression and neoadjuvant therapy in esophageal adenocarcinoma. Exp Mol Pathol. 2012;93:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14:2370-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Chang CK, Mulholland HG, Cantwell MM, Anderson LA, Johnston BT, McKnight AJ, Thompson PD, Watson RG, Murray LJ; FINBAR study group. Vitamin d receptor gene variants and esophageal adenocarcinoma risk: a population-based case-control study. J Gastrointest Cancer. 2012;43:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |