Copyright
©The Author(s) 2024.
World J Gastrointest Oncol. Jun 15, 2024; 16(6): 2439-2448
Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2439
Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2439
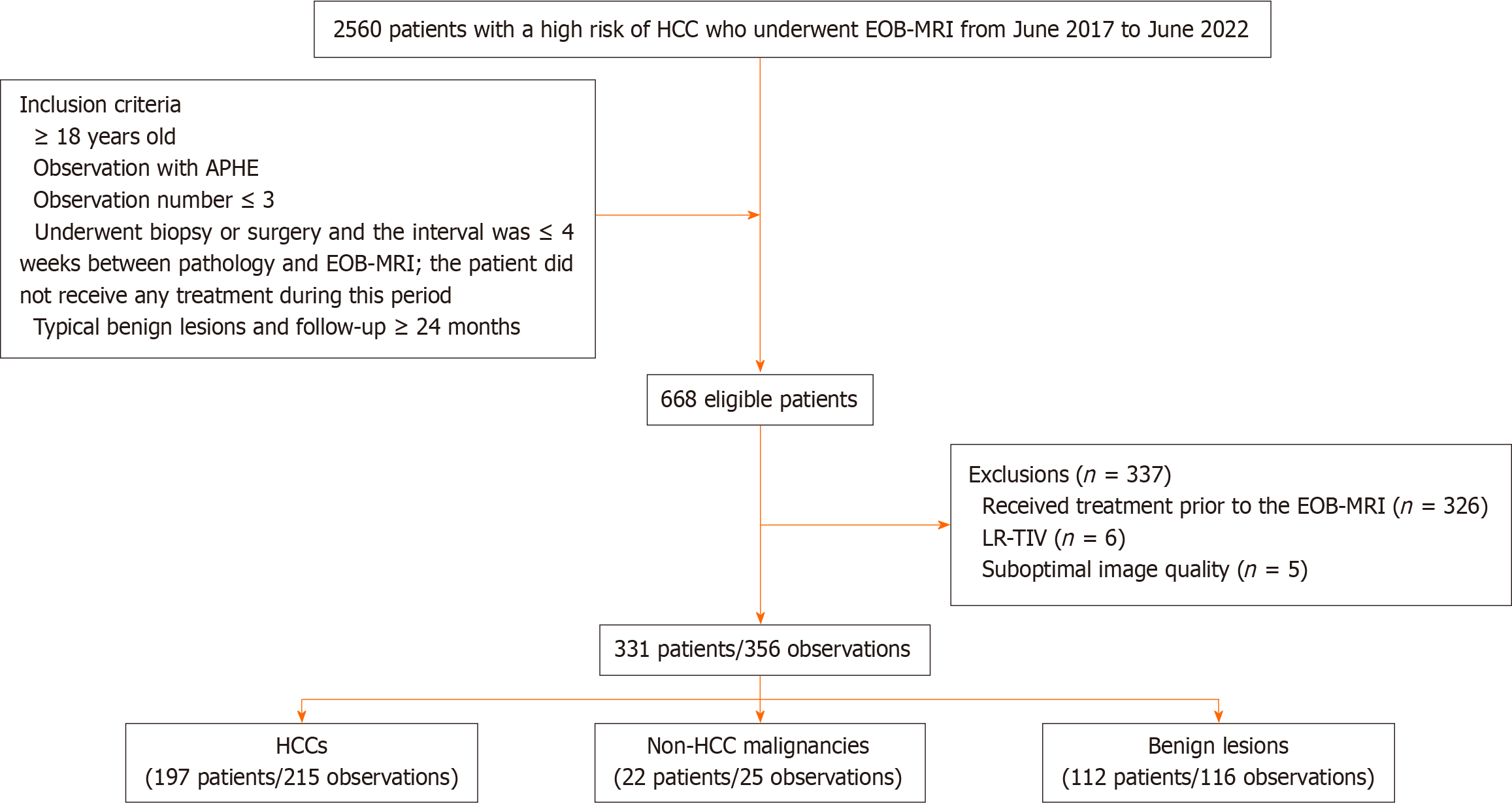
Figure 1 Flow chart of patient and observation selection.
HCC: Hepatocellular carcinoma; EOB-MRI: Gadoxetic acid-enhanced magnetic resonance imaging; APHE: Arterial phase hyperenhancement; LR (LI-RADS): Liver imaging reporting and data system; TIV: Tumour-in-vein.
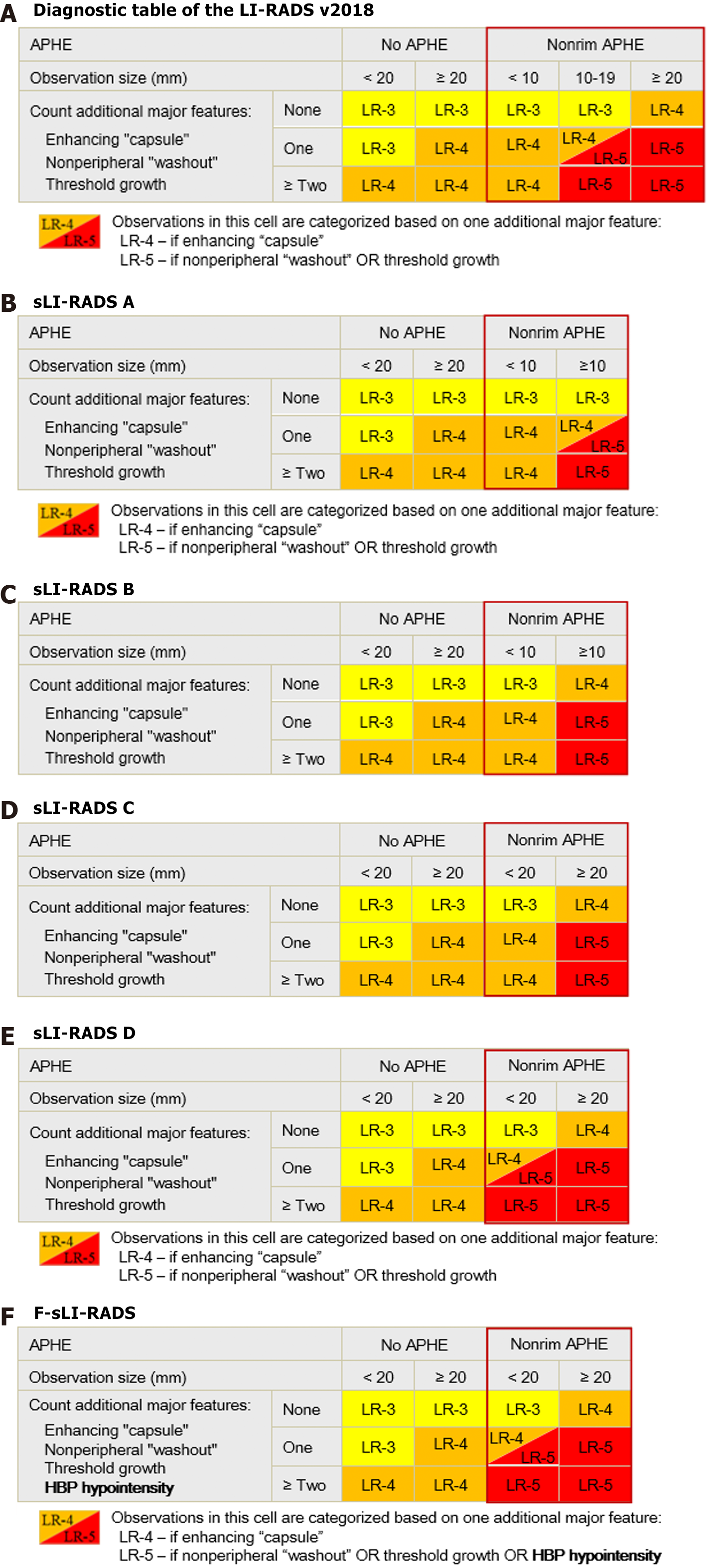
Figure 2 Illustration of the liver imaging reporting and data system diagnostic table.
A: The first diagnostic table shows the liver imaging reporting and data system (LI-RADS) version 2018; B-E: Show the simplified LI-RADS (sLI-RADS) A-D; F: Shows the final sLI-RADS. HBP: Hepatobiliary phase. APHE: Arterial phase hyperenhancement; F-sLI-RADS: Final simplified liver imaging reporting and data system; sLI-RADS: Simplified liver imaging reporting and data system; LI-RADS: Liver imaging reporting and data system; LR: LI-RADS.
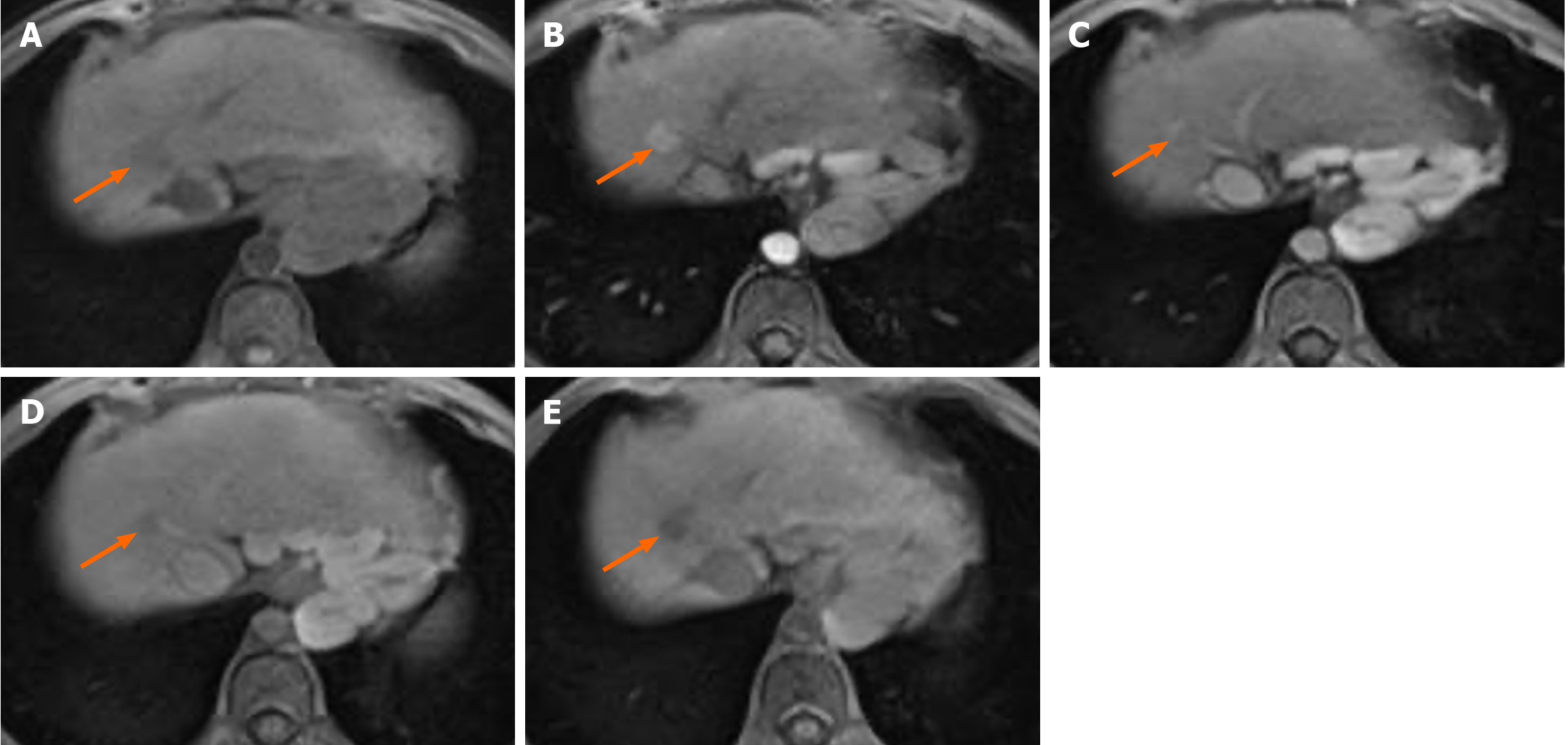
Figure 3 Gadoxetic acid-enhanced magnetic resonance image of a 67-year-old female patient with hepatocellular carcinoma confirmed by biopsy.
A: T1WI showing a 12 mm hypointense nodule (arrow) in hepatic segment VII; B: The nodule (arrow) exhibits arterial phase hyperenhancement; C: It has an enhanced “capsule” and no washout appearance on the portal vein phase image; D and E: In the transitional phase (D) and hepatobiliary phase (HBP) (E), the nodule (arrow) exhibited hypointensity. This nodule was assessed with liver imaging reporting and data system-4 (LR-4) according to whether liver imaging reporting and data system (LI-RADS) version 2018 or simplified LI-RADS (sLI-RADS) D was adopted. When HBP hypointensity was used as a major feature and according to sLI-RADS D, the nodule was recategorized as LR-5.
- Citation: Lyu R, Hu WJ, Wang D, Wang J, Ye YB, Jia KF. Simplified liver imaging reporting and data system for the diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced magnetic resonance imaging. World J Gastrointest Oncol 2024; 16(6): 2439-2448
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2439.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2439