Copyright
©The Author(s) 2025.
World J Gastrointest Oncol. Jan 15, 2025; 17(1): 97831
Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.97831
Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.97831
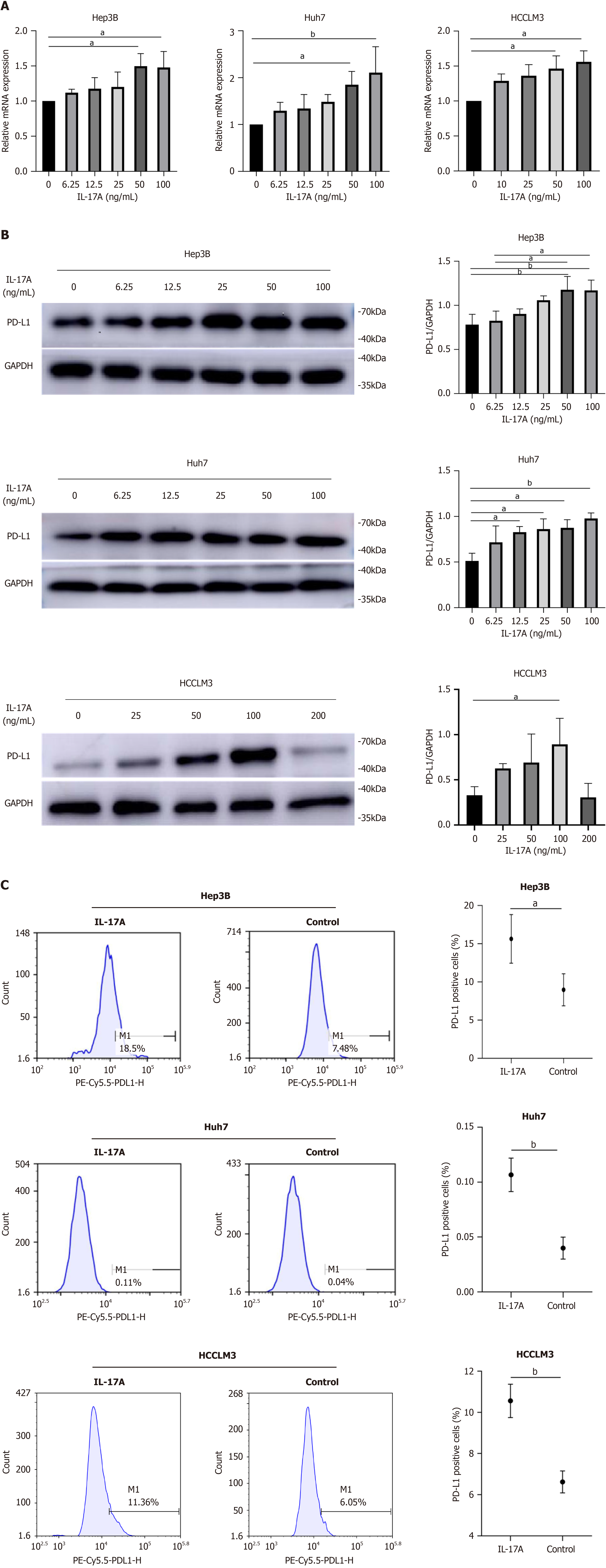
Figure 1 Interleukin-17A regulated programmed cell death ligand-1 expression at the RNA and protein levels in a dose-dependent manner.
A: Programmed cell death ligand-1 (PD-L1) mRNA levels were detected by reverse transcription PCR in hepatocellular carcinoma (HCC) cells after treatment with different concentrations of interleukin-17A (IL-17A) for 24 h; B: PD-L1 protein levels were determined by western blotting in HCC cells after treatment with different concentrations of IL-17A for 24 h; C: PD-L1 protein levels in HCC cells were tested by flow cytometry after treatment with 100 ng/mL IL-17A for 24 h. aP < 0.05; bP < 0.01. PE: Phycoerythrin.
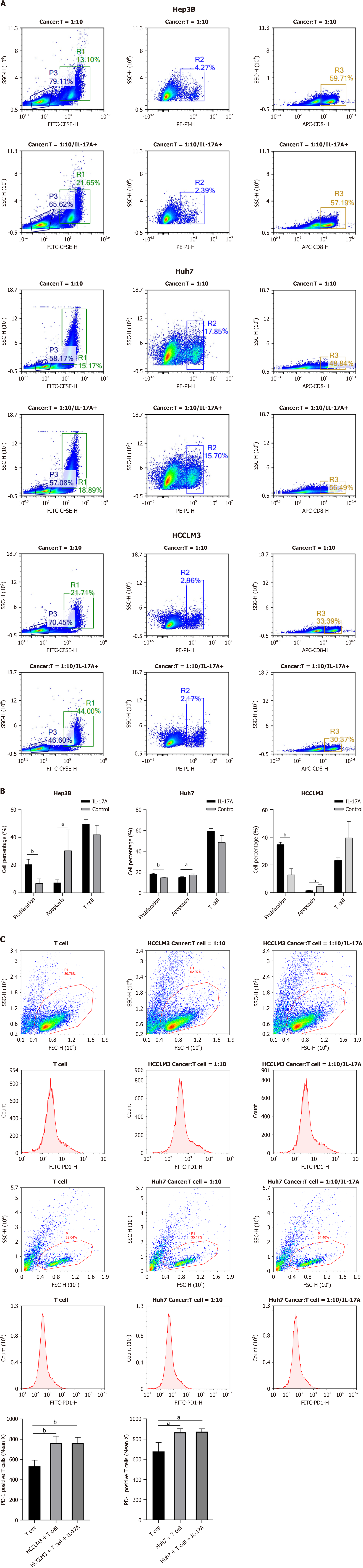
Figure 2 Interleukin-17A weakened the killing effect of T cells on hepatocellular carcinoma cells.
A: Hepatocellular carcinoma cell proliferation and apoptosis were tested by flow cytometry after coculture with or without interleukin-17A (IL-17A) for 24 h. The number of T cells was ten times greater than the number of tumor cells; B: Histogram showing the percentage of proliferating and apoptotic hepatocellular carcinoma cells and the percentage of T cells in the coculture model; C: The expression of programmed death-1 on T cells in coculture models. aP < 0.05; bP < 0.01; cP< 0.001. APC-CD8-H: Allophycocyanin-cluster of differentiation 8-height; FITC-CFSE-H: Fluorescein isothiocyanate-carboxy fluorescein diacetate succinimidyl ester-height; FITC-PD1-H: Fluorescein isothiocyanate-programmed cell death 1-height; FSC-H: Forward scatter-height; PE-PI-H: Phycoerythrin-propidium iodide-height; SSC-H: Side scatter-height.
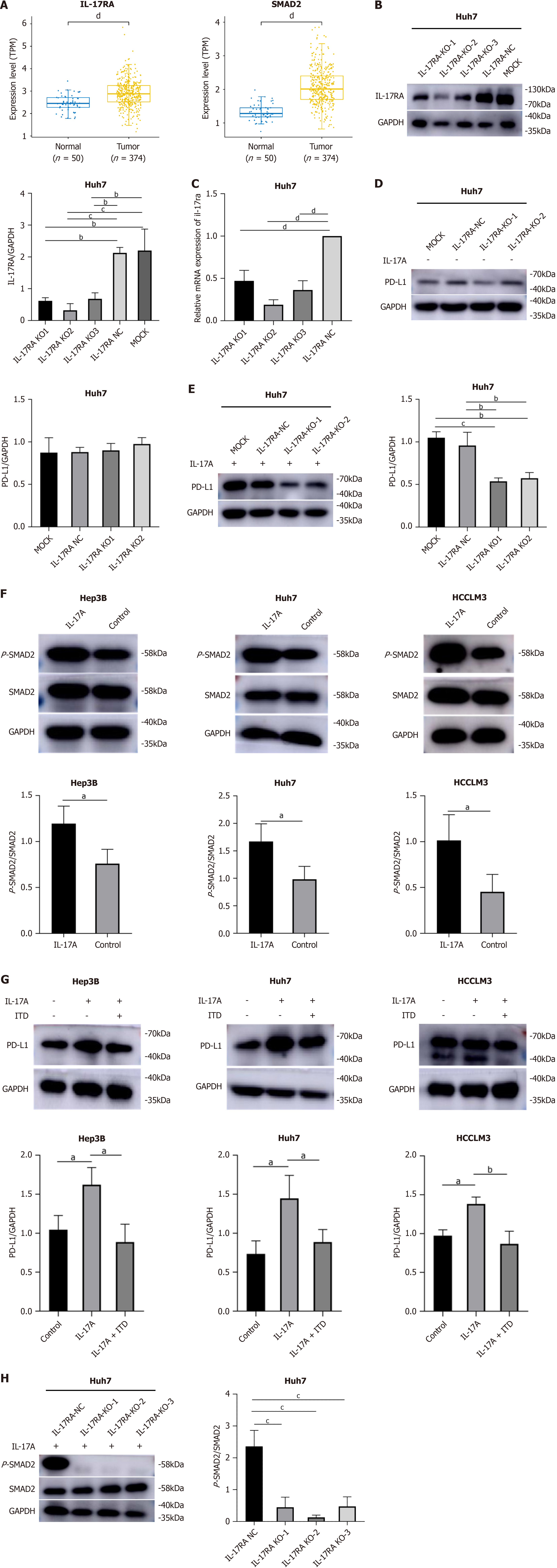
Figure 3 Interleukin-17A upregulated programmed cell death ligand-1 expression via the interleukin-17A receptor/p-small mothers against decapentaplegic 2 signaling pathway in hepatocellular carcinoma cells.
A: The Cancer Genome Atlas results revealed that interleukin-17A (IL-17A) and small mothers against decapentaplegic 2 (SMAD2) expression was increased in hepatocellular carcinoma (HCC) samples compared with normal tissue samples; B: Interleukin-17A receptor (IL-17RA) protein expression level after knockout (KO) of IL-17RA in Huh7 cells; C: IL-17RA mRNA levels after KO of IL-17RA in Huh7 cells; D: IL-17RA KO alone did not affect programmed cell death ligand-1 (PD-L1) expression in Huh7 cells; E: IL-17A-induced PD-L1 expression was reversed by IL-17RA KO in Huh7 cells; F: IL-17A increased the level of phosphorylated (p)-SMAD2 in HCC cells; G: IL-17A-induced PD-L1 expression was inhibited by pretreatment with transforming growth factor-beta signal pathway inhibitor in HCC cells; H: IL-17RA KO sharply reversed IL-17A-induced p-SMAD2 expression in Huh7 cells. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. ITD: TGF-beta signal pathway inhibitor; MOCK: Blank control; NC: Negative control.
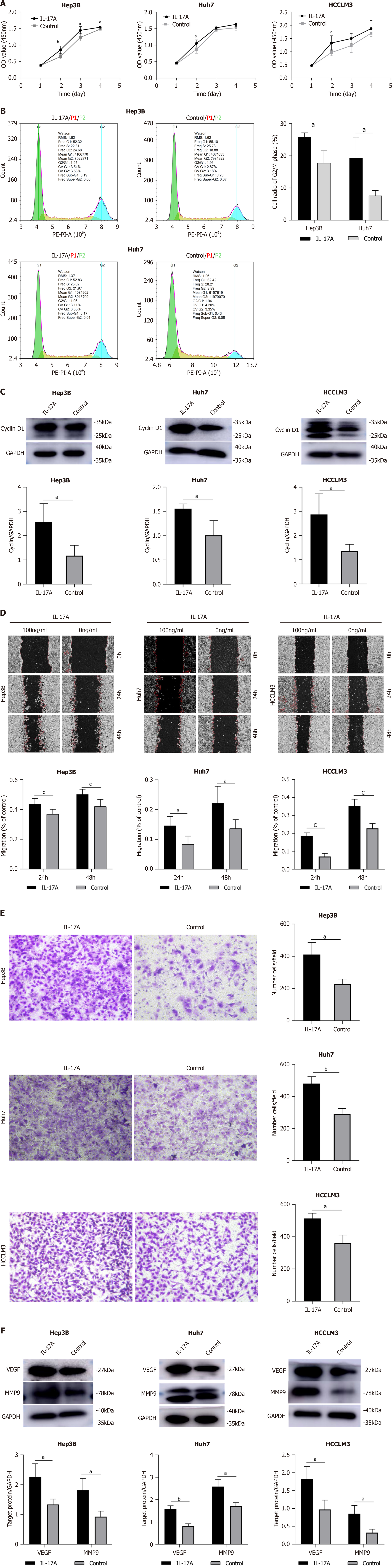
Figure 4 Interleukin-17A promoted hepatocellular carcinoma cell proliferation, migration, and angiogenesis.
A: Growth curve showed that interleukin-17A (IL-17A) stimulated cell proliferation, a significant difference that was detected at 24 h after treatment; B: Flow cytometry revealed that, compared with the control, IL-17A significantly increased the proportion of cells in G2/M phase; C: IL-17A elevated cyclinD1 expression in hepatocellular carcinoma (HCC) cells; D and E: Scratch tests and Transwell assays revealed that IL-17A promoted the migration of HCC cells (× 100 magnification); F: IL-17A increased the protein expression of VEGF and MMP9 in HCC cells. aP < 0.05; bP < 0.01; cP < 0.001. PE: Phycoerythrin; PI: Propidium iodide.
Figure 5 Interleukin-17A inhibited hepatocellular carcinoma cell apoptosis.
A: Flow cytometry analysis revealed that interleukin-17A (IL-17A) suppressed hepatocellular carcinoma (HCC) cell apoptosis; B: IL-17A decreased the expression of BAX and increased the expression of Bcl-2 in HCC cells. aP < 0.05; bP < 0.01. FITC-A: Fluorescein isothiocyanate-area; PE-A: Phycoerythrin-area.
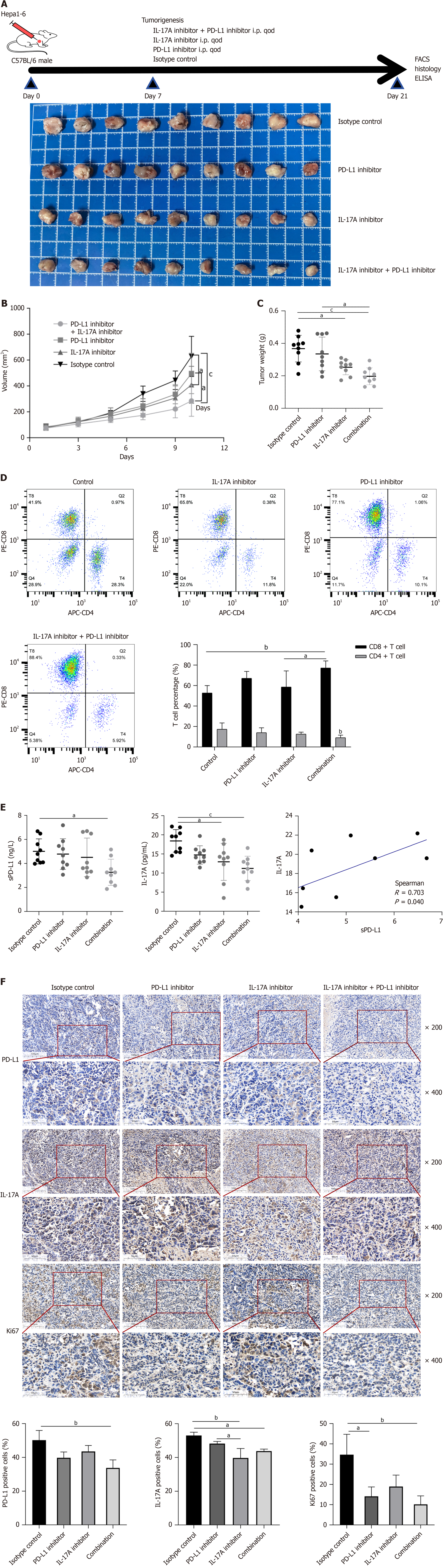
Figure 6 An interleukin-17A inhibitor suppressed tumor growth and enhanced the therapeutic efficacy of an anti-programmed cell death ligand-1 antibody.
A: Flow diagram of the construction of the mouse model and treatment scheme for programmed cell death ligand-1 (PD-L1) inhibitors and/or interleukin-17A (IL-17A) inhibitors in mice. Images of the tumors in different treatment groups (the control group, PD-L1 inhibitor group, IL-17A inhibitor group, and combined group) are shown. Each group contained nine mice; B: Tumor growth curves of the mice after PD-L1 inhibitor and/or IL-17A inhibitor treatment. A PD-L1 inhibitor combined with an IL-17A inhibitor demonstrated a much greater antitumor effect on the hepatocellular carcinoma mouse model; C: Tumor weights of mice in the four groups after treatment. The tumor weights of mice in the combined group were significantly lower than those of mice in the control group; D: Flow cytometry analysis of the differences in the infiltration of cluster of differentiation 8+ T cells and cluster of differentiation 4+ T cells in tumor tissues among the four groups; E: The levels of serum soluble (s)PD-L1 and IL-17A were compared among the four groups. The sPD-L1 and IL-17A levels were lower in the combined group than in the control group. The sPD-L1 level was positively correlated with the serum IL-17A level in the control group; F: Immunohistochemical staining revealed the expression of PD-L1, IL-17A, and Ki67 in tumor tissues. Scale bars, 100 μm (× 200) and 50 μm (× 400). The number of positive cells was determined using ImageJ2x software. Boxplots displaying the expression of PD-L1, IL-17A, and Ki67 in the four groups. aP < 0.05; bP < 0.01; cP < 0.001. APC-CD4: Allophycocyanin-cluster of differentiation 4; FACS: Fluorescence-activated cell sorting; PE-CD8: Phycoerythrin-cluster of differentiation 8.
- Citation: Yang ZX, Zhang LT, Liu XJ, Peng XB, Mao XR. Interleukin-17A facilitates tumor progression via upregulating programmed death ligand-1 expression in hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(1): 97831
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/97831.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.97831