Copyright
©The Author(s) 2025.
World J Gastrointest Oncol. Apr 15, 2025; 17(4): 102258
Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.102258
Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.102258
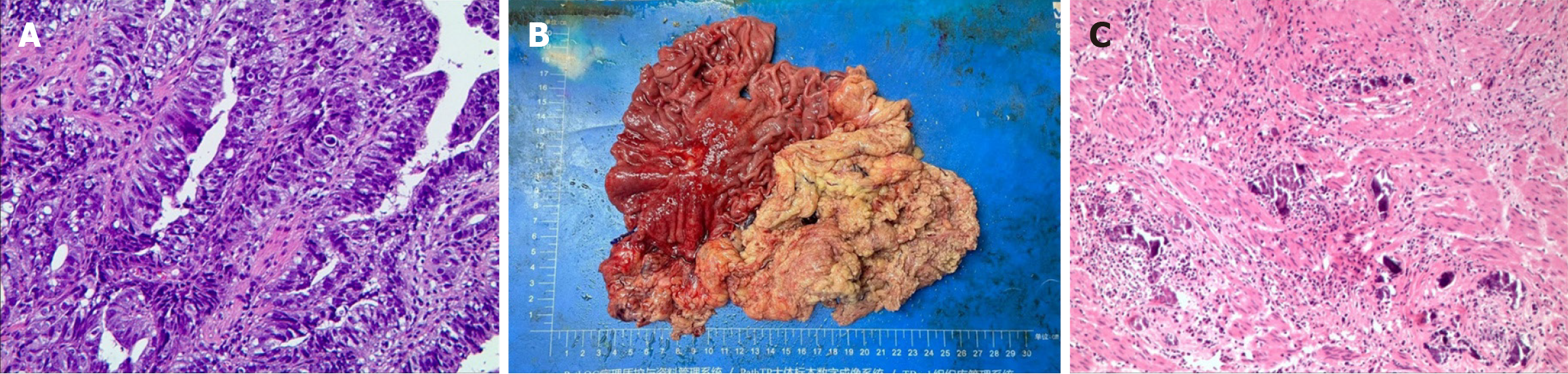
Figure 1 Hematoxylin-eosin staining and immunohistochemical evaluation of the obtained using a pathological biopsy.
A: The preoperative pathology image showed poorly differentiated adenocarcinoma of the gastric body; B: The specimen of the entire stomach, lymph node and tumor tissue removed during surgery; C: The postoperative pathology image showed no residual tumor cells.
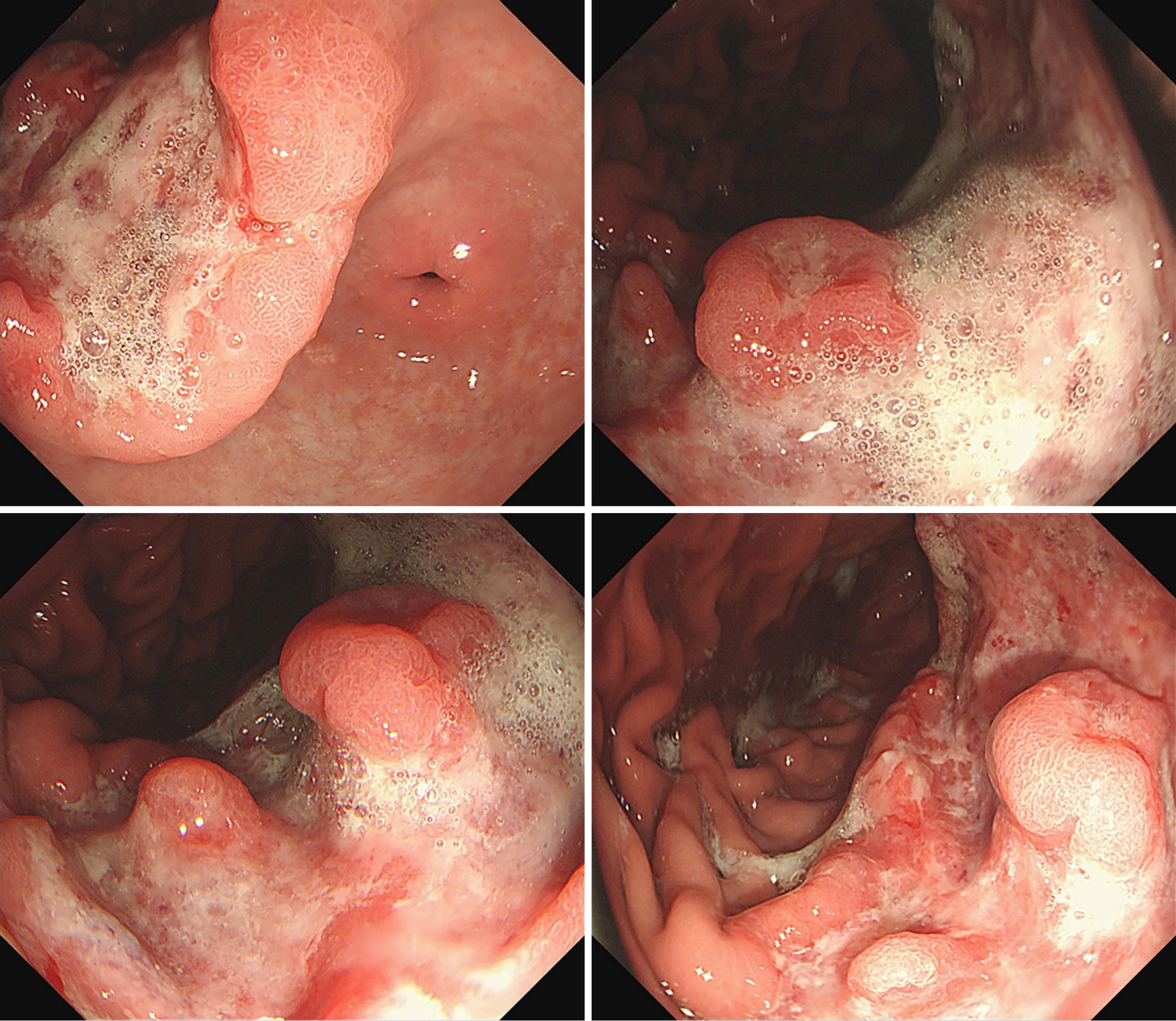
Figure 2 Endoscopic images.
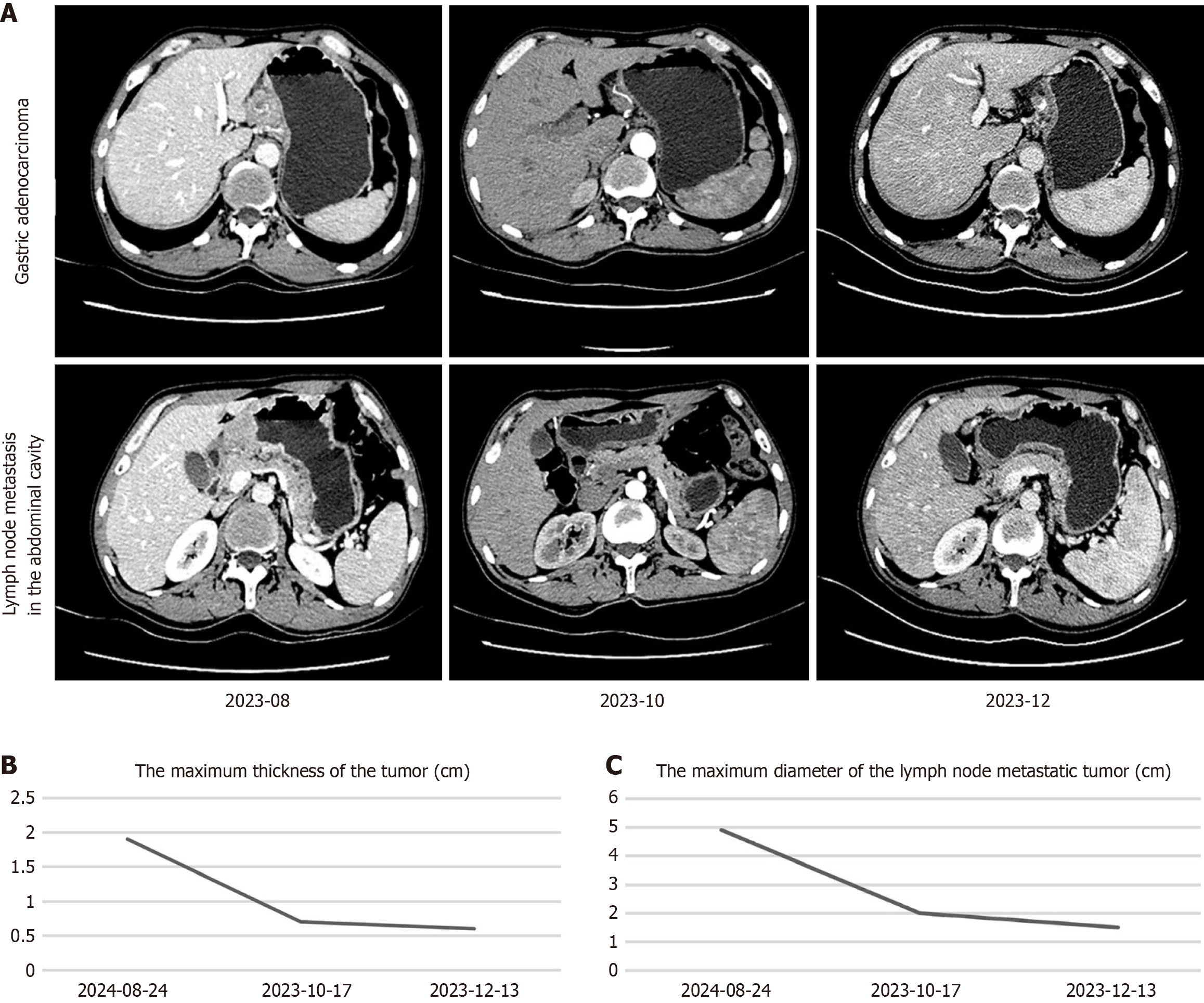
Figure 3 Enhanced abdominal computed tomography images for evaluating the treatment response.
A: Upper-row images show the changes in the size of the adenocarcinoma tumor tissue at the gastric body; Lower-row images show the changes in the size of the lymph node metastasis in the abdominal cavity; B: The maximum thickness of the tumor at the gastric body; C: The maximum diameter of the lymph node metastatic tumor.
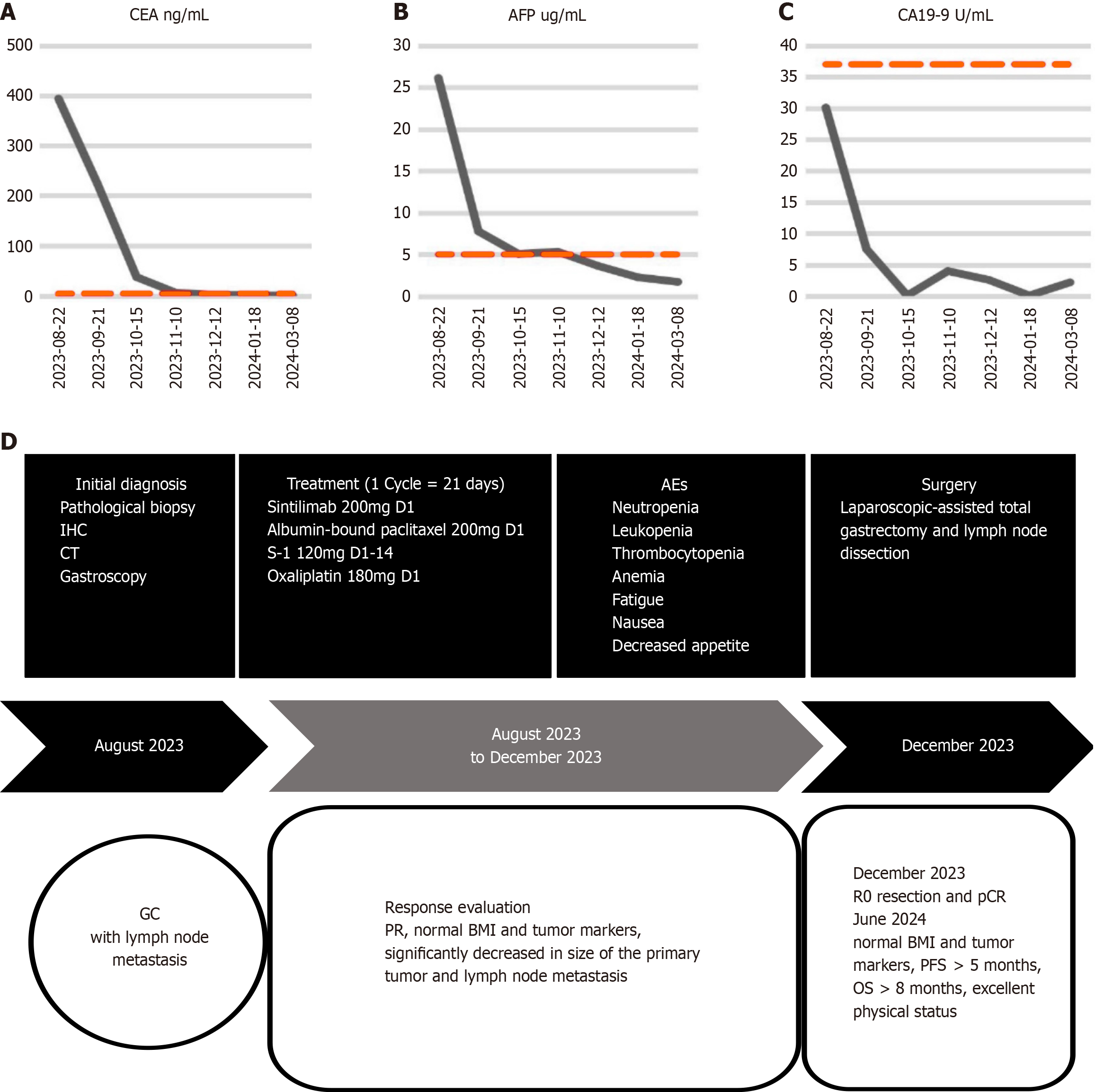
Figure 4 Dynamics of carcinoembryonic antigen, alpha-fetoprotein, and cancer antigen 19-9 and timeline of treatment options.
A-C: Diagram depicting the dynamic of carcinoembryonic antigen (A), alpha-fetoprotein (B) and cancer antigen 19-9 (C); D: The timeline of the treatment protocol. CEA: Carcinoembryonic antigen; AFP: Alpha-fetoprotein; CA19-9: Cancer antigen 19-9; IHC: Immunohistochemistry; CT: Computed tomography; AEs: Adverse events; GC: Gastric cancer; PR: Partial response; BMI: Body mass index; pCR: Pathological complete response; PFS: Progression-free survival; OS: Overall survival.
- Citation: Du XY, Xia RJ, Shen LW, Ma JG, Yao WQ, Xu W, Lin ZP, Ma LB, Niu GQ, Fan RF, Xu SM, Yan L. Quadruple therapy with immunotherapy and chemotherapy as first-line conversion treatment for unresectable advanced gastric adenocarcinoma: A case report. World J Gastrointest Oncol 2025; 17(4): 102258
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/102258.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.102258