Copyright
©The Author(s) 2024.
World J Gastrointest Oncol. May 15, 2024; 16(5): 2253-2260
Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2253
Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2253
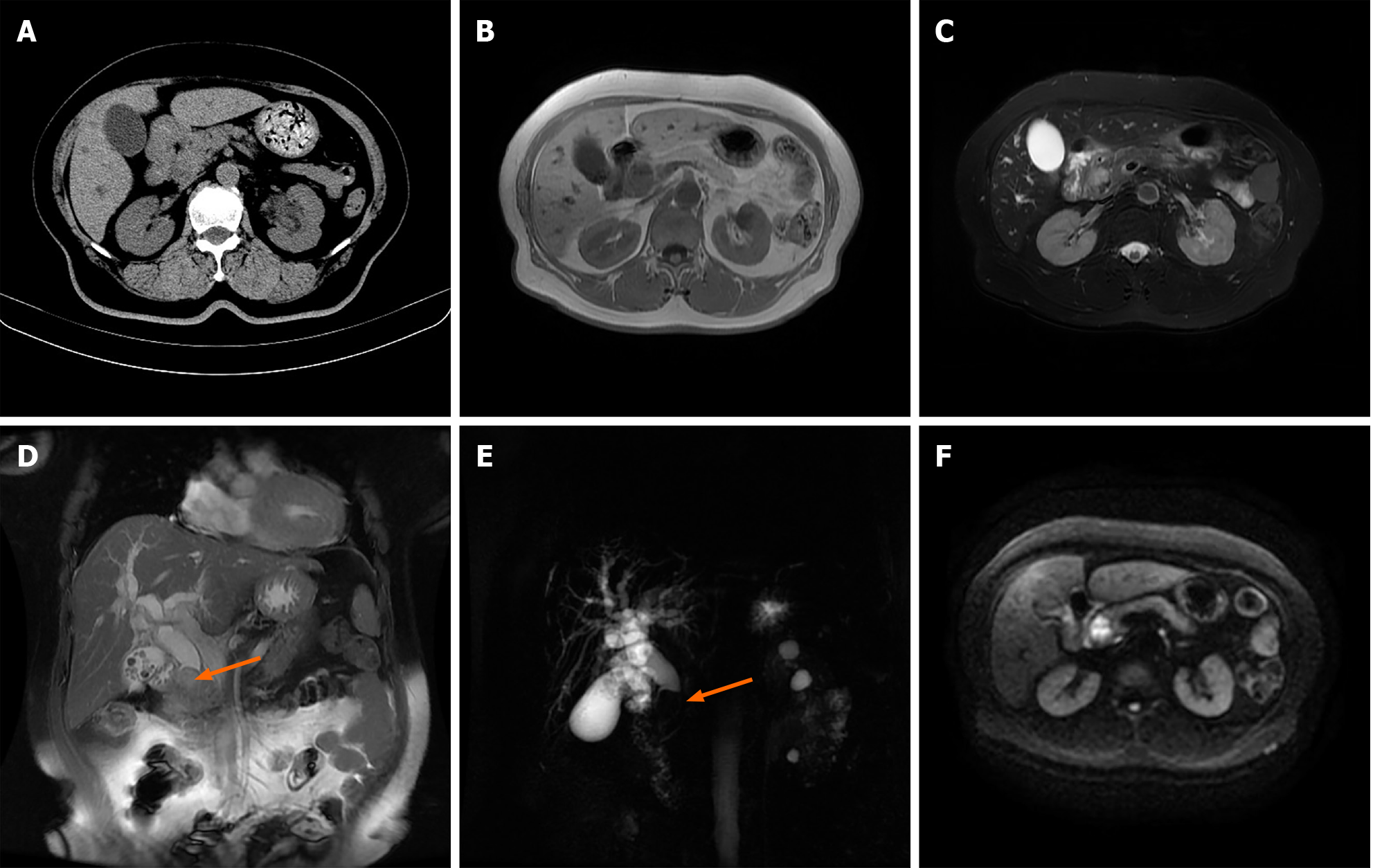
Figure 1 Imaging revealed calculous cholecystitis, intrahepatic and extrahepatic bile duct dilation with extrahepatic bile duct calculi, and a space-occupying lesion of the distal common bile duct.
A: Computed tomography showed calculous cholecystitis, and a soft tissue density shadow at the distal common bile duct (CBD) with intrahepatic and extrahepatic bile duct dilatation; B-F: Magnetic resonance imaging by the (B) T1-weighted image, (C) T2-weighted image, (D) coronal plane sequence, (E) magnetic resonance cholangiopancreatography and (F) diffusion-weighted imaging revealed calculous cholecystitis, intrahepatic and extrahepatic bile duct dilation with extrahepatic bile duct calculi, and a soft tissue mass signal at the distal CBD with limited diffusion.
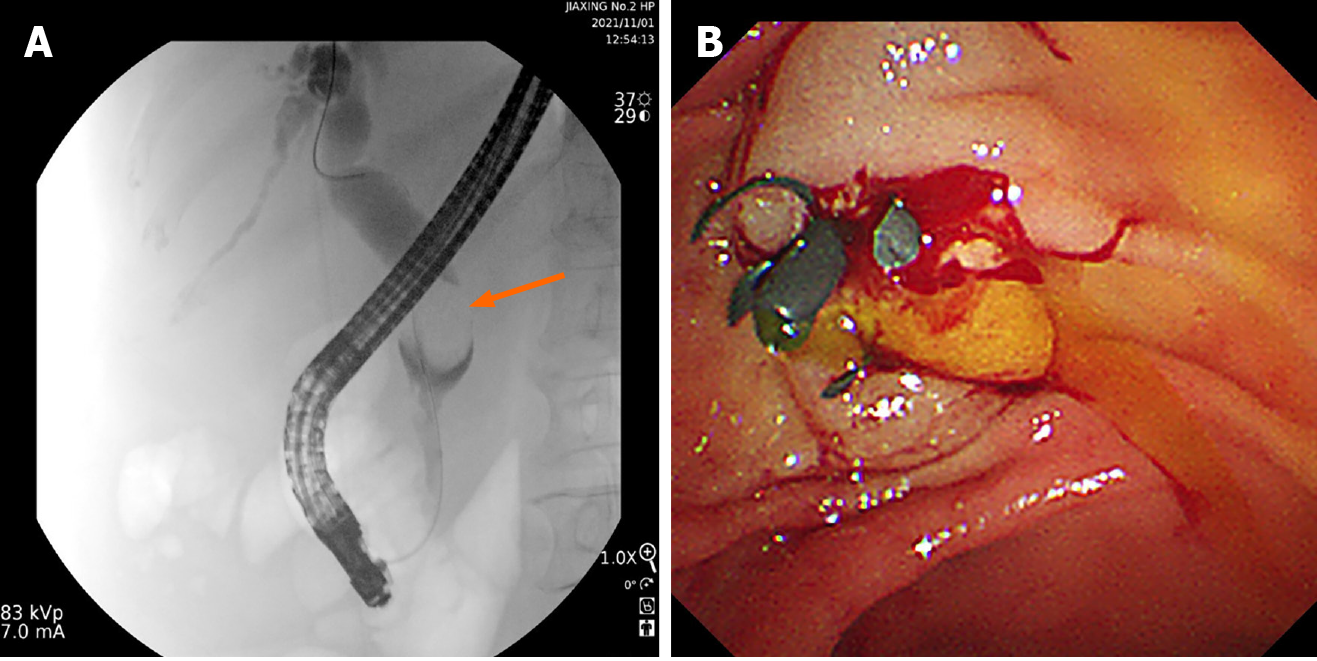
Figure 2 Endoscopic retrograde cholangiopancreatography was recommended and a biliary plastic stent was implanted for alleviating jaundice.
A: Endoscopic retrograde cholangiopancreatography revealed a space-occupying lesion of the distal common bile duct; B: Endoscopic biliary plastic stent implantation.
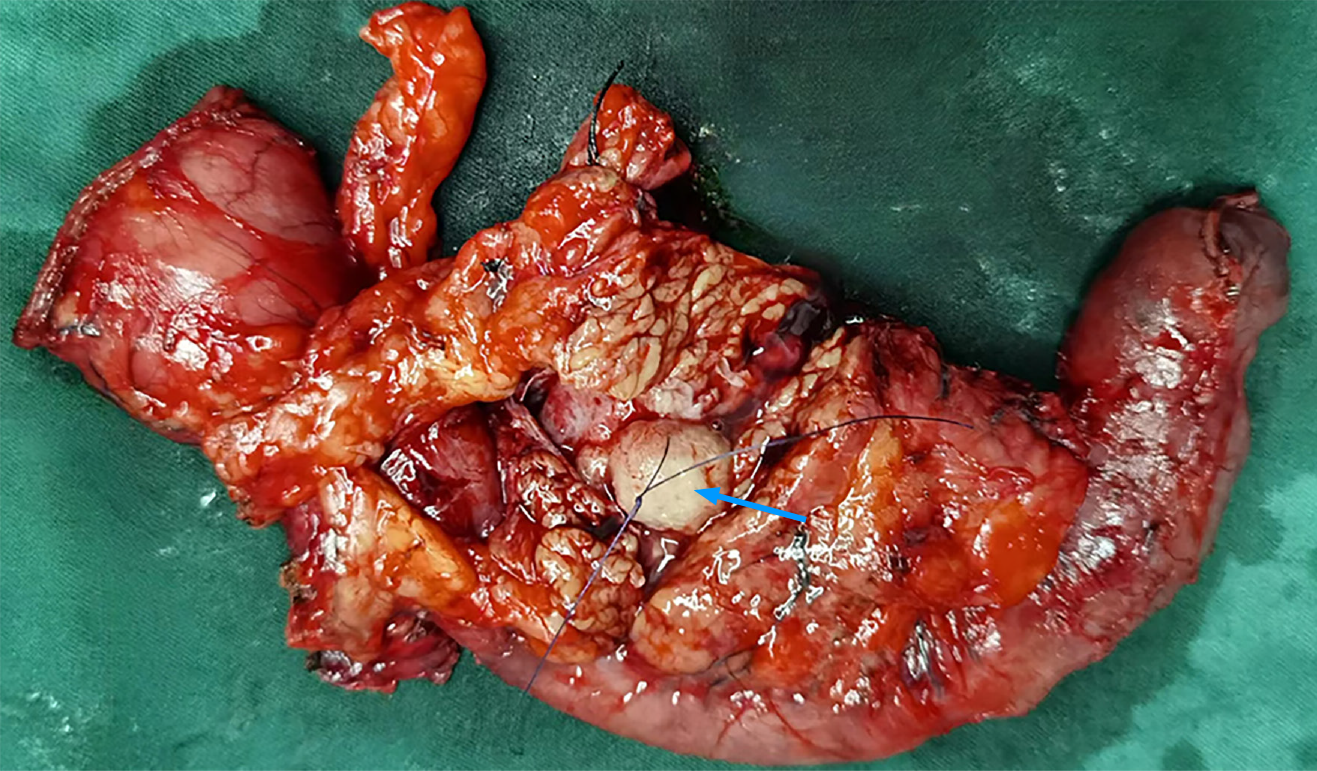
Figure 3 In situ, the neoplasm was located in the distal common bile duct, measuring approximately 2.
2 cm × 1.8 cm in size, and exhibited clear boundaries.
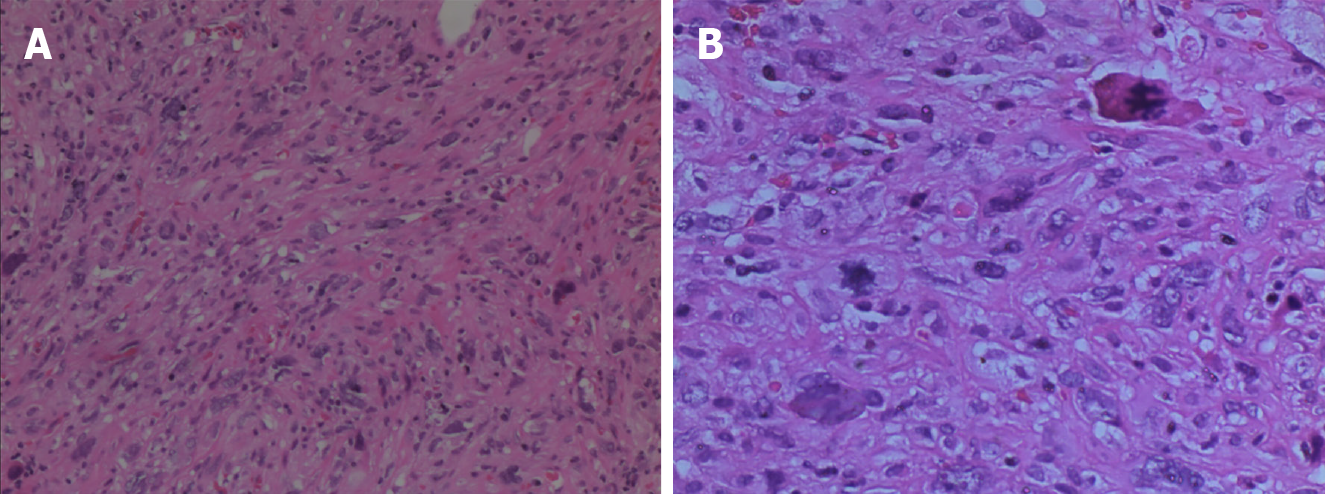
Figure 4 Pathological examination findings.
The tumor cells were spindle-shaped or pleomorphic, arranged in sarciniform structure, with deeply stained nuclei, singular nuclei and nuclear division, abundant cytoplasm, and scattered inflammatory cells infiltration. A: Hematoxylin & eosin (H&E) staining, magnification: 100 ×; B: H&E staining, magnification: 200 ×.
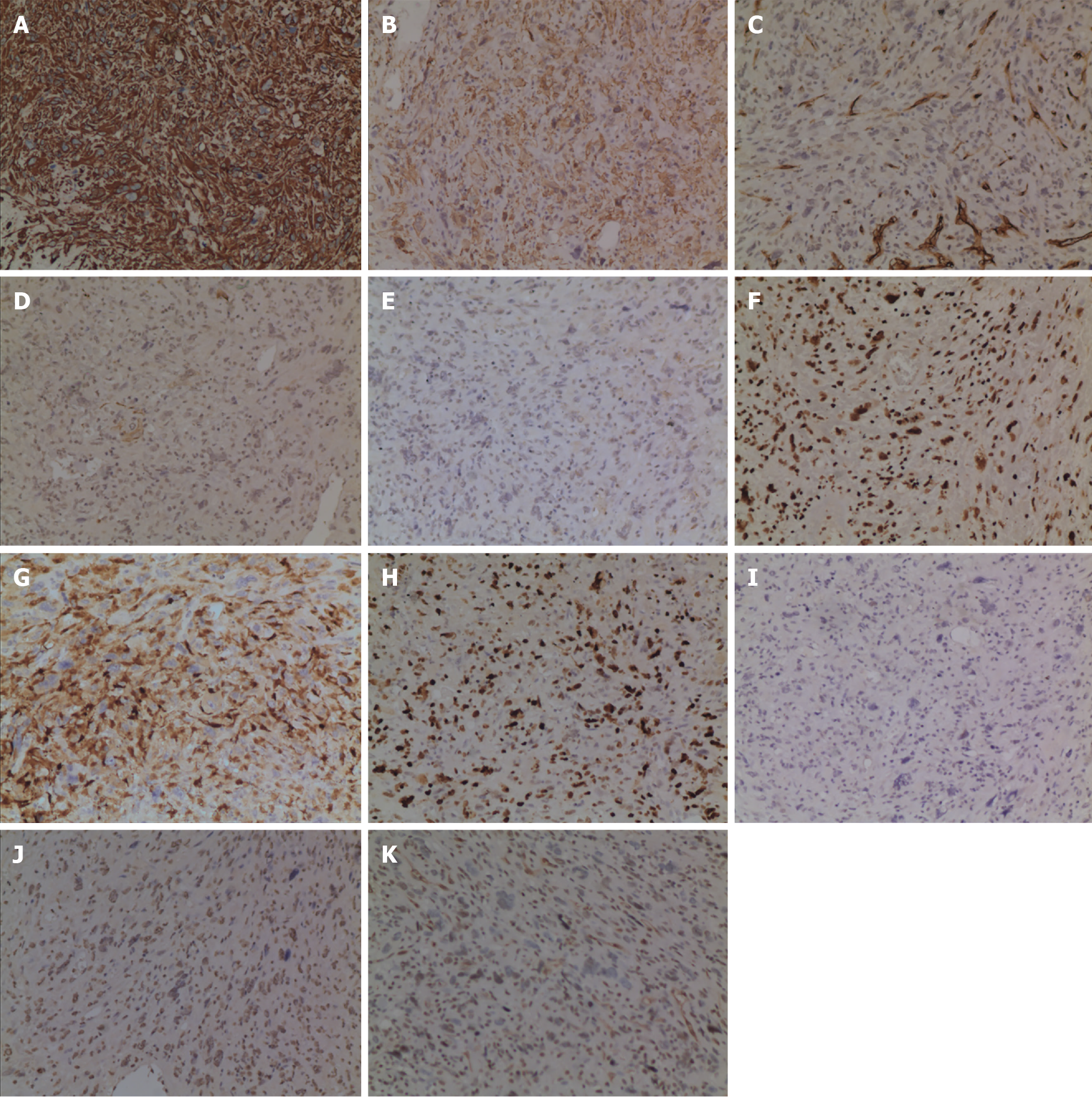
Figure 5 Immunohistochemical examination findings.
A: The tissue was diffusively positive for Vimentin; B: The tissue was partially positive for SMA; C: The tissue was partially positive for CD34; D: The tissue was scatteredly positive for CK (AE1/AE3); E: The tissue was scatteredly positive for EMA; F: The tissue was partially positive for H3K27Me3; G: The tissue was partially positive for S-100; H: Ki-67 was approximately 60%; I: The tissue was negative for CK7; J: The tissue was negative for Desmin; K: The tissue was negative for CD117.
- Citation: Zheng LP, Shen WY, Hu CD, Wang CH, Chen XJ, Wang J, Shen YY. Undifferentiated high-grade pleomorphic sarcoma of the common bile duct: A case report and review of literature. World J Gastrointest Oncol 2024; 16(5): 2253-2260
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2253.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2253